Abstract
Mitochondria are dynamic organelles that constantly undergo fission and fusion. The balance between fission and fusion determines the fate of the cell. In this study, we show that mitochondrial fission factor (MFF) is upregulated upon hydrogen peroxide treatment or ischemia/reperfusion (I/R) injury. Knockdown of MFF attenuated hydrogen peroxide- and I/R injury-induced cardiomyocyte apoptosis and myocardial infarction. We found that MFF is a direct target of miR-761, and miR-761 inhibits mitochondrial fission and cardiomyocyte apoptosis by repressing MFF. This study reveals a novel model of mitochondrial fission regulation, which is composed of miR-761 and MFF. Modulation of their levels may provide a new approach for tackling apoptosis and myocardial infarction.
Keywords: MicroRNAs, Mitochondrial fission, Apoptosis, MFF, Hydrogen peroxide, Ischemia/reperfusion, Free radicals
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression by inhibiting target mRNA translation or promoting mRNA degradation [1–3]. In the apoptotic cascades, some miRNAs have been found to be proapoptotic. miR-34a induces apoptosis by suppressing the expression of silent information regulator 1 (SIRT1) [4]. miR-29b downregulates Mcl-1 and promotes apoptosis [5]. Overexpression of miR-320 can increase cardiac apoptosis and infarction sizes upon ischemia/reperfusion injury [6]. In contrast, some miRNAs can be antiapoptotic. For example, miR-21 inhibits apoptosis [7]. miR-155 suppresses apoptosis by targeting tumor protein 53-induced nuclear protein 1 [8]. Thus, different miRNAs exert distinct effects on apoptosis.
Mitochondria are highly dynamic organelles that possess two opposing activities. They divide and fuse constantly, and the balance between mitochondrial fission and fusion affects the morphology of mitochondria. Various proteins participate in the regulation of mitochondrial dynamics [9–13]. Dysregulation of mitochondrial dynamics is closely correlated with apoptosis. Overexpression of mitofusin 1 (MFN1) and mitofusin 2 (MFN2) promotes mitochondrial fusion and inhibits apoptosis [14]. Dynamin-related protein 1 (Drp1) is necessary for mitochondrial fission and inhibition of Drp1 suppresses apoptosis [15,16]. Overexpression of Fis1 induces mitochondrial fission and cytochrome c release resulting in apoptosis [17]. Abnormality in mitochondrial dynamics can provoke the occurrence of severe diseases. Opa1 mutation was observed in dominant optic atrophy [18,19]. Mutation in MFN2 results in Charcot-Marie-Tooth neuropathy type 2A [20]. Mitochondrial fission factor (MFF) is located in the outer membrane of mitochondria and participates in the regulation of mitochondrial fission [21]. However, the role of MFF in the heart remains unknown.
Our present work revealed that MFF plays a key role in regulating mitochondrial dynamics. miR-761 inhibits the mitochondrial fission program and apoptosis by targeting MFF. Our data reveal a novel model regulating the mitochondrial network, which is composed of miR-761 and MFF.
Materials and methods
Cell culture and treatment
Neonatal rat cardiomyocytes were isolated from 2-day-old Wistar rats as we have described [22]. In brief, the dissected hearts were washed and minced in HEPES-buffered saline solution containing 130 mM NaCl, 3 mM KCl, 1 mM NaH2PO4, 4 mM glucose, and20 mMHEPES(pHadjustedto7.35withNaOH). Tissueswerethen dispersed in a series of incubations at 37 °C in HEPES-buffered saline solutioncontaining1.2 mg/mlpancreatinand0.14 mg/mlcollagenase (Worthington). After centrifugation, the cells were resuspended in Dulbecco’s modified Eagle medium/F-12 (Invitrogen) containing 5% heat-inactivated horse serum, 0.1 mMascorbate, insulin-transferring sodium selenitemedium supplement,100 U/ml penicillin,100 μg/ml streptomycin, and 0.1 mMbromodeoxyuridine. The dissociated cells were preplated at 37 °C for 1 h. After that, the cardiomyocytes were diluted to 1 × 106 cells/ml and plated in 10 μg/ml laminin-coated culture dishes according to the specific experimental requirement. Hydrogen peroxide treatment was performed as described [23]. In brief, cardiomyocyteswere incubated at 37 °C in Hanks’ balanced salt solution containing 100 μM hydrogen peroxide for the indicated periods of time. The treatment was terminated by removing the Hanks’ balanced salt solution containing hydrogen peroxide.
Adenovirus construction and infection
The adenovirus containing β-galactosidase (β-gal) was as we described elsewhere [24]. The MFF RNAi target sequence was 5′-GACCAGCAGATCTTGACCT-3′; the scramble MFF RNAi target sequence was 5′-ACAGTCAGCAGTCATCGTC-3′. They were cloned into a pSilencer adeno 1.0-CMV vector (Ambion) according to the manufacturer’s instructions.
To generate adenoviruses expressing miR-761, rat genomic sequence harboring the pre-miR-761 was amplified using the primers forward, 5′-TTCATTCTACCCAGATGCGTT-3′, and reverse, 5′-GAGGATTCACGTGGAGAAGTT-3′, and then cloned into the adenovirus system. All adenoviruses were amplified in HEK293 cells. Adenoviral infection of cardiomyocytes was performed as described [23].
Transfection of miR-761 mimic and antagomir
miR-761 mimic, the mimic negative control (NC), miR-761 antagomir, and antagomir NC were synthesized by GenePhama Co. Ltd. They were transfected at 50 nM. The transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s guidelines.
Reporter construction and luciferase assay
The MFF 3′UTR was amplified from mouse genomic DNA by PCR. The forward primer was 5′-CCTCAGCATTCACATATATTG-3′. The reverse primer was 5′-AAAACAGCATTGCACACAGAC-3′. The PCR products were gel-purified and ligated into the pGL3 reporter vector (Promega) immediately downstream of the stop codon of the luciferase gene. To generate the mutated MFF 3′UTR construct, the mutations were introduced using a QuickChange II XL site-directed mutagenesis kit (Stratagene).
Luciferase activity assay was performed as described [25]. In brief, cells were cultured in 24-well plates. They were infected with adenoviral miR-761 or β-gal and then transfected with the plasmid construct pGL3-MFF-3′UTR or pGL3-MFF-3′UTR-mut at a concentration of 200 ng/well using Lipofectamine 2000 (Invitrogen). Renilla luciferase plasmid was also cotransfected at 2.5 ng/well, serving as the internal control. Forty-eight hours after transfection, the cells were lysed and the luciferase activity was detected with a Dual Luciferase Reporter Assay kit (Promega).
Quantitative real-time PCR (qRT-PCR)
Stem-loop qRT-PCR for mature miR-761 was performed as described [26]. Total RNA was extracted using the TRIzol reagent (Invitrogen). After DNase I treatment (Takara, Japan), the RNA was reverse transcribed with reverse transcriptase (ReverTra Ace, Toyobo). The samples were run in triplicate in an Applied Biosystems ABI 7000 sequence detector system according to the manufacturer’s instructions. The results of qRT-PCR were normalized to that of U6. The primers for U6 were forward, 5′-GCTTCGGCAGCACATATACTAA-3′, and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The specificity of the PCR amplification was confirmed by agarose gel electrophoresis.
Immunoblot
Immunoblot was performed as described [27]. In brief, the cells were lysed for 1 h at 4 °C in a lysis buffer (20 mM Tris, pH 7.5, 2 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 250 mM sucrose, 0.1 mM phenylmethylsulfonyl fluoride, 1% Triton X-100, and a protease inhibitor mixture). The samples were subjected to 12% SDS–PAGE and transferred to nitrocellulose membranes. Equal protein loading was controlled by Ponceau red staining of membranes. The blots were probed using the primary antibodies. Antibody to MFF was from Abcam.
Mitochondrial staining
Mitochondria were stained as described [16,28]. In brief, cells were plated onto the coverslips coated with 0.01% poly-L-lysine. After treatment, they were stained for 20 min with 0.02 μM MitoTracker Red CMXRos (Molecular Probes). Mitochondria were imaged using a laser-scanning confocal microscope (Zeiss LSM510 META).
Target protector preparation and transfection
The target protector was designed and named as others and we have described [24,29]. In brief, the MFF-TPmiR-761 sequence was 5′-GATGCAAAGACAGAAACAATGAAGT-3′. MFF-TPcontrol was 5′-CCTCTTACCTCAGTTACAATTTATA-3′. They were obtained from Gene Tools. Transfection of the target protector was performed using an Endo-Porter kit (Gene Tools) according to the manufacturer’ s instructions.
Electron microscopy
Heart ultrastructural analysis was performed to quantify mitochondrial fission. Sample preparations and conventional electron microscopy were carried out as described [30]. Samples were examined at a magnification of 15,000 with a JEOL JEM-1230 transmission electron microscope. For comparison of mitochondrial fission, electron microscopy micrographs of thin sections were evaluated. The sizes of individual mitochondria were measured by Image-Pro Plus software. We determined the mitochondria smaller than 0.6 μm2 to be fission mitochondria.
Animal experiments
Adult male C57BL/6 mice (8 weeks of age) were purchased from the Institute of Laboratory Animal Science of the Chinese Academy of Medical Sciences (Beijing, China). Experiments were performed according to the protocol assigned by the Institute Animal Care Committee. For three consecutive days, the mice received intravenous injections of miR-761 mimic or its control at a dose of 30 mg/kg body wt in a small volume (0.2 ml) per injection.
To perform ischemia/reperfusion (I/R) surgery, the mice were anesthetized and the chest was opened. An 8-0 silk suture was passed around the left anterior descending coronary artery (LAD) at the inferior border of the left auricle. The LAD was occluded by snaring with the vinyl tube through which the ligature had been passed. The coronary artery was occluded by pulling the snare tight and securing it with a hemostat. Forty-five minutes later, the ligature was released and the heart was reperfused. The sham-operated group experienced the same procedure except the snare was left untied. After 24 h of reperfusion, the mice were anesthetized. The chest of each mouse was reopened and the suture was religated. Evans blue dye (1 ml of a 2.0% solution; Sigma–Aldrich) was injected into the jugular vein into the heart for delineation of the ischemic area from the nonischemic area. The heart was sliced and immersed in 1.0% 2,3,5-triphenyltetrazolium chloride (Sigma–Aldrich) at 37 °C for 15 min to discriminate viable from nonviable myocardium within the risk zone. The area of infarction (INF), area at risk (AAR), and nonischemic left ventricle (LV) were assessed with computer-assisted planimetry (NIH Image 1.57) by an observer blinded to sample identity. The ratios of AAR/LV and INF/AAR were calculated. The AAR in the center of the territory of the left anterior descending coronary artery and the remote area in the posterior part of the left ventricle far from the AAR were prepared as described [31].
To perform intracoronary delivery of adenovirus, 5 days before the I/R operation, the mice were anesthetized. The chest was opened and MFF small interfering RNA (siRNA) adenoviruses (2 × 1010 m.o.i.) were injected with a catheter from the apex of the left ventricle into the aortic root while the aorta and pulmonary arteries were cross-clamped. The clamp was maintained for 20 s when the heart pumped against a closed system. The chest was closed and the animal was transferred back to its cage for recovery.
Echocardiographic assessment
Echocardiography was performed as we have described [24]. Generally, the mice were mildly anesthetized and transthoracic echocardiography was performed using a Vevo 770 high-resolution system (Visualsonics, Toronto, Canada). Two-dimensional guided M-mode tracings were recorded in both parasternal long and short axis views at the level of papillary muscles. Systolic left-ventricular internal diameter (LVIDs) and diastolic left-ventricular internal diameter (LVIDd) were measured. We calculated fractional shortening of left-ventricular diameter using the standard equation [(LVIDd − LVIDs)/LVIDd] × 100. All of the measurements were made from more than three beats and averaged.
Histology
The harvested hearts were fixed in 10% formalin, embedded in paraffin, and sectioned at 6 μm thickness. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) was performed according to the manufacturer’s instructions (Roche). The cardiomyocytes were stained with antibody to α-actinin (Sigma) and the total nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Statistical analysis
Data are expressed as means ± SEM. The statistical comparison among groups was performed by one-way ANOVA. Paired data were evaluated by Student’s t test. P<0.05 was considered statistically significant.
Result
MFF regulates mitochondrial fission and apoptosis in vitro
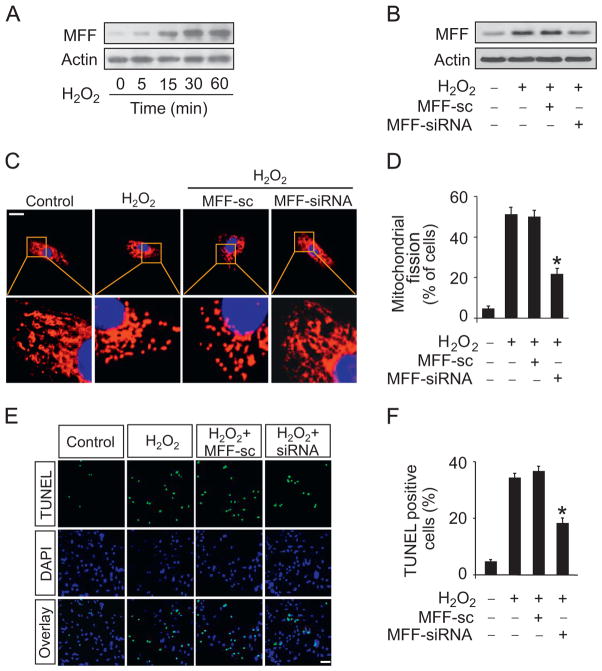
To study the role of MFF in cardiomyocytes, we treated cardiomyocytes with hydrogen peroxide. The expression levels of MFF were upregulated after hydrogen peroxide treatment (Fig. 1A). To study the functional role of MFF, MFF siRNA adenoviruses were constructed and they efficiently attenuated the elevations of MFF upon hydrogen peroxide insult (Fig. 1B). Knockdown of MFF attenuated mitochondrial fission induced by hydrogen peroxide as revealed by the analysis of mitochondrial morphology (Fig. 1C) and the counting of the cells with fission (Fig. 1D). Further, knockdown of MFF attenuated hydrogen peroxide-induced apoptosis (Fig. 1E and F). Taken together, these results suggest that MFF participates in the cascades of mitochondrial fission and apoptosis in cardiomyocytes.
Fig. 1.
MFF regulates mitochondrial fission and apoptosis in vitro. (A) MFF is upregulated upon hydrogen peroxide treatment. Cardiomyocytes were treated with 100 μM hydrogen peroxide, and MFF was detected by immunoblot. (B) MFF siRNA can efficiently knock down MFF expression. Cardiomyocytes were infected with adenoviral constructs of MFF siRNA or its scrambled form (sc) at a m.o.i. of 100 and then exposed to hydrogen peroxide. MFF was analyzed by immunoblot. (C and D) Knockdown of MFF attenuated mitochondrial fission induced by hydrogen peroxide. Cardiomyocytes were infected with adenoviruses harboring MFF siRNA or its sc form and then treated with hydrogen peroxide. (C) Mitochondria were stained by MitoTracker red. Scale bar, 20 μm. (D) The nuclei were visualized by DAPI. Mitochondrial fission was counted and calculated. *P<0.05 versus hydrogen peroxide alone. (E and F) Knockdown of MFF reduces apoptosis induced by hydrogen peroxide. Cardiomyocytes were treated as described for (C) and (D). (E) TUNEL was employed to analyze apoptotic cells. (F) TUNEL-positive cells were counted and calculated. *P<0.05 versus hydrogen peroxide alone.
MFF regulates mitochondrial fission and apoptosis in vivo
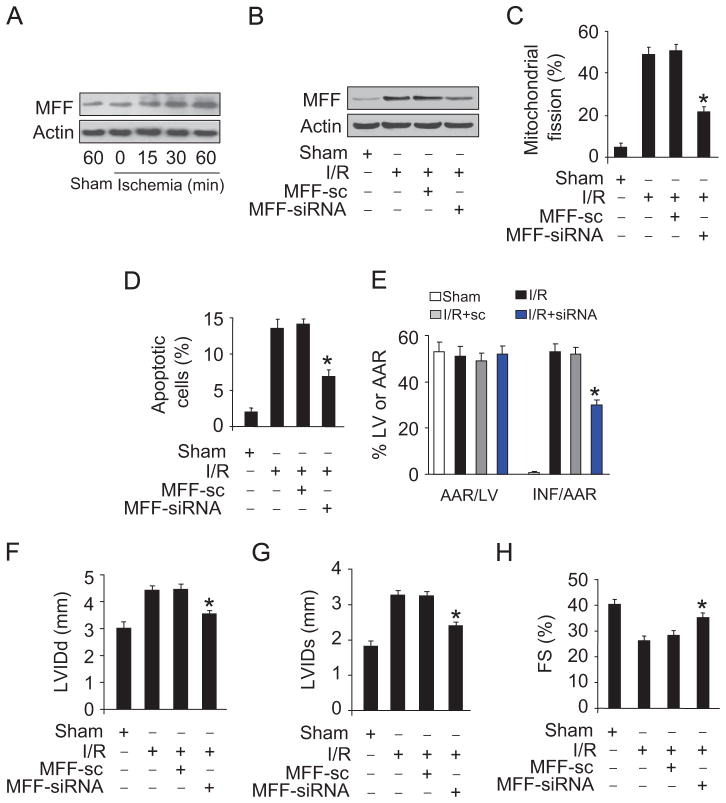
To understand the role of MFF in myocardial infarction, we employed the I/R model. Ischemia induced an elevation in MFF levels (Fig. 2A). The administration of MFF siRNA adenoviruses downregulated its elevated expression upon I/R (Fig. 2B). Knockdown of MFF resulted in a reduction in mitochondrial fission (Fig. 2C), apoptosis (Fig. 2D), and infarct size (Fig. 2E) upon I/R. Echocardiography showed that the cardiac function was ameliorated (Fig. 2F–H). These data suggest that MFF participates in mediating the signal for mitochondrial fission and apoptosis in the heart.
Fig. 2.
MFF regulates mitochondrial fission and apoptosis in vivo. (A) MFF is upregulated in response to ischemia. Mice were subjected to various time periods of ischemia, and MFF was detected by immunoblot. (B) MFF siRNA can efficiently knock down MFF in the animal model. After intracoronary delivery of adenoviruses harboring MFF siRNA or the scrambled form, the mice were subjected to I/R injury. MFF was detected by immunoblot. (C and D) Knockdown of MFF attenuates I/R-induced mitochondrial fission and apoptosis. Mice were treated as described for (B). (C) Mitochondrial fission was analyzed. (D) TUNEL assay was employed to detect apoptotic cells. *P<0.05 versus I/R alone. (E) Knockdown of MFF decreases myocardial infarct sizes in response to I/R. Mice were treated as described for (B), and infarct sizes were calculated. *P<0.05 versus I/R alone. (F–H) Echocardiographic analysis. Mice were treated as described for (B), and echocardiography was employed to test heart function. (F) Diastolic left-ventricular internal diameter (LVIDd), (G) systolic left-ventricular internal diameter (LVIDs), and (H) fractional shortening (FS) were calculated. *P<0.05 versus I/R alone.
miR-761 participates in the regulation of MFF expression
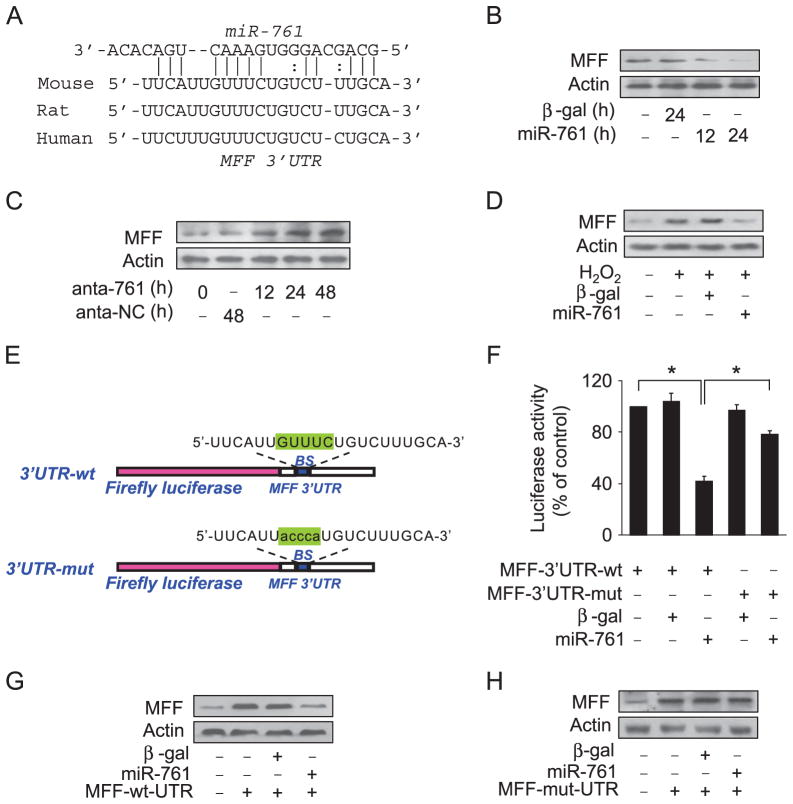
miRNA is able to suppress gene expression. To explore the underlying mechanism by which MFF is upregulated upon hydrogen peroxide and I/R insult, we tested whether miRNA can control MFF expression. We analyzed the 3′UTR of MFF using the target scan program and found some potential miRNAs. To understand which miRNA is involved in the regulation of MFF, we employed RT-PCR to detect miRNA levels in response to hydrogen peroxide treatment. Among several miRNAs, miR-761 was substantially reduced. In addition, two other programs including RNA22 and RNAhybrid also showed that miR-761 has a potential binding site in the MFF 3′UTR (Fig. 3A). Accordingly, we focused on miR-761. To test whether miR-761 is involved in the regulation of MFF expression, several approaches were employed. Enforced expression of miR-761 led to a reduction in MFF levels (Fig. 3B). Administration of the miR-761 antagomir could elevate the expression of MFF (Fig. 3C). Enforced expression of miR-761 could attenuate the increase in MFF levels in response to hydrogen peroxide treatment (Fig. 3D).
Fig. 3.
miR-761 targets mitochondrial protein MFF. (A) Analysis of MFF 3′UTR potential binding site for miR-761. (B) miR-761 suppresses the expression of MFF. Adenoviruses harboring miR-761 or β-gal were used to infect cardiomyocytes at a multiplicity of infection of 100. The expression of MFF was detected by immunoblot. (C) Knockdown of miR-761 elevates MFF levels. Cardiomyocytes were transfected with antagomir miR-761 (anta-761) or antagomir negative control (anta-NC). The expression of MFF was detected by immunoblot. (D) miR-761 attenuates hydrogen peroxide-induced MFF upregulation. Cardiomyocytes were infected with adenoviruses encoding miR-761 or β-gal at a multiplicity of infection of 100 and then treated with 100 μM hydrogen peroxide. MFF levels were detected by immunoblot. (E) miR-761 binding site was mutated in the MFF 3′UTR. (F) miR-761 inhibits the luciferase activity of MFF with wild-type 3′UTR. HEK293 cells were infected with adenoviral miR-761 or β-gal and then transfected with MFF with a wild-type 3′UTR or mutated 3′UTR. The cells were harvested, and luciferase activity was measured. *P<0.05. (G and H) miR-761 can inhibit the expression of MFF with wild-type 3′UTR. Cardiomyocytes were co-infected with adenoviral miR-761 or β-gal together with MFF with a wild-type 3′UTR or mutated 3′UTR. The expression of MFF was assayed by immunoblot.
Further, we employed the luciferase assay system to test whether miR-761 can influence the translation of MFF (Fig. 3E). As shown in Fig. 3F, the wild-type 3′UTR of MFF exhibited a low translation level in the presence of miR-761, whereas the mutated 3′UTR did not show a significant response to miR-761. We finally tested whether miR-761 regulates MFF expression by targeting its 3′UTR. MFF with wild-type 3′UTR was expressed at a low level in the presence of miR-761 (Fig. 3G). Introduction of mutations into the miR-761 binding site abolished the inhibitory effect of miR-761 on MFF expression (Fig. 3H). Taken together, these results suggest that MFF is a specific target of miR-761.
miR-761 can inhibit mitochondrial fission and apoptosis
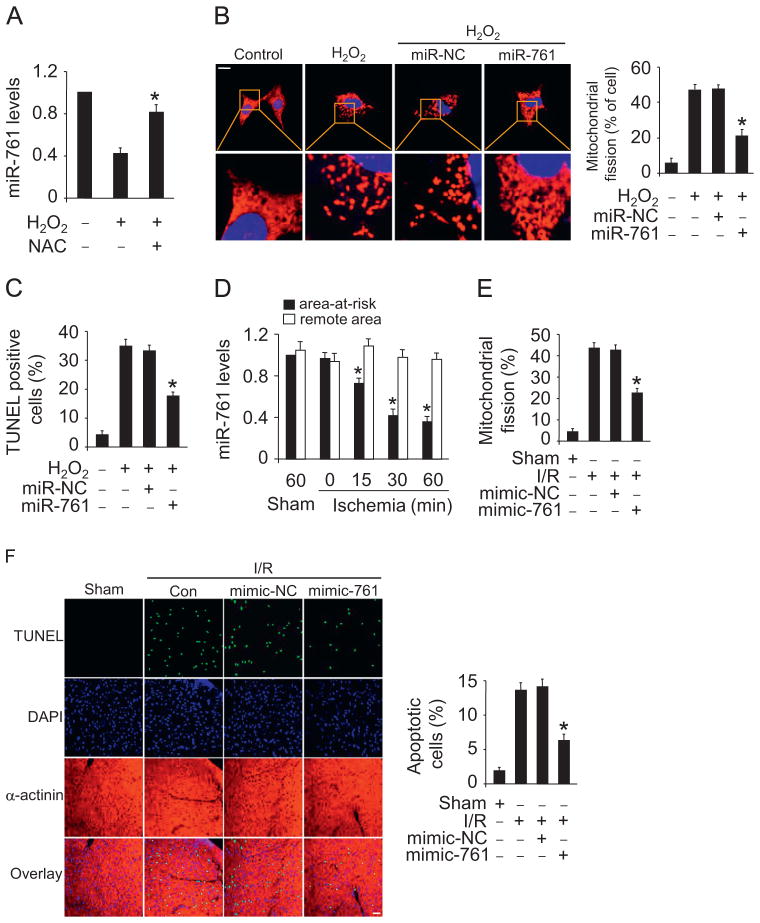
We explored the functional role of miR-761 in mitochondrial fission and apoptosis. miR-761 is downregulated in response to hydrogen peroxide treatment. Administration of the antioxidant N-acetylcysteine (NAC) prevented miR-761 reduction induced by hydrogen peroxide (Fig. 4A). Enforced expression of miR-761 inhibited mitochondrial fission (Fig. 4B) and apoptosis (Fig. 4C) induced by hydrogen peroxide. To understand the pathophysiological role of miR-761, we detected whether miR-761 is involved in the pathogenesis of myocardial infarction in the animal model. miR-761 was reduced in response to ischemia (Fig. 4D). Delivery of miR-761 mimics resulted in a reduction in mitochondrial fission (Fig. 4E) and apoptosis (Fig. 4F) upon I/R injury. Thus, miR-761 plays a functional role in regulating mitochondrial fission and apoptosis.
Fig. 4.
miR-761 can inhibit mitochondrial fission and apoptosis. (A) miR-761 is downregulated upon hydrogen peroxide treatment and addition of NAC abolished downregulation of miR-761 induced by hydrogen peroxide. Cardiomyocytes were pretreated with 10 mM NAC and then treated with 100 μM hydrogen peroxide. miR-761 levels were detected by qRT-PCR. (B and C) miR-761 suppresses hydrogen peroxide-induced mitochondrial fission and apoptosis. Cardiomyocytes were transfected with miR-761 mimic or NC. After that, they were treated with 100 μM hydrogen peroxide. (B) Mitochondrial fission and (C) apoptosis were assayed. *P<0.05 versus hydrogen peroxide alone. (D) Analysis of the expression levels of miR-761. Mice were subjected to various periods of ischemia and the expression of miR-761 was assayed by qRT-PCR. *P<0.05 versus sham or 0 min group. (E and F) miR-761 attenuates mitochondrial fission and apoptosis upon I/R injury. Adult C57BL/6 mice received miR-761 mimic or mimic-NC for 3 days. They were then subjected to I/R. (E) The percentage of mitochondrial fission was calculated. (F) Apoptosis was detected by TUNEL. *P < 0.05 versus I/R alone.
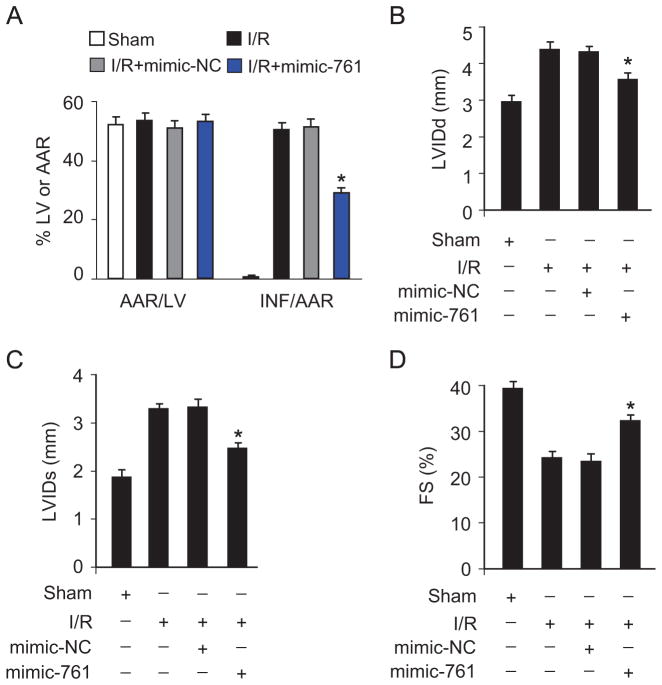
miR-761 attenuates myocardial infarction in mice
Subsequently, we tested whether miR-761 affects the size of myocardial infarction and cardiac function. Administration of miR-761 mimics could significantly reduce infarct sizes upon I/R injury (Fig. 5A). Concomitantly, the heart function was ameliorated when the mice were treated with miR-761 mimics as assessed by echocardiography (Fig. 5B–D).
Fig. 5.
miR-761 attenuates myocardial infarction in mice. (A) miR-761 attenuates myocardial infarction in response to I/R injury. Adult C57BL/6 mice received miR-761 mimic or mimic-NC for 3 days. They were then subjected to 45 min of ischemia and 24 h of reperfusion. Myocardial infarction sizes were detected. *P<0.05 versus I/R alone. (B–D) miR-761 improves heart function assessed by echocardiography. (B) Diastolic left-ventricular internal diameter, (C) systolic left-ventricular internal diameter, and (D) fractional shortening were calculated. *P<0.05 versus I/R alone.
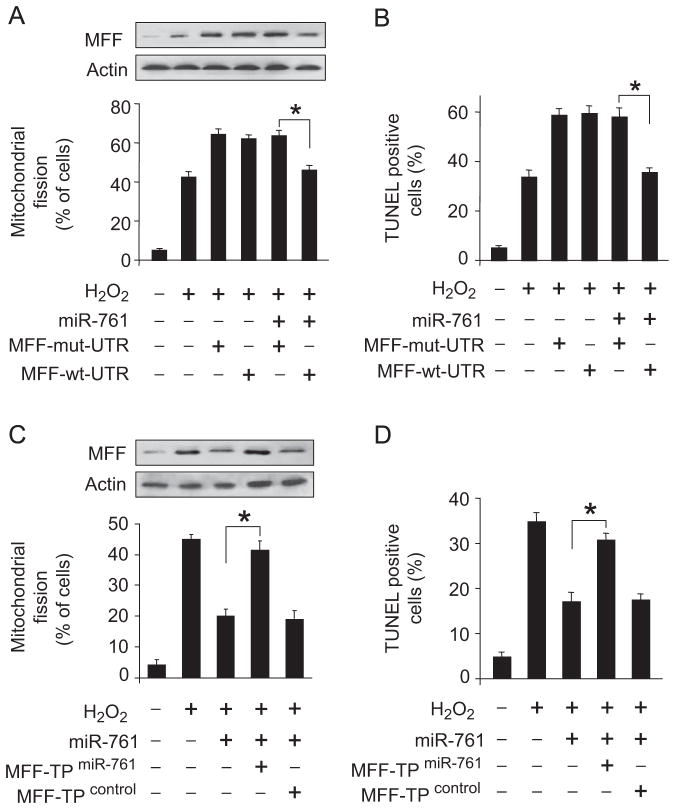
miR-761 regulates mitochondrial fission and apoptosis by targeting MFF
We explored how miR-761 exerts its effect on the mitochondrial network. As miR-761 is able to suppress MFF expression, we thus tested whether MFF is a mediator of miR-761. MFF with mutated 3′UTR, but not the wild-type MFF, attenuates the inhibitory effect of miR-761 on mitochondrial fission and apoptosis (Fig. 6A and B). To further confirm the relationship between miR-761 and MFF in mitochondrial fission machinery, we employed the target protector technology in which a target protector is able to disrupt the specific interaction of miRNA–mRNA pairs. To this end, we produced MFF target protector and observed that the inhibitory effect of miR-761 on mitochondrial fission and apoptosis was reduced in the presence of the MFF target protector (Fig. 6C and D). These data suggest that miR-761 targets MFF in the cascades of mitochondrial fission and apoptosis.
Fig. 6.
miR-761 regulates mitochondrial fission and apoptosis through MFF. (A and B) miR-761 suppresses the expression of MFF, mitochondrial fission, and apoptosis in the presence of MFF with wild type 3′UTR rather than mutated 3′UTR. Cardiomyocytes were infected with adenoviral miR-761, along with constructs of MFF-wt-UTR or MFF-mut-UTR. (A) Mitochondrial fission and (B) apoptosis were assayed. MFF levels were detected by immunoblot. *P<0.05. (C and D) The inhibitory effect of miR-761 on mitochondrial fission and apoptosis is abolished by MFF target protector. Cardiomyocytes were infected with adenoviruses harboring miR-761 and then transfected with MFF target protector or the protector control. (C) Mitochondrial fission and (D) apoptosis were detected. MFF levels were detected by immunoblot. *P<0.05.
Discussion
Heart failure is a great threat to health worldwide. It is important to find out the molecular mechanism leading to cardiac apoptosis and the resulting heart failure. Our present study revealed that MFF is able to regulate the mitochondrial network and apoptosis. We found that miR-761 is responsible for the downregulation of MFF and it can suppress mitochondrial fission and apoptosis by targeting MFF. Our results provide novel evidence demonstrating that miR-761 and MFF constitute an axis in the machinery of the mitochondrial network and apoptosis.
Mitochondrial fission and fusion are mediated by a multitude of proteins [32,33]. These proteins have important roles in a variety of fields. For mitochondrial fusion, the dynamin-related guanosine triphosphatases (GTPases) MFN1 and MFN2 are involved in mitochondrial outer membrane fusion through their active GTPase domain. Opa1 participates in inner mitochondrial membrane fusion. Drp1 is mainly located in the cytosol and can be recruited to the outer membrane of mitochondria during mitochondrial fission. Fis1 functions as a receptor protein for Drp1 in mitochondria. Recently, it was found that Mid49, Mid51 [34], and MFF [35] are needed for the recruitment of Drp1 to mitochondria during mitochondrial fission. Mitochondrial fission is correlated with apoptosis [36]. It is reported that knockdown of MFF could inhibit mitochondrial fission, cytochrome c release, and apoptosis [21]. In this study, we demonstrate that knockdown of MFF can inhibit mitochondrial fission and apoptosis in cardiomyocytes. Administration of MFF siRNA can reduce myocardial infarct size. The relationship between MFF and other fusion/fission factors in the apoptotic pathway of cardiomyocytes needs to be determined in the future study.
miRNAs are small noncoding RNAs that negatively regulate gene expression. They play an important role in the process of cardiac apoptosis by suppressing the expression of the target genes [2,37]. For example, loss of miR-214 in mice causes increased apoptosis upon I/R injury [38]. miR-20a inhibits apoptosis in cardiomyocytes in response to treatment with hypoxia/reoxygenation in vitro [39]. miR-210 can inhibit apoptosis during myocardial infarction in mice [40]. miR-195 promotes palmitate-induced apoptosis in cardiomyocytes by targeting SIRT1 [41]. In this study, we found that miR-761 can directly inhibit the expression of MFF by base-pairing with the 3′UTR of MFF in cardiomyocytes. miR-761 protects cardiomyocytes from hydrogen peroxide and ischemia/reperfusion-induced apoptosis and myocardial infarction. A miRNA may have multiple targets. It would be interesting to know whether other genes can be regulated by miR-761 under specific conditions.
The expression levels of miRNAs remain constant under physiological conditions. Their alteration may cause pathological disorders. Our results showed that miR-761 was downregulated in response to pathological insult. Nevertheless, the molecular mechanism governing miR-761 expression during apoptosis remains unknown. Our previous work has shown that the expression of a miRNA can be regulated at the transcriptional level [24]. Other reports also show that miRNAs can be regulated by transcription factors. For example, the transcription factor AP-1 plays a key role in tumorigenesis, and it can induce miR-21 expression [42]. miR-34a causes dramatic reprogramming of gene expression and promotes apoptosis, and it is transactivated by the tumor suppression gene p53 [43]. Serum response factor can directly bind to the promoters of miR-1-1 and miR-1-2 and activate their expression [44]. Because the function of miR-761 is closely related to its expression level, it is critically important to identify transcription factors that participate in the regulation of miR-761 in future studies.
In summary, this study reveals that miR-761 is integrated into the machinery of mitochondrial dynamics and it can affect the mitochondrial apoptotic pathway by targeting MFF. Thus, modulation of miR-761 may represent a novel approach for interventional treatment of heart disease, including myocardial infarction.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31010103911, 81000034) and the U.S. National Institutes of Health (5R01HL102202).
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 3.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 4.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 8.Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci USA. 2007;104:16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 11.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 12.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 14.Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 15.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 17.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 18.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 19.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 20.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 21.Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan WQ, Wang K, Lv DY, Li PF. Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem. 2008;283:29730–29739. doi: 10.1074/jbc.M805514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YZ, Lu DY, Tan WQ, Wang JX, Li PF. p53 initiates apoptosis by transcriptionally targeting the antiapoptotic protein ARC. Mol Cell Biol. 2008;28:564–574. doi: 10.1128/MCB.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106:12103–12108. doi: 10.1073/pnas.0811371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Long B, Zhou J, Li PF. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem. 2010;285:11903–11912. doi: 10.1074/jbc.M109.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murtaza I, Wang HX, Feng X, Alenina N, Bader M, Prabhakar BS, Li PF. Down-regulation of catalase and oxidative modification of protein kinase CK2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J Biol Chem. 2008;283:5996–6004. doi: 10.1074/jbc.M706466200. [DOI] [PubMed] [Google Scholar]
- 28.Wang JX, Li Q, Li PF. Apoptosis repressor with caspase recruitment domain contributes to chemotherapy resistance by abolishing mitochondrial fission mediated by dynamin-related protein-1. Cancer Res. 2009;69:492–500. doi: 10.1158/0008-5472.CAN-08-2962. [DOI] [PubMed] [Google Scholar]
- 29.Choi WY, Giraldez AJ, Schier AF. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science. 2007;318:271–274. doi: 10.1126/science.1147535. [DOI] [PubMed] [Google Scholar]
- 30.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, et al. Hydrogen sulfide attenuates myocardial ischemia–reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90:E25–33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 32.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123(Pt 15):2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12:565–573. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 37.Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. 2012;94:284–292. doi: 10.1093/cvr/cvr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R, Sadek HA, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank D, Gantenberg J, Boomgaarden I, Kuhn C, Will R, Jarr KU, Eden M, Kramer K, Luedde M, Mairbaurl H, et al. MicroRNA-20a inhibits stress-induced cardiomyocyte apoptosis involving its novel target Egln3/PHD3. J Mol Cell Cardiol. 2012;52:711–717. doi: 10.1016/j.yjmcc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122(11 Suppl):S124–131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92:75–84. doi: 10.1093/cvr/cvr145. [DOI] [PubMed] [Google Scholar]
- 42.Talotta F, Cimmino A, Matarazzo MR, Casalino L, De Vita G, D’Esposito M, Di Lauro R, Verde P. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28:73–84. doi: 10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
- 43.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]