Abstract
Background
While numerous studies have shown that severe to moderate wasting at the time of antiretroviral therapy (ART) initiation is strongly predictive of mortality, it remains unclear whether nutritional interventions at or prior to ART initiation will improve outcomes. This review examines data on nutrition assessment, counseling, and support (NACS) interventions in resource-limited settings.
Methods
We identified articles published between 2005 and 2014 on the effectiveness of NACS interventions, particularly its impact on five outcomes: mortality, morbidity, retention in care, quality of life, and/or prevention of ongoing HIV transmission. We rated the overall quality of individual articles and summarized the body of evidence and expected impact for each outcome.
Results
Twenty-one articles met all inclusion criteria. The overall quality of evidence was weak, predominantly due to few studies being designed to directly address the question of interest. Only two studies were randomized trials with proper control groups. The remainder were randomized studies of one type of food support versus another, cohort (non-randomized) studies, or single-arm studies. Ratings of individual study quality ranged from “medium” to “weak,” and the quality of the overall body of evidence ranged from “fair” to “poor.” We rated the expected impact on all outcomes as “uncertain.”
Conclusion
Rigorous, better designed studies in resource-limited settings are urgently needed to understand the effectiveness of nutrition assessment and counseling alone, as well as studies to understand better modalities of food support (targeting, timing, composition, form, and duration) to improve both short- and long-term patient retention in care and treatment, and clinical outcomes.
Introduction
The care and support of people living with HIV (PLHIV) has evolved rapidly with the widespread introduction of effective antiretroviral therapy (ART). Despite substantial improvements in morbidity and mortality, ART alone has not eliminated the need to be concerned about the nutritional status of PLHIV. While uncontrolled or advanced HIV infection is associated with weight loss and severe wasting, numerous studies in low-resource settings have shown that clinical under-nutrition [as indicated by low body mass index (BMI)] at the time of ART initiation is a strong and independent predictor of mortality.1–11 In addition, HIV infection exists in geographical areas where there is high prevalence of non-communicable diseases (diabetes, cancer, and cardiovascular disease), food insecurity, and other endemic infections (e.g., malaria, TB, and diarrheal diseases). The overlap of these conditions, which all have significant nutritional consequences and often occur within the same patient, requires a comprehensive approach to nutritional assessment and care.
While ART initiation is associated with weight gain12 and early weight gain on ART is associated with survival, particularly among those with low BMI at baseline,11,12 it remains unclear whether nutritional interventions to improve weight/BMI prior to or at ART initiation will improve subsequent clinical outcomes. In 2003, the World Health Organization (WHO) provided the following guidance on nutrient requirements for adults living with HIV/AIDS: 1) adequate nutrition, which is best achieved through consumption of a balanced healthy diet, is vital for health and survival for all individuals regardless of HIV status; 2) energy requirements are likely to increase by 10% to maintain body weight and physical activity in asymptomatic HIV-infected adults; and 3) during symptomatic HIV, and subsequently during AIDS, energy requirements increase by approximately 20% to 30% to maintain adult body weight.13 However, this guidance was largely based on evidence from studies conducted in resource-rich settings prior to the widespread use of ART.
In 2006, Kenya initiated a Food by Prescription (FBP) program in which eligible PLHIV (generally based on an assessment of their nutritional status) were prescribed specialized food products to treat severe to moderate under-nutrition. The specialized food products were provided to the individuals in fixed portions (to discourage household sharing) until they reached “nutritional recovery” based on another nutritional assessment. This FBP model was later scaled up nationally in Kenya and now has been adapted by more than a dozen other countries supported by the President’s Emergency Fund for AIDS Relief (PEPFAR). In 2009, in order to limit the inclination of FBP programs to focus mainly on the specialized food products, PEPFAR began promoting the term “NACS” (nutrition assessment, counseling, and support) to encompass the entire spectrum of interventions needed to identify, prevent, and treat malnutrition (including both under- and over-nutrition). The extent of the implementation of NACS interventions and the impact on clinical outcomes remains unclear.
The aim of this systematic review is to evaluate the body of evidence on the effectiveness of NACS interventions among HIV-infected adolescents and adults in clinical care in low-resource settings. The review focuses on five outcomes, as mentioned in the introductory article14: mortality, morbidity, retention in care, quality of life, and on-going HIV transmission. This article is one of 13 papers in this supplement addressing specific HIV care and support interventions.
METHODS
Literature searches were conducted in six medical literature databases [Medline, Embase, Global Health, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Sociological Abstracts (SOCA), and African Index Medicus (AIM)] to identify articles relevant to NACS interventions from January 1995 to May 2014. The overall search strategy is described in detail in the introduction to this supplement14. Table 1 shows the lists of intervention-specific search terms used for each database. Although unpublished studies, clinical trial registries or grey literature (e.g. government or organization reports) were not searched, one relevant unpublished report was identified by USAID and was included in this review15.
Table 1.
Search Strategy and Keyword Terms Used for Each Database
| Medline | EMBASE | Global Health | CINAHL |
|---|---|---|---|
|
MeSH terms, Explode and Focus: dietary services food, fortified nutrition assessment nutrition policy nutritional status nutritional support parenteral nutrition starvation thinness MeSH terms, Focus only: anthropometry body mass index dietary supplements hiv wasting syndrome malnutrition nutrition disorders nutrition therapy weight loss Textwords: anthropometry dietary assessment or dietary counseling dietary supplement* or food supplement* or supplementary food* fortified food* fortified blended foods or corn soya blend or rutf or ready-to-use therapeutic food or plumpy nut therapeutic food* nutrient replacement or “food by prescription” nutrient deficienc* or dietary deficienc* nutrition* assessment starvation therapeutic feeding or nutrition counseling undernourished underweight |
Emtree terms, Explode and Focus: anthropometry body mass nutrition assessment nutritional support starvation Emtree terms, focus only: hiv wasting syndrome nutritional status Textwords: anthropometry dietary assessment or dietary counseling food supplement* or supplementary food* fortified blended foods or corn soya blend or rutf or ready-to-use therapeutic food or plumpy nut fortified food* or nutrient replacement or “food by prescription” nutrient deficienc* or dietary deficienc* nutrition* assessment starvation or undernourished therapeutic food* therapeutic feeding or nutrition counseling underweight |
Thesaurus terms, explode and focus: body mass index nutrition assessment nutrition policy nutritional state nutritional support starvation thinness wasting disease Textwords: anthropometry food supplement* fortified blended foods or corn soya blend or rutf or ready-to-use therapeutic food or plumpy nut nutrition* assessment nutrient replacement or “food by prescription” or fortified food* nutrient deficienc* or dietary deficienc* starvation therapeutic feeding or nutrition counseling or dietary assessment or dietary counseling therapeutic food* undernourished underweight |
Text words: Anthropometry Body mass index Fortified food Nutrition* assessment Nutrition* counseling Nutrition* support Nutrition* status Therapeutic feeding |
|
SOCA Thesaurus terms, explode: HIV OR Acquired Immune Deficiency Syndrome AND Nutrition OR body mass index | |||
|
African Index Medicus (AIM) Thesaurus terms: HIV or AIDS AND Food or food insecurity or malnutrition |
Study Selection and Eligibility Criteria
All abstracts identified through the literature search were screened independently by at least two investigators on the basis of predetermined eligibility criteria with a low threshold to exclude irrelevant abstracts. Full-text articles were retrieved for all abstracts that were deemed eligible by at least one investigator. Articles were then evaluated independently by teams of two investigators to determine if they met the criteria for inclusion. Cases in which there was disagreement between the reviewers were resolved by discussion and consensus. Reference lists of all full-text articles were manually screened for additional publications.
We included studies in HIV-positive adolescents and adults (>14 years of age) in clinical care in low-resource settings. Included studies had to address a NACS intervention and report effects on at least one of the five designated outcomes (stated above). We operationalized the definition of nutrition support as either conventional food or macronutrient supplements. Macronutrient supplements included specialized food products such as ready-to-use supplementary or therapeutic foods (RUSF/RUTF) and fortified blended flours which require cooking [e.g., corn-soya blend (CSB), CSB+, or high energy protein supplements (HEPS)]. For the purpose of this review, we did not include studies of livelihood or household food security interventions. In addition, given the recently updated Cochrane review of micronutrient supplementation in children and adults with HIV,16 we excluded micronutrient interventions from this review as well. Recognizing the ethical complications of conducting randomized clinical trials (RCTs) of food support in malnourished populations living in resource-limited settings, we included other study designs such as cohort studies, quasi-experimental designs, and single-arm studies.
For the morbidity outcome, we included effects on CD4+ cell counts, hemoglobin concentration, number of severe clinical events (death or hospitalization), and WHO stage. For the outcome of retention in HIV care, we included effects on ART adherence, and for quality of life outcomes we included Karnofsky score, measures of functional ability (physical, emotional, or social), and perceived health. While some may consider HIV viral load as a morbidity outcome, we considered effects on viral load as an indicator of risk of HIV transmission given the importance of HIV treatment and viral load suppression as a method of HIV prevention. Full-text articles from abstracts focusing only on nutrition-related outcomes (e.g., change in weight or BMI) were also retrieved and reviewed for any relevant secondary outcomes analyzed that may not have been included in the abstract. Upon full review, articles that did not meet these eligibility criteria were excluded from further analysis.
Data Synthesis and Presentation
Study data from included papers were abstracted using a standardized data collection form to record key data elements, including study design, study period, number of participants, details of NACS intervention, and relevant outcomes assessed. Standardized criteria were used to assess internal validity of individual studies based on study design. For RCTs and cohort studies, the following criteria were used: initial assembly of comparable groups; clear definition of interventions; maintenance of comparable groups over follow-up period (includes attrition, crossovers, adherence, and contamination); differential loss to follow-up or overall loss to follow-up rate; measurements of outcomes being equal, reliable, and valid (includes masking of outcome assessment); and appropriate statistical analysis (e.g., adjustment for confounders in cohort studies, or intention to treat analysis for RCTs). Single group designs (before and after comparisons, use of historical controls, implicit comparisons) were also included in this review and separate criteria were used to assess internal validity for each single group design.17 For before and after comparisons, the following criteria were used: intervention was the only change across the time period; influence of adjunctive therapies or interventions administered concurrently; presence of carryover effects from therapies administered before the intervention of interest; possibility of natural recovery, reduction, or disappearance of symptoms; patients selected into the study represented a relatively extreme subset of the patient population with respect to disease severity and symptoms; and whether patients with more or less favourable outcomes were lost-to-follow up. For single group studies that used historical controls, we judged internal validity based on the following criteria: changes or differences in factors other than intervention across time periods, availability of information on the variability of effect estimates from the historical control group, and adequacy of reporting of data sources for historical response rates.
Assessment of external validity was based on our judgment of how well the study population reflected the target population of HIV-positive adolescents and adults in clinical care in low-resource settings and if the study results could be generalized beyond each study’s specific eligibility criteria. In addition, we abstracted information on the key findings for the relevant outcomes, timing of the intervention by WHO staging/CD4 count and ART status, and any information on cost-effectiveness. For each individual study, the overall quality of evidence was rated as strong, medium, or weak based on the criteria described above and as described in the introductory article14.
Data from all eligible studies were then grouped according to outcome and the quality of the entire body of evidence for that outcome was rated as good, fair, or poor, also as described in the introductory article14. Finally, the expected impact of the intervention by outcome was rated as high, moderate, low, or uncertain based on the magnitude of effect demonstrated in individual studies, the quality of the body of evidence, and consistency across the studies.
RESULTS
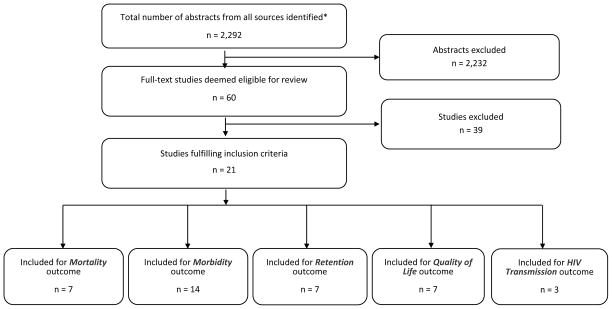
Figure 1 displays the summary of our literature search and study selection process. The literature search yielded 2,292 potentially relevant abstracts that were screened, resulting in a total of 60 studies that were considered for full-text retrieval. After reviewing the full texts, 39 were subsequently excluded because they did not meet the eligibility requirements stated above. The remaining 21 studies are included in this systematic review. Table 2 summarizes the study design, sample size, key findings, and quality of evidence rating for each of these 21 studies.
Figure 1.
Study Flow Diagram for NACS Review
Table 2.
Summary Table: Assessment of Individual Studies by Outcome
| Study Characteristics | Key Findings [Magnitude of effect (HR, OR, RR, RD & 95% CI) or other description] |
Quality of evidence for individual studies |
Evidence from Economic Evaluation (e.g., cost- effectiveness) (Yes or No; if Yes, brief description and rating of quality of study (Level 1, 2, or 3)** |
Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Citation | Study Design (e.g., RCT) |
Study Period, Country |
NACS Inter- vention |
No. Partici- pants |
External and Internal Validity (1=Good; 2=Fair; 3=Poor) |
Overall Quality of Evidence Rating* (1=Strong; 2=Medium; 3=Weak) |
||||
| Internal Validity (Bias) |
External Validity (Generaliz- ability) |
|||||||||
| Mortality | ||||||||||
| Bowie et al, 200521 | Single-arm, historical controls | 2003–2004, Malawi | WFP ration of maize, beans, and CSB; Half received oil | 360 | 6-mo mortality pre-food was not different from post-food (logrank p = 0.65); Mortality rate was lower in those who received oil compared to those who did not (logrank p = 0.0001) | 3 | 2 | 3 | No | <5% not HIV-infected; Random assignment for oil not strictly adhered to; Dist. of food rations not reliable; High mortality overall (~33%); None on ART |
| Cantrell et al, 200819 | Cohort | 2004–2005, Zambia | WFP ration of maize, beans, veg oil, and CSB | 636 (442 food, 194 controls) | 12-mo mortality: 6% food group vs. 8% controls (p = 0.23) | 2 | 2 | 2 | No | More food insecure and CD4<50 in food clinics; Low mortality overall; All subjects initiating ART |
| Manary et al, 201022 | Single-arm, historical controls | 2006–2007, Malawi | RUSF or CSB | 491 in RCT, # of controls not stated | Supplementary food use was not directly associated with improved survival. | 3 | 2 | 3 | No | No data presented in paper; All subjects initiating ART. |
| Ndekha et al, 200918 | RCT | 2006–2007, Malawi | RUSF vs. CSB | 491 (245 RUSF, 246 CSB) | 14-wk mortality: 27% RUSF vs. 26% CSB; HR (RUSF vs. CSB) = 0.91 (0.73 –1.14) | 1 | 1 | 3 | No | High mortality overall; All subjects initiating ART. |
| Sadler et al, 201220 | Cohort | 2010+, Ethiopia | RUTF | 2,595 (1,956 RUTF, 639 no RUTF) | Mortality rates = 1.5% for RUTF and 0.8% for no RUTF group (p = 0.18) | 2 | 2 | 2 | No | Low mortality overall; ~80% on ART for average of 30 mos. (RUTF) and 28 mos. (no RUTF) |
| Serrano et al, 201024 | Single-arm, historical controls | 2006–2007, Niger | WFP ration of cereal, legumes and veg oil | 180 (62 food; 118 controls) | 6 mo. mortality significantly lower in food group vs. controls (mortality ratio = 0.19; p<.05) | 2 | 2 | 3 | No | Food group had lower BMI; Subjects on ART for varying lengths of time (median duration = 6 mos.) |
| Van Oosterhout et al, 201023 | Single-arm, historical controls | 2005–2007, Malawi | RUSF or CSB | 593 (245 CSB, 244 RUSF, 104 controls) | No difference in mortality between groups: OR (RUSF vs. controls) = 1.2 (0.8, 1.9) after 14 wk and 1.15 (0.73, 1.8) after 26 wk; OR (CSB vs. controls) = 1.2 (0.78, 1.96) after 14 wk and 1.18 (0.76, 1.82) after 26 wk | 3 | 2 | 3 | No | Food scarcity and use of co-trimoxazole were different between time periods; Unclear if tracing efforts similar across time periods; All subjects initiating ART. |
| Morbidity | ||||||||||
| Change in CD4 (in cells/mm3) | ||||||||||
| Azabji-Kenfack et al, 201125 | RCT | 2008–2009, Cameroon | Spirulina vs. soya beans | 52 (26 spirulina, 26 soya beans) | 12 wk CD4 change: +99 spirulina vs. +46 soya beans. | 3 | 2 | 3 | No | All subjects initiating ART. |
| Cantrell et al, 200819 | Cohort | 2004–2005, Zambia | WFP ration of maize, beans, veg oil, and CSB | 404 (279 food, 125 controls) | 6 mo. CD4 change: +154 food vs. +171 controls (p = 0.50). 12 mo. CD4 increase: +182 food vs. +180 controls (p = 0.96). |
2 | 2 | 2 | No | More food insecure and CD4<50 in food clinics; Low mortality overall; all subjects initiating ART |
| Chakravarty et al, 200927 | Cohort (with PSM) | 2002–2007, Kenya | Vegetables, staples, beans, oil, yogurt, eggs | 1977 (548 non-food, 1429 food) | No difference in CD4 change between groups | 3 | 2 | 3 | No | Poor-quality electronic medical record data; not all eligible actually receive food; poor screening process for food eligibility; all subjects initiating ART |
| KEMRI, 201215 | RCT, stratified by ART and pre-ART | 2006–2008, Kenya | Nutrition counseling vs. Nutrition counseling plus food (300 g/day of FBF) |
ART: 626 (348 food, 278 controls) Pre-ART: 432 (239 food, 192 controls) |
ART: No difference in CD4 change between groups Pre-ART: 3 mo. CD4 change: +7 food vs. −33 controls (p = 0.04). |
2 | 3 | 2 | No | Overall very high rates of attrition; Losses to follow-up differed by study arm for some time points; Subjects in ART arm all initiated ART within 5 weeks of recruitment. |
| Ivers et al, 201434 | RCT | 2010–2011, Haiti | RUSF vs. CSB+ | 524 (285 RUSF, 239 CSB+) | No difference in CD4 change between groups at 6 or 12 mos. | 2 | 2 | 3 | No | CSB+ group more likely to be missing CD4 data at 12 months than RUSF group; Median ART duration: 12 mos. (CSB+) and 10 mos. (RUSF). |
| Ndekha et al, 200918 | RCT | 2006–2007, Malawi | RUSF vs. CSB | 491 (245 RUSF, 246 CSB) | No difference in CD4 change between groups | 1 | 1 | 3 | No | All subjects initiating ART. |
| Nyamathi et al, 201328 | Cohort | Not stated, India | High protein vs. standard protein supplements | 68 (34 High protein, 34 low protein) | 6 mo. CD4 change: +275 intervention vs. −31 controls (p = 0.0001) | 3 | 3 | 3 | No | In addition to protein supplements, intervention group received intense monitoring and interactive group sessions; Subjects on ART for varying lengths of time; Half were on ART for >18 mos. |
| Rawat et al, 201429 | Cohort (with PSM) | 2008–2009, Uganda | WFP ration of maize, pulses, veg oil, salt, and CSB | 640 (319 food, 322 no food) | No difference in CD4 change between groups | 2 | 1 | 2 | No | All subjects ART naive (participants who started ART during the study were subsequently excluded − 23% food group and 19% no food group) |
| Sadler et al, 201220 | Cohort | 2010+, Ethiopia | RUTF | 481 (428 RUTF, 53 no RUTF) | Median CD4 change: +29 RUTF vs. no change in no RUTF (p = 0.02); Controlling for baseline differences, RUTF had an increase in CD4 of 75.2 cells/mm3 more than no RUTF group (p = 0.001). | 2 | 2 | 2 | No | CD4 data available for only 21% of RUTF and 8% of no RUTF; 80% in both groups on ART for average of 30 mos (RUTF) and 28 mos (no RUTF); Effects of food on CD4 were more apparent among those stable on ART. |
| Scarcella et al, 201137 | Single-arm, before-after | 2002+, Mozambique | WFP ration of cereals, pulses, peanuts, sugar, sunflower seed oil, and CSB | 106 (84 on ART, 22 not on ART) |
ART: 12 mo. CD4 change: +104, p < 0.001; No ART: 12 mo. CD4 change: +48, p>.05 |
3 | 2 | 3 | No | 84 on ART >6 mos |
| Serrano et al, 201024 | Single-arm, historical controls | 2006–2007, Niger | WFP ration of cereal, legumes and veg oil | 160 (60 food; 100 controls) | 6 mo. CD4 change: +114 food vs. +68 controls (p<.05) | 2 | 2 | 3 | No | Median ART duration = 6 mos.; ART adherence significantly better in food group vs. controls (98% vs. 77%; p < 0.005) |
| Swaminathan et al, 201030 | Cohort | 2005–2007, India | Indiamix -WFP ration of FBF | 361 (281 food, 79 no food) | 6 mo. CD4 change: +12.5 food vs. −60 no food (not significant after adjusting for baseline differences) | 2 | 2 | 2 | No | High rate of non-completers (43%); 40% initiated ART during study and became ineligible. |
| # of severe clinical events, change in WHO stage, Hemoglobin (Hgb) levels, self-reported HIV-related symptoms | ||||||||||
| Alo et al, 201431 | RCT | Not stated, Nigeria | Monthly, individual nutrition counseling | 84 (42 intervention, 42 controls) | Mean Hgb levels: 12.1 mg/dL (intervention) vs. 11.2 mg/dL (controls), p = 0.0015). | 2 | 2 | 2 | No | All patients on ART |
| Rawat et al, 201032 | Cohort (with PSM) | 2002–2007, Uganda | WFP ration (CSB, vegetable oil, pulses & maize or rice) or USAID ration (CSB & veg oil | 14,481 (3370 food; 11,111 no food) |
No ART: Food group was 3 percentage points less likely to progress to worse WHO stage than matched controls. ART: No impact of food on WHO stage |
2 | 2 | 2 | No | Only 14,481 out of 195,676 (7%) had necessary data for inclusion; 12% on ART; 70% WHO Stage 2. |
| Rawat et al, 201429 | Cohort (with PSM) | 2008–2009, Uganda | WFP ration of maize, pulses, veg oil, salt, and CSB | 641 (319 food, 322 no food) |
Overall: No difference in Hgb levels between groups; Significant reduction in number of reported HIV-related symptoms (−4.1± 0.54, p<.01) CD4 > 350 cells/mm3: Significant increase in Hgb levels (+1.0 g/dL) |
2 | 1 | 2 | No | All participants ART-naive. ~30% lost to follow-up, died, or excluded due to 1) initiating ART (21%) or 2) receiving non-WFP food assistance in the no-food comparison group (5%). |
| Serrano et al, 201024 | Single-arm, historical controls | 2006–2007, Niger | WFP ration of cereal, legumes and veg oil | 158 (61 food; 97 controls) | No difference in improvement in WHO stage: 44% (food) and 45% (controls), p>.05 | 2 | 2 | 3 | No | Median ART duration = 6 mos.; ART adherence significantly better in food group vs. controls (98% vs. 77%; p < 0.005) |
| Van Oosterhout et al, 201023 | Single-arm, historical controls | 2005–2007, Malawi | RUSF or CSB | 593 (245 CSB, 244 RUSF, 104 controls) | No. severe clinical events (hospitalization or death: 14 wks: RUSF: 41%, CSB: 38%, Controls: 34% 26 wks: RUSF: 50%, CSB: 49%, Controls: 38% (no p-values given) |
3 | 2 | 3 | No | Food scarcity and co-trimoxazole use different between time periods; Unclear if tracing efforts similar across time periods; All subjects initiating ART. |
| Retention in Care (ART adherence) | ||||||||||
| Cantrell et al, 200819 | Cohort | 2004–2005, Zambia | WFP ration of maize, beans, veg oil, and CSB | 532 (366 food, 166 no food) | RRadj for MPR ≥ 95% = 1.5 (1.2 to 1.8) | 2 | 2 | 2 | No | 84% had ART adherence data; all subjects initiating ART |
| Ivers et al, 201026 | Cohort | 2006, Haiti | WFP ration of cereal, legumes, veg oil, salt, and CSB |
6 mos: 488 (237 food, 251 no food) 12 mos: 340 (215 food, 125 no food) |
No. visits attended by 6 mos: 5.5 (food) vs. 2.8 (no food), p < 0.0001 No. visits attended by 12 mos: 9.7 (food) vs. 8.3(no food), p = 0.007 |
3 | 2 | 3 | No | Food vs. no food groups were inherently different at baseline; many switched from no food to food during follow-up; Subjects on ART for varying lengths of time. |
| Ivers et al, 201434 | RCT | 2010–2011, Haiti | RUSF vs. CSB+ | 524 (285 RUSF, 239 CSB+) | No difference between groups in change in % with suboptimal adherence at 6 or 12 mos. | 2 | 2 | 3 | No | CSB+ group more likely to be missing interview data at 12 months than RUSF group; Median ART duration: 12 mos. (CSB+) and 10 mos. (RUSF). |
| Ndekha et al, 200918 | RCT | 2006–2007, Malawi | RUSF vs. CSB | 491 (245 RUSF, 246 CSB) | No difference in ART adherence between groups | 1 | 1 | 3 | No | No data shown in paper; findings noted in abstract only; all subjects initiating ART. |
| Nyamathi et al, 201328 | Cohort | Not stated, India | High protein vs. standard protein dal | 68 (34 high protein, 34 low protein) | 6 mo. Change in % adherent: +59% (intervention) vs. +14% (usual care), p = 0.0001 | 3 | 3 | 3 | No | In addition to protein supplements, intervention group received intense monitoring and interactive group sessions focused on improving ART adherence and skills development; subjects on ART for varying lengths of time; half were on ART for >18 mos. |
| Serrano et al, 201024 | Single-arm, historical controls | 2006–2007, Niger | WFP ration of cereal, legumes and veg oil | 158 (61 food; 97 controls) | Adherence at 6 mos: 98% (food) vs. 77% (controls) remained adherent to ART (p < 0.005). | 3 | 2 | 3 | No | Subjects on ART for varying lengths of time |
| Tirivayi et al, 201233 | Cohort (with PSM) | 2009, Zambia | WFP ration of maize, veg oil, peas, and CSB | 168 (61 food, 107 no food) |
Overall: MPR=98% (food) vs. 89% (no food), p<.01. The following subgroups had greater improvements in adherence: those on ART <231 days; those with BMI <18.5; those in WHO stages III/IV; and those with CD4≤350. |
1 | 2 | 2 | No | Mean ART duration: 777 days (food) and 864 days (no food) |
| Quality of Life | ||||||||||
| Bahwere et al, 200935 | Single-arm, before-after | 2005, Malawi | Chickpea sesame-based RUTF | 42 | Proportion of patients able to walk to clinic increased: 42% to 78% (p < 0.001); Karnofsky score improved for 73.3% |
3 | 3 | 3 | No | Cotrimoxazole given concurrently; 75% WHO Stage 4 and 25% Stage 3; 13% initiated ART 1–2 mos. prior |
| KEMRI, 201215 | RCT, stratified by ART and pre-ART | 2006–2008, Kenya | Nutrition counseling vs. Nutrition counseling plus food (300 g/day of FBF) |
ART: 626 (348 food, 278 controls) Pre-ART: 432 (239 food, 192 controls) |
ART: no difference in perceived health between groups; Pre-ART: More improvement and fewer declines in perceived health in food group at one month (p = 0.02), but not at other time points. |
2 | 3 | 2 | No | Overall high rates of attrition; LTFU differed by study arm for some time points. |
| Greenaway et al, 201236 | Single-arm, before-after | Not stated, Zambia | RUTF plus HEPS | 91 (84 adults) | ‘Fully active’ clients: 5% pre-intervention to 51% post-intervention. | 3 | 3 | 3 | No | Clients self-reported QOL pre- and post-intervention at the post-intervention time point; Small purposive sample selected from each of 11 clinics, not all ART clinics; No info on ART or disease status. |
| Ivers et al, 201026 | Cohort | 2006, Haiti | WFP ration of cereal, legumes, veg oil, salt, and CSB | 488 (251 no food, 237 food) |
6 mos: no difference in role-functioning QOL. 12 mos change in role-functioning QOL score: +3.7 (food) vs. −3.8 (no food) (p = 0.13). |
3 | 2 | 3 | No | Food vs. no-food groups were inherently different at baseline; many switched from no food to food during follow-up; Subjects on ART for varying lengths of time. |
| Ivers et al, 201434 | RCT | 2010–2011, Haiti | RUSF vs. CSB+ | 524 (285 RUSF, 239 CSB+) | No difference between groups in change in general health perceptions score at 6 or 12 mos. | 2 | 2 | 3 | No | CSB+ group more likely to be missing interview data at 12 months than RUSF group; Median ART duration: 12 mos. (CSB+) and 10 mos. (RUSF). |
| Ndekha et al, 200918 | RCT | 2006–2007, Malawi | RUSF vs. CSB | 491 (245 RUSF, 246 CSB) | No difference between groups in QOL measures at 14 weeks | 2 | 2 | 3 | No | All subjects initiating ART. |
| Sadler et al, 201220 | Cohort | 2010+, Ethiopia | RUTF | 350 (334 RUTF, 16 no RUTF) | % showing improvement in functional status: 21.9% (RUTF) vs. 3.8% (no RUTF). | 2 | 2 | 2 | No (cost effectiveness determined for outcome of BMI recovery only) | Small number of original participants had this outcome assessed; ~80% on ART. |
| HIV Transmission (Change in Viral Load (VL)) | ||||||||||
| Azabji-Kenfack, 201125 | RCT | 2008–2009, Cameroon | Spirulina vs. soya beans | 52 (26 spirulina, 26 soya beans) | LogVL significantly lower in spirulina vs. soya beans at end of follow-up (4.5±0.49 vs. 4.8±0.36; p=.02). | 3 | 2 | 3 | No | All subjects initiating ART |
| Ndekha, 200918 | RCT | 2006–2007, Malawi | RUSF vs. CSB | 491 (245 RUSF, 246 CSB) | No difference in VL between groups at end of 14 weeks | 2 | 2 | 3 | No | All subjects initiating ART. |
| Scarcella, 201137 | Single arm, before-after | 2002+, Mozambique | WFP ration of cereals, pulses, peanuts, sugar, sunflower seed oil, and CSB | 106 (84 on ART, 22 not on ART) |
ART: −102,200 copies, p<.001 No ART: −29,000 copies, p>.05 |
3 | 2 | 3 | No | 84 on ART >6 mos |
WFP = World Food Program; RUSF = ready-to-use supplementary food; CSB = corn soya blended flour; HR = hazard ratio; OR = odds ratio; RR = Relative Risk; FBF = fortified blended flour; RUTF = ready-to-use therapeutic food; HEPS = high energy protein supplement; QOL = quality of life; PSM = propensity score matching; RCT = randomized clinical trial; ART = antiretroviral therapy; veg = vegetable; Hgb=Hemoglobin; MPR = Medication Possession Ratio
Mortality
There were seven studies that examined the effect of a NACS intervention on mortality. Only one used a randomized design;18 however, this trial did not have a control group. Instead, the comparison was between an RUSF and CSB. Mortality rates were high in both groups (26% to 27%) and there was no difference in survival between the groups after 14 weeks. There were two cohort studies that compared mortality rates between PLHIV receiving a food supplement and PLHIV in communities that were not yet receiving food supplements due to a phased rollout of the food supplementation program. In one study, participants received a monthly ration of CSB, vegetable oil, maize meal, and beans,19 while in the other study, participants received an RUTF (Plumpy’Nut™).20 Neither study found a significant difference in mortality between those receiving food supplements and those who did not. The remaining four studies all used a single-arm design compared with historical controls. Three of these studies observed no difference in mortality rates,21–23 while the fourth study reported a significantly lower mortality rate for those on nutrition support (mortality ratio=0.19, p<0.05).24 Overall, we rated the body of evidence for mortality outcomes as “fair” and concluded that rolling out NACS will have an uncertain impact on mortality (Table 3).
Table 3.
Summary of Evidence Table
| Overall Quality of Evidence | Impact of the intervention | Evidence from Economic Evaluation | Comments | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Studies (# Studies addressing each outcome and references) |
Overall Quality of the Body of Evidence
(for all studies addressing each outcome) (1=Good; 2=Fair; 3=Poor) (Score and narrative) |
Expected Impact of the intervention* (Based on the main findings from good quality studies addressing the intervention) (1=High; 2=Moderate; 3=Low; 4=Uncertain) |
Studies (# Studies with cost effectiveness data addressing each outcome) |
Quality of evidence from economic evaluation (Summary assessment) |
||
|
| ||||||
| Mortality | 1 RCT18, 2 Cohort,19,20 and 4 single-arm studies21–24 involving 4,855 PLHIV (3 studies from the same research group). | Fair | Uncertain | None | Evidence suggests that giving food to PLHIV with advanced disease is unlikely to provide a mortality benefit. In populations with low rates of mortality, food supplementation is unlikely to reduce mortality rates further.19,20 | |
|
| ||||||
| Morbidity | 4 RCT,15,18,25,34 6 cohort,19,20,27–30 and 2 single- arm studies24,37 involving 6,322 PLHIV for the outcome of CD4 change. | Poor | Uncertain | None | Evidence suggests no benefit of food supplementation on CD4 levels. However, the effect of NACS on the outcome of CD4 change can be confounded by the effect of ART initiation if NACS and ART are initiated concurrently. | |
| 1 RCT,31 2 cohort,29,32 and 2 single-arm studies23,24 involving 15,956 PLHIV for the outcomes of # severe clinical events, change in WHO stage, Hgb levels, and HIV- related symptoms. | Poor | Evidence from one study32 suggests that among those not on ART, those receiving food were slightly less likely to progress to worse WHO stage, relative to matched controls. | ||||
|
| ||||||
| Retention in Care | 2 RCT,18,34 4 cohort,19,26,28,33 and 1 single-arm study24 involving 2,429 PLHIV for the outcome of ART adherence. | Fair | Uncertain | None | Evidence suggests that food rations can help to improve various measures of ART adherence, including pill counts, MPR, and self-report. | |
|
| ||||||
| Quality of Life | 3 RCT,15,18,34 2 cohort,20,26 and 2 single-arm studies35,36 involving 3,062 PLHIV for various quality of life outcomes. | Fair | Uncertain | None | Evidence suggests that food support can improve various QOL outcomes, including mobility, Karnofsky score, perceived health, and functional status. | |
|
| ||||||
| HIV Transmission | 2 RCT18,25 and 1 single-arm study37 involving 649 PLHIV were included in this review for the outcome of viral load. | Poor | Uncertain | None | Evidence shows an unclear effect of food supplementation on viral load. Effects observed are likely due to ART and not food in these studies. | |
Morbidity
Change in CD4+ cell counts
We identified 12 studies that examined the impact of a NACS intervention on change in CD4+ cell count, which we considered to be a surrogate outcome for morbidity. Four of these studies were RCTs: three comparing one type of food supplement against another type of food supplement18,25,26 and one unpublished report comparing 12 months of nutrition counseling alone to 12 months of nutrition counseling plus six months of food support (300 g/d of fortified blended flour).15 The results from these four trials were mixed. In a small study randomizing 26 PLHIV to spirulina (a blue-green alga with a very high protein content) and 26 PLHIV to soya beans, the authors reported that, on average, the spirulina group had a statistically significant larger increase in CD4+ cell counts than the soya bean group (+99 vs. +46 cells/mm3, p<0.05).25 All participants initiated ART at the same time they were enrolled in the trial. In a Kenya Medical Research Institute (KEMRI) trial, participants were stratified by ART (n=626) or pre-ART (n=432)status.15 Although change in CD4+ counts did not differ between the food and no-food groups for those on ART, there was a small but statistically significant difference among those in the pre-ART strata. CD4+ counts increased by seven cells/mm3 in the food group whereas it decreased by 33 cells/mm3 in the no-food group (p=0.04). The remaining two trials both found no difference in increase in CD4+ count when comparing a RUSF with either CSB or CSB+.18,26
There were six cohort studies examining the effect of a food supplement on change in CD4+ counts.19,20,27–30 Four of these found no effect.19,27,29,30 In a study conducted in India, Nyamathi et al reported that high-protein supplements plus intense support from Ashas (accredited social health activists) resulted in significant improvements in CD4+ cell counts over six months compared to usual care.28 However, since the intervention group received weekly intense monitoring of ART adherence from Ashas along with the high protein supplements, the larger increase in CD4+ cell counts cannot be directly attributed to the effect of the protein supplement alone. In Ethiopia, Sadler et al found that participants at sites offering a food support program and prescribed Plumpy Nut™ showed an increase in CD4+ count of 75 cells/mm3 more than participants at sites that did not offer the food support program, and this difference was most significant for those who were not on ART.20 However, pre- and post-CD4 data were only available for a small subset of participants in this study (21% of FBP group and 8% of control group). Overall, we rated the body of evidence for change in CD4 outcomes as “poor” and concluded that rolling out NACS will have an uncertain impact on CD4+ cell counts (Table 3).
Other morbidity outcomes
Five studies included results on morbidity outcomes other than change in CD4+ counts. These outcomes included effects on hemoglobin concentrations,29,31 number of severe clinical events (including hospitalizations and death),23 change in WHO stage,24,32 and number of self-reported HIV-related symptoms.29 One of these studies was an RCT in Nigeria comparing six months of nutrition counseling to a control group who were not provided nutrition counseling. The authors reported a significantly higher hemoglobin concentration in participants who were assigned to monthly, individualized dietary and food hygiene counseling sessions compared to controls (12.1 vs. 11.2 mg/dL, p=0.0015),31 suggesting that individualized counseling to improve intake of iron-rich foods that are locally available and affordable can help to improve or prevent HIV-related anemia. In Uganda, Rawat et al found no effect of food supplementation on hemoglobin concentrations in the overall cohort, but reported a more significant impact among the subset of individuals with CD4 counts >350 cells/mm3.29 In the same study, Rawat et al found a significant reduction in the number of reported HIV-related symptoms in those receiving a food supplement compared to propensity score matched controls. In an earlier cohort, Rawat and colleagues reported that participants not on ART and given household food rations were slightly less likely to progress to a worse WHO stage than propensity score matched controls, while there was no impact of food on WHO stage among those on ART32. In the two single-arm studies, one found higher rates of clinical events (hospitalization or death) in those given an RUSF or CSB compared to historical controls,23 while the other found no difference in improvement in WHO stage among those given a family food ration compared to historical controls.24 Overall, we rated the body of evidence for other morbidity outcomes as “poor” and concluded that rolling out NACS will have an uncertain impact on morbidity (Table 3).
Retention in care
Seven studies examined the effect of food supplementation on adherence to ART, which was the only outcome related to retention in care that came up in the literature search. The majority of these studies found that food supplementation had a positive impact on adherence as measured by medication possession ratio,19,33 pill counts,24,28 or clinic visit attendance.26 There were two RCTs in this group but neither found an effect on adherence.18,34 However, both trials were comparing RUSF versus CSB/CSB+ (i.e., neither trial had a no-food control group); therefore, it is not surprising that no differences in ART adherence were observed between the two trial arms. Overall, we rated the body of evidence for adherence outcomes as “fair” and concluded that rolling out NACS will have an uncertain impact on ART adherence (Table 3).
Quality of Life
Seven studies examined the effect of food supplementation on quality of life. Two of these were single-arm studies with a before-after design35,36 and both reported improvements in quality of life measures (ability to walk, Karnofsky score, and proportion “fully active”) from pre-RUTF to post-RUTF. The two RCTs of RUSF versus CSB/CSB+ also found improvements in quality of life in both study arms given food support.18,34 The RCT of 12 months of nutrition counseling plus food supplementation versus nutrition counseling alone in Kenya found a greater improvement in perceived health after one month of food supplementation in those who were pre-ART.15 However, this effect did not persist at later time points, nor was any effect observed among those on ART. The results from the two cohort studies were mixed. In Haiti, there was no effect of a food support program on quality of life after 12 months.26 In contrast, the cohort study in Ethiopia found that the food support group showed greater improvements in functional status compared to controls, although only a very small number of the original participants had this outcome assessed.20 Overall, we rated the body of evidence for this outcome as “fair” and concluded that rolling out NACS will have an uncertain impact on quality of life (Table 3).
Prevention of HIV transmission
Only three studies assessed the impact of food supplementation on the outcome of ongoing transmission, which we operationalized as HIV viral load levels. Two were RCTs comparing two types of food supplements18,25 and the third was a single-arm before-after study.37 In all studies, ART was initiated at the same time as the food supplements so changes in viral load are difficult to interpret. Azabji-Kenfack et al found that participants randomized to a spirulina supplement had a significantly larger decrease in log viral load after 12 weeks compared to those randomized to soya beans. This was a small trial (n=52) and therefore results should be replicated in a larger trial before firm conclusions can be made. Overall, we rated the body of evidence for this outcome as “poor” and concluded that rolling out NACS will have an uncertain impact on HIV viral load (Table 3).
DISCUSSION
A 2007 Cochrane review examined the effects of macronutrient supplementation on morbidity and mortality in PLHIV and found no relevant clinical trials in low-resource settings.38 Our paper updates this review of clinical trials and expands the scope to include other study designs that could inform our research question (e.g., cohort studies, quasi-experimental designs, and single-arm studies). We identified 21 articles, published between 2005 and 2014, reporting data on the clinical impact of a NACS intervention among PLHIV in resource-limited settings. Based on our review of the literature, we conclude that the data are inconclusive with regards to the impact of NACS programming on all five outcomes of interest and that significant knowledge and research gaps remain.
Limitations
Our review highlights the fact that the overall quality of evidence for the impact of NACS on clinical outcomes is extremely weak, predominantly due to the fact that very few studies were designed to directly address our questions of interest. Although NACS encompasses the entire spectrum of nutritional interventions, all but one of the studies we reviewed evaluated only a single component of NACS, i.e. providing therapeutic and/or supplementary foods to those with severe to moderate under-nutrition. Furthermore, within the realm of food support programs, very few of the studies were adequately designed to evaluate the effectiveness of food support on clinical outcomes in PLHIV.
While RCTs would be the ideal study design to test the effectiveness of food support, they are difficult to conduct in resource-limited settings given the ethical considerations of randomizing nutritionally compromised individuals to a study arm that does not provide optimal nutrition services. Only one of the four studies we identified with randomized food support could directly address our questions of interest. This was the study by KEMRI which compared nutrition counseling plus food support to nutrition counseling alone.15 The other RCTs compared one type of food support to another18,25,34 and although most were well-conducted studies, the lack of a proper control group (one without food support) limits the ability to attribute any effects on outcomes to food support in general.
Several studies used a quasi-experimental (non-randomized) prospective (cohort) study design, taking advantage of the common programmatic context in which food support programs are rolled out across a country in phases. These researchers were able to study individuals living in districts/villages receiving food support with individuals living in districts/villages not yet receiving food support.19,20,29 Similarly, others conducted cohort studies comparing patients from clinics that distributed food support to patients from clinics (some within the same district) that did not distribute food support.30,33 This type of study assumes that clinics providing food support are similar to clinics not providing food support, an assumption that is oftentimes not met. The third type of cohort study design compared individuals within the same clinic or district who were eligible for food support to those who were not eligible for food support.26,27,32 This type of design, however, is somewhat weaker as those who are eligible for food support are likely to be sicker, more food insecure, and/or more nutritionally compromised at baseline compared to those who are not eligible for food support. Two of these studies accounted for this non-comparability of study groups by using propensity score matching, a method of statistical analysis that compares food support recipients to non-recipients who most closely match their (measured) baseline characteristics,27,32 while the third study used traditional multivariate techniques to adjust for baseline differences. All of these designs and analytic strategies circumvent the ethical considerations of randomizing individuals to food versus no food, but they are still limited by their inability to adjust and control for unmeasured differences that are likely to exist between groups.
The third type of study design we encountered were those that used a single-arm design, either comparing outcomes before and after food intervention35–37 or comparing results to historical controls.21–23,37 Again, these types of study designs are far from ideal in that changes other than the intervention may occur during the intervention period (before/after design) or across time periods (historical controls). This makes it impossible to attribute findings to the effect of the intervention alone. However, these types of studies can be useful for generating hypotheses or demonstrating the feasibility and acceptability of an intervention.
In addition to study design, many of the studies we reviewed were further limited by the choice of populations in terms of disease stage and timing of intervention with respect to ART. For example, our review suggests that giving food to PLHIV is unlikely to have an impact on mortality. However the studies evaluating mortality as an outcome enrolled populations that were either extremely advanced in their disease at the time of ART initiation, or were stable on ART with therefore already low mortality rates. It would be difficult to expect NACS to provide a significant short-term survival advantage in either of these extreme circumstances. It may be more realistic to expect a survival advantage for PLHIV given food support at earlier stages of disease (prior to severe wasting) or to follow participants for a longer period after ART initiation.
In terms of morbidity, our review suggests no benefit of food supplementation on CD4 levels; however, many of the studies evaluated the effects of food support on changes in CD4 counts at the time of ART initiation or among subjects who were on ART for varying lengths of time. Since ART has such a strong and direct effect on CD4 counts at initiation, it is not surprising that additional benefits of food support are not observed during this stage of clinical care.
There is evidence from our review that food rations may help to improve ART adherence, though most of the studies used indirect measures of ART adherence such as clinic or pharmacy visits; therefore it is not surprising that the promise of food motivated patients to attend their scheduled visits. What remains unknown is what happens to adherence between clinic visits, how long the promise of food will continue to be a motivating factor, and whether different types of food support could make a difference in adherence rates (i.e., whether a less palatable food supplement would have less effect on adherence).
Limited data are available examining the effect of food rations on ART adherence using the best measure of adherence, namely HIV viral load. Further, the three studies examining the effect of food support on HIV viral load were difficult to interpret. In all three, participants initiated ART at the same time they were given food support so it is impossible to distinguish the effects of ART versus food. Food support could provide PLHIV with nutrients essential for mounting an immune response to retard viral replication, but none of the studies were designed to determine the effects of food support on viral replication beyond the effects of ART.
Knowledge gaps
Typically, approximately 10–15% of adult patients entering clinical care and treatment require food support for an average of 3–5 months to achieve a BMI>18.5. While providing FBP to these clinically undernourished patients remains a medical need per WHO guidance (irrespective of HIV status), the far greater scope of NACS lies in the long term assessment and counseling of patients to manage their diets and lifestyles to tolerate and adhere to their medications, restore their immune response and health, and prevent the early onset of non-communicable diseases. As stated earlier, none of the studies we reviewed evaluated a comprehensive NACS program. Given the expansion of ART eligibility criteria and the expected decline in proportions of adult PLHIV who will present with clinical wasting, the longer term effectiveness of nutrition assessment, counseling and other types of support, including economic strengthening, livelihood and food security support linked to adherence to clinical care and treatment, remains an important research gap. While ART leads to initial weight recovery among those who are under-nourished, many PLHIV on ART continue to gain weight with the belief that being heavier is healthier; but obesity can further elevate their risk for cardiovascular disease, stroke and diabetes associated with HIV infection and chronic use of ARVs. Nutrition assessment and counseling could play an important role in the chronic care and treatment of PLHIV who now have life expectancies of decades, rather than months or years. Although nutrition assessment and counseling can be effective in improving energy intake, weight, and body composition,39 there is no evidence yet that it has any effect on long-term survival or other clinical/functional outcomes in PLHIV in resource-limited settings. Additional studies are needed to assess the effects of nutrition assessment and counseling over the short and longer term.
We identified only one study that focused on nutrition counseling as an intervention. This was the trial by Alo et al, which tested the effectiveness of monthly, individualized nutrition counseling in Nigeria. The authors found that hemoglobin concentrations, the only outcome of this study that was relevant to our review, were significantly higher among those who were counseled versus controls. While promising, this was a relatively small study (n=84); therefore, results need to be replicated to include other clinical outcomes in larger populations before any firm conclusions can be drawn.
A scientific and data-driven approach to improve counseling techniques and modalities (e.g., individual vs group, frequency and duration of counseling, as well as the potential roles of different counsellors including physicians, nurses, auxiliary and community health workers and expert patients) is urgently needed to strengthen NACS, both at the level of the clinic and in the community. This would also include studies to develop and test non-traditional approaches to nutrition counseling that are less resource-intensive and replicable.
Programmatic Considerations for Implementation
NACS is a multi-sectoral, systems approach to integrating nutrition care within health services, linking clinics and communities, and embracing interventions that are nutrition-specific (those that address the immediate determinants of malnutrition) and nutrition-sensitive (those that address the underlying and systemic causes of malnutrition). NACS is a framework that provides opportunities to not only treat malnutrition, but to prevent malnutrition across the continuum of care.
NACS also provides an important opportunity to connect patients in clinical care to economic strengthening and livelihood support to improve individual and household food security and resilience, which is a major concern among populations affected by HIV, particularly in low-resource settings. Thus, a successful model for nutrition counseling and other support in resource-limited settings could be more cost-effective, equitable, and sustainable in the long-term than traditional food support programs that focus solely on provision of specialized food products at the severe wasting end of the spectrum.
A number of issues pose challenges to effective implementation of NACS. Traditional methods of effective nutrition assessment and counseling can be complicated, as well as time- and resource-intensive. ART clinics are often understaffed and the existing staff may feel unqualified or be unable to find the time to conduct proper nutrition assessment and provide adequate counseling to their clients, including counseling on adherence and retention in clinical care and treatment. The application of quality improvement approaches has been critical to finding efficiencies in service delivery, defining roles and responsibilities of staff, and establishing performance standards and supervision that allow integration of nutrition care within the clinical management of patients.
ART clinics also may not have the proper equipment or tools (e.g., scales and stadiometers) in working order to conduct nutrition assessments. Ministries of Health have been challenged to establish procurement, supply chain management and inventory control systems that can assure provision of appropriate therapeutic and supplementary foods to clinically malnourished adults and children. In addition, health systems are challenged to routinely and consistently provide micronutrient supplements for PLHIV whose diets are likely inadequate to meet vitamin and mineral requirements. Continued focus on quality improvement and strengthening procurement and supply chain systems will be key to assure availability of necessary equipment and supplies.
Conclusions
The variable and largely poor quality of the studies we reviewed should not diminish concerns about nutrition status or the importance of NACS for PLHIV. There is an abundance of evidence that poor nutritional status at ART initiation is associated with increased mortality and other adverse outcomes. WHO, providing guidance on nutrition for adult PLHIV, underscores the importance of adequate nutrition, best achieved through consumption of a balanced healthy diet, as vital for health and survival for all individuals regardless of HIV status.13 By definition, essential nutrients are essential because of their established requirements for health, including immune function, which is significantly compromised by HIV infection. Studies addressing the need for food support among PLHIV should be appropriately designed to answer the questions of who should be prioritized to get the food, what the composition of the food should be, when they should get it, and for how long, particularly with regard to early infection or as a long-term adjunct to ART to maintain nutritional status and improve clinical outcomes. In addition, as PLHIV are surviving longer, the impact of nutrition and the effectiveness of counseling to prevent or ameliorate non-communicable diseases (heart disease, stroke, diabetes, osteoporosis etc.) in resource-limited settings should also be at the forefront of research efforts.
Acknowledgments
We would like to thank Gail Bang and Emily Weyant for conducting the literature search for this review.
Footnotes
Disclaimer: The findings and conclusions of this article are those of the authors and do not necessarily represent the official positions of the USAID Office of HIV/AIDS or the U.S. Department of State’s Office of the U.S. Global AIDS Coordinator.
Financial disclosures: This work was supported by USAID/FANTA III (AID-OAA-A-12-00005) and the National Institutes of Health (P30AI042853).
References
- 1.Liu E, Spiegelman D, Semu H, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis. 2011 Jul 15;204(2):282–290. doi: 10.1093/infdis/jir246. [DOI] [PubMed] [Google Scholar]
- 2.Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS. 2006 May 12;20(8):1163–1169. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- 3.Toure S, Kouadio B, Seyler C, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008 Apr 23;22(7):873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006 Aug 16;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 5.Severe P, Leger P, Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005 Dec 1;353(22):2325–2334. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 6.Sieleunou I, Souleymanou M, Schonenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009 Jan;14(1):36–43. doi: 10.1111/j.1365-3156.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 7.Marazzi MC, Liotta G, Germano P, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008 Apr;24(4):555–560. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 8.Zachariah R, Tayler-Smith K, Manzi M, et al. Retention and attrition during the preparation phase and after start of antiretroviral treatment in Thyolo, Malawi, and Kibera, Kenya: implications for programmes? Trans R Soc Trop Med Hyg. 2011 Aug;105(8):421–430. doi: 10.1016/j.trstmh.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Srasuebkul P, Lim PL, Lee MP, et al. Short-term clinical disease progression in HIV-infected patients receiving combination antiretroviral therapy: results from the TREAT Asia HIV observational database. Clin Infect Dis. 2009 Apr 1;48(7):940–950. doi: 10.1086/597354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth RE, van der Meer JT, Hoepelman AI, et al. Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis. 2008 Oct;27(10):977–984. doi: 10.1007/s10096-008-0534-2. [DOI] [PubMed] [Google Scholar]
- 11.Koethe JR, Lukusa A, Giganti MJ, et al. Association between weight gain and clinical outcomes among malnourished adults initiating antiretroviral therapy in Lusaka, Zambia. Journal of acquired immune deficiency syndromes. 2010 Apr 1;53(4):507–513. doi: 10.1097/QAI.0b013e3181b32baf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madec Y, Szumilin E, Genevier C, et al. Weight gain at 3 months of antiretroviral therapy is strongly associated with survival: evidence from two developing countries. AIDS. 2009 Apr 27;23(7):853–861. doi: 10.1097/QAD.0b013e32832913ee. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Nutrient requirements for people living with HIV/AIDS. Report of a technical consultation; 13–15 May 2003; Geneva, Switzerland. 2003. [Google Scholar]
- 14.Kaplan JE, Hamm TE, Forhan S, et al. The Impact of HIV Care and Support Interventions on Key Outcomes in Low and Middle-Income Countries: A Literature Review. Introduction. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000495. (this suppl):XXX-XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KEMRI. Randomized Controlled Trial of the Impacts of Supplementary Food on Malnourished Adult ART Clients and Adult pre-ART Clients in Kenya, Final Report. 2012. [Google Scholar]
- 16.Irlam JH, Siegfried N, Visser ME, Rollins NC. Micronutrient supplementation for children with HIV infection. Cochrane Database Syst Rev. 2013;10:CD010666. doi: 10.1002/14651858.CD010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ip S, Paulus JK, Balk EM, Dahabreh IJ, Avendano EE, Lau J. Role of Single Group Studies in Agency for Healthcare Research and Quality Comparative Effectiveness Reviews. Research White Paper. (Prepared by Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I.) Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 18.Ndekha MJ, van Oosterhout JJ, Zijlstra EE, Manary M, Saloojee H, Manary MJ. Supplementary feeding with either ready-to-use fortified spread or corn-soy blend in wasted adults starting antiretroviral therapy in Malawi: randomised, investigator blinded, controlled trial. Bmj. 2009;338:b1867. doi: 10.1136/bmj.b1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantrell RA, Sinkala M, Megazinni K, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. Journal of acquired immune deficiency syndromes. 2008 Oct 1;49(2):190–195. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadler K, Bontrager E, Rogers B, et al. Food by Prescription: Measuring the impact and cost-effectiveness of prescribed food on recovery from malnutrition and HIV disease progression among HIV+ adult clients in Ethiopia. Boston, USA: Feinstein International Center, Friedman School of Nutrition Science and Policy, Tufts University; 2012. [Google Scholar]
- 21.Bowie C, Kalilani L, Marsh R, Misiri H, Cleary P, Bowie C. An assessment of food supplementation to chronically sick patients receiving home based care in Bangwe, Malawi: a descriptive study. Nutrition journal. 2005;4:12. doi: 10.1186/1475-2891-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manary M, Ndekhat M, van Oosterhout JJ. Supplementary feeding in the care of the wasted HIV infected patient. Malawi medical journal: the journal of Medical Association of Malawi. 2010 Jun;22(2):46–48. doi: 10.4314/mmj.v22i2.58792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Oosterhout JJ, Ndekha M, Moore E, Kumwenda JJ, Zijlstra EE, Manary M. The benefit of supplementary feeding for wasted Malawian adults initiating ART. AIDS care. 2010 Jun;22(6):737–742. doi: 10.1080/09540120903373581. [DOI] [PubMed] [Google Scholar]
- 24.Serrano C, Laporte R, Ide M, et al. Family nutritional support improves survival, immune restoration and adherence in HIV patients receiving ART in developing country. Asia Pacific journal of clinical nutrition. 2010;19(1):68–75. [PubMed] [Google Scholar]
- 25.Azabji-Kenfack M, Dikosso SE, Loni EG, et al. Potential of Spirulina Platensis as a Nutritional Supplement in Malnourished HIV-Infected Adults in Sub-Saharan Africa: A Randomised, Single-Blind Study. Nutr Metab Insights. 2011;4:29–37. doi: 10.4137/NMI.S5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivers LC, Chang Y, Gregory Jerome J, Freedberg KA. Food assistance is associated with improved body mass index, food security and attendance at clinic in an HIV program in central Haiti: a prospective observational cohort study. AIDS Res Ther. 2010;7:33. doi: 10.1186/1742-6405-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakravarty S. Harvesting Health: Fertilizer, Nutrition and AIDS Treatment in Kenya. New York: Graduate School of Arts and Sciences, Columbia University; 2009. [Google Scholar]
- 28.Nyamathi A, Sinha S, Ganguly KK, Ramakrishna P, Suresh P, Carpenter CL. Impact of protein supplementation and care and support on body composition and CD4 count among HIV-infected women living in rural India: results from a randomized pilot clinical trial. AIDS Behav. 2013 Jul;17(6):2011–2021. doi: 10.1007/s10461-013-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawat R, Faust E, Maluccio JA, Kadiyala S. The impact of a food assistance program on nutritional status, disease progression, and food security among people living with HIV in Uganda. Journal of acquired immune deficiency syndromes. 2014 May 1;66(1):e15–22. doi: 10.1097/QAI.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 30.Swaminathan S, Padmapriyadarsini C, Yoojin L, et al. Nutritional supplementation in HIV-infected individuals in South India: a prospective interventional study. Clin Infect Dis. 2010 Jul 1;51(1):51–57. doi: 10.1086/653111. [DOI] [PubMed] [Google Scholar]
- 31.Alo C, Ogbonnaya LU, Azuogu BN. Effects of nutrition counseling and monitoring on the weight and hemoglobin of patients receiving antiretroviral therapy in Ebonyi State, Southeast Nigeria. Hiv/Aids. 2014;6:91–97. doi: 10.2147/HIV.S60429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawat R, Kadiyala S, McNamara PE. The impact of food assistance on weight gain and disease progression among HIV-infected individuals accessing AIDS care and treatment services in Uganda. BMC Public Health. 2010;10:316. doi: 10.1186/1471-2458-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirivayi N, Koethe JR, Groot W. Clinic-Based Food Assistance is Associated with Increased Medication Adherence among HIV-Infected Adults on Long-Term Antiretroviral Therapy in Zambia. J AIDS Clin Res. 2012;3(7):171. doi: 10.4172/2155-6113.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivers LC, Teng JE, Jerome JG, Bonds M, Freedberg KA, Franke MF. A randomized trial of ready-to-use supplementary food versus corn-soy blend plus as food rations for HIV-infected adults on antiretroviral therapy in rural Haiti. Clin Infect Dis. 2014 Apr;58(8):1176–1184. doi: 10.1093/cid/ciu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahwere P, Sadler K, Collins S. Acceptability and effectiveness of chickpea sesame-based ready-to-use therapeutic food in malnourished HIV-positive adults. Patient Prefer Adherence. 2009;3:67–75. doi: 10.2147/ppa.s4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenaway KA, Jere EC, Zimba ME, Masi C, Kawana BM. Examining the integration of food by prescription into HIV care and treatment in Zambia. Field Exchange Emergency Nutrition Network. 2012;(42):30–31. http://fex.ennonline.net/42/examining.
- 37.Scarcella P, Buonomo E, Zimba I, et al. The impact of integrating food supplementation, nutritional education and HAART (Highly Active Antiretroviral Therapy) on the nutritional status of patients living with HIV/AIDS in Mozambique: results from the DREAM Programme. Ig Sanita Pubbl. 2011 Jan-Feb;67(1):41–52. [PubMed] [Google Scholar]
- 38.Mahlungulu S, Grobler LA, Visser ME, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database Syst Rev. 2007;3:CD004536. doi: 10.1002/14651858.CD004536.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Baldwin C, Weekes CE. Dietary advice with or without oral nutritional supplements for disease-related malnutrition in adults. Cochrane Database Syst Rev. 2011;9:CD002008. doi: 10.1002/14651858.CD002008.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]