Abstract
Therapy of cancer can be achieved by artificially stimulating anti-tumor T and NK lymphocytes with agonist monoclonal antibodies. T and NK cells express several members of the Tumor Necrosis Factor-receptor (TNFR) family specialized in delivering a costimulatory signal on their surface. Engagement of these receptors is typically associated with proliferation, elevated effector functions, resistance to apoptosis, and differentiation into memory cells. These receptors lack any intrinsic enzymatic activity and their signal transduction relies on associations with TRAF adaptor proteins. Stimulation of CD137 (4-1BB), CD134 (OX40), and GITR (CD357) promotes impressive tumor-rejecting immunity in a variety of murine tumor models. The mechanisms of action depend on a complex interplay of cytolytic T lymphocytes, helper T cells, regulatory T cells, dendritic cells, and vascular endothelium in tumors. Agonist monoclonal antibodies (mAbs) specific for CD137 have shown signs of objective clinical activity in metastatic melanoma patients while anti-OX40 and anti-GITR mAbs have entered clinical trials. Preclinical evidence suggests that engaging TNFR members would be particularly active with conventional cancer therapies and additional immunotherapeutic approaches. Indeed, T cell responses elicited to tumor antigens by means of immunogenic tumor cell death are amplified by these immunostimulatory agonist mAbs. Furthermore, anti-CD137 mAbs have been shown to enhance NK-mediated cytotoxicity elicited by rituximab and trastuzumab. Combinations with other immunomodulatory mAb that block T cell checkpoint blockade receptors such as CTLA-4 and PD-1 are also promising.
INTRODUCTION
TNFR family members provide costimulation to T and NK cells
Lymphocyte activation integrates multiple signals carried and delivered across immune synapses. Critical signals for activation are dependent on specific antigens, such as T-cell antigen receptor (TCR) ligation on T cells or on recognition of antibody-coated target cells sensed by FcRγIII (CD16) on NK cells. Costimulatory molecules will subsequently determine the outcome of the primary antigen recognition by providing signals that will amplify, complement, and modulate those elicited from the TCR or CD16. Costimulation(1) is therefore a pathway of intercellular communication that depends on the expression of complementary glycoproteins on the surface of interacting cells.
Four families of molecules play important roles in immune synapses: the immunoglobulin superfamily, the integrin superfamily, C-type lectins and the tumor necrosis factor/tumor necrosis factor receptor families. Receptor-ligand interactions in the immune synapse are important for maintaining structure (adhesion), conveying bidirectional biochemical signals for activation or inhibition, reorganizing the cytoskeleton, and reorienting the secretory machinery. The role of the costimulatory members of the TNFR family seems to be related to signalling. However, it should be noted that many molecular players are acting in a structured and concerted fashion at the synapse including receptors, signalling adaptors, cytoskeletal components and the distribution of lipids in the interacting plasma membranes(2).
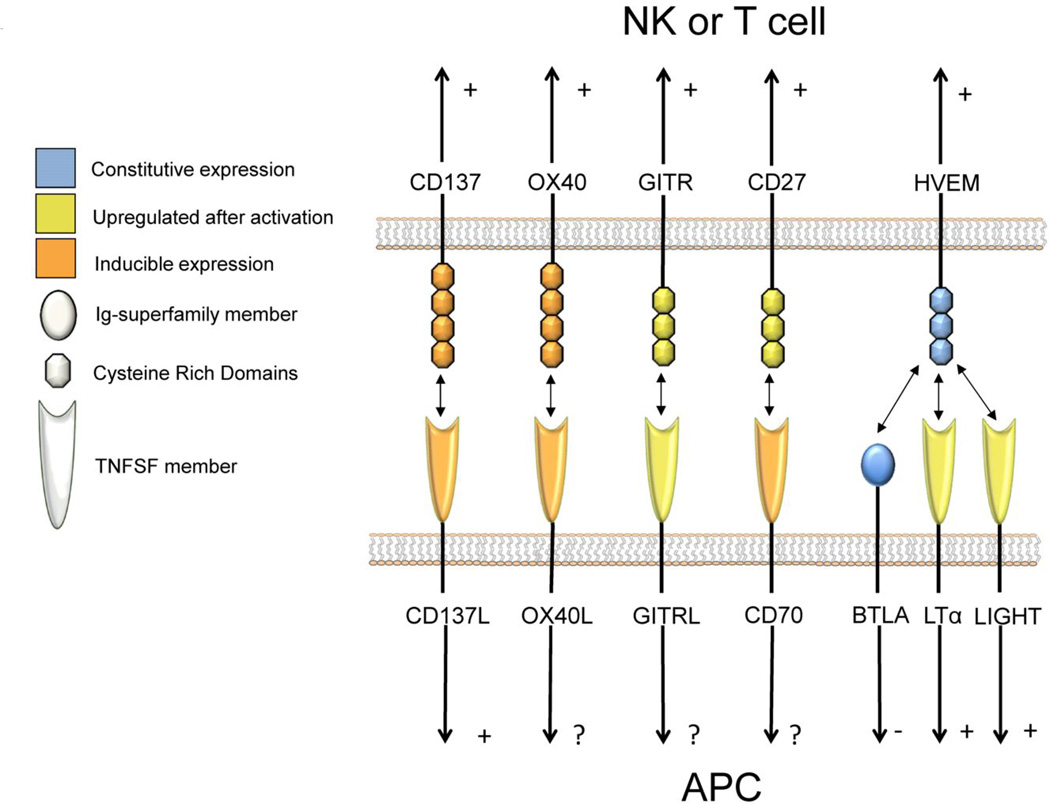
T and NK cells express a panoply of cell surface members belonging to the TNFR family (Figure 1 and Table 1). Some TNFR members such as CD27 are constitutively expressed. However, the expression of other members such as CD137, OX40, and GITR are expressed at low levels or not at all in the resting state but are upregulated upon activation (color-coded in Figure 1). The respective ligands for the TNFR molecules are type II transmembrane proteins, primarily expressed on antigen-presenting cells such as macrophages, dendritic cells, and activated B cells(3,4). Structural studies have demonstrated that TNFR ligands form trimmers and multimerization is essential for cross-linking the receptors(4,5).
Figure 1. Cell surface-attached costimulatory members of the TNF and TNFR superfamilies.
Schematic receptor-ligand pair interactions of the costimulatory members of the TNF and TNFR families at immune synapses. Receptors are color-coded for activation-dependent inducibility and the family of molecules is shape-coded. Plus (+), minus (-) and question mark (?) signs placed at the side of the arrows indicate activatory and inhibitory signals or unknown functional effects.
The TNF family with at least 18 described members and TNFR family that encompass at least 27 members play functions in many other biological functions beyond costimulation (Table 1) of T and NK responses. It is well known that some of the TNF members act as cell surface-attached molecules and some as soluble cytokines that in some cases can hetero trimerize. Soluble forms of the costimulatory members depicted in the figure have been described but their functional importance remains elusive. We can classify TNFR family members depending on the presence absence of a death domain in the cytoplasmic tail. This death domain recruits apoptosis inducing molecules upon ligation of the receptor and is absent from the costimulatory members whose main function is to convey proinflamatory and activatory signals. The pair CD40/CD40 ligand has not been included since the main role of CD40 is activating antigen-presenting cells and has been reviewed in detail in an accompanying review (100).
Table 1.
Members of TNFR superfamily.
| Without death-domain (costimulatory and proinflamatory) | OX40 (CD34) (TNFRSF4) |  |
| CD40 (TNFRSF5) | ||
| CD27 (TNFRSF7) | ||
| CD30 (TNFRSF8) | ||
| CD137 (4-1BB) (TNFRSF9) | ||
| HVEM (CD270) (TNFRSF14) | ||
| GITR (CD357) (TNFRSF18) | ||
| TNFR1B (CD120b) (TNFRSF1B) | ||
| Lymphotoxin beta receptor (CD18) (TNFRSF3) | ||
| DCR3 (TNFRSF6B) | ||
| DCR1 or TRAILR3 (CD263) (TNFRSF10C) | ||
| RANK (CD265) (TNFRSF11A) | ||
| Fn14 or TWEAKR (CD266) (TNFRSF12A) | ||
| TACI (CD267) (TNFRSF13B) | ||
| BAFFR (CD268) (TNFRSF13C) | ||
| BCM or BCMA (CD269) (TNFRSF17) | ||
| TRADE (TNFRSF19) | ||
| EDA2R (TNFRSF27) | ||
| Death-domain (apoptosis inducing) | TNFR1A (CD120a) (TNFRSF1A) | |
| FAS or APO-1 (CD95) (TNFRSF6) | ||
| DR4 or APO-2 or TRAILR (CD261) (TNFRSF10A) | ||
| DR5 or KILLER (CD 262) (TNFRSF10B) | ||
| DCR2 or TRAILR4 (CD264) (TNFRSF10D) | ||
| Osteoprotegerin (TNFRSF11B) | ||
| NGFR (CD271) (TNFRSF16) | ||
| DR6 (CD358) (TNFRSF21) | ||
| APO-3 or DR3 (TNFRSF25) | ||
Knock-out mice for TNFR molecules and their ligands show relatively mild phenotypes with partial loss in the ability to fight viral infections controlled by cellular immune response(6). However, cells artificially exposed to a TNFR stimulus via mAbs show a highly activated phenotype. Most of the basic knowledge of the TNFR molecules comes from T cell studies, but additional cell lineages such as NK cells and myeloid cells are known to express TNFR molecules. While the primary function of TNFR family is to provide adequate costimulation, back-signalling by the ligands can convey a proinflammatory stimuli(7). Therefore, using artificial ligands such as mAb to engage TNFR molecules forces the receptor system to a point that probably is never reached under physiological conditions when these molecules are acting confined to immune synapses during transient cell-cell interactions(8).
These families of receptor-ligand pairs are susceptible to multiple layers of regulation because of the following following mechanistic facts:
The level of surface expression depends on the activation state of the lymphocyte: For the immunomodulatory mAb to be effective, expression of the target molecule on tumor infiltrating lymphocytes or other anti-tumor T cells is critical.
Differential expression, distribution, and function on naïve versus memory T cell subsets.
Differential recruitment to the cytoplasmic tail of members of the TRAF family of signalling adaptors where expression is regulated upon activation.
The level of expression of the ligands is controlled by the activation/maturation state of the antigen presenting cells.
The existence and regulation of negative feedback mechanisms such as deubiquitinases and phosphatases that quench signals from the receptors.
TNFR family member signalling in immune cells
The immunologic outcome of costimulation can be determined by the nature and intensity of reversible biochemical signals. Specifically, integrated signals from multiple accessory receptors dictate, in a coordinated fashion, the intensity, duration, and quality of the immune response(1). Most costimulatory signalling is regulated at the transcriptional level; however, additional mechanisms such as chromatin remodelling, stability of mRNAs, and miRNAs are very likely to play a role.
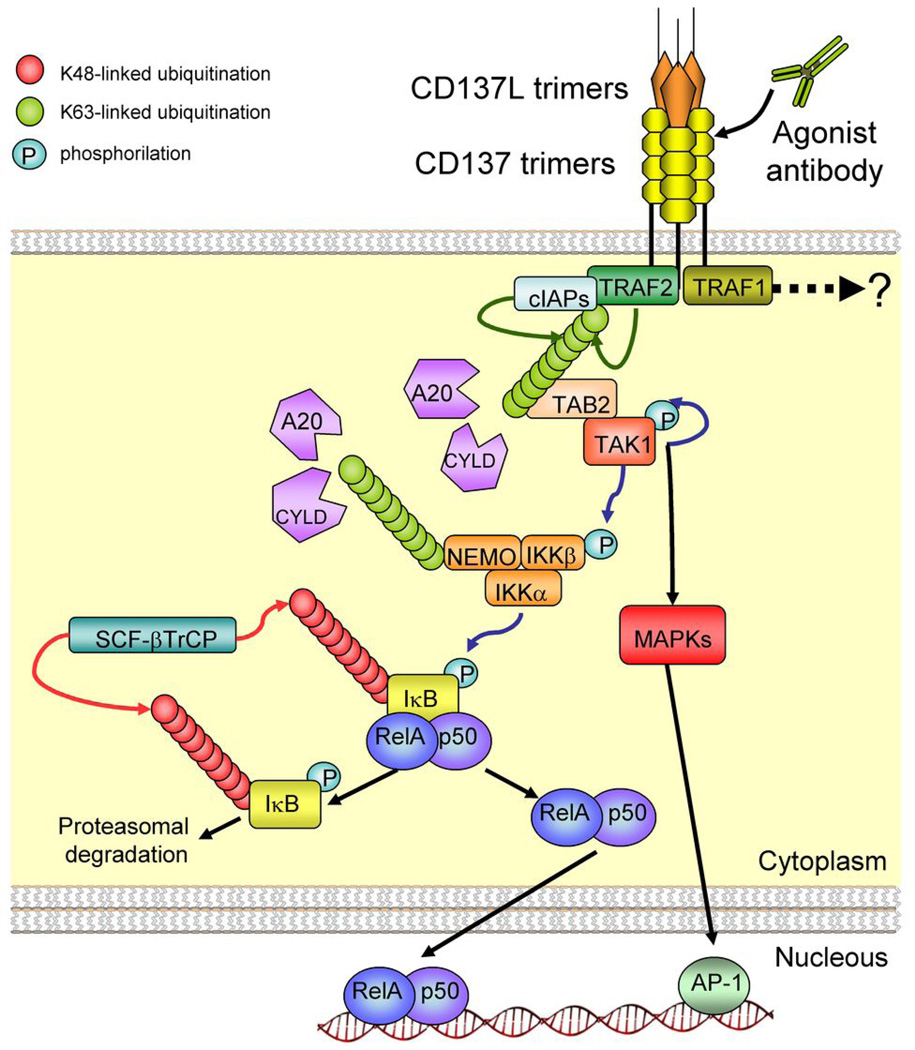
The TNF Receptor family is a large group of over 27 members that share sequence homology with the tumor necrosis factor (TNF) and lymphotoxin receptors (Table 1). Some of the members of the TNFR family were originally discovered in T cells (Figure 1). Biochemically, TNFR family member signalling begins by multimerization of the receptors that eventually lead to the formation of multiprotein complexes important for conveying downstream signalling(9,10). The cytoplasmic tail of these molecules contain TNF receptor associated factor (TRAF) binding domains that recruit TRAFs upon receptor-ligand binding (Figure 2). TRAF2, TRAF1 and TRAF5 are the primary TRAF adaptors reported to interact with the intracellular tails of the costimulatory receptors of the TNFR family (CD137, CD134, and GITR)(11). In addition, TRAF3 and TRAF6 may play a role for some of these receptors(12). TRAF molecules form heterodimers that associate with the receptors and signalling from homo and heterotrimers reportedly have different quantitative and qualitative outcomes(11). For instance, TRAF1 is upregulated upon T cell activation and would displace homotrimers of TRAF2 generating TRAF2:TRAF1 heterotrimers with functional consequences.
Figure 2. Early signal transduction events from CD137.
Schematic representation of TRAF-2 and TRAF-1 recruitment by CD137 surface molecules perturbed by the natural ligand or agonist mAb. TRAF-2 has associated ubiquitin ligase activity (E3) that dictate self ubiquitination and presumably ubiquitination of other protein targets. These events lead to recruitment of TAB1/2-TAK1 complexes that downstream activate NF-κB and MAP-Kinases. Signals controlled or modulated by TRAF-1 are less well understood. K63 polyubiquitin chains are removed by deubiquitinases (i.e: CYLD and A20) which keep the pathway under control and therefore offer potential therapeutic targets.
TRAF2 has been reported to exert E3 ubiquitin ligase activity through its RING domain(13,14) (Figure 2). TRAF2 is constitutively associated with cIAP1 and 2 which are endowed with E3 ubiquitin ligase activities(15). Upon ligation of the TNFR molecules, TRAF2-asociated E3 activity forms polyubiquitin chains linked via their lysine 63 residue(16). These polyubiquitins become attached to TRAF2 and additional protein substrates and may act as second messengers. K-63-polyubiquitins act as docking sites for downstream signalling molecules through recruitment of the TAB1/2-TAK1 complexes that ultimately activate the MAP kinase pathway to form Fos/Jun AP1 transcription factors. In addition, polyubiquitination promotes NEMO-IKKβ complexes to unleash the canonical NF-κB pathway transcription factors (Figure 2). K63 ubiquitin chains are kept at bay by specific deubiquitinases such as CYLD and A20 whose functional control is not well understood(17) (Figure 2). It is clear however, that the deficiency of these enzymes in mice causes autoimmunity and hyperinflammation. TRAF5 also contains a RING ubiquitin ligase catalytic domain and presumably operates in a similar manner. TRAF1 is induced upon T cell activation and complexes with the receptor. Even though the biochemical functions of TRAF1 are not well understood(18), this adaptor is known to be critical for optimal T cell memory(19).
CD137-based cancer immunotherapy
CD137 (4-1BB, TNFRSF9) is a surface protein originally discovered on activated, but not resting, T cells by B Kwon(20,21). CD137 has only one confirmed ligand (CD137-Ligand, TNFSF9) expressed primarily on macrophages, activated B cells and dendritic cells. In the mouse, NK cells express CD137 when activated by cytokines in contrast to human cells where surface expression requires ligation of CD16(22). Expression of CD137 is also found on activated B cells, dendritic cells, myeloid precursors, mast cells, and endothelial cells in tumors or inflamed tissues(8). CD137 and CD137-Ligand deficiency do not cause an overt immune deficiency but only mild alterations in T cell activation and memory. Mice deficient in CD137 signalling weakly control virulent viral infections(23,24).
CD137 agonists such as mAb and soluble forms of the ligand have been shown to enhance cytokine production, proliferation, cytolytic effector functions, and protect lymphocytes from programmed cell death by upregulating BCL-xL and downregulating BIM(25,26). CD137 is also expressed by activated Tregs and ligation of CD137 on Tregs limits the suppressive function by a mechanism yet to be elucidated(27). Paradoxically, CD137 ligation on Tregs can cause pro-mitogenic effects. Importantly, ligation of CD137 on NK cells enhances cytokine release (including IFNγ)(28) and potentiates antibody-dependent cellular cytotoxicity (ADCC)(29,30).
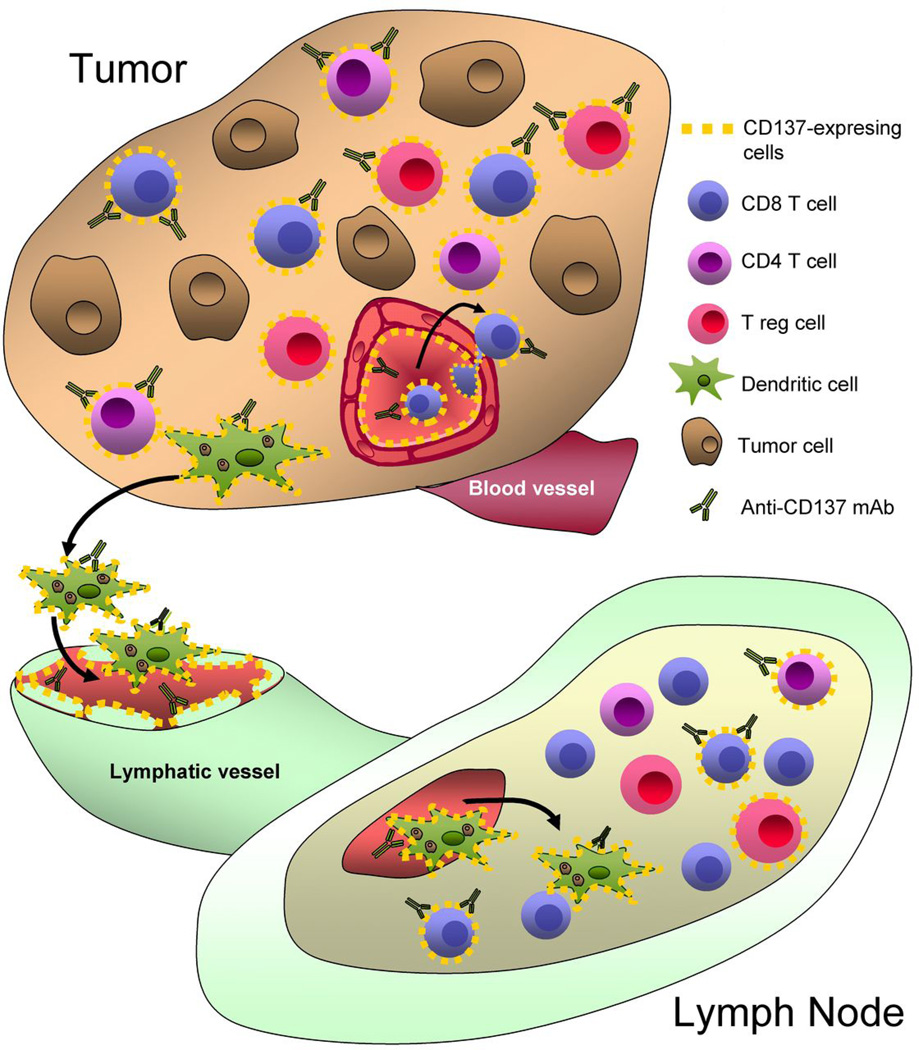
CD137 ligation was first used to treat mouse tumors by means of agonist antibodies(31). As a monotherapy, CD137 mAbs are effective in controlling tumor growth or promoting complete rejection in a variety of transplantable rodent tumors including sarcomas, matocytomas, colon carcinomas, and lymphomas. The mechanism clearly involves enhancement of cytotoxic T lymphocyte (CTL) function(31,32) and cross-priming of tumor antigens by dendritic cells(33) (Figure 3). Interestingly, CD4+ T cells seem to be first stimulated and then eliminated by activation-induced cell death (AICD)(34). This is one of the explanations for the paradox that the very same mAbs that successfully treat tumors, can ameliorate autoimmune diseases by removing autoreactive CD4+ T lymphocytes(35).
Figure 3. Schematic representation of the sites and cells on which agonist anti-CD137 mAbs act as example of TNFR mAb mechanism.
The main mechanism of action is costimulation of CD8+ CTLs crossprimed by DC against tumor antigens. CD137 expression on dendritic cells, activated CD4 T cells (including Tregs) and on tumor blood and lymphatic vessels could offer additional sites and mechanisms of action. Similar mode of action settings can be envisioned for other TNFR members suitable for targeted cancer immunotherapy.
Recent reports have shown that CD137 is present on the surface of capillaries in the tumor bed but not in healthy vasculature. One of the reasons hypothesized for the ectopic CD137 expression on vascular cells is hypoxia(36). CD137, on endothelial cells, promotes leukocyte infiltration by upregulating the expression of adhesion molecules. Interestingly, CD137 expression by effector and regulatory T cells in the tumor microenvironment is dependent on the HIF1α pathway, which senses hypoxia. Local costimulation may be effective in treating tumors because of the selective CD137 expression at this hypoxic peri-tumoral location. This point opens up the possibility for local or targeted delivery of CD137 agonists(37).
Apart from immunostimulatory mAbs, CD137-based immunotherapy has been achieved by transfecting tumor cells to express CD137 ligand(38) or membrane-bound single chain antibodies(39). Multimerizing RNA aptamers (but not monomers), binding selectively to CD137, has also shown anti-tumor efficacy(40) particularly when targeted to a surface tumor antigens(41).
A potentially promising aspect of anti-CD137 mAb immunotherapy is combination with other treatments (both conventional and immunotherapeutic). Combination with chemotherapy(42,43) and radiotherapy(44) is clearly synergistic in pre-clinical models and likely dependent on eliciting immunogenic cell death with subsequent cross-priming of tumor antigens. Synergistic combinations with vaccines(45) and virotherapy(46,47) also rely on the principle that CD137 costimulation must act on ongoing tumor-specific immune responses encompassing CD137+ activated lymphocytes. Recently, some intriguing pre-clinical studies have shown that anti-CD137 mAb therapy synergizes with NK-mediated ADCC elicited by antibodies targeting the surface antigens CD20 or HER-2(30,48).
Preclinical toxicity of CD137 ligation, mainly mediated by polyclonal T cell infiltrates in the liver (dominated by CD8 T cells), results in mild and reversible transaminase elevations(49). In addition, TNFα-mediated myelosupression has been reported(49,50). Fully human and chimeric monoclonal antibodies against CD137 have been produced (BMS-663513, PF-05082566, GTC biotherapeutics). These reagents effectively upregulate cellular immune functions and show tolerable toxicity levels in non-human primates(51).
Clinical trials have only been carried out to completion with BMS-663513 (Table 2). This drug has been used in phase I and in multiple dose phase II clinical trials. Indications of objective clinical activity in melanoma were reported(50). About 10% of the patients developed liver inflammation limiting treatment and two fatalities were reported due to liver toxicity at doses greater 1 mg/kg. This speaks to the need to reconsider dose ranges for agonist antibodies, which likely require lower doses for efficacy compared with antagonist antibodies. Both BMS and Pfizer have resumed clinical trials, implementing dose escalation studies that focus on safety (NCT01471210 and NCT01307267).
Table 2.
Clinical trials with antiCD137, antiOX40, antiCD27 and antiGITR mAb.
| Study agent (Clinical Trial Gov identifier) |
Indication disease | Status (Oct 2012) |
Doses | Phase | |
|---|---|---|---|---|---|
| antiCD137 | BMS-663513 (NCT00309023) | Metastatic solid tumors | Terminated | BMS-663513 (0.3, 1, 3, 6, 10, 15 mg/kg) | I/II |
| BMS-663513 (NCT00351325) | Metastatic solid tumors | Terminated | BMS-663513 (0.3, 1, 3, 10 mg/kg) every 3 or 6 week | I | |
| BMS-663513 (NCT00461110) | Metastatic Non small cell lung cancer | Terminated | BMS-663513 (0.3, 1,3,6,10 mg/kg) | I | |
| BMS-663513 (NCT00612664) | Unresectable Stage III or stage IV Malignant Melanoma | Completed | BMS-663513 (0.1,1,5 mg/kg) | II | |
| BMS-663513 plus Ipilimumab (NCT00803374) | Unresectable Stage III or stage IV Malignant Melanoma | Withdrawn | BMS-663513 (0.1, 0.3,1, 3,10 mg/kg) Ipilimumab (10 mg/kg) | I | |
| PFZ-05082566 plus rituximab ( NCT01307267) | Series A. Solid tumor Series B. Non-Hodgkin's Lymphoma. | Recruiting | A. PFZ-05082566 Dose escalation B. Rituximab 375 mg/m2 PFZ-05082566 Dose escalation | I | |
| BMS-663513 (NCT01471210) | Metastatic solid tumors | Recruiting | BMS-663513 (0.03,0.1,0.3 mg/kg) | I | |
| antiOX40 | antiOX40 plus cyclophosphamide (NCT01303705) | Metastatic prostate Cancer | Recruiting | antiOX40 (0.4 mg/kg) cyclophosphamide (300, 600, 900 mg/m2) | I/II |
| antiOX40 plus radiation (NCT01642290) | Metastatic breast Cancer | Recruiting | antiOX40 (0.4 mg/kg) | I | |
| antiOX40 plus KLH and Tetanus toxoid vaccine (NCT01644968) | Metastatic solid tumors | Active, not recruiting | antiOX40 (0.1, 0.4, 2 mg/kg d1,3,5) | I | |
| antiCD27 | CDX-1127 (NCT01460134) | Hematologic Malignancies Metastatic Solid Tumors | Recruiting | CDX-1127 (0.1–10 mg/kg) | I |
| antiGITR | TRX518 (NCT01239134) | Unresectable Stage III or stage IV Malignant Melanoma | Recruiting | Dose escalation | I |
In the trial with PF-05082566 sponsored by Pfizer (NCT01307267), a combination with rituximab is formally planned as an extension to exploit the ADCC potentiating effect. If active and safe doses are clinically defined, this will open opportunities for local delivery and combinatorial approaches.
OX40 based cancer immunotherapy
OX40 (CD134 or TNFRSF4) is a costimulatory molecule discovered on the surface of activated CD4+ T cells in rats(52). Expression of OX40 was later found to be restricted to activated CD4+ T cells 24–72 hours after TCR engagement. Subsequent studies revealed that OX40 is also found on CD8+ T lymphocytes and other cells such as NK, NKT, and neutrophils(53). CD4+ Foxp3+ Tregs constitutively express OX40 in mice, but human cells upregulate its expression.
Signalling through OX40 increases T cell survival, promotes clonal expansion, and augments pro-inflammatory cytokine production(54). Ligation of OX40 is known to recruit TRAF2 and 3, leading to activation of the canonical and non canonical NF-κB pathways(55,56). OX40 mediated NF-κB activation subsequently leads to enhanced expression of anti-apoptotic molecules such as Bcl-2, Bcl-xl, and survivin which provide the basis for clonal expansion and expanded memory pool of activated T cells(57).
Given that OX40 engagement can expand T cell populations, promote cytokine secretion, and support T cell memory, agonists including monoclonal Abs and soluble forms of OX40L have been used successfully in a variety of preclinical tumor models. The first studies, in which anti-OX40 antibodies showed antitumor activity, were pioneered by Andy Weinberg’s group. Mirroring early studies where monoclonal Abs recognizing inhibitory and costimulatory molecules induce tumor immunity in rodents (anti-CTLA4 and 4-1BB(31,58)), an anti-OX40 antibody was shown to be effective in a number of tumor models including MC303 sarcomas, CT26 colon carcinomas, SM1 breast cancer, and small B16 melanoma(59). Subsequent studies confirmed these observations in additional preclinical models (60–62).
As a monotherapy, OX40 engagement has been effective in eradicating primarily immunogenic tumors while failing to provide adequate antitumor immunity in established and more clinically relevant poorly-immunogenic tumors. Therefore, a variety of combinatorial strategies to increase anti-OX40 antibody therapy have been explored. Given that OX40 ligation upregulates cytokine receptors on T cells, OX40 antibodies synergize with cytokines such as IL-2 or IL-12 alone or with vaccination(63,64). Combining OX40 agonists with GM-CSF-secreting syngeneic irradiated tumor cells or DNA vaccination promotes the expansion of tumor-specific T cells, leading to protection or eradication of established cancers (Murata et al(65) and unpublished observations). Furthermore, anti-OX40 antibodies have been combined with other clinically relevant monoclonal antibodies against inhibitory and costimulatory molecules to treat lymphomas and sarcomas(66,67).
Modalities with direct cytolytic capability, such as chemotherapy or radiation have proven particularly effective in treating established tumors when used concomitantly with OX40 agonists(68,69). In combination with cyclophosphamide, engagement of OX40 not only expands anti-tumor T effector cells but also reduces Foxp3+ regulatory T cells by promoting activation induced cell death. Of interest, elimination and deactivation of Tregs by anti-OX40 antibodies has been important in the antitumor response in some preclinical models(62,68,70,71). Furthermore, given that OX40 ligation can potently stimulate CD4+ T cells, adoptive transfer of anti-melanoma CD4+ T cells can eliminate very advanced melanomas when combined with an anti-OX40 antibody and cyclophosphamide. The potency of the therapy is in part attributed to the newly described ability of OX40 engagement to trigger a cytolytic program in CD4+ T cells(72,73).
Given the substantial evidence from mouse models showing that OX40 agonists can potentiate an anti-tumor immune response in multiple settings, a clinical grade reagent is now being developed and tested. A mouse anti-human OX40 monoclonal antibody has shown activity in nonhuman primates with induction of enlarged lymph nodes and spleens and increased T cell responses(74). This antibody was further tested in phase I clinical trials in 30 patients where the mouse anti-human OX40 antibody was given on days 1, 3, and 5 at 0.1, 0.4, and 2.0 mg/kg (Table 2). The antibody was well tolerated with minimal toxicity and observation of some tumor size reduction, although none of the patients demonstrated an objective response by RECIST criteria. However, specific proliferation and activation of T cells against KLH or tetanus toxin were observed when these model antigens were co-injected the anti-OX40 antibodies(75). Given that patients showed elevated levels of neutralizing human anti-mouse antibodies, the clinical effectiveness of this antibody is significantly limited. For that reason, humanized anti-OX40 antibodies are being prepared for future clinical trials.
GITR-based cancer immunotherapy
The glucocorticoid induced tumor necrosis factor receptor (GITR; TNFRSF18) was originally discovered on T cell hybridomas that were treated with dexamethasone(76). Glucocorticoid treatment, however, was later shown to have no effect on GITR expression on human T cells and was not necessary in mice(77,78). GITR is upregulated on T cells 24–72 hours after activation, although basal expression of GITR is found both on human and mouse T cells(79). GITR expression has also been found on NK cells, eosinophils, basophils, macrophages, and B cells, particularly upon activation(80).
Similarly to OX40 and CD137, GITR modulates T cell activation by providing a costimulatory signal. Unique for a TNFR family member, GITR signals through a complex of a single TRAF5 and two TRAF2 molecules, suggesting a non-redundant role for this molecule(11). GITR, as a costimulatory molecule, increases proliferation, activation, and cytokine production of CD4+ and CD8+ T cells after TCR engagement. Furthermore, it appears that GITR engagement supports a Th1 response in CD4+ T cells in a variety of disease models(80).
Initial studies with an agonist monoclonal rat anti-mouse GITR antibody (DTA-1) show that it can potently stimulate effector T cells while decreasing the suppressive function of regulatory T cells leading to autoimmunity (81–83). Subsequently, it was shown that DTA-1 overrides the suppressive effects of Tregs on T effector cells(84). Thus, anti-GITR can potentially overcome tolerance to self and tumor antigens, making it an attractive target for development as a cancer immunotherapy. Indeed, DTA-1 has been shown to be effective in treating small established B16 tumors(85,86) and 8-day established Meth-A sarcomas(87), CT26(88) and A20 lymphoma (unpublished data). An interesting antitumor property of DTA-1 is its capacity to promote concomitant immunity(89), suggesting the potential for GITR-induced tumor immunity in treating metastatic disease.
DTA-1 has also been successful as an immunologic adjuvant in variety of combinatorial settings. Notably, DTA-1 has shown to substantially enhance the potency of xenogenic DNA vaccines in a melanoma model where protection is marginal(90). Similarly, dendritic cells engineered to express a melanoma antigen showed higher therapeutic potency when co-administered with DTA-1 or when DCs are engineered to secrete DTA-1 or soluble GITRL(91). Moreover, an adenovirus-based vaccine against human papillomavirus failed to provide complete protection unless it was combined with GITR engagement(92).
A humanized agonist anti-human GITR monoclonal antibody (TRX518) has been developed by Tolerex Inc (Now GITR Inc). and, similarly to DTA-1, provides potent costimulation to human lymphocytes in vitro. A dose escalation phase I clinical trial has been initiated at Memorial Sloan-Kettering Cancer Center using TRX518 (Table 2).
Future perspective
While it is clear that agonist antibodies against members of TNFR family can significantly increase anti-tumor immune responses based on preclinical data, these agents are not realistically expected to induce complete regressions in cancer patients as monotherapies. Therefore, combinatorial modalities should be explored in future clinical trials. One attractive strategy is to combine cytolytic chemotherapeutic agents with TNFR agonists. In addition to directly killing tumor cells, these agents can lead to release of self antigens and TLR agonists that can expand antitumor T cells. In one study, stereotactic radiation is being combined with anti-OX40 in patients with metastatic breast cancer (NCT01642290). Furthermore, anti-OX40 is being combined with cyclophosmamide and radiation in patients with metastatic prostate cancer (NCT01301705).
Another interesting approach is the combination of agonist anti-TNFR antibodies in combination with checkpoint blockading antibodies, such as anti-CTLA-4 (ipilimumab) or anti-PD-1 (93). Anti-CTLA-4 and anti-PD-1 have shown anti-tumor activity in about 20–30% of patients tested (94–98). Given the non-redundant signalling of the TNFR and checkpoint blockade pathways, it is conceivable that combinations of agonist and antagonist antibodies against these pathways can synergize to yield higher response rates.
Safety is a concern when considering agonist immunomodulatory antibody therapy. While the phase I anti-human OX40 antibody was well tolerated with low toxicity, trials of anti-CD137 mAb were temporarily suspended after fatal hepatic events were observed. Such studies have now been successfully re-opened using lower doses of agonist antibody therapy. Conversely, in some models, the use of TNFR antibodies can cause hyperactivation and death of antigen-specific effector T cells(99,90) with the potential of hampering antitumor immunity. Therefore, careful design of future clinical trials, identification of biomarkers, and lessons from pre-clinical studies will be necessary to guide future therapies in our quest to develop potent and well-tolerated treatments.
Acknowledgements
We are grateful for scientific discussions with doctors Stacie Goldberg, David Feltquate, Maria Jure-Kunkel. IM receives a grant (SAF2011-22831) from Ministerio de Economía y Competitividad and from UTE for project CIMA. MFS receives a Rio Hortega contract and AMK a FPI scholarship both from Ministerio de Economía y Competitividad. DHC receives a National Cancer Institute R01 CA56821, Swim Across America, and Ludwig Trust.
JDW and DH are supported by National Cancer Institute (R01 CA56821, P01 CA33049, and P01 CA59350), Swim Across America, the Lita Annenberg Hazen Foundation, the T.J. Martell Foundation, the Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Cancer Foundation for Research, the Ludwig Trust, and the Experimental Therapeutics Center of MSKCC.
Conflict of interest
IM and JDW are consultants for Bristol Myers Squibb and receive research funding.
References
- 1.Zhu Y, Yao S, Chen L. Cell surface signaling molecules in the control of immune responses: a tide model. Immunity. 2011;34:466–478. doi: 10.1016/j.immuni.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nat. Rev. Immunol. 2011;11:672–684. doi: 10.1038/nri3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An H-J, Kim YJ, Song DH, Park BS, Kim HM, Lee JD, et al. Crystallographic and mutational analysis of the CD40-CD154 complex and its implications for receptor activation. J. Biol. Chem. 2011;286:11226–11235. doi: 10.1074/jbc.M110.208215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Won E-Y, Cha K, Byun J-S, Kim D-U, Shin S, Ahn B, et al. The structure of the trimer of human 4-1BB ligand is unique among members of the tumor necrosis factor superfamily. J. Biol. Chem. 2010;285:9202–9210. doi: 10.1074/jbc.M109.084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 7.Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J. Leukoc. Biol. 2011;89:21–29. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- 8.Melero I, Murillo O, Dubrot J, Hervás-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol. Sci. 2008;29:383–390. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Wu H. Assembly of post-receptor signaling complexes for the tumor necrosis factor receptor superfamily. Adv. Protein Chem. 2004;68:225–279. doi: 10.1016/S0065-3233(04)68007-7. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay K, Ramagopal UA, Mukhopadhaya A, Malashkevich VN, Dilorenzo TP, Brenowitz M, et al. Assembly and structural properties of glucocorticoid-induced TNF receptor ligand: Implications for function. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19452–19457. doi: 10.1073/pnas.0709264104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snell LM, Lin GHY, McPherson AJ, Moraes TJ, Watts TH. T-cell intrinsic effects of GITR and 4-1BB during viral infection and cancer immunotherapy. Immunol. Rev. 2011;244:197–217. doi: 10.1111/j.1600-065X.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 12.Hauer J, Püschner S, Ramakrishnan P, Simon U, Bongers M, Federle C, et al. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2874–2879. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Forero I, Rouzaut A, Palazon A, Dubrot J, Melero I. Lysine 63 polyubiquitination in immunotherapy and in cancer-promoting inflammation. Clin. Cancer Res. 2009;15:6751–6757. doi: 10.1158/1078-0432.CCR-09-1225. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J. Biol. Chem. 2006;281:1080–1090. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- 16.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed N, Zeng M, Sinha I, Polin L, Wei W-Z, Rathinam C, et al. The E3 ligase Itch and deubiquitinase Cyld act together to regulate Tak1 and inflammation. Nat. Immunol. 2011;12:1176–1183. doi: 10.1038/ni.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing roles for TRAF1 in the alternative versus classical NF-κB pathway in T cells. J. Biol. Chem. 2012;287:23010–23019. doi: 10.1074/jbc.M112.350538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, McPherson AJ, Jones RB, Kawamura KS, Lin GHY, Lang PA, et al. Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J. Exp. Med. 2012;209:77–91. doi: 10.1084/jem.20110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 21.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin W, Voskens CJ, Zhang X, Schindler DG, Wood A, Burch E, et al. Fc-dependent expression of CD137 on human NK cells: insights into «agonistic» effects of anti-CD137 monoclonal antibodies. Blood. 2008;112:699–707. doi: 10.1182/blood-2007-11-122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J. Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 24.Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, et al. Immune responses in 4-1BB (CD137)-deficient mice. J. Immunol. 2002;168:5483–5490. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 25.Kroon HM, Li Q, Teitz-Tennenbaum S, Whitfield JR, Noone A-M, Chang AE. 4-1BB costimulation of effector T cells for adoptive immunotherapy of cancer: involvement of Bcl gene family members. J. Immunother. 2007;30:406–416. doi: 10.1097/CJI.0b013e31802eecc6. [DOI] [PubMed] [Google Scholar]
- 26.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J. Immunol. 2008;180:8093–8101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 27.So T, Lee S-W, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19:253–262. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilcox RA, Tamada K, Strome SE, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J. Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 29.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117:2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J. Clin. Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 32.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murillo O, Dubrot J, Palazón A, Arina A, Azpilikueta A, Alfaro C, et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur. J. Immunol. 2009;39:2424–2436. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Maris CH, Foell J, Whitmire J, Niu L, Song J, et al. Immune suppression or enhancement by CD137 T cell costimulation during acute viral infection is time dependent. J. Clin. Invest. 2007;117:3029–3041. doi: 10.1172/JCI32426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Subudhi SK, Fu Y-X. Co-stimulation agonists as a new immunotherapy for autoimmune diseases. Trends Mol Med. 2003;9:483–489. doi: 10.1016/j.molmed.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Palazón A, Teijeira A, Martínez-Forero I, Hervás-Stubbs S, Roncal C, Peñuelas I, et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011;71:801–811. doi: 10.1158/0008-5472.CAN-10-1733. [DOI] [PubMed] [Google Scholar]
- 37.Palazón A, Martínez-Forero I, Teijeira A, Morales-Kastresana A, Alfaro C, Sanmamed MF, et al. The HIF-1α hypoxia response in tumor-infiltrating T lymphocytes induces functional CD137 (4-1BB) for immunotherapy. Cancer Discov. 2012;2:608–623. doi: 10.1158/2159-8290.CD-11-0314. [DOI] [PubMed] [Google Scholar]
- 38.Melero I, Bach N, Hellström KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur. J. Immunol. 1998;28:1116–1121. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 39.Ye Z, Hellström I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellström KE. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat. Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 40.McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, et al. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pastor F, Kolonias D, McNamara JO, 2nd, Gilboa E. Targeting 4-1BB costimulation to disseminated tumor lesions with bi-specific oligonucleotide aptamers. Mol. Ther. 2011;19:1878–1886. doi: 10.1038/mt.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ju S-A, Cheon S-H, Park S-M, Tam NQ, Kim YM, An WG, et al. Eradication of established renal cell carcinoma by a combination of 5-fluorouracil and anti-4-1BB monoclonal antibody in mice. Int. J. Cancer. 2008;122:2784–2790. doi: 10.1002/ijc.23457. [DOI] [PubMed] [Google Scholar]
- 43.Kim YH, Choi BK, Kim KH, Kang SW, Kwon BS. Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res. 2008;68:7264–7269. doi: 10.1158/0008-5472.CAN-08-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 45.Melero I, Martinez-Forero I, Dubrot J, Suarez N, Palazón A, Chen L. Palettes of vaccines and immunostimulatory monoclonal antibodies for combination. Clin. Cancer Res. 2009;15:1507–1509. doi: 10.1158/1078-0432.CCR-08-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.John LB, Howland LJ, Flynn JK, West AC, Devaud C, Duong CP, et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res. 2012;72:1651–1660. doi: 10.1158/0008-5472.CAN-11-2788. [DOI] [PubMed] [Google Scholar]
- 47.Quetglas JI, Dubrot J, Bezunartea J, Sanmamed MF, Hervas-Stubbs S, Smerdou C, et al. Immunotherapeutic Synergy Between Anti-CD137 mAb and Intratumoral Administration of a Cytopathic Semliki Forest Virus Encoding IL-12. Mol. Ther. 2012;20:1664–1675. doi: 10.1038/mt.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alizadeh AA, Gentles AJ, Alencar AJ, Liu CL, Kohrt HE, Houot R, et al. Prediction of survival in diffuse large B-cell lymphoma based on the expression of 2 genes reflecting tumor and microenvironment. Blood. 2011;118:1350–1358. doi: 10.1182/blood-2011-03-345272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J. Immunol. 2007;178:4194–4213. doi: 10.4049/jimmunol.178.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubrot J, Milheiro F, Alfaro C, Palazón A, Martinez-Forero I, Perez-Gracia JL, et al. Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ. Cancer Immunol. Immunother. 2010;59:1223–1233. doi: 10.1007/s00262-010-0846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher TS, Kamperschroer C, Oliphant T, Love VA, Lira PD, Doyonnas R, et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer immunology immunotherapy. 2012;61:1721–1733. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol. Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 53.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu. Rev. Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J. Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 55.Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol. Cell. Biol. 1998;18:558–565. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J. Biol. Chem. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 57.Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J. Immunol. 2008;180:7240–7248. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 59.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 60.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 61.Ndhlovu LC, Ishii N, Murata K, Sato T, Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J. Immunol. 2001;167:2991–2999. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 62.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J. Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 64.Redmond WL, Triplett T, Floyd K, Weinberg AD. Dual anti-OX40/IL-2 therapy augments tumor immunotherapy via IL-2R-mediated regulation of OX40 expression. PLoS ONE. 2012;7:e34467. doi: 10.1371/journal.pone.0034467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murata S, Ladle BH, Kim PS, Lutz ER, Wolpoe ME, Ivie SE, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J. Immunol. 2006;176:974–983. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 66.Lee S-J, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J. Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 67.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J. Exp. Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT, et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J. Immunother. 2010;33:798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitamura N, Murata S, Ueki T, Mekata E, Reilly RT, Jaffee EM, et al. OX40 costimulation can abrogate Foxp3+ regulatory T cell-mediated suppression of antitumor immunity. Int. J. Cancer. 2009;125:630–638. doi: 10.1002/ijc.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burocchi A, Pittoni P, Gorzanelli A, Colombo MP, Piconese S. Intratumor OX40 stimulation inhibits IRF1 expression and IL-10 production by Treg cells while enhancing CD40L expression by effector memory T cells. Eur. J. Immunol. 2011;41:3615–3626. doi: 10.1002/eji.201141700. [DOI] [PubMed] [Google Scholar]
- 72.Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose M-C, Ramanarasimhaiah R, Ménoret A, et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J. Immunol. 2011;187:3555–3564. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, et al. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med. 2012;209:2113–2126. doi: 10.1084/jem.20120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, Wong S, et al. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J. Immunother. 2006;29:575–585. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 75.Jensen SM, Maston LD, Gough MJ, Ruby CE, Redmond WL, Crittenden M, et al. Signaling through OX40 enhances antitumor immunity. Semin. Oncol. 2010;37:524–532. doi: 10.1053/j.seminoncol.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, et al. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gurney AL, Marsters SA, Huang RM, Pitti RM, Mark DT, Baldwin DT, et al. Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr. Biol. 1999;9:215–218. doi: 10.1016/s0960-9822(99)80093-1. [DOI] [PubMed] [Google Scholar]
- 78.Kwon B, Yu KY, Ni J, Yu GL, Jang IK, Kim YJ, et al. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J. Biol. Chem. 1999;274:6056–6061. doi: 10.1074/jbc.274.10.6056. [DOI] [PubMed] [Google Scholar]
- 79.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur. J. Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 80.Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr. Opin. Immunol. 2012;24:217–224. doi: 10.1016/j.coi.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 82.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 83.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 84.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 85.Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, Terwey TH, Kochman AA, Lu S, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J. Immunol. 2006;176:6434–6442. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 86.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS ONE. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tapmeier TT, Fearn A, Brown K, Chowdhury P, Sacks SH, Sheerin NS, et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78:351–362. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- 89.Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen AD, Diab A, Perales M-A, Wolchok JD, Rizzuto G, Merghoub T, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boczkowski D, Lee J, Pruitt S, Nair S. Dendritic cells engineered to secrete anti-GITR antibodies are effective adjuvants to dendritic cell-based immunotherapy. Cancer Gene Ther. 2009;16:900–911. doi: 10.1038/cgt.2009.39. [DOI] [PubMed] [Google Scholar]
- 92.Hoffmann C, Stanke J, Kaufmann AM, Loddenkemper C, Schneider A, Cichon G. Combining T-cell vaccination and application of agonistic anti-GITR mAb (DTA-1) induces complete eradication of HPV oncogene expressing tumors in mice. J. Immunother. 2010;33:136–145. doi: 10.1097/CJI.0b013e3181badc46. [DOI] [PubMed] [Google Scholar]
- 93.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013:19. doi: 10.1158/1078-0432.CCR-12-2214. xx-xx. [DOI] [PubMed] [Google Scholar]
- 94.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robert C, Thomas L, Bondarenko I, O’Day S, MD JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 96.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013:19. doi: 10.1158/1078-0432.CCR-12-2063. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muriglan SJ, Ramirez-Montagut T, Alpdogan O, Van Huystee TW, Eng JM, Hubbard VM, et al. GITR activation induces an opposite effect on alloreactive CD4(+) and CD8(+) T cells in graft-versus-host disease. J. Exp. Med. 2004;200:149–157. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013:19. doi: 10.1158/1078-0432.CCR-12-2064. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]