Abstract
While adjusting flow-mediated dilation (FMD), a measure of endothelial function, for shear rate may be important when evaluating endothelial-dependent vasodilation, the relationship of FMD with shear rate in study populations with cardiovascular risk factors is unclear. We aimed to investigate the association of 4 measures of shear rate (peak shear rate (SRpeak) and shear rate area under the curve through 30 seconds (SRAUC 0-30), 60 seconds (SRAUC 0-60), and time to peak dilation (SRAUC 0-ttp)) with FMD in 50 study subjects with type 2 diabetes and mild hypertension undergoing baseline FMD testing for an exercise intervention trial. Associations among measures of shear rate and FMD were evaluated using Pearson's correlations and R2. The 4 measures of shear rate were highly correlated within subjects, with Pearson's correlations ranging from 0.783 (p<0.001) to 0.972 (p<0.001). FMD was associated with each measure of shear rate, having a correlation of 0.576 (p<0.001) with SRAUC 0-30, 0.529 (p<0.001) with SRAUC 0-60, and 0.512 (p<0.001) with SRpeak. Nine of 50 subjects (18%) did not dilate following the shear stimulus. Among the 41 responders, FMD had a correlation of 0.517 (p<0.001) with SRAUC 0-ttp and similar correlations to those found in the full sample for SRAUC 0-30, SRAUC 0-60, and SRpeak. In conclusion, shear rate appears to explain up to a third of between-person variability in FMD response and our results support the reporting of shear rate and FMD with and without adjustment for shear rate in similar clinical populations with CVD risk factors.
Keywords: endothelial function, vascular responsiveness, shear rate, diabetes
Introduction
Assessment of brachial artery flow-mediated dilation (FMD) is perhaps the most commonly used, non-invasive technique to assess vascular endothelial function.1 In this method, the diameter of the brachial artery is measured before and after a period of limb ischemia induced by a blood pressure cuff.2 Endothelium-dependent vasodilation is estimated based on the percentage increase of the arterial diameter following the release of the blood pressure cuff. Previous research has established an association between the vasodilatory capacity of the brachial and coronary arteries.3 Endothelial dysfunction is believed to be involved in the pathogenesis of atherosclerosis4 and predicts cardiovascular events independently of traditional cardiovascular risk factors.5-7 Thus, a diminished capacity of the brachial artery to respond to the hyperemic stimulus is thought to be a reflection of compromised global vascular health.8
Vascular responsiveness is understood to be determined, in part, by the magnitude of the shear stress stimulus imposed on the vessel wall.9,10 Experiments conducted by Pyke and Tschakovsky support normalization of FMD to shear rate calculated as the area under the shear rate response curve to the time of peak diameter.11 However, several analytical methods for shear rate quantification and adjustment exist in the literature,12-17 and the most effective and appropriate way to account for shear stimulus when reporting FMD remains the subject of considerable debate.18,19 Further, recent evidence indicates that some adults with type 2 diabetes do not dilate in response to a standard reactive hyperemic stimulus and these ‘non-responders’ have significantly lower peak shear stress compared to those subjects who exhibited a FMD response.20 This qualitative lack of response may further obscure the shear rate – FMD relationship in patients with type 2 diabetes or other cardiovascular disease risk factors.
Despite the growing body of literature emphasizing the importance of FMD normalization or adjustment by the shear stimulus, we are aware of only one study which examines the relationships between FMD and four commonly used methods of shear rate quantification in a sample of healthy volunteers.17 However, the quantification method which correlates most strongly with FMD in a clinical population is not known. Therefore, we studied 50 consecutive men and women who underwent baseline FMD testing for the Sugar, Hypertension and Physical Exercise (SHAPE2) trial (NCT00212303), a 6-month clinical trial designed to investigate the effects of exercise training on parameters of cardiac and peripheral vascular function in persons who have both type 2 diabetes and high normal blood pressure or mild hypertension. The primary aim of the present investigation was to compare cross-sectional associations of four measures of shear rate with FMD in this clinical sample. A secondary objective was to identify non-responders and evaluate the impact of non-response on these associations.
Methods
Participants
Participants were between 40 and 65 years old with blood pressure in the range of high normal or mild hypertension (Stage 1) according to the JNC VII criteria,21 with systolic blood pressure 120-159 mmHg or diastolic blood pressure between 80-99 mmHg or taking anti-hypertensive medications. Type 2 diabetes was verified by test results using the 1997 ADA criteria of fasting glucose > 126 mg/dl, symptoms of hyperglycemia with casual plasma glucose > 200 mg/dl, or two-hour plasma glucose > 200 mg/dl after a 75 gram oral glucose load.22 Key exclusion criteria included fasting blood glucose > 400 mg/dl, HbA1c >11%, use of insulin for diabetes treatment, resting blood pressure exceeding 160/100 mmHg, participation in regular exercise,23 history of myocardial infarction, prior CABG or PTCA, chronic heart failure, cigarette smoking in the previous 6 months, or self-reported substance abuse. Informed consent was obtained from all participants before enrollment into the study and the Johns Hopkins Institutional Review Board approved the protocol.
FMD Testing Procedures
Participants reported to a quiet, climate controlled laboratory (22-24°C) in a fasted state between the hours of 8:00AM and 10:00AM for examination. In accordance with previously published guidelines,2 participants were asked to refrain from taking vasoactive medications, participating in physical exercise, or consuming foods high in nitrates or fat as well as caffeine 24 hours prior to testing and to avoid consuming alcohol 48 hours before testing. Brachial artery ultrasound measures were obtained with participants resting in the supine position with the left arm immobilized by a foam support and extended laterally. Heart rate was monitored continuously using a three lead ECG and blood pressures were taken in the opposite arm with a mercury sphygmomanometer. A rapid inflation/deflation pneumatic cuff (Hokanson E-10, D.E. Hokanson, Inc, Bellevue, WA) was placed just proximal to the ultrasound transducer, which was held in place by a mechanical clamp. Images were captured using a 10 MHz multi-frequency linear array transducer attached to a high-resolution Toshiba Aplio Ultrasound system. Images were obtained in the longitudinal view and image depth was initially set at 4 cm. Gain settings were adjusted to provide the optimal view of the anterior and posterior intimal interfaces of the brachial artery. Blood velocity was acquired simultaneously using pulsed wave Doppler at an angle of insonation of <60°. Baseline brachial artery diameter and velocity were assessed for 30 seconds following 20 minutes of supine rest. Immediately following acquisition of baseline values, the occlusion cuff was inflated to > 200 mmHg for 5 minutes to establish an ischemic condition in the lower arm. Brachial diameter images and velocities during hyperemia were recorded continuously from the final 30 seconds of occlusion until 180 seconds (3 minutes) after deflation of the cuff. Twenty minutes following the hyperemic test, the resting brachial artery diameter was measured immediately before and for 4 minutes after the administration of 2.5 mg sublingual nitroglycerin.
Data analysis
Brachial artery diameters were analyzed off-line using the Brachial Imager software (Medical Imaging Applications, LLC, Coralville, IA). A region of interest was identified on a clear portion of the vessel and diameters were calculated as the mean distance between the anterior and posterior wall at the blood vessel interface with the image in diastole, defined as peak of the r-wave. Brachial diameters were collected at a frequency of 8 frames per second and measures at diastole were identified as local minima with at least 2 measures before and after of greater or equal value. Resting diameter was calculated as the average of diameters at diastole over 30 seconds of data obtained following the 20 minute rest period. Peak diameter during hyperemia was calculated as the maximal value of a third order polynomial fit to brachial diameters measured at diastole, to reduce to influence of outliers. Peak dilation values and time-to-peak dilation were confirmed by visual inspection of the arterial diameter curve. FMD was calculated as the percent change from baseline to maximal diameter. An intraclass correlation coefficient between fitted and visually measured FMD was calculated to check model fit. Subjects with a ≤ 0% dilation of the brachial artery following the shear stimulus were categorized as ‘non-responders’. Nitroglycerin-mediated dilation (NMD) was calculated using similar procedures to FMD.
Peak shear rate (SRpeak) was assessed prospectively and was calculated as 4 • maximal velocity/diameter at the time of peak velocity.18 For measurement of blood velocity, a region of interest was selected around the Doppler spectra located along the Doppler strip. The velocity-time integral was traced and the area under this tracing was integrated to calculate mean peak velocity. The time course for velocities was determined using a 5 second average. The mean diameter measure and the velocity measure were synchronized and, in turn, used to calculate shear rate (4 • blood velocity (cm/s)/ brachial diameter (mm)). Baseline shear rate was calculated from measurements taken prior to cuff deflation. Shear rate area under the curve was then calculated by summing shear rate for each interval up to 30 seconds (SRAUC0-30), 60 seconds (SRAUC0-60), and until the time of peak dilation (SR0-ttp). SR0-ttp could only be measured in responders, since non-responders did not have a peak diameter or time-to-peak.
In our laboratory, we performed repeated readings of FMD images on 5 subjects by the same observer (S.L). The intraclass correlation coefficient for repeated readings was r=0.90 with a coefficient of variation of 12%.
Statistical Analysis
Statistical analyses were performed using STATA 10.1 (College Station, Texas). All variables were checked for normality, and each measure of shear rate was log transformed. Pearson's correlations were calculated between each measure of shear rate to assess collinearity. Pearson's correlations were also calculated to evaluate associations of SRAUC 0-30, SRAUC 0-60, and SRpeak with FMD in all subjects. After exclusion of non-responders, the association of SRAUC0-ttp was calculated and associations of SRAUC0-30, SRAUC0-60, and SRpeak with FMD were recalculated to evaluate the impact of these individuals on the relationship between shear rate and FMD. Differences among the correlations between each shear rate measure and FMD were tested after Fisher's Z transformation. Two additional sensitivity analyses were performed, including: 1) repeated analysis after subtracting baseline shear rate from shear rate area under the curve and 2) repeated analysis with adjustment for baseline brachial artery diameter using partial correlations. We also evaluated relationships between FMD and measures of shear rate with NMD. Data are reported as means ± SD and the type 1 error rate was set at α ≤ 0.05.
Results
Subject Characteristics
Clinical characteristics and vascular measures of the study sample are reported in Table 1. Subjects had type 2 diabetes and were, on average, middle-aged, obese, with mildly elevated systolic blood pressure, lipids within target ranges for individuals with type 2 diabetes, and good glycemic control. Approximately half the study population was male and non-white.
Table 1.
Characteristics and Vascular Measures of Study Participants (n=50)
| Demographics | |
| Age (years) | 55 ± 7 |
| Men, % | 46 |
| Non-white, % | 48 |
| Clinical characteristics | |
| BMI, kg·m−2 | 34.6 ± 4.8 |
| Body fat, % | 41 ± 8 |
| Total abdominal fat, cm2 | 632 ± 150 |
| Systolic blood pressure, mmHg | 126 ± 15 |
| Diastolic blood pressure, mmHg | 71 ± 11 |
| Total cholesterol, mg·dl−1 | 177 ± 43 |
| HDL cholesterol, mg·dl−1 | 48 ± 14 |
| Triglycerides, mg·dl−1 | 150 ± 115 |
| HbA1c, % | 6.7 ± 1.5 |
| VO2peak ml·kg−1·min−1 | 22.2 ± 6.3 |
| VO2peak L-min- | 2.22 ± 0.83 |
| Vascular Measures | |
| Baseline diameter, mm | 4.34 ± 0.85 |
| Peak diameter, mm | 4.56 ± 0.82 |
| Change from baseline, mm | 0.22 ± 0.15 |
| Change from baseline, FMD% | 5.56 ± 4.14 |
| Time-to-peak dilation, sa | 64 ± 28 |
| Change from baseline, NMD% b | 11.6 ± 6 |
| SRAUC 0-30, s−1 ×103 | 13.3 ± 9.6 |
| SRAUC 0-60, s−1 ×103 | 19.8 ± 12.1 |
| SRAUC 0-60, s−1 ×103a | 23.8 ± 18.4 |
| SRpeak, s−1×103 | 1.13 ± 0.52 |
Values are means ± SD; BMI, Body mass index; HDL, High density lipoprotein; FMD%, flow-mediated dilation as % change from baseline diameter; NMD%, nitroglycerin-mediated dilation as % change from baseline diameter. Post deflation shear rate area-under-the-curve (SRAUC) presented as shear rate 0-30, 0-60 and 0-time to peak. Peak shear rate presented as SRpeak.
N/A in 9 participants classified as non-responders.
Unavailable in 4 participants due to contraindication or poor image quality
Vascular Measures
Brachial artery diameters, percent dilation, and shear rates for the participants are also presented in Table 1. FMD is expressed as both an absolute and a percent change in vessel diameter from baseline. The average percent change in vessel diameter was 5.56%, ranging from 0% to 15.07%. FMD from the model was nearly identical to values measured by visual inspection with an intraclass correlation coefficient of 0.99 (p<0.001). Among responders, average time-to-peak diameter following the hyperemic stimulus was 64 seconds with a standard deviation of 28 seconds (median 68 seconds, range18 to 110 seconds). The largest SRAUC was SRAUC 0-ttp, followed by SRAUC 0-60, SRAUC 0-30 and SRpeak, respectively.
Correlations among Measures of Shear Rate and with Flow-Mediated Dilation
Intra-person Pearson's correlation coefficients (P) among the four different shear rate stimuli were very high, with Ρ>0.78 (p<0.001) between all measures (Table 2). The greatest correlation was between SRAUC 0-30 and SRAUC 0-60 (Ρ=0.972). SRpeak was slightly less correlated with the other measures of shear rate, but the correlations were still excellent, ranging from 0.783 to 0.832.
Table 2.
Pearson's Correlations (P) between measures of shear ratea in 50 Participants with Type 2 Diabetes
| SRAUC 0-30, s−1×103 | SRAUC 0-60, s−1×103 | SRAUC 0-ttp, s−1×103a | SRpeak, s−1×103 | |
|---|---|---|---|---|
| SRAUC 0-30, s−1, ×103 | 1.0 | - | - | - |
| SRAUC 0-60, s−1 ×103 | 0.972c | 1.0 | - | - |
| SRAUC 0-ttp, s−1 ×103b | 0.931c | 0.937c | 1.0 | - |
| SRpeak, s−1 ×103 | 0.827c | 0.832c | 0.783c | 1.0 |
Data presented as P
All measures of shear rate were log transformed
N/A in 9 participants classified as non-responders
(p< 0.001)
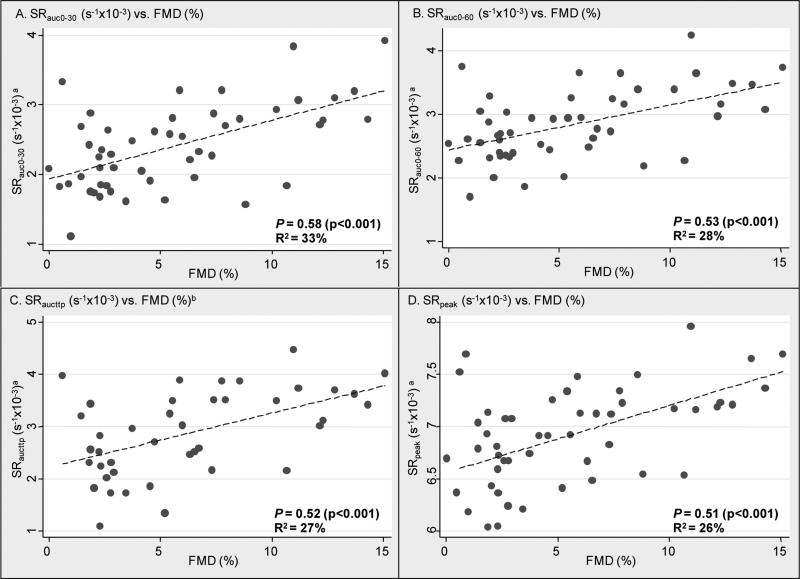
Scatter plots of measures of shear rate stimuli (log transformed) by FMD with Pearson's correlation coefficients and R2 are presented in the Figure 1. All measures of shear rate (SRAUC 0-30, SR0-60, SRAUC 0-ttp, and SRpeak) were significantly correlated with FMD with Ρ ranging from 0.512 to 0.576 (Table 3), thus 26% to 33% of FMD variability was explained. There were no statistical differences between the correlation coefficients. When we repeated the above analyses using shear rate area under the curve above baseline and with adjustment for resting brachial diameter, we found similar though slightly attenuated associations (Table 3). NMD was not associated with FMD or any measure of shear rate.
Figure 1.
Associations of measures of shear rate and brachial artery flow-mediated dilation, presented as Pearson's correlations (Ρ) between % change from baseline diameter (FMD%), and A) SRAUC 0-30a , B) SRAUC 0-60a C) SRAUC 0-ttp a,b and D) SRpeaka in adults with type 2 diabetes (n=50).
a measures of shear rate are log transformed.
b SRAUC 0-ttp available in responders only (n=41)
Table 3.
Pearson's Correlations (P) of FMD with measures of shear ratea in 50 Participants with Type 2 Diabetes
| Overall (n=50) | Responders only (n=41) | With adjustment for resting brachial diameter (n=50) | With subtraction of baseline shear from SRAUC (n=50) | |
|---|---|---|---|---|
| SRAUC 0-30, s−1, ×103 | 0.576 (<0.001) | 0.572 (<0.001) | 0.489 (<0.001) | 0.515 (<0.001) |
| SRAUC 0-60, s−1 ×103 | 0.529 (<0.001) | 0.516 (<0.001) | 0.434 (0.002) | 0.420 (0.004) |
| SRAUC 0-ttp, s−1 ×103 | N/Ab | 0.517 (<0.001) | 0.471 (<0.001)b | 0.412 (0.010)b |
| SRpeak, s−1 ×103 | 0.512 (<0.001) | 0.569 (<0.001) | 0.390 (0.006) | N/Ac |
Data presented as P
All measures of shear rate were log transformed
N/A in 9 participants classified as non-responders
N/A based on formula used to calculate SRpeak
Effect of Non-responders on the Relationship between Shear Rate and Flow-Mediated Dilation
Nine of the 50 subjects (18%) in our sample met our criterion of non-response (≤0% dilation of the brachial artery after release of the blood pressure cuff). Analyses were repeated after excluding non-responders to determine the effect non-response on the relationship of shear rate with FMD in subjects with type 2 diabetes. Demographics and clinical characteristics were similar between responders and non-responders, with the exception of lower total cholesterol in responders vs. non-responders (171 ± 41 mg·dL−1 vs. 203 ± 43 mg·dL−1, p=0.04). In addition, SRAUC 0-30 (14.6 ± 10.0s−1 × 10−3 vs. 7.5 ± 3.2s−1 × 10−3, p=0.016) and SRAUC 0-60 (21.4 ±12.7 s−1 × 10−3 vs. 12.3±4.2 s−1 × 10−3, p=0.022) were significantly higher in responders compared to non responders. SRpeak, baseline brachial diameter, and NMD were not different between groups (data not shown).
Correlations of measures of shear rate (SRAUC 0-30, SRAUC 0-60, and SRpeak) with FMD in responders only (n=41) were nearly identical to that calculated in the full study sample (Table 3).
Discussion
Assessment of brachial artery FMD is widely used as an index for endothelial function in human studies. Pyke and Tschakovsky10 advocated normalization of the FMD response according to the shear stimulus and these same authors recently published data suggesting that shear rate area under the curve from post cuff deflation until time to peak FMD be used for normalization purposes.11 Still, several different shear rate methods have been reported in the literature.24-27 Thijssen and colleagues17 systematically evaluated relationships of four different shear rate measures (SRpeak, SRAUC 0-30, SRAUC 0-60 and SRAUC 0-ttp) with FMD in healthy children and younger and older adults. When pooled across the entire sample, no significant correlation was found between SRpeak and FMD, whereas SRAUC 0-30, SRAUC 0-60, and SR0-ttp correlated significantly. When the age groups were analyzed separately, SR0-ttp only correlated significantly with FMD in younger adults and not in children or older adults, suggesting effect modification by age group. The present investigation is novel to the extent that it examines relationships across these same measures of shear rate stimuli and FMD in a sample of men and women with cardiovascular risk factors (type 2 diabetes and mild hypertension), who are likely to exhibit a diminished FMD response to hyperemic stimuli.28,29
We observed strong and consistent correlations among all measures of shear rate stimuli and FMD in our sample, with no advantage of SRAUC 0-ttp as found in the report by Pyke and Tschakovsky.11 The uniformity of shear rate correlations with FMD likely stems from the high correlations across the four measures of shear rate, although we acknowledge that some of the shear rate may not be relevant for peak dilation (for example, SRAUC 0-60 in individuals with peak diameter prior to 60 seconds). Our findings are in direct contrast to the lack of association observed in similarly aged, apparently healthy older adults 17 and support a more consistent relationship between FMD and shear rate in a sample of adults with type 2 diabetes. While our study was not designed to address potential mechanisms to explain the discrepancy between studies, we speculate that underlying pathophysiological changes characteristic of diabetes30 may become superimposed on age-related changes in vascular structure and function.31 Based on the high correlations in our study, shear rate does appear to explain a fair amount of the variability in FMD response and thus should be reported and used for FMD normalization or covariate adjustment in statistical analyses. Atkinson and colleagues13 recently demonstrated that normalization of FMD by SRAUC 0-ttp was not statistically sound in a sample of 79 healthy males, and our data agree with this analysis in that measures of shear rate were not normally distributed and the intercept of the association was not zero. Additionally, the persistence of FMD – shear rate associations in our sensitivity analyses bolsters our findings of a strong correlation between these two measures. Taken together, we advocate reporting FMD with and without covariate adjustment for measures of shear rate, but not normalization.
Given the inherent limitations of cross-sectional analyses, we cannot draw firm conclusions with regard to how an intervention (e.g., exercise training) that alters regional vascular function will impact the relationship of shear stress with FMD. It is clear that FMD and shear rate represent distinct regional vascular functions (FMD representing macrovascular, shear rate representing microvascular), with FMD being partly dependent on the shear rate. Presumably, an intervention which alters the magnitude of the shear stimulus would result in improved FMD, yet recent studies employing localized handgrip training reveal a complex time course for these vascular adaptations.32,33 Specifically, Tinken et al. demonstrated that over 8 weeks of localized handgrip training, FMD was increased at 2 weeks, independent of a change in shear rate, but returned to baseline level by 8 weeks.33 These results suggest that vascular adaptations may begin with enhanced NO-mediated function of the larger conduits, whereas longer training periods (up to 8 weeks) result in microvessel remodeling and a subsequent return of FMD to baseline values.
The extent to which a prolonged (≥ 6 months) exercise intervention that will likely change fitness, body composition, and cardiovascular risk factors impacts the association between shear rate and FMD remains an important, unanswered question. Previous research has demonstrated that accounting for shear rate measures may alter the interpretation of FMD across static groups (e.g. low vs. moderate cardiovascular risk).34 Further, recent evidence suggests that more direct measures of vascular function, like shear rate or blood flow, may be independently prognostic for cardiovascular disease outcomes35,36 and therefore could augment FMD in the assessment of treatment effects. We aim to study the relationship between changes in shear rate, FMD, and the relationship between these measures in exercise and control participants from the SHAPE2 study once the 6-month follow-up is complete.
Interestingly, we observed a lack of dilation during reactive hyperemia in 18% of the subjects in our study, which is comparable to the report by Irace et al. who observed non-response in 27% of men with type 2 diabetes.20 The authors postulated that the lack of response in this group of individuals may be the result of inadequate shear stimulus subsequent to 5 minutes of lower limb ischemia. This could potentially explain our results, although we observed this non-response while employing an upper arm cuff placement (a technique which leads to recruitment of more resistance vessels and perhaps a more intense shear stimulus). Still, whereas Irace et al. assessed the shear stress stimulus only during the first 15 seconds after cuff release,20 we collected data over the entire vascular response. Analysis of our own data revealed no difference in SRpeak between those who exhibited a measurable FMD (responders) vs. nonresponders. Conversely, SRAUC0-30 and SRAUC0-60 were significantly attenuated in the nonresponders. These data are suggestive of divergent shear rate profiles in which the shear stimulus measured in the non-responders returns to baseline more rapidly than that observed in the responders. Thus, it appears that the responders where exposed to elevated shear for a longer duration, and this difference in microvascular function may contribute to FMD response (or lack of thereof) in populations with cardiovascular risk factors. Future studies should examine this and other mechanisms which could potentially explain these differences.
The presence of ‘non-responders’ among adults with type 2 diabetes calls into question not just which measure of shear rate should be used to quantify the shear signal, but more importantly, how to evaluate and report FMD in this population. Since SRAUC0-ttp was not calculable in this group and did not associate more strongly than other measures of shear in the remaining sample, it may not be as useful in a population with type 2 diabetes as the other shear rate measures. If other measures of shear rate are used, it may be appropriate to include all subjects since we saw no difference in the correlations between shear rate and FMD when excluding nonresponders. This practice should be confirmed in future studies. Further, data from the Framingham study37 indicate that adjustment for shear stress (quantified by hyperemic flow for the initial 15 seconds after cuff deflation) attenuated associations between cardiovascular risk factors and FMD and that shear stress in itself was significantly associated with cardiovascular risk factors. These data highlight the potential utility of shear stress, or more generally, peak hyperemic blood velocity, as an independent vascular measure and predictor of cardiovascular disease.
The primary intent of the current research was to identify the best measure of shear rate to use in research for clinical populations with cardiovascular risk factors. Since we observed no clear advantage for any measure of the shear stimulus, we put forth that SRpeak may have merit for several reasons. First, it avoids the need to simultaneously obtain B-mode images and PW-mode Doppler data throughout the entire vasodilatory response, a limitation of some ultrasound equipment.38 SRpeak can be assessed in PW-mode and the technician can switch to B-mode imaging without missing the peak diameter, if it occurs. Second, SRAUC 0-ttp is simply not relevant in those who do not dilate, as we have shown in 18% of our sample. Lastly, shear stress assessed during peak hyperemic blood flow may have important prognostic implications.37 We recognize that SRAUC 0-30 and SRAUC 0-60 are also calculable in non-responders, and we urge future studies in clinical populations to confirm our findings, particularly in longitudinal designs.
We acknowledge that comparisons of previous work with our own are not direct given the differences in methodology. In the current study, ischemia was induced by placing the occlusion cuff on the upper arm. It is argued that proximal placement of the occlusion cuff evokes a dilatory response that is only partially mediated by nitric oxide, suggesting that the flow-mediated response to distal occlusion is a better index of NO-mediated endothelial function.39,40 For this reason, many investigations have adopted a forearm occlusion model. However, we contend that while the upper arm cuff approach is less often reported in the literature, FMD measured by this technique has been shown to be impaired in the presence of risk factors,41 is reduced following the ingestion of a high fat meal,42 and is predictive of cardiac events,35,43 and is thus a valid measure of risk. Still, it is important to consider that the shear rate profile and the relationship of shear rate measures with FMD could vary with cuff position.
Conclusion
In conclusion, we found that quantification of the shear stimulus following cuff deflation measured as peak shear rate (SRpeak) as well as shear rate area under the curve through 30 seconds (SRAUC 0-30), 60 seconds (SRAUC 0-60) and individual time to peak dilation (SRAUC 0-ttp) were each highly and similarly correlated with FMD in a clinical population with type 2 diabetes and mild hypertension. We additionally found that 18% of our study population had no FMD response. SRpeak is statistically similar to other shear rate measures, easier to aquire, and relevant regardless of FMD response. Our data support the reporting of shear rate as well as FMD with and without covariate adjustment for shear rate in future investigations of similar populations.
Acknowledgements and Grants
This work was supported by grants from the National Heart Lung and Blood Institute (R21-HL095157, 12/01/2008 – 12/01/2010) and the National Institute for Diabetes, Digestive, and Kidney Disorders (R01 DK062368-04, 02/02/04 – 12/31/10).
References
- 1.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010 May;55(5):1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002 Jan 16;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 3.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995 Nov 1;26(5):1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. 2003 Dec 8;115(Suppl 8A):99S–106S. doi: 10.1016/j.amjmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Muiesan ML, Salvetti M, Paini A, et al. Prognostic role of flow-mediated dilatation of the brachial artery in hypertensive patients. J Hypertens. 2008 Aug;26(8):1612–1618. doi: 10.1097/HJH.0b013e328304b083. [DOI] [PubMed] [Google Scholar]
- 7.Kuvin JT, Patel AR, Sliney KA, et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol. 2001 Dec;38(7):1843–1849. doi: 10.1016/s0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- 8.Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005 Feb;23(2):233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Silber HA, Ouyang P, Bluemke DA, Gupta SN, Foo TK, Lima JA. Why is flow-mediated dilation dependent on arterial size? Assessment of the shear stimulus using phase-contrast magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2005 Feb;288(2):H822–828. doi: 10.1152/ajpheart.00612.2004. [DOI] [PubMed] [Google Scholar]
- 10.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005 Oct 15;568(Pt 2):357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007 Apr;102(4):1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 12.Widlansky ME. Shear stress and flow-mediated dilation: all shear responses are not created equally. Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H31–32. doi: 10.1152/ajpheart.01187.2008. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson G, Batterham AM, Black MA, et al. Is the ratio of flow-mediated dilation and shear rate a statistically sound approach to normalization in cross-sectional studies on endothelial function? J Appl Physiol. 2009 Dec;107(6):1893–1899. doi: 10.1152/japplphysiol.00779.2009. [DOI] [PubMed] [Google Scholar]
- 14.de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2004 Jul;287(1):H374–380. doi: 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- 15.McGowan CL, Visocchi A, Faulkner M, et al. Isometric handgrip training improves local flow-mediated dilation in medicated hypertensives. Eur J Appl Physiol. 2006 Nov;98(4):355–362. doi: 10.1007/s00421-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 16.Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol. 2006 Dec;291(6):H3043–3049. doi: 10.1152/ajpheart.00190.2006. [DOI] [PubMed] [Google Scholar]
- 17.Thijssen DH, Bullens LM, van Bemmel MM, et al. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H57–64. doi: 10.1152/ajpheart.00980.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker BA, Trehearn TL, Meendering JR. Pick Your Poiseuille: Normalizing the Shear Stimulus In Studies of Flow-Mediated Dilation. J Appl Physiol. 2008 Nov 13; doi: 10.1152/japplphysiol.91302.2009. [DOI] [PubMed] [Google Scholar]
- 19.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irace C, Tschakovsky ME, Carallo C, Cortese C, Gnasso A. Endothelial dysfunction or dysfunctions? Identification of three different FMD responses in males with type 2 diabetes. Atherosclerosis. 2008 Oct;200(2):439–445. doi: 10.1016/j.atherosclerosis.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 22.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997 Jul;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 23.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007 Aug;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 24.Chironi G, Craiem D, Miranda-Lacet J, Levenson J, Simon A. Impact of shear stimulus, risk factor burden and early atherosclerosis on the time-course of brachial artery flow-mediated vasodilation. J Hypertens. 2008 Mar;26(3):508–515. doi: 10.1097/HJH.0b013e3282f3adc4. [DOI] [PubMed] [Google Scholar]
- 25.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol. 2006 Apr;290(4):H1446–1453. doi: 10.1152/ajpheart.00771.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kooijman M, Thijssen DH, de Groot PC, et al. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008 Feb 15;586(4):1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol. 2007 Sep;103(3):843–851. doi: 10.1152/japplphysiol.00273.2007. [DOI] [PubMed] [Google Scholar]
- 28.Malik AR, Kondragunta V, Kullo IJ. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension. 2008 Jun;51(6):1512–1518. doi: 10.1161/HYPERTENSIONAHA.107.106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996 Mar 1;27(3):567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 30.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002 May 15;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 31.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003 Jan 7;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 32.Dobrosielski DA, Greenway FL, Welsh DA, Jazwinski SM, Welsch MA. Modification of vascular function after handgrip exercise training in 73- to 90-yr-old men. Med Sci Sports Exerc. 2009 Jul;41(7):1429–1435. doi: 10.1249/MSS.0b013e318199bef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010 Feb;55(2):312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 34.Padilla J, Johnson BD, Newcomer SC, et al. Adjusting flow-mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res. 2009;46(6):592–600. doi: 10.1159/000226227. [DOI] [PubMed] [Google Scholar]
- 35.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011 Mar;57(3):363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 36.Huang AL, Silver AE, Shvenke E, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007 Oct;27(10):2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004 Aug;44(2):134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 38.McGowan CL, Levy AS, McCartney N, MacDonald MJ. Isometric handgrip training does not improve flow-mediated dilation in subjects with normal blood pressure. Clin Sci (Lond) 2007 Jun;112(7):403–409. doi: 10.1042/CS20060195. [DOI] [PubMed] [Google Scholar]
- 39.Doshi SN, Naka KK, Payne N, et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 2001 Dec;101(6):629–635. [PubMed] [Google Scholar]
- 40.Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005 Sep;99(3):1233–1234. doi: 10.1152/japplphysiol.00601.2005. discussion 1237-1238. [DOI] [PubMed] [Google Scholar]
- 41.Vogel RA. The Mediterranean diet and endothelial function: why some dietary fats may be healthy. Cleve Clin J Med. 2000 Apr;67(4):232, 235–236. doi: 10.3949/ccjm.67.4.232. [DOI] [PubMed] [Google Scholar]
- 42.Plotnick GD, Corretti MC, Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997 Nov 26;278(20):1682–1686. [PubMed] [Google Scholar]
- 43.Gokce N, Keaney JF, Jr., Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002 Apr 2;105(13):1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]