Abstract
Objectives
To assess the prevalence and changes over time of ideal Life’s Simple Seven (LSS) in African-Americans.
Methods
Prospective cohort of 5301 African-Americans from the Jackson Heart Study (JHS) from 2000 to 2013. Each of the LSS metrics was categorized as poor, intermediate, or ideal.
Results
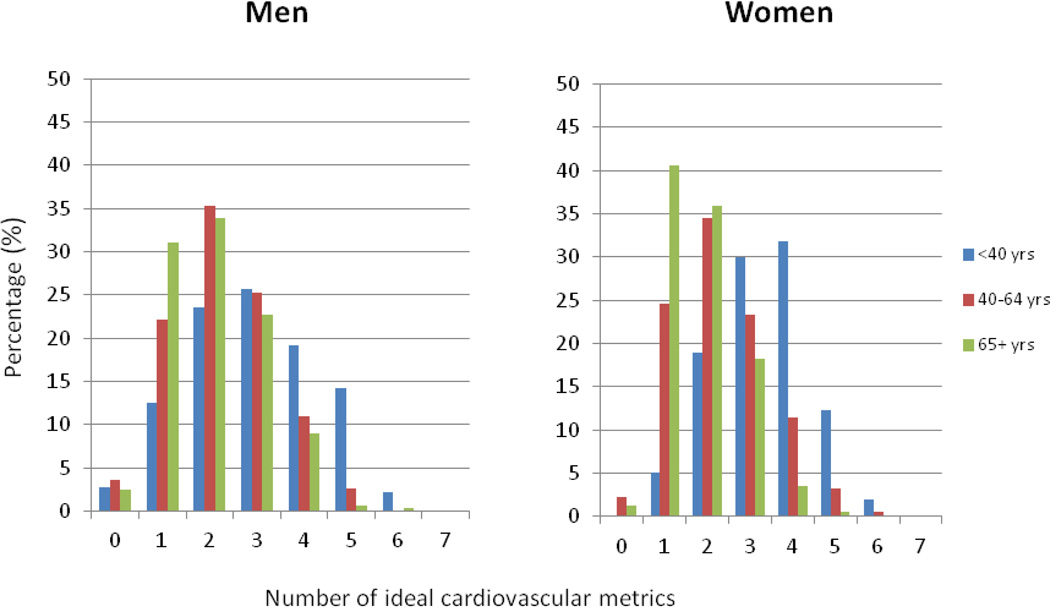
Among men, the prevalence of having 0, 1, 2, 3, 4, 5, 6, and 7 ideal LSS was 3.3%, 23.0%, 33.5%, 24.7%, 11.6%, 3.6%, 0.3%, and 0%, respectively. Corresponding values for women were 1.7%, 26.3%, 33.1%, 22.8%, 11.9%, 3.7%, 0.6%, and 0%. Prevalence of ideal diet was 0.9%. The proportions of those meeting LSS ideal recommendations for cholesterol and fasting glucose declined from the first through third JHS visits across all age groups, whereas prevalence of ideal BMI declined only in participants <40 years at a given visit. Prevalence of ideal blood pressure did not change over time and being ideal on physical activity improved from the first [18.3% (95% CI: 17.3% to 19.3%)] to third visit [24.8% (95% CI: 23.3% to 26.3%)].
Conclusions
Our data show a low prevalence of ideal LSS (especially diet, physical activity, and obesity) in the JHS and a slight improvement in adherence to physical activity recommendations over time.
Keywords: Epidemiology, cardiovascular disease, risk factors, diet
Despite the identification of major cardiovascular risk factors in the early 1960s1 and advances in biomedical research, cardiovascular disease (CVD) remains the leading cause of death in the US2. People who maintain ideal levels of physical activity, diet, adiposity, blood pressure, lipids, etc, have fewer adverse health outcomes (i.e., CVD)3–5. Hence, the American Heart Association (AHA)’s goal is to improve the cardiovascular health of all Americans by 20% by year 2020 while reducing deaths from cardiovascular diseases by 20%6. To monitor such goals, AHA developed a simple metric based on 4 health [adiposity, total cholesterol, blood pressure (BP) and fasting plasma glucose (FPG)] and 3 behavioral (smoking, exercise and diet) factors, subsequently referred to as life’s simple seven (LSS). These 7 factors are then used to define the concept of poor, intermediate, and ideal cardiovascular health6. Recent data from a US representative sample showed that fewer than 1% of adult Americans met all 7 metrics, with ideal healthy diet met by the fewest7. Another report showed variations in the prevalence of meeting ideal LSS, with age-standardized prevalences varying from 1.2% to 6.6% across the 50 US states8. Of note is that Mississippi – home state of the JHS – was among the states with the lowest age-standardized prevalence of ideal LSS.
There is a disproportionate burden of CVD in the African-American population, with higher prevalences of major risk factors including hypertension, overweight/obesity, type 2 diabetes, and physical inactivity, compared with other ethnic groups2,9. While one publication from the National Health and Nutrition Examination Survey (NHANES)2 reported comparable overall prevalences across ethnic groups, detailed data on the prevalence of poor, intermediate, and ideal dietary components among African-Americans have not been reported. In addition, there are no data on changes over time in LSS in a large cohort of African-Americans. Such data are critical in monitoring milestones towards achieving the AHA 2020 goals and reduce the burden of chronic diseases in a high-risk population. The JHS is unique in its ability to address the above gaps among middle-aged and elderly African Americans, given the availability of data on all seven health and behavioral factors and repeated measurements on most of those factors (BMI, cholesterol, FPG, BP, and physical activity) during its initial three clinic visits. Hence, the current project examines the prevalence and changes over time of LSS in a large cohort of African-Americans.
Methods
Population
The JHS is a prospective cohort study designed to investigate determinants of CVD among African-Americans living in the tri-county area (Hinds, Madison, and Rankin counties) of the Jackson, Mississippi metropolitan area. Detailed descriptions of the JHS have been previously published10,11. Of the 5,301 JHS participants who completed the baseline clinic visit (2000–2004), we excluded 1169 subjects who had missing data on one or more LSS. This resulted in a final sample of 4,132 individuals with complete data at the baseline JHS visit. The JHS visit two was conducted between 2005 and 2009, and visit three was completed from 2009 to 2013. Each participant gave written informed consent, and the study protocol was approved by the institutional review boards of each of the participating institutions.
Assessment of LSS
A definition of LSS (poor, intermediate, and ideal) based on AHA guidelines is provided in supplemental Table 1.
Table 1.
Baseline Characteristics of African Americans adults in the Jackson Heart Study*
| Men | Women | Total | |
|---|---|---|---|
| Characteristics | N=1934 | N=3367 | N=5301 |
| Age (y) | 54.6 ± 13.0 | 55.8 ± 12.8 | 55.4 ± 12.8 |
| Body mass index (kg/m2) | 29.9 ± 6.1 | 32.8 ± 7.6 | 31.8 ± 7.2 |
| Waist circumference (cm) | 101 ± 15 | 100 ± 17 | 101 ± 16 |
| Education (%) | |||
| Less than high school | 21.0 | 19.9 | 20.3 |
| High school graduate/GED | 17.7 | 18.8 | 18.4 |
| >High School but < Bachelor’s degree | 29.1 | 28.4 | 28.6 |
| Bachelor degree or higher | 31.9 | 32.6 | 32.3 |
| Current alcohol use (%) | 58.7 | 38.1 | 45.6 |
| Current smoking (%) | 18.1 | 10.2 | 13.1 |
| Comorbidity | |||
| Prevalent coronary heart disease (%) | 9.5 | 6.4 | 7.6 |
| Prevalent type 2 diabetes (%) | 20.2 | 22.6 | 21.7 |
| Prevalent hypertension (%) | 58.4 | 63.1 | 61.4 |
| Occupation (%) | |||
| Production | 23.6 | 9.8 | 14.8 |
| Service | 16.0 | 30.5 | 25.2 |
| Management/professional | 30.9 | 38.1 | 35.4 |
| Have health insurance (%) | 86.0 | 86.3 | 86.2 |
Few participants had missing data: BMI (n=9), waist circumference (n=9), education (n=20), current alcohol use (n=30), smoking (n=48), prevalent diabetes (n=61), hypertension (n=6), occupation (n=6), health insurance (n=25)
Body mass index (BMI)
Standing height was measured without shoes and recorded to the nearest centimeter. Weight was measured at baseline on a scale. BMI was computed by dividing weight (kg) by height squared (m2). Briefly, ideal, intermediate, and poor BMI were defined as BMI <25; 25 to 29.9; and ≥ 30 kg/m2, respectively.
Physical activity
At baseline, participants completed an interviewer-administered physical activity survey. The instrument used was similar to the Kaiser physical activity survey, and derived from the Baeke physical activity and Atherosclerosis Risk in Communities (ARIC) surveys12,13. Reported activity was organized into four domains: sports and exercise; active living; occupational activity; and home, family, yard and garden activity. To maintain comparability with Bell et al.14, who used ARIC physical activity survey data, only activity compiled by the sport and exercise component of the instrument was used in the current analysis. Sport and exercise was reported by named activity and the average amount of time per week spent at that activity. Metabolic equivalent (MET) levels for each named activity were taken from the most current version of the national Compendium of Physical Activity15. Activities identified as either vigorous (>6 METs) or moderate (3–6 METS)16 contributed to the participant’s physical activity score for the purpose of this analysis. The average time per week spent engaged in all activities at either a vigorous or moderate level was tallied for each participant. Each participant was then scored as having one of the three AHA recommended levels of physical activity6: 1) Recommended : ≥150 min/wk of moderate activity or ≥ 75 min/wk of vigorous activity or ≥150 min / wk of moderate + vigorous activity; Intermediate: 1–149 min/wk of moderate activity or 1–74 min/wk of vigorous activity or 1–149 min/wk of moderate + vigorous activity; Poor: 0 min/wk of physical activity.
Dietary assessment
Dietary intakes in the JHS were assessed using a regional and culturally appropriate, 158 food item, semi quantitative food frequency questionnaire (FFQ) that was designed specifically for the study population17. The FFQ has been validated in a subset of the JHS cohort using multiple 24-hr recalls and nutrient biomarkers18,19. We had FFQ information for 5065 of the 5301 JHS participants at baseline. We excluded 304 participants with extreme energy intakes (defined as ≤ 600 kcal/d or ≥ 4800 kcal/d) and included 4761 participants for the computation of diet score based on AHA guidelines. Individuals were given one point for each of 5 dietary goals. These included: 1) At least 4.5 cups/day of fruit and vegetables (fruit included whole fruit and 100% fruit juice; vegetables included orange and green leafy vegetables, root and starchy vegetables (including sweet potatoes and potatoes), tomatoes, and other vegetables such as peppers and onions—however, fried preparations including French fried potatoes and fried onion rings were excluded); 2) at least two 3.5 oz servings/week of fish (shellfish and any fried or fast food preparations such as fried catfish and fish sandwiches were excluded); 3) at least three 1oz servings/day of fiber rich whole grains (estimated by identifying all foods on the FFQ which contained grains, and calculating the total exposure to whole grains); 4) no more than 36 fluid oz/week of sugar-sweetened beverages (including non-diet soda, fruit drinks sweet tea and sweetened coffee); 5) less than 1500 mg of sodium/day [from direct nutrient analysis of the FFQ, using the Nutrition Data System for Research, NDS-R, version 4.04, 2001 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN)].
Smoking
At baseline, participants were asked if they had smoked at least 400 cigarettes in their life, if they currently smoked cigarettes, and the number of years since they last smoked, if participants indicated that they no longer smoked cigarettes. Participants were then classified as a current, former, or never smoker. Former smokers were further divided into those who had quit smoking less than 12 months or ≥ 12 months prior to the interview. Ideal smoking was defined as never smokers or former smoker who had quit ≥ 12 months ago. Intermediate smoking was defined as former smokers who had quit within the past year, and poor smoking was defined as current smokers.
Assessment of blood pressure (BP), fasting plasma glucose (FPG), and cholesterol
BP was calculated the average of two sitting BP measures using an appropriate cuff size20. FPG was measured by glucose oxidase colorimetric method using a Vitros 950 or 250, Ortho-Clinical Diagnostics analyzer at baseline and by Roche Modular P Chemistry analyzer at clinic visits 2 and 3. Total cholesterol was measured by the cholesterol oxidase method as described previously21.
Participants were asked to bring all medications they had been taking during the two weeks prior to each clinic visit. Medications were defined as antihypertensive, hypoglycemic, or statin, based on the Therapeutic Classification System. Medication accountability was also assessed to determine if all medications were brought to the clinic visit. If needed, follow-up telephone calls were performed to obtain medication information.
Poor blood pressure was defined as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg; intermediate as systolic BP ≥ 120 and < 140 mmHg or diastolic BP ≥ 89 and < 90 mmHg, untreated, or systolic BP < 120 mmHg and diastolic BP < 80 mmHg if treated; and ideal as systolic BP < 120 and diastolic BP < 80 mmHg, if untreated. Poor glucose was defined as FPG ≥ 126 mg/dL or HbA1c ≥ 6.5% or reported diabetes medication use; intermediate as FPG ≥ 100 mg/dL and < 126 mg/dL, or HbA1c ≥ 5.7% and < 6.5%,untreated; and ideal as FPG < 100 mg/dL and HbA1c < 5.7% untreated. Poor cholesterol was defined as total cholesterol ≥ 240 mg/dL; intermediate as ≥ 200 mg/dL and < 240 md/dL untreated, or < 200 mg/dL treated; and ideal as < 200 mg/dL untreated.
Repeated assessment of LSS over time
Assessments of BMI, BP, FPG and total cholesterol were repeated during the JHS Visit 2 (2005–2009) and Visit 3 (2009–2013), while physical activity was reassessed only during the JHS Visit 3. Dietary intake information was available only at the JHS baseline examination.
Other important variables
Information on demographics, education, occupation, alcohol intake, healthcare access, and anthropometrics was obtained during the baseline clinic visit and during the JHS Visit 2 and Visit 3, for some factors. Waist circumference was measured at each visit in cm, at the iliac crest.
Statistical analysis
Baseline characteristics are presented for men and women as means ± standard deviations, for continuous variables, or as proportions, for categorical variables. The prevalence of ideal cardiovascular health metrics was defined as the total number of metrics that met AHA recommendations. Results were calculated among participants with complete information for all seven metrics, and were stratified by age at a given JHS visit (< 40 years, 40 – < 65 years, or ≥ 65+ years). This means that participants were allowed to age into or out of an age group for these analyses. For JHS visits 2 and 3, prevalence of poor, intermediate, and ideal metrics were calculated while accounting for missing data on each metric. Specifically, we used logistic regression to generate the odds of a missing value according to age group. Cluster probability was then used to create a weighted probability for missing. Finally, generalized estimating equations, weighted by missing probability, were used to generate probabilities with (95% CI) for ideal adjusted for gender, age group, and visit. Analysis was completed using SAS version 9.3 (SAS Institute, Cary, North Carolina), and Stata version 13 (StataCorp, College Station, TX).
Results
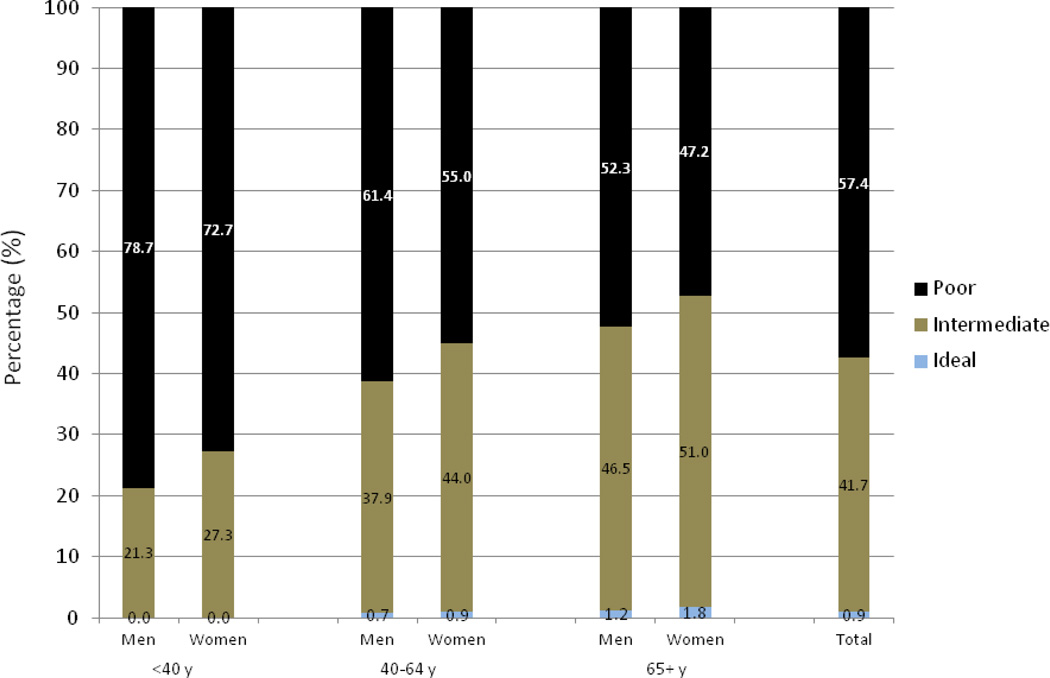
We analyzed data from 5301 participants of the JHS of whom (63.5%) were women. The mean age was 55.3±12.7 years at baseline, and 60.0±12.3 during the JHS visit 3. A total of 4194 subjects provided data during JHS visit 2 and 3815 during JHS visit 3. Table 1 presents baseline characteristics. Only 88.0% of the JHS participants were ideal on smoking, followed by having ideal levels of FPG (45.3%), cholesterol (45.2%), physical activity (19.3%), BP (17.8%), BMI (13.7%), and healthy diet (0.9%) (supplemental Fig. 1). The median number of ideal LSS components was only two (out of seven) in this population. Overall, only 7.3% participants were ideal on all three health factors [4.9% of men and 8.7% of women] and almost none were ideal on all four behavioral factors [0.07% of men and 0.04% of women].
Among men, the prevalence of having 0, 1, 2, 3, 4, 5, 6, and 7 ideal cardiovascular health metrics was 3.3%, 23.0%, 33.5%, 24.7%, 11.6%, 3.6%, 0.3%, and 0%, respectively (Fig 1). Corresponding values for women were 1.7%, 26.3%, 33.1%, 22.8%, 11.9%, 3.7%, 0.6% and 0% (Fig 1). There was a shift toward fewer LSS being met with age (the median ideal LSS was 4 for those < 40, and 3 for those ≥ 65 years at baseline, Fig 1). The prevalence of ideal diet (meeting 3–5 dietary components) was extremely low (<2%) across all age groups, in both men and women (Fig 2). Among individual dietary factors, recommendations for sodium (0.2%) and whole grains (4.1%) were the least likely to be achieved (supplemental Fig 2).
Figure 1.
Number of ideal cardiovascular health components in the JHS
Figure 2.
Prevalence of ideal, intermediate, and poor dietary score by age and sex
From JHS visit 1 through JHS visit 3, the prevalence of being ideal for cholesterol and FPG declined, while the prevalence of being ideal on BMI declined only among participants aged <40 y (Table 2). Over time, there was a slight increase in prevalence of ideal physical activity in both men and women aged 40+ years and no change in prevalence of ideal blood pressure (Table 2).
Table 2.
Prevalence of ideal LSS at each JHS visit according to attained age at a given visit and accounting for missing data
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 3 | Visit 1 | Visit 2 | Visit 3 | |
| Ideal physical activity* | ||||||
| Age (y) | ||||||
| <40 | 36.4 (30.8–42.0) | - | 34.5 (25.3–43.7) | 22.5 (18.4–26.6) | - | 17.1 (8.6–25.7) |
| 40–<65 | 22.5 (20.3–24.7) | - | 31.6 (28.6–34.6) | 17.7 (16.1–19.3) | - | 23.4 (21.1–25.6) |
| 65+ | 15.9 (12.9–19.0) | - | 26.7 (23.4–29.8) | 12.4 (10.3–14.5) | - | 19.7 (17.5–21.9) |
| Ideal body mass index | ||||||
| Age (y) | ||||||
| <40 | 19.5 (15.7–23.3) | 10.8 (5.8–15.7) | † | 17.2 (14.2–20.2) | 10.7 (6.3–15.1) | † |
| 40–<65 | 17.9 (16.1–19.8) | 14.5 (12.6–16.4) | 14.7 (12.7–16.7) | 11.7 (10.5–12.9) | 10.6 (9.3–11.8) | 9.7 (8.3–11.1) |
| 65+ | 19.5 (16.6–22.4) | 18.6 (16.0–21.1) | 20.7 (18.2–23.2) | 11.5 (9.6–13.4) | 12.9 (11.2–14.6) | 13.9 (12.3–15.6) |
| Ideal fasting glucose | ||||||
| Age (y) | ||||||
| <40 | 59.0 (53.7–64.3) | 40.4 (32.4–48.4) | 34.9 (25.2–44.6) | 69.0 (65.0–72.9) | 51.0 (43.9–58.1) | 46.1 (37.0–55.3) |
| 40–<65 | 43.4 (40.9–45.9) | 27.1 (24.4–29.9) | 20.9 (18.2–23.6) | 46.2 (44.2–48.2) | 30.6 (28.3–32.8) | 25.0 (22.8–27.3) |
| 65+ | 32.7 (29.2–36.2) | 24.7 (21.6–27.9) | 23.9 (21.2–26.7) | 29.1 (26.5–31.7) | 21.8 (19.7–24.0) | 21.6 (19.7–23.6) |
| Ideal cholesterol | ||||||
| Age (y) | ||||||
| <40 | 52.3 (46.8–57.7) | 52.0 (43.9–60.1) | 48.2 (39.4–57.0) | 61.5 (57.3–65.7) | 62.6 (55.6–69.6) | 58.9 (51.0–66.9) |
| 40–<65 | 49.6 (46.9–52.3) | 45.6 (42.3–48.9) | 40.5 (37.3–43.6) | 45.3 (43.2–47.3) | 42.7 (40.1–45.3) | 37.6 (35.2–40.1) |
| 65+ | 41.7 (37.7–45.8) | 41.1 (36.8–45.5) | 33.4 (30.0–36.8) | 32.2 (29.3–35.2) | 33.1 (30.0–36.1) | 25.4 (23.0–27.7) |
| Ideal blood pressure | ||||||
| Age (y) | ||||||
| <40 | 28.6 (23.4–33.88) | 28.4 (21.0–35.8) | 25.5 (16.4–34.5) | 43.5 (39.1–47.9) | 39.3 (32.5–46.0) | 36.7 (28.0–45.5) |
| 40–<65 | 15.3 (13.5–17.04) | 15.5 (13.3–17.6) | 14.1 (12.0–16.3) | 18.7 (17.1–20.2) | 14.9 (13.2–16.6) | 14.0 (12.2–15.7) |
| 65+ | 5.9 (3.8–8.1) | 9.7 (7.7–11.7) | 8.3 (6.6–10.1) | 7.1 (5.8–8.4) | 6.8 (5.6–8.1) | 5.9 (4.8–7.0) |
Physical activity was assessed at JHS visit 1 and 3 only; data restricted to LSS assessed at more than one visit to assess any change over time. Smoking and diet were only assessed at visit 1.
Unable to estimate confidence interval due to very small numbers.
Sample sizes included 1934 male participants at JHS visit 1 (2000–2004); 1474 participants at JHS visit 2 (2005–2009); and 1377 people at JHS visit 3 (2009–2013).
Corresponding numbers for female were 3367, 2720, and 2438, respectively.
Discussion
In this cohort, the prevalence of meeting most of the seven LSS was low at baseline, especially among older adults (65+ years). While only 88.0% of JHS participants were ideal for smoking, fewer than half met the ideal for FPG (45.3%) or total cholesterol (45.2%); and fewer than a quarter for physical activity (19.3%), blood pressure (17.8%); or BMI (13.7%). Meeting ideal dietary intakes was rare (0.9%) in this cohort, with extremely low prevalence of achieving sodium (0.2%) and whole grain (4.1%) recommendations. We observed mixed results over time of LSS. From the baseline clinic visit (2000–2004) through the JHS visit 3 (2009–2013), the proportions of participants that were ideal on total cholesterol and FPG declined across all ages, whereas a decline in prevalence of ideal BMI was mostly observed in people aged <40 y. While the prevalence of ideal BP was stable over time, we observed a slight increase in prevalence of ideal physical activity among participants aged 40+ y.
Few data are available on the prevalence of LSS in African-Americans. In the Heart Strategies Concentrating on Risk Evaluation study22, none of the 855 African-American participants was ideal on all seven cardiovascular health metrics. Other investigators have reported similarly low prevalence of ideal LSS from other ethnic groups. In the 2003–2008 NHANES data7, none of the African-American adults met all 7 LSS, and the number of ideal LSS was inversely related with age, consistent with the pattern reported here in the JHS. The prevalence of meeting all seven LSS has also been shown to be low in a variety of predominantly non-Hispanic white populations ranging from 0% to 1% only23–26. Studies in Chinese populations also show low prevalence of meeting all 7 LSS (0.1% to 1.5%)27–29.
Among individual LSS, having an ideal dietary score was the least achieved among JHS participants (0.9%), partly due to 0.2% and 4.1% prevalence for meeting sodium and whole grain recommendations, respectively. This observation is not unique to the JHS, as data from the NHANES 2003–2008 reported a low prevalence for ideal diet score among Americans (range of 0.2% to 2.6% across various sex-, ethnic-, and age groups)7. Data from the ARIC cohort also showed that being ideal on diet score was least likely to be met [5.3% among total ARIC participants and 4.4% among African-American participants of ARIC study]30. In a Spanish cohort of 11,408 adults, the prevalence of having an ideal diet score was 0.4% in men and 0.6% in women and did not vary by age or educational attainment31. Consistent with JHS data, sodium was the item least likely to be met in the Young Finns study (~4%)25 or Spanish cohort (<10%)31. Other investigators have reported prevalences of ideal diet score from 0% to 5%32,33.
This is the first study to examine longitudinal changes in LSS in a large cohort of African-Americans. We observed a decline over time in the likelihood of meeting most LSS ideals, including BMI, cholesterol, and FPG while prevalence of ideal BP did not change. Given that many of these health changes are consistent with expectation during this period of aging, it is encouraging that the measure of physical activity tended to improve during the same period of time in adults aged 40+. Unfortunately, the JHS did not have repeated data on diet or smoking to assess changes in those factors. A low prevalence of being ideal on diet, especially sodium intake underscores the need for future repeated dietary assessment in JHS.
As noted above, we only had baseline data on smoking (without information on duration since quitting smoking) and dietary habits for current analyses. This precludes us from evaluating any possible changes in dietary and smoking habits among JHS participants over time. Furthermore, self-reported physical activity was only assessed at baseline and during the JHS visit 3 and we were not able to collect data on all domains of physical activity. We cannot exclude misclassification of LSS measured via self-report. Nonetheless, it is less likely that such misclassification would change the conclusion of our report as subjects might have been more likely to report the desirable behavior (ideal factor) more often than the poor behavior. In addition, several individuals had missing data on LSS. Although we used a statistical approach to account for missing data, we acknowledge the fact that such approach is not perfect and cannot exclude an under- or overestimation of LSS prevalence in this study. The apparent contradiction between observed decline in CVD rates and outcomes over time in the US in all ethnic groups and our extreme low prevalence of ideal LSS in JHS could be due to inaccuracy associated with LSS assessment. Alternatively, drug treatment (i.e., statin use) and other treatments may be more related to CVD outcomes than LSS.
Strengths of this study include a large sample size, availability of repeated measurements on many of the LSS, use of standardized protocol to collect key data, and generalizability of the results among African-Americans in the southern US.
Our data are consistent with a low prevalence of ideal LSS in the JHS. Because adherence to LSS has been associated with lower incidence of CVD30, cancer32, mortality26,34,35, and lower risk of subclinical disease24,25, it is likely that improvement of LSS in the African-American community could help reduce the burden of CVD. Future endeavors to improve LSS, including dietary patterns and various measures of adiposity, are necessary to improve cardiovascular health in African-Americans.
Perspectives
The very low prevalence of ideal cardiovascular health among participants of the JHS, in particular extremely low prevalence of ideal BMI, physical activity, and diet, underscores the need for reassessment of tools and strategies available to achieve the AHA 2020 goal among African-Americans; provides specific targets for clinical and public health interventions (i.e., diet, physical activity, and adiposity); and might help explain the burden of CVD in stroke-belt regions of the US. The current findings of extremely low attainment of ideal diet score (<1%) may inform the design and implementation of novel strategies that are unique to African-Americans. The high prevalence of sodium intake above 1.5 g/d and the very low consumption of whole grains in this cohort also provide a second prevention target and emphasize the need for repeated assessment of sodium intake (i.e. via urine collection) and other dietary components during future JHS examination cycles.
Supplementary Material
Highlights.
About 60% of African-Americans from the Jackson Heart Study met <3 ideal LSS
Prevalence of ideal diet was only 0.9%
Prevalence of ideal BMI declined only in participants <40 years at a given visit
Prevalence of ideal blood pressure did not change over time
Being ideal on physical activity improved from the first to third visit
Acknowledgements
We thank the participants and the staff of the Jackson Heart Study for their long term commitment to the study.
Funding:
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. This project was also supported by grant R21 NR013231 from the National Health Institutes. Dr. Talegawkar was supported by grant 13BGIA17080036 from the American Diabetes Association.
Abbreviation list
- AHA
American Heart Association
- BMI
Body mass index
- BP
blood pressure
- CI
Confidence interval
- CVD
Cardiovascular disease
- FFQ
Food frequency questionnaire
- FPG
Fasting plasma glucose
- JHS
Jackson Heart Study
- LSS
Life’s simple seven
- MET
Metabolic equivalent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
References
- 1.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes JI. Factors of risk in the development of coronary heart disease-- six year follow-up experience; the Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 4.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 5.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects--Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 7.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang J, Yang Q, Hong Y, Loustalot F. Status of cardiovascular health among adult Americans in the 50 States and the District of Columbia, 2009. J Am Heart Assoc. 2012;1:e005371. doi: 10.1161/JAHA.112.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 10.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6–S17. [PubMed] [Google Scholar]
- 11.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6–S29. [PubMed] [Google Scholar]
- 12.Dubbert PM, Carithers T, Ainsworth BE, Taylor HA, Jr, Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis. 2005;15:S6–S61. [PubMed] [Google Scholar]
- 13.Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the kaiser physical activity survey in women. Med Sci Sports Exerc. 2000;32:1327–1338. doi: 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in Atherosclerosis Risk in Communities. Med Sci Sports Exerc. 2013;45:901–907. doi: 10.1249/MSS.0b013e31827d87ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassetts DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. Compendium of Physical Activities Tracking Guide. Healthy Lifestyles Research Center, College of Nursing & Health Innovation, Arizona State University; 2011. [Accessed Nov. 27, 2013]. [ https://sites.google.com/site/compendiumofphysicalactivities/.]. 2011. [Google Scholar]
- 16.Centers for Disease Control. General Physical Activities Defined by Level of Intensity. [Accessed Nov. 29, 2013];2013 http://www.cdc.gov/nccdphp/dnpa/physical/pdf/PA_Intensity_table_2_1.pdf.
- 17.Tucker KL, Maras J, Champagne C, Connell C, Goolsby S, Weber J, Zaghloul S, Carithers T, Bogle ML. A regional food-frequency questionnaire for the US Mississippi Delta. Public Health Nutr. 2005;8:87–96. [PubMed] [Google Scholar]
- 18.Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, Taylor HA, Jr, Tucker KL. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc. 2009;109:1184–1193. doi: 10.1016/j.jada.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talegawkar SA, Johnson EJ, Carithers TC, Taylor HA, Bogle ML, Tucker KL. Carotenoid intakes, assessed by food-frequency questionnaires (FFQs), are associated with serum carotenoid concentrations in the Jackson Heart Study: validation of the Jackson Heart Study Delta NIRI Adult FFQs. Public Health Nutr. 2008;11:989–997. doi: 10.1017/S1368980007001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickson DA, Diez Roux AV, Wyatt SB, Gebreab SY, Ogedegbe G, Sarpong DF, Taylor HA, Wofford MR. Socioeconomic position is positively associated with blood pressure dipping among African-American adults: the Jackson Heart Study. Am J Hypertens. 2011;24:1015–1021. doi: 10.1038/ajh.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of"ideal cardiovascular health" in a community-based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123:850–857. doi: 10.1161/CIRCULATIONAHA.110.980151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JI, Sillah A, Boucher JL, Sidebottom AC, Knickelbine T. Prevalence of the American Heart Association's "ideal cardiovascular health" metrics in a rural, cross-sectional, community-based study: the Heart of New Ulm Project. J Am Heart Assoc. 2013;2:e000058. doi: 10.1161/JAHA.113.000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alman AC, Maahs DM, Rewers MJ, Snell-Bergeon JK. Ideal cardiovascular health and the prevalence and progression of coronary artery calcification in adults with and without type 1 diabetes. Diabetes Care. 2013 doi: 10.2337/dc13-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oikonen M, Laitinen TT, Magnussen CG, Steinberger J, Sinaiko AR, Dwyer T, Venn A, Smith KJ, Hutri-Kahonen N, Pahkala K, Mikkila V, Prineas R, Viikari JS, Morrison JA, Woo JG, Chen W, Nicklas T, Srinivasan SR, Berenson G, Juonala M, Raitakari OT. Ideal cardiovascular health in young adult populations from the United States, Finland, and Australia and its association with cIMT: the International Childhood Cardiovascular Cohort Consortium. J Am Heart Assoc. 2013;2:e000244. doi: 10.1161/JAHA.113.000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artero EG, Espana-Romero V, Lee DC, Sui X, Church TS, Lavie CJ, Blair SN. Ideal cardiovascular health and mortality: Aerobics Center Longitudinal Study. Mayo Clin Proc. 2012;87:944–952. doi: 10.1016/j.mayocp.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, Li K, Jin C, Cai J. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5:487–493. doi: 10.1161/CIRCOUTCOMES.111.963694. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q, Dong SY, Song ZY, Zheng YS, Wu HY, Mao LN. Ideal cardiovascular health in Chinese urban population. Int J Cardiol. 2013;167:2311–2317. doi: 10.1016/j.ijcard.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Wu HY, Sun ZH, Cao DP, Wu LX, Zeng Q. Cardiovascular health status in Chinese adults in urban areas: analysis of the Chinese Health Examination Database 2010. Int J Cardiol. 2013;168:760–764. doi: 10.1016/j.ijcard.2012.09.235. [DOI] [PubMed] [Google Scholar]
- 30.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. Journal of the American College of Cardiology. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graciani A, Leon-Munoz LM, Guallar-Castillon P, Rodriguez-Artalejo F, Banegas JR. Cardiovascular health in a southern Mediterranean European country: a nationwide population-based study. Circ Cardiovasc Qual Outcomes. 2013;6:90–98. doi: 10.1161/CIRCOUTCOMES.112.967893. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forget G, Doyon M, Lacerte G, Labonte M, Brown C, Carpentier AC, Langlois MF, Hivert MF. Adoption of American Heart Association 2020 ideal healthy diet recommendations prevents weight gain in young adults. J Acad Nutr Diet. 2013;113:1517–1522. doi: 10.1016/j.jand.2013.06.346. [DOI] [PubMed] [Google Scholar]
- 34.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.