Abstract
There is a well-described association between childhood epilepsy and pervasive cognitive and behavioral deficits. Often these children not only have ictal EEG events, but also frequent interictal abnormalities. The precise role of these interictal discharges in cognition remains unclear. In order to understand the relationship between frequent epileptiform discharges during neurodevelopment and cognition later in life, we developed a model of frequent focal interictal spikes (IIS). Postnatal day (p) 21 rats received injections of bicuculline methiodine into the prefrontal cortex (PFC). Injections were repeated in order to achieve 5 consecutive days of transient inhibitory/excitatory imbalance resulting in IIS. Short-term plasticity (STP) and behavioral outcomes were studied in adulthood. IIS is associated with a significant increase in STP bilaterally in the PFC. IIS rats did not show working memory deficits, but rather showed marked inattentiveness without significant alterations in motivation, anxiety or hyperactivity. Rats also demonstrated significant deficits in social behavior. We conclude that GABAergic blockade during early-life and resultant focal IIS in the PFC disrupt neural networks and are associated with long-term consequences for behavior at a time when IIS are no longer present, and thus may have important implications for ADHD and autism spectrum disorder associated with childhood epilepsy.
Keywords: Pediatric epilepsy, Interictal spikes, Attention, Social behavior, Short-term plasticity, Prefrontal cortex
Introduction
Almost 40% of children with epilepsy have comorbid behavioral deficits (Brunquell et al., 2002; Holmes, 2009) including ADHD, autism, anxiety and OCD, as well as general learning and memory impairment (Brooks-Kayal, 2010; Brunquell et al., 2002; Cabaleiro, 1969; Dunn et al., 2003; Holmes, 2009; Kaufmann et al., 2009; Levin and Duchowny, 1991). Many children with these co-morbidities have frequent overt seizures and it is hypothesized that these seizures are partially responsible for adverse outcomes. The association between frequent generalized early life seizures and hippocampal-dependent cognition has been well studied in rodent models and support the view that epileptic discharges disrupt normal development of hippocampal networks (Chang et al., 2005; Huang et al., 1999, 2002; Liu et al., 1994). However, conclusions drawn from these models may be confounded by the effects of general brain injury due to hypoxia/ischemia and metabolic imbalance that occur at the time of the generalized seizure (McCabe et al., 2001). Furthermore these models often focus on the hippocampus, thereby neglecting the neocortical brain regions associated with many of the most common behavioral deficits seen in children with epilepsy. From a clinical perspective, these models also do not adequately model a large group of children with epilepsy who have infrequent seizures despite frequent focal neocortical interictal spikes (IIS) associated with serious cognitive and behavioral impairments, e.g. children with Landau–Kleffner Syndrome or Continuous Spike-wave in Sleep (Gordon, 1990, 1997; Praline et al., 2003). Therefore, in order to understand these effects it is essential to develop an animal model that recapitulates the human condition. As no such model currently exists, we set out to develop a model that recapitulates the phenotype of frequent neocortical epileptiform activity through focal alteration of the inhibitory/excitatory balance during childhood in order to test if this is sufficient to generate later life network abnormalities and behavioral deficits.
We chose microinjections of bicuculline, a competitive GABAA antagonist, into the prefrontal cortex to create a model of inhibitory/excitatory network imbalance leading to focal IIS activity in adolescent rats. This model is clinically relevant as a large body of evidence implicates GABAergic system dysfunction in children with autism and ADHD that present with or without focal IIS on the EEG (Edden et al., 2012; Fatemi et al., 2009; Mori et al., 2012; Samaco et al., 2005). The PFC was chosen based on the preponderance of PFC-specific deficits in children with epilepsy, particularly deficits in executive functioning and social behaviors.
We hypothesize that transient GABA signaling disruption and resultant frequent focal IIS during development will result in long-term neural network disruptions and impairments in PFC-dependent behavior. We show that focal IIS during adolescence is associated with long-lasting alterations in short-term plasticity (STP) in the PFC, a type of plasticity that is thought to underlie information processing and synaptic filtering important for cognition (Jääskeläinen et al., 2011; Rotman et al., 2011). Using delayed-non-match to sample, sociability, motivation and open arena tasks, we also show selective behavioral deficits in measures of attention and social behavior in animals with frequent focal epileptiform discharges.
Methods
Animals
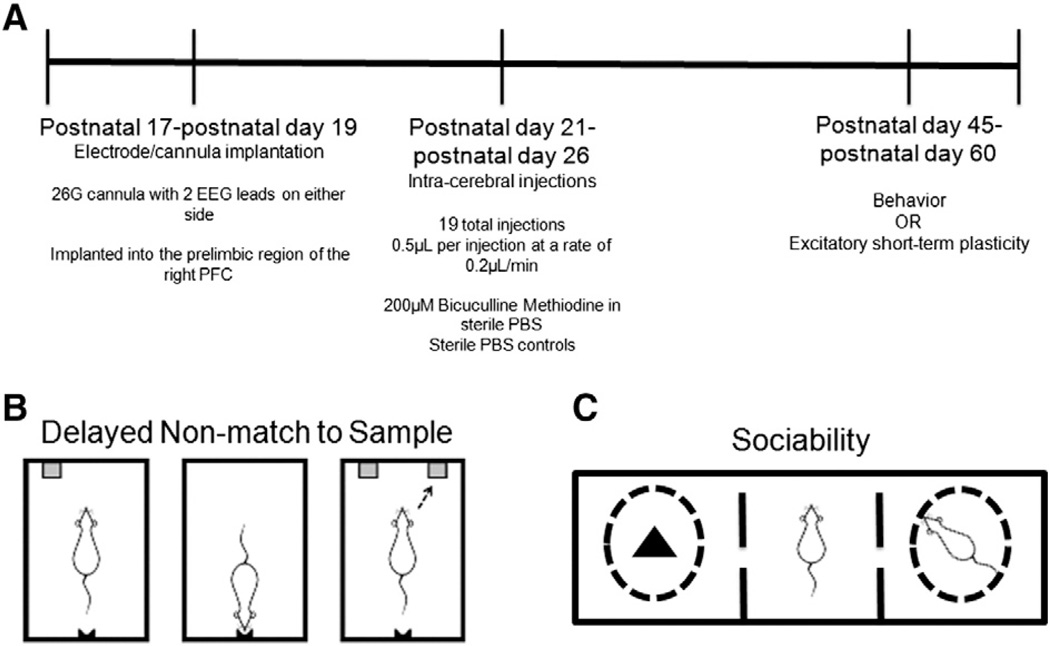
All experiments were performed in accordance with the guidelines set down by the National Institutes of Health and the Geisel School of Medicine at Dartmouth for the humane treatment of animals (see Fig. 1A for experimental design). The animal protocol was approved by the Institutional Animal Care and Use Committee of Dartmouth College.
Fig. 1.
Experimental Design is shown in (A). Behavioral tasks conducted on animals were the delayed non-match to sample task (B) and sociability (C). Fig. 1B is modified from (Kleen et al., 2011).
Surgery
Male Sprague–Dawley rat pups were anesthetized at p17–p19 with isoflurane (2–3% in oxygen) and implanted with custom-made cannula/EEG electrodes (Plastics One, Roanoke, VA). Each implant had a 26G guide cannula fitted with a 33G dummy cannula. The guide cannula was 6 mm in length, the dummy cannula 6.2 mm in length. Injection cannulas measured 6 mm in length as well and were also 33G. Affixed to either side in the medial/lateral direction were two EEG wires, 7 mm each in length, which was attached to a Mill–Max connector (Mill–Max Manufacturing Corporation, Oyster Bay, NY). The electrode/cannula set up was first dipped in 70% ethanol and allowed to air dry completely before implanting into the right prefrontal cortex such that the tip of the cannula sat at the following coordinates; 3.0 mm anterior to bregma, 0.8 mm to the right of midline, 2.5 mm ventral to the surface of the brain. Two bone screws were attached to the skull, a ground wire was soldered to one of these screws and a reference wire was inserted into a small hole above the cerebellum between the skull and the brain parenchyma. All holes were filled with sterile petroleum jelly and implants were held in place with grip cement (DentSply International, York, PA). All animals were treated with 5 mg/kg ketoprofen every 4 hours as needed during the post-surgical recovery phase.
Early-life epileptiform activity model
After a 2–4 day recovery period, animals were injected intracortically with of 0.5–0.7 µL of 200 µM bicuculline methiodine using a pump that delivered fluid at 0.2 µL/minute. At p21, rat pups received 3 injections, with 4 injections per day every day thereafter until p25 (total of 19 injections over the course of 5 days). Littermate controls were also chronically implanted with an electrode/cannula and injected with sterile saline. An injection cannula was left in place for 2 min after the injection had finished in order to allow time for the solution to diffuse into the tissue. Continuous intracranial EEG was recorded from a location adjacent to the injection site and referenced to a common cerebellar electrode for at least 20 min post-injection in order to ensure IIS activity was achieved. Presence of behavioral seizures was visually monitored in real time and video recorded.
Histology
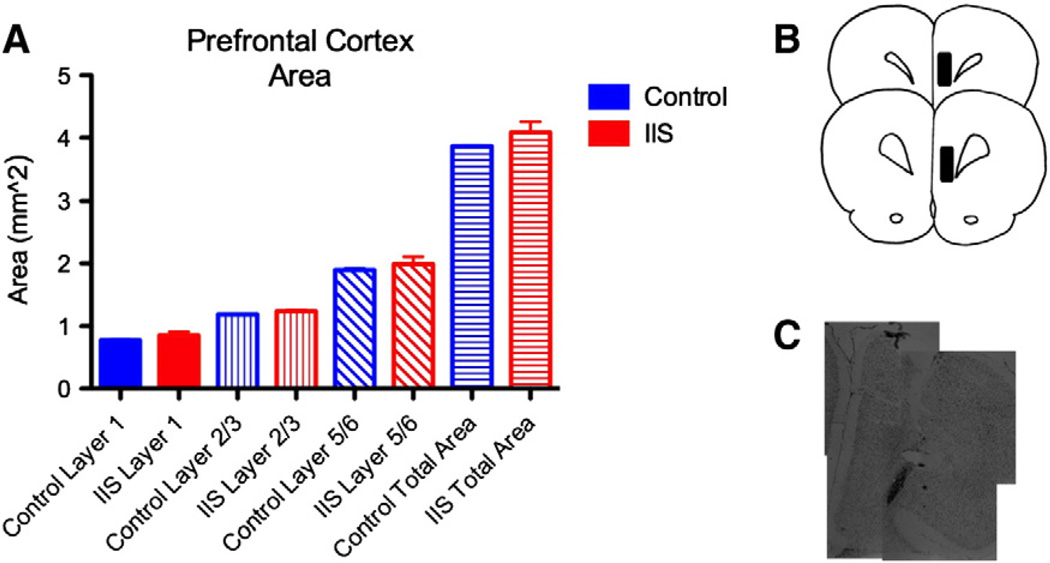
Following completion of the studies, animals were sacrificed and brain sections were cut and stained with thionin. Electrode/cannula placement was verified post-mortem (Fig. 5B). A group of animals were used to examine potential structural abnormalities in the PFC after early life IIS.
Fig. 5.
PFC structure is unaltered after early life IIS. We measured layer areas in histological sections from animals that had experienced early life IIS, littermate controls. There were no alterations in the areas of any of the layers of the PFC after early life IIS (A). (B) Shows the location of the injection site in the cohort. (C) shows thionin stained images of the electrode track lesion taken at p65.
Electrophysiology
Characterization of the model (In vivo electrophysiology)
Operational amplifiers (unity gain) were connected to a Mill–Max connector on the animal, and EEG signals were transmitted via a custom cable connected to a rotating commutator (Dragonfly Research and Development Corporation, Frostburg, MD). EEG signals were referenced to the cerebellar lead and each signal was filtered between 0.1 Hz and 1 kHz and amplified at 1000× gain. Recordings were sampled at 2315 Hz using an analog-to-digital converter Digidata 1320 (Axon Instruments, Foster City, CA).
Short-term plasticity (In vitro electrophysiology)
Brain slices were prepared from P45–P60 rats (Controls N = 4, IIS N = 4; n = 24 Control slices for LII–LV experiments, n = 25 IIS slices for LII–LV experiments. n = 23 Control slices for LV–LV, n = 22 IIS slices for LV–LV) as previously described (Hernan et al., 2012). Types of excitatory short-term plasticity assessed were post-tetanic potentiation (PTP), paired pulse plasticity and activity dependent plasticity. Stimulus amplitudes were set at a level that produced 50% of the maximal field excitatory postsynaptic potential (fEPSP) amplitude for each slice. In order to assess PTP, a tetanic stimulation (15 pulses at 50 Hz) was delivered following stabilization of the baseline and a post-tetanic response was collected at 0.1Hz. Layer II/III (LII/III) or Layer V (LV) of the mPFC was stimulated and amplitudes of resultant fEPSPs were recorded in LV. For paired pulse experiments, 2 pulses were delivered to either LII/III or LV at varying interstimulus intervals. Activity-dependent plasticity was assessed through administration of 15 pulses delivered at 50Hz to either LII/III or LV. Recording electrodes were made of borosilicate glass capillaries, which were pulled to a resistance of 2–5 mΩ and filled with aCSF (in mM: 126 NaCl, 3.5 KCl, 2.0 CaCl2, 1.3 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, 11 glucose pH = 7.27–7.30) containing 50 µM gabazine.
Behavior
Apparatus
An operant box (Med Associates Inc., St. Albans, VT) enclosed in a dimly lit, sound-attenuating chamber was used for delayed non-match to sample (DNMS) experiments. Inside the box, two retractable levers were located on one wall separated by a pellet dispenser. The opposite wall contained a nose-poke hole that contained infrared beams that are broken when the animal inserts his nose into the hole. Stimulus lights were located above each lever, the food cup and the nose-poke hole. Behavioral software (Med Associates Inc., St. Albans, VT) reported number of presses on each lever, number of rewards dispensed, and number of nose-pokes. Food rewards were used in all tasks (45 mg Noyes food pellet; Research Diets Inc, New Brunswick, NJ). For all behavioral experiments, animals were acclimated to the testing room for at least 2 hours before testing.
Delayed non-match to sample (DNMS)
The DNMS task was performed using methods previously described (Kleen et al., 2011). At p45, rats (N = 5 Control, N = 5 IIS) housed in pairs were food deprived to approximately 85–90% of starting weight and were rewarded during the task with dustless precision pellets from a pellet-feeder (45 mg Noyes precision pellets, Research Diets Inc., Brunswick, NJ). Behavioral software (Med Associates Inc., St. Albans, VT) reported the number of correct trials completed and the number of omission errors made in the entire session. An error of omission was counted as any event where a response was expected and the animal did not respond; in the event of an omissions error, the trial reset and began again.
The DNMS task involved several training steps before a full trial could be executed. These sessions were done one session per day, 7 days a week at approximately the same time each day. Rats were initially trained to associate either of both freely available levers with food reward. They were then trained to press only one extended lever, and subsequently trained to place their snout in the nose hole when the light above it was illuminated. This last training part was first performed while the operant box lights were switched off entirely to encourage the rat to follow the illuminated light about the nosepoke hole and then in a dimly-lit operant box. Criteria for advancing through these training sessions were as follows; bar presses: >25 rewards received, with both levers pressed at least once each; nose-poke dark: >25 rewards received, nose poke light: >10 rewards. If <10 rewards were given in a session, rats were demoted to the previous session stage.
Rats were then trained in a forced DNMS task. The rat was presented with one lever (sample phase), had to press the lever, break the nose-poke beam in the opposite wall, and was then presented only the lever (test phase) that was not presented before (non-match lever). A successful press of the test lever in less than 10 seconds was associated with a food reward. Once animals were able to perform this type of trial with an 80% response rate (>40 trials), they were trained in the full DNMS paradigm with a 0 second delay. Here rats were presented with both test levers and had to press the one that was not presented in the sample phase. During this training paradigm, an error during one trial (i.e., the rat chose the match lever instead of the non-match lever) resulted in a subsequent forced DNMS trial (lever-nose poke-opposite lever). Once criterion (80% correct on the DNMS trials) was achieved within the DNMS training session, full DNMS sessions began (Fig. 1B).
DNMS was done in blocks with increasing delay times between the nosepoke and test (non-match) phases; block 0: 0 s delay, block 1: 0–5 s; block 2: 0–10 s and block 2A 5–10 s delays assigned randomly. Criteria to pass to the next block include accuracy of 80% or more (40 or more correct trials out of a total of 50 trials per session) during the session and no clear lever preference. Lever preference or less than 60% accuracy (fewer than 30 correct trials) resulted in regression to the previous block or phase of DNMS training. In the DNMS task, the number of days to completion was used as a measure of learning in the working memory component of the task.
Operant lever-pressing motivation task
At p45, rats (Control N = 5; IIS N = 6)were food deprived to approximately 85–90% of starting weight and were rewarded during the task with sucrose pellets from the pellet-feeder. Animals were placed in the operant box with free access to both levers, with both lever presses providing food rewards at a fixed ratio of one-to-one (FR1). Total number of presses and total number of rewards were assessed during a 30-min session.
Sociability
The sociability experiment was based on a previously described mouse sociability task (Moy et al., 2004). Rats (Control N = 5; IIS N = 5) were placed in a plexiglass box 48″ in length and 16″ in height that was separated into three smaller chambers (Fig. 1C) and filled with wood shavings; a central chamber measuring 10″ in width, with adjacent right and left chambers measuring 18.5″ in width each that were separated from the center chamber by a plexiglass wall with a removable door cutout. After a 5-minute acclimation period in the center chamber the panels blocking the doors were lifted and the experimental animal could move freely into both the right and left chambers for 10 min. The right chamber contained an age-matched novel male rat isolated in a small round wire cage so that all social interaction was initiated by the experimental rat and not the novel rat. The same novel rat was used throughout the experiment. Wire caging around the novel rat had large enough holes to allow for nose-to-nose contact but no aggression or sexual behaviors could be initiated. The left chamber contained a novel object in an identical isolation cage. Right and left chambers were just large enough to allow for the wire cages and the experimental rat to prevent rodents from disengaging from the task during the entire 10-minute session. Sociability was operationally defined as spending more time in the chamber with the novel rat than in the chamber with the novel object. This approach was validated in the original manuscript describing the sociability protocol (Nadler et al., 2004), and is corroborated by our visual observations of the video recordings of the experiments. Under control conditions wild-type rodents are generally sociable (Moy et al., 2004).
Open arena
Rats (Control N = 5; IIS N = 5) were placed in a circular chamber 1 meter in diameter in an isolated room and allowed to explore freely for 10 minutes. Sessions were recorded and analyzed for total locomotion as well as relative time spent along the perimeter of the chamber vs. the center in order to obtain measures for hyperactivity and anxiety, respectively (Carola et al., 2002; Prut and Belzung, 2003).
Statistics
Statistics were done using Prism (GraphPad, La Jolla, CA) and SPSS v.20 (IBM Armonk, NY). For STP, DNMS and sociability experiments we carried out repeated measures regression using generalized estimating equations in order to assume the correct distribution for the data and to test the impact of potential confounding variables. The effects of litter, and seizures were tested and were not significant in any of our experiments. Open arena and center arm entries were analyzed using a two-tailed Student's t-test.
Results
Characterization of the model and the long-term effects of IIS on PFC neural networks
Developmental IIS model
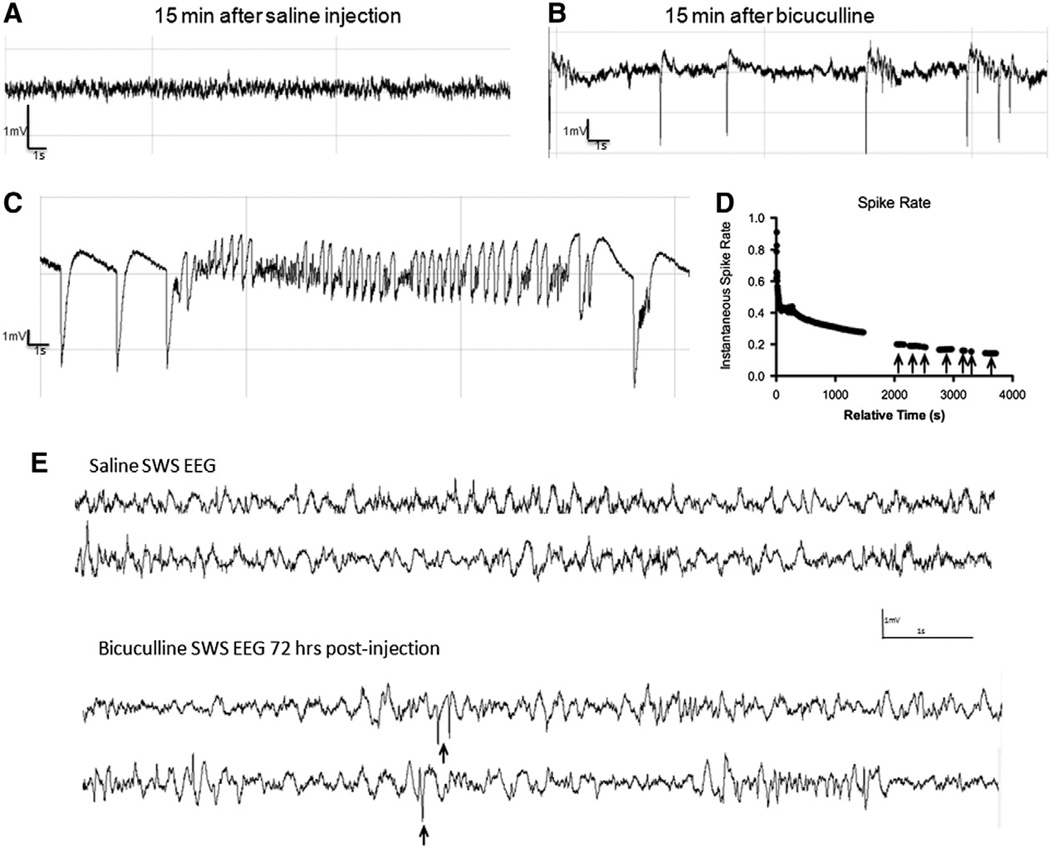
We first wanted to determine whether injection of bicuculline results in IIS in the absence of seizures. Immediately after each bicuculline injection, local EEG was recorded for 30 min. This 30 min window was chosen based on preliminary experiments indicating that if IIS was to develop into ictal EEG activity, this would occur within 10–20 min post-injection. Animals were visually monitored in real time and video recorded for signs of seizures during this time. Saline-injected animals never developed IIS (Fig. 2A compared to B).
Fig. 2.
Bicuculline injection produces robust IIS. EEG traces from saline (A) and bicuculline-injected animals (B). Epileptiform discharges appear as polyspikes shown in (B) or spike-and-wave discharges that are prominently seen during the awake EEG for approximately 25 min post-injection. Representative example of an electrographic seizure in (C), the average length of each seizure events was under 15 s. Hour-long recordings in a representative animal show changes in spike rate over time immediately post-injection (D). IIS activity decreases exponentially with time from 0 to 25 min post injection, with IIS returning during slow wave sleep periods marked with arrows in D. IIS during slow wave sleep continue for 72 h post-injection (E, compare top two traces of saline SWS EEG to bottom two traces of SWS EEG from two bicuculline-injected animals. IIS events shown with arrows.).
During a pilot experiment, we found that injections initially induced IIS activity at a rate of approximately 0.85 spikes per s, followed a by sharp decrease in the rate of IIS on the order of 15–30 s. This was followed by a period with a slower decay rate in the minutes post injection, with robust spiking during the awake period ending approximately 25 min post-injection (Fig. 2D).
Although IIS activity decreased rapidly 20–25 min post-injection, it reappeared during slow-wave sleep at a rate of approximately 0.2 spikes per s (Fig. 2D, arrows). IIS activity was observed during the sleep period up to 24 h after the last bicuculline injection and 1/4 animals experienced IIS during slow wave sleep periods 72 h after the last injection (Fig. 2E). One animal had 31 total spikes in a 75-min recording session. 30-min segments of EEG were used to estimate differences in the duration of slow wave sleep; no significant differences were noted with controls spending 5.1 ± 2.6 min in slow wave sleep compared to animals in the IIS group that spent 3.3 ± 2.1 min in slow wave sleep (p = 0.6). The fact that IIS occur at all at a time when bicuculline has likely washed out entirely suggests that there are long-term changes to these networks.
In experiments where unilateral injection was paired with bilateral depth EEG recordings in the PFC, it was determined that IIS activity propagated to the contralateral PFC as evidenced by a phase delay and a different morphology of the IIS. However, in experiments where unilateral injection was paired with recording in ipsilateral PFC and ipsilateral somatosensory cortex, IIS were not seen in somatosensory cortex.
Although our goal was to develop a model without seizures, brief electrographic seizures lasting less than 15 s (average length 14.9 ± 0.3 s) did occur in 2 of the 13 of the bicuculline-exposed animals in these studies. Electrographic seizures were easily distinguished from IIS activity based on EEG characteristics (compare Fig. 2B to C). While it is possible that we may have missed electrographic seizures not occurring within the 30 min recording window, presence or absence of seizures was modeled into all statistics and there was no significant effect of the seizures that were observed in any of the behavioral or plasticity outcomes.
Short-term plasticity is altered after early life IIS
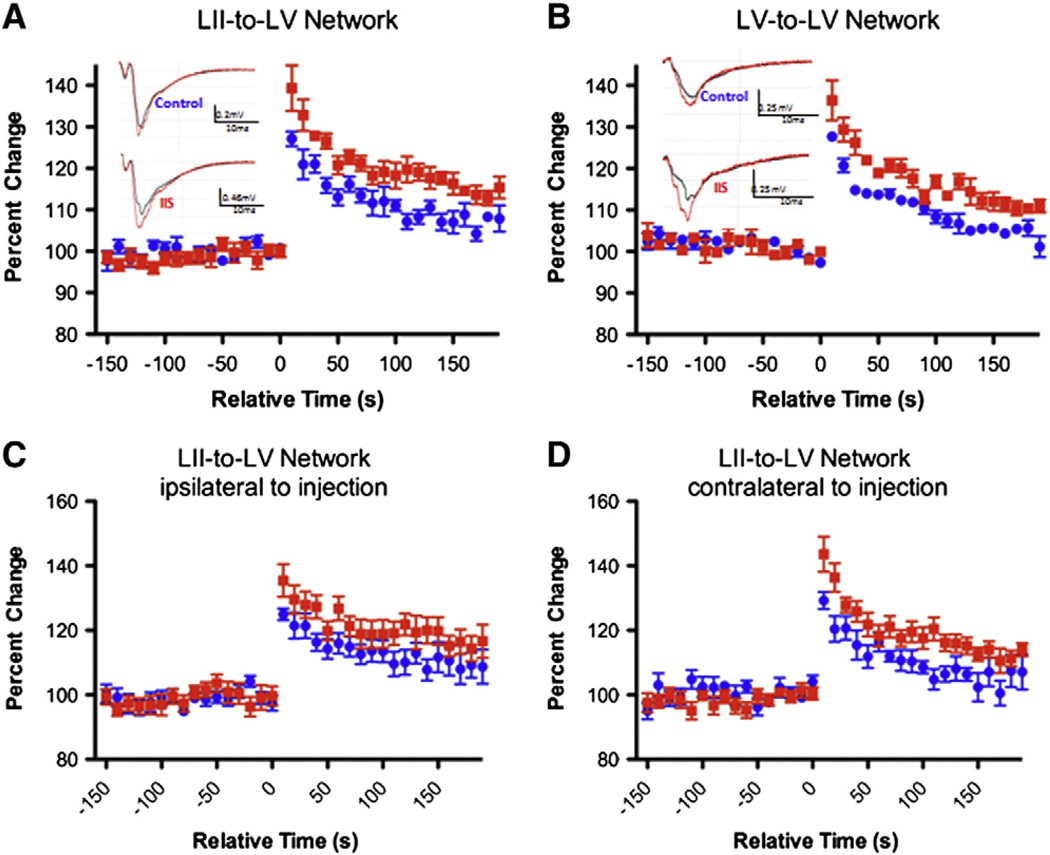
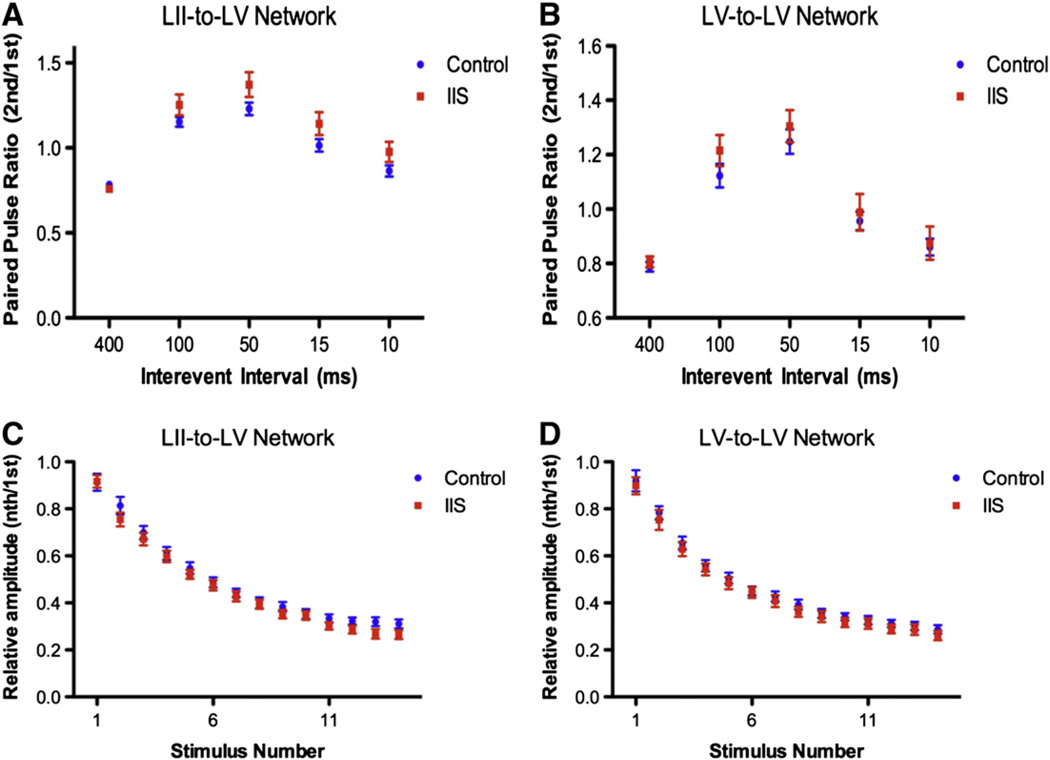
In order to determine whether IIS lead to long-lasting alterations in neural networks in the PFC we evaluated short-term synaptic plasticity (STP). We chose to evaluate two networks in the PFC, the LII/III-to-LV and LV-to-LV network, in order to determine if the IIS insult caused more broad alterations to the PFC as seen after early life seizures (Hernan et al., 2012) or if only one network was preferentially altered. In LII/III-to-LV networks, maximum PTP was 127.1 ± 1.8% of baseline in control and 139.4 ± 5.5% of baseline in IIS animals (p < 0.001; Fig. 3A). In LV-to-LV networks, the maximum PTP was 127.7 ± 0.4% in controls and 136.4 ± 4.8% in IIS animals (p < 0.001; Fig. 3B). This increase is significant regardless of the side of the brain the PTP was recorded on (ie- ipsi- or contralateral to the original bicuculline methiodine injection), suggesting that the entire PFC is affected by the IIS activity despite the fact that initial injections were only unilateral. Other forms of STP were also assessed; paired-pulse facilitation was not altered by IIS in either LII/III–LV or LV–LV networks (Fig. 4A; p = 0.10 LII to LV, p = 0.42 LV to LV) nor were there alterations in activity-dependent plasticity in either LII/III–LV or LV–LV in our focal IIS model (Fig. 4B; p = 0.33 LII to LV, p = 0.44 LV to LV).
Fig. 3.
IIS during development lead to long-lasting alterations in PFC networks. Post-tetanic plasticity (PTP), a form of short-term plasticity, was increased after early life IIS (IIS data points shown in red compared to littermate controls in blue) in two networks in the PFC, LII/III-to-LV and LV-to-LV (A and B). Example traces (insets in A and B) show larger relative increase in fEPSP amplitudes after tetanic stimulation (red trace compared to black trace in insets) in IIS group (bottom) compared to controls (top). Interestingly, PTP was increased in IIS animals both contralaterally and ipsilaterally to injection in the LII/III-to-LV network (C and D). Graph values represent mean ± SEM.
Fig. 4.
Other forms of STP are unaltered after early-life IIS. At interpulse intervals ranging from 10 to 400 ms, the relative ratio of the amplitude of the first fEPSP to the second fEPSP (paired pulse facilitation) is not changed in animals with a history of early life IIS activity (red) in either LII-to-LV (A) or LV-to-LV (B) compared to littermate controls (blue). Activity-dependent plasticity after early-life IIS. Individual points represent mean values of fEPSP amplitudes normalized to the first fEPSP amplitude during train of 15 stimuli delivered at 50 Hz to LII/III (C) or LV (D) and recorded in LV at ELS (red) and control (blue) groups. No significant differences were noted in either the LII-to-LV or the LV-to-LV network. Graph values are means ± SEM: N = 4 Control, N = 4 IIS; 22–25 slices per group.
Thus, unilateral intracortical injection of bicuculline methiodine into the PFC results in frequent epileptic discharges on the EEG and long-term alterations in the plasticity of neural networks bilaterally.
PFC structure after developmental IIS
We examined whether there were any gross structural changes in the PFC layers after early life IIS. Using thionin-stained sections to evaluate LI, LII/III and LV/VI areas, we found no significant change in the area of any of the individual layers of the prelimbic/infralimbic region of the mPFC (Fig. 5A).
Impact of IIS on working memory, attention and social behaviors
Long-term alterations of PFC networks suggest that behaviors dependent upon this structure (working memory, attention and sociability) will also be affected by IIS. To test this, we used several behavioral tasks giving us output measures on a variety of PFC-dependent behaviors.
The effect of developmental IIS on working memory and attention
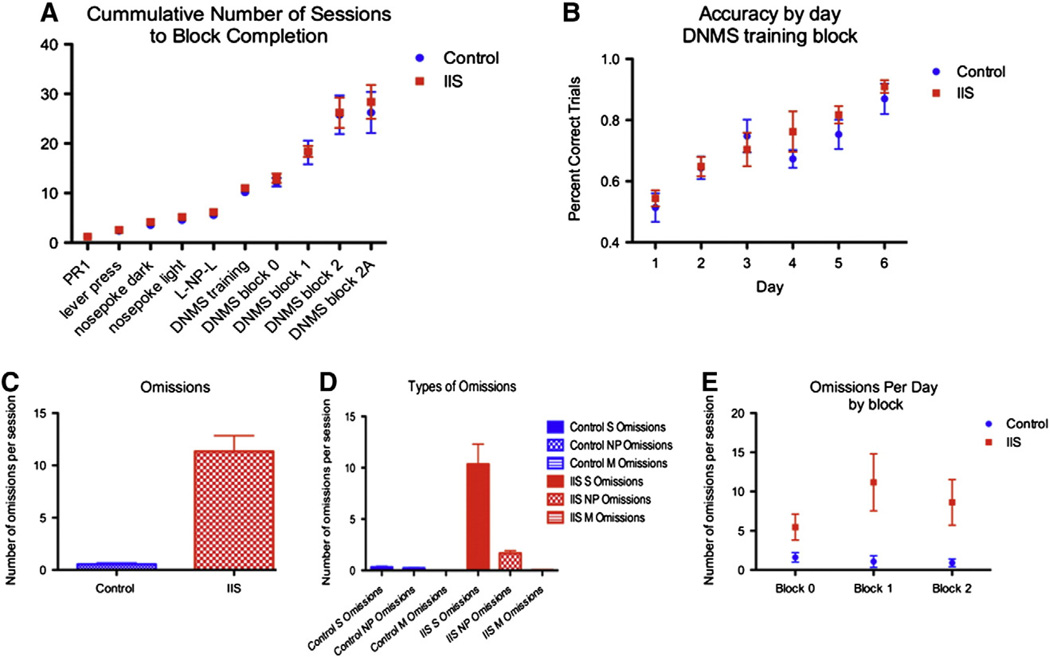
During the DNMS task, all animals were fed 14.5 g of food per day; weights did not significantly differ between the groups during the experiment (242.9 ± 3.6 g in control group, 232.6 ± 4.0 in IIS group, p = 0.37). We found no significant difference in number of sessions to completion (Fig. 6A), or in accuracy by day during any of the blocks (accuracy in the DNMS training block show in Fig. 6B), suggesting that working memory is largely intact in IIS animals. However, we visually observed that the IIS animals appeared more easily distracted and therefore we evaluated this quantitatively as the number of omissions errors made per session. IIS animals made significantly more omissions per session than control animals in all 28 days of testing, with the average number of errors of omission per animal per day equaling 0.55 ± 0.1 in control animals and 11.33 ± 1.5 in IIS animals (p < 0.01, Fig. 6C), indicating that IIS animals had a significant increase in the number of omissions errors made during the task. These omissions were largely in the sample phase when rats failed to press the initial lever (0.32 ± 0.09 errors made per session in controls vs. 10.35 ± 2.0 errors made per session in the IIS group) and in the nose poke phase (0.23 ± 0.05 omissions per session in controls, vs. 1.68 ± 0.25 omissions per session in IIS animals). Virtually no match lever omissions were made in either group (0 made in the control group, 0.04 ± 0.02 omissions per session in the IIS group; see Fig. 6D). Previous research has suggested that omissions are indicative of an attention deficit (Dalley et al., 2004; Risbrough et al., 2002). As omissions in this task were observed on all sessions or blocks (Fig. 6E), they are not directly related to difficulty in the working memory component of the task.
Fig. 6.
Early life epileptiform activity selectively alters attention. Cumulative number of sessions to task completion (A) and accuracy in the training session block (B) in the DNMS task were not different after IIS. However, IIS animals (red)made significantly more omissions in the task than their control (blue) counterparts (p < 0.01). This is shown in the average number of omissions per session (C).Most of these omissions were sample lever press omissions (“S Omissions”), with fewer nose poke omissions (“NP Omissions”) and almost no match lever press omissions (“M Omissions”) (D). The number of omissions errors per day did not vary based on the difficulty in the working memory component of the task (E). Data points on all graphs represent mean ± SEM.
The effect of developmental IIS on motivation, hyperactivity and anxiety measures
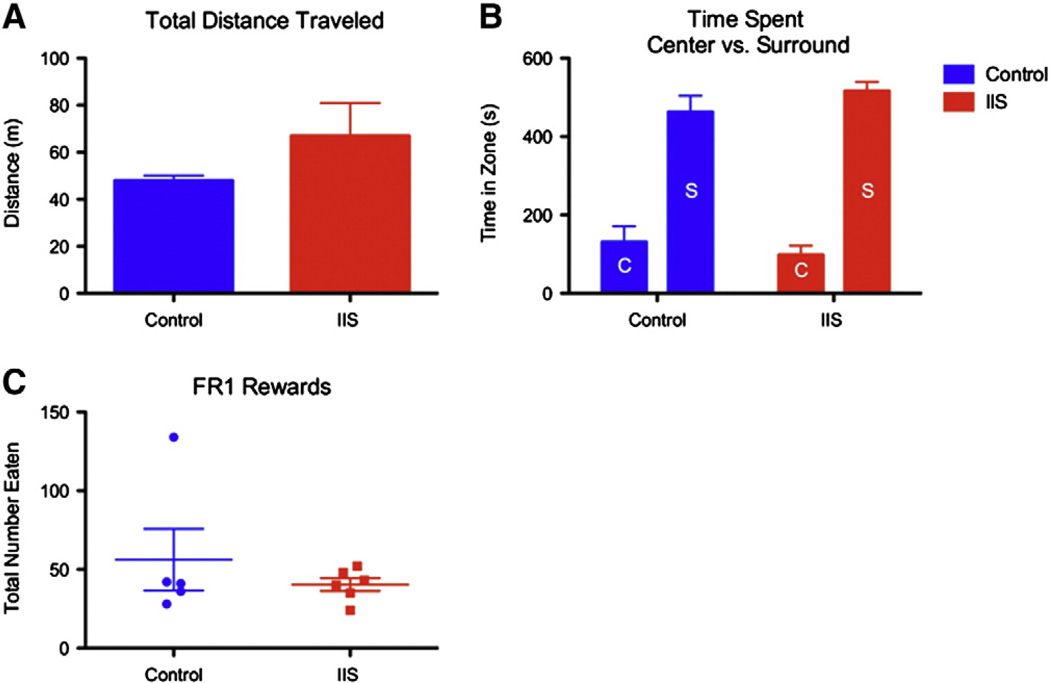
In order to further understand the implications of the omissions errors, a standard open arena test was used to give us outcome measures of anxiety and hyperactivity, both of which could offer insight as to why animals with a history of IIS were making significantly more omissions in the DNMS task than their control counterparts. In a cohort of 5 animals with a history of IIS and 5 littermate controls, no significant differences in total distance traveled during the 10-min session (47.95 ± 2.18 min in controls, 66.93 ± 14.01 min in IIS; p = 0.18; Fig. 7A), or in the ratio between time spent in the center vs. time spent along the perimeter in the circular arena (controls: 131.30 ± 39.97 s in center, 462.92 ± 41.56 s in surround; IIS: 98.04 ± 23.95 s in center vs. 516.24 ± 23.59 s in surround; p = 0.21, interaction term; Fig. 7B) were found.
Fig. 7.
Early life IIS does not alter activity level, anxiety or motivation. An open arena task was used to assess anxiety and hyperactivity. Total distance traveled during the 10-minute session (A) was not different between controls (blue bars, A) and animals with a history of IIS (red bars, A) suggesting over all levels of activity were similar between groups. Additionally, the relative amount of time spent in the center (bars labeled “C” in B) vs. the surround (bars labeled “S” in B) of the arena (B) was not different between groups (red bars: IIS animals, blue bars: control animals). Finally, total number of sucrose rewards eaten during a simple FR1 schedule motivation task did not differ between the groups (C).
Additionally, we performed a FR1 schedule operant task to assess motivation for the sucrose pellet rewards awarded during the DNMS task. This task was conducted in order to eliminate potential differences between the groups in food-driven operant responding as a potential source of omissions errors. During 30-minute sessions, the groups did not differ in the number of total presses made or the number of rewards eaten (59.3 ± 22.3 rewards and presses in N = 5 control animals, 40.3 ± 4.1 in N = 6 IIS animals; Fig. 7C). Taken together, these data suggest that the interpretation of the omissions errors as an attention deficit most likely correct.
The effect of developmental IIS on sociability
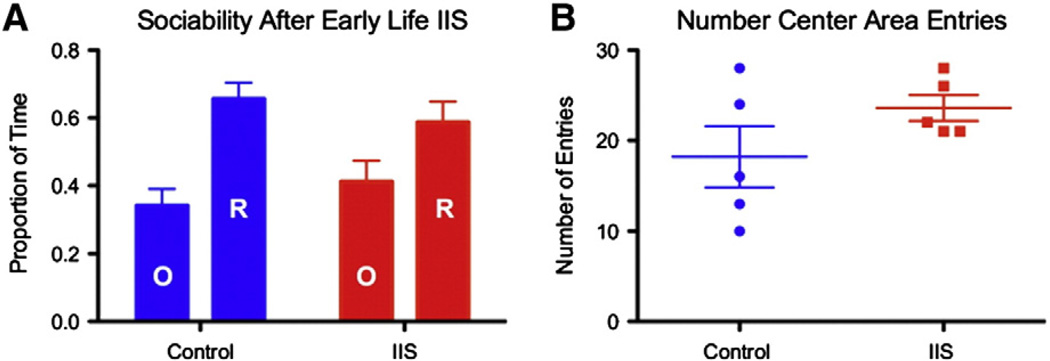
Early life IIS significantly altered sociability (p < 0.05, group by time spent effect) in adult rodents. Control animals spent 65.7 ± 4.7% of the total time in either arm with the rat and 34.3 ± 4.7% of time with the novel object. Animals with a history of IIS were not significantly sociable, spending 58.8 ± 6.1% of the time in either arm with the novel rat and 41.2 ± 6.1% of time with the novel object (Fig. 8A, red bars compared to blue bars). These alterations in time spent in each compartment occurred alongside no change (p = 0.34) in the total number of center chamber entries (Fig. 8B), suggesting that the animals were not simply crossing more frequently from one arm to the other and therefore not showing a hyperactive phenotype during the sociability task, but instead were spending less time with the novel rat. These data show that IIS alone during neurodevelopment are associated with significant deficits in sociability.
Fig. 8.
Animals with a history of IIS are less sociable than their control counterparts. Sociability as defined by the relative amount of time spent with a novel rat (bars labeled “R”) over a novel object (bars labeled “O”), was also altered by early life IIS. The group by time effect can be seen as a reduction in the ratio of the amount of time spent with the rat compared to the amount of time spent with the object. Control animals (blue) spent a significantly greater proportion of time with the novel rat than the novel object compared to IIS rats (red) (A). The number of entries into the center compartment did not differ between the groups (B). All bars are means ± SEM.
Discussion
We show for the first time that prefrontal IIS during development are associated with both long-lasting alterations in short-term synaptic plasticity and deficits attention and sociability in rodents. This effect is independent of the presence or absence of seizures observed during a 30-minute post-injection recording session, demonstrating that transient GABAergic signaling disruption and resultant IIS activity in the developing brain can lead to deleterious behavioral and network-level outcomes even after the IIS activity has subsided.
IIS resulted in an increase in a specific type of short-term synaptic plasticity, PTP. These changes are similar to those that we have previously observed after early life generalized seizures induced using flurothyl inhalation and occur without alterations in shorter forms of STP (Hernan et al., 2012). It may seem counterintuitive that increases in STP can be associated with deleterious cognitive outcomes, however it is expected that alterations in STP in either direction would interfere with the induction and maintenance of sustained activity and synaptic filtering properties of the neural network, thereby leading to alterations in information processing in the PFC (Abbott and Regehr, 2004; Deng and Klyachko, 2011; Rotman et al., 2011). Although no direct link between STP and behavior has been made, STP is thought to underlie decision making, attention and temporal encoding in the PFC (Buonomano, 2000; Deco et al., 2010; Deng and Klyachko, 2011). Therefore, long-lasting disruptions in STP are indicative of long-term changes in PFC networks, thereby providing strong justification for evaluating behavior.
The first task we chose was a DNMS test of working memory. We found no changes in working memory in animals with a history of early life IIS. This finding is not entirely surprising, as proper working memory function relies heavily on the interaction between the PFC and the hippocampus, and not just one of these brain structures alone (Laroche et al., 2000). Given the focal nature of the GABAergic disruption and resultant IIS insult, we therefore hypothesize that the undamaged hippocampus was able to compensate for the PFC damage in our IIS animals. We did, however, find a significant increase in the number of omissions errors made during each session in the DNMS task, specifically sample press omissions and nose poke omissions, with very few match lever press omissions. These data indicate that once the animal is actively engaged in the task, it will see the task through to completion, suggesting a deficit in attention instead of complex behavioral planning or motivation. The use of the omissions outcome measure as an attention surrogate is not without precedent (Dalley et al., 2004; Risbrough et al., 2002).
To test whether this increase in omissions was caused by hyperactivity or anxiety, we performed an open field test. No differences in total distance traveled or relative time spent in the center versus the surround of the arena were found, suggesting that IIS animals do not have a straightforward hyperactive or anxious phenotype, respectively. This is an interesting observation, as the ADHD with a predominantly inattentive phenotype is more often associated with epilepsy and epileptiform activity than ADHD with hyperactivity or ADHD with both hyperactivity and inattention (Dunn et al., 2003; Hermann and Seidenberg, 2007).
Other investigators have interpreted omission as a change in motivation to complete food-driven operant responses (Grottick and Higgins, 2000). However, both groups of animals were food deprived to the same degree and mean group weights did not differ throughout the task. Additionally, animals did not differ in the total number of presses made and the total number of sucrose rewards eaten in a FR1 schedule test of motivation.
Interestingly although the present study was not adequately powered to address the question of whether or not inattention and social behavior deficits correlate within animal, more recent thinking suggests that there is much overlap in the symptoms of ADHD and autism in patient populations diagnosed with one or the other disorder (Gillberg, 2010). Given that the PFC seems to be involved in attention and social behaviors, it is not unsurprising that symptoms are often shared between these two disorders and future directions should include investigation of this link in our model.
Due to the fact that all animals had lost their implants before the end of behavioral experiments, EEG was not recorded during behavioral tasks. Reimplantation is logistically difficult as scar tissue has formed and the skull has been weakened by chronic implantation. These data would have been valuable as they could have verified the absence of all epileptiform activity at this time point, however we feel confident that all IIS activity had ended before behavioral testing given that preliminary data failed to find evidence of IIS 2-weeks post-injection.
As with many epilepsy models and pharmacological disease models in general, it is difficult to dissociate the effect of the seizure and the drug used to induce the seizures (Loscher, 2011). However, this distinction is not critical in this model. The drug bicuculline methiodine was chosen because it has a well-established mechanism of action as a GABAergic antagonist. GABAergic drugs are often used to create well-accepted and validated animal models of generalized pediatric epilepsy. Although these models are not identical to human epilepsy, they offer insight into aspects of human epilepsies that cannot be disentangled in patients. It is widely accepted that IIS are a function of a pathophysiological process that disrupts the excitatory/inhibitory balance in focal brain regions. Our ‘etiology’ in this context was modulation of GABAergic signaling using bicuculline methiodide. Thus, although bicuculline methiodide is not an etiology in humans, the consequential GABAergic dysfunction likely is (Baulac et al., 2001; Calcagnotto, 2005; McDonald et al., 1991; Wallace et al., 2001), and may also be involved in ADHD (Edden et al., 2012) and autism(Fatemi et al., 2009). Although it would be almost impossible to say with certainty, both the etiology and the epileptiform activity itself are likely to influence later cognition given that we see STP changes bilaterally when injections were unilateratal but IIS propagate bilaterally. Thus regardless of whether the downstream consequences of the bicuculline injection rather than the IIS activity alone are playing a role in the observed effects, we suggest this model is clinically relevant and significant.
Taken together, these data show that GABAergic blockade and resultant early life IIS, independently of overt generalized seizures, can lead to long-lasting alterations in the PFC on a network and a behavioral level. Previous studies have shown long-lasting effects of epileptiform activity in the lateral geniculate nucleus and the superior colliculus of neonatal rabbits (Baumbach and Chow, 1981; Crabtree et al., 1981; Ostrach et al., 1984). We extend this work, creating for the first time a rodent model of neonatal focal IIS in a brain region that is relevant to co-morbidities common in children with epilepsy. Further investigation of the mechanisms underpinning PFC dysfunction in patients with frequent focal epileptiform discharges may lead to novel therapeutic approaches that ultimately improve quality of life.
Acknowledgments
Acknowledgments/Conflicts of interest
This work was supported by 1R01NS073083 (awarded to GLH), Emmory R. Shapses Research Fund (GLH), R01NS075249 (RCS) and R01NS076763 (PPLS). RCS is funded by Great Ormond Street Children's Charity. EI is funded by State Foundation of Fundamental Research of Ukraine F46.2/001.
Abbreviations
- IIS
interictal spike
- STP
short term plasticity
- PFC
prefrontal cortex
Contributor Information
Amanda E. Hernan, Email: Amanda.Hernan@uvm.edu.
Rod C. Scott, Email: Rodney.Scott@med.uvm.edu.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat. Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- Baumbach HD, Chow KL. Visuocortical epileptiform discharges in rabbits: differential effects on neuronal development in the lateral geniculate nucleus and superior colliculus. Brain Res. 1981;209:61–76. doi: 10.1016/0006-8993(81)91172-0. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal A. Epilepsy and autism spectrum disorders: are there common developmental mechanisms? Brain Dev. 2010;32:731–738. doi: 10.1016/j.braindev.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Brunquell PJ, Glennon CM, DiMario FJ, Lerer T, Eisenfeld L. Prediction of outcome based on clinical seizure type in newborn infants. J. Pediatr. 2002;140:707–712. doi: 10.1067/mpd.2002.124773. [DOI] [PubMed] [Google Scholar]
- Buonomano DV. Decoding temporal information: a model based on short-term synaptic plasticity. J. Neurosci. 2000;20:1129–1141. doi: 10.1523/JNEUROSCI.20-03-01129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaleiro G. Obsessive syndrome of epileptic etiology in children and pre-adolescents] Arch. Neurobiol. 1969;32:521–534. [PubMed] [Google Scholar]
- Calcagnotto ME. Dysfunction of synaptic inhibition in epilepsy associated with focal cortical dysplasia. J. Neurosci. 2005;25:9649–9657. doi: 10.1523/JNEUROSCI.2687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- Chang Y-C, Kuo Y-M, Huang A-M, Huang C-C. Repetitive febrile seizures in rat pups cause long-lasting deficits in synaptic plasticity and NR2A tyrosine phosphorylation. Neurobiol. Dis. 2005;18:466–475. doi: 10.1016/j.nbd.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Crabtree JW, Chow KL, Ostrach LH, Baumbach HD. Development of receptive field properties in the visual cortex of rabbits subjected to early epileptiform cortical discharges. Brain Res. 1981;227:269–281. doi: 10.1016/0165-3806(81)90113-9. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls ET, Romo R. Synaptic dynamics and decision making. Proc. Natl. Acad. Sci. U.S.A. 2010;107:7545–7549. doi: 10.1073/pnas.1002333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Klyachko V. The diverse functions of short-term plasticity components in synaptic computations. Commun. Integr. Biol. 2011;4:543–548. doi: 10.4161/cib.4.5.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn DW, Austin JK, Harezlak J, Ambrosius WT. ADHD and epilepsy in childhood. Dev. Med. Child Neurol. 2003;45:50–54. [PubMed] [Google Scholar]
- Edden RAE, Crocetti D, Zhu H, Gilbert DL, Mostofsky SH. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2012;69:750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J. Autism Dev. Disord. 2009;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C. The ESSENCE in child psychiatry: Early Symptomatic Syndromes Eliciting Neurodevelopmental Clinical Examinations. Res. Dev. Disabil. 2010;31:1543–1551. doi: 10.1016/j.ridd.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Gordon N. Acquired aphasia in childhood: the Landau–Kleffner syndrome. Dev. Med. Child Neurol. 1990;32:270–274. doi: 10.1111/j.1469-8749.1990.tb16935.x. [DOI] [PubMed] [Google Scholar]
- Gordon N. The Landau–Kleffner syndrome: increased understanding. Brain Dev. 1997;19:311–316. doi: 10.1016/s0387-7604(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav. Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M. Epilepsy and cognition. Epilepsy Curr. 2007;7:1–6. doi: 10.1111/j.1535-7511.2007.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan AE, Holmes GL, Isaev D, Scott RC, Isaeva E. Altered short-term plasticity in the prefrontal cortex after early life seizures. Neurobiol. Dis. 2012;50:120–126. doi: 10.1016/j.nbd.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL. The long-term effects of neonatal seizures. Clin. Perinatol. 2009;36:901–914. doi: 10.1016/j.clp.2009.07.012. (vii–viii). [DOI] [PubMed] [Google Scholar]
- Huang L, Cilio MR, Silveira DC, McCabe BK, Sogawa Y, Stafstrom CE, Holmes GL. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Brain Res. Dev. Brain Res. 1999;118:99–107. doi: 10.1016/s0165-3806(99)00135-2. [DOI] [PubMed] [Google Scholar]
- Huang L-T, Yang SN, Liou C-W, Hung P-L, Lai M-C, Wang C-L, Wang T-J. Pentylenetetrazol-induced recurrent seizures in rat pups: time course on spatial learning and long-term effects. Epilepsia. 2002;43:567–573. doi: 10.1046/j.1528-1157.2002.29101.x. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Andermann ML, Belliveau JW, Raij T, Sams M. Short-term plasticity as a neural mechanism supporting memory and attentional functions. Brain Res. 2011;1422:66–81. doi: 10.1016/j.brainres.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann R, Goldberg-Stern H, Shuper A. Attention-deficit disorders and epilepsy in childhood: incidence, causative relations and treatment possibilities. J. Child Neurol. 2009;24:727–733. doi: 10.1177/0883073808330165. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Wu EX, Holmes GL, Scott RC, Lenck-Santini PP. Enhanced oscillatory activity in the hippocampal–prefrontal network is related to short-term memory function after early-life seizures. J. Neurosci. 2011;31:15397–15406. doi: 10.1523/JNEUROSCI.2196-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Levin B, Duchowny M. Childhood obsessive–compulsive disorder and cingulate epilepsy. Biol. Psychiatry. 1991;30:1049–1055. doi: 10.1016/0006-3223(91)90124-5. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gatt A, Werner SJ, Mikati MA, Holmes GL. Long-term behavioral deficits following pilocarpine seizures in immature rats. Epilepsy Res. 1994;19:191–204. doi: 10.1016/0920-1211(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, Holmes GL. Reduced neurogenesis after neonatal seizures. J. Neurosci. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Garofalo EA, Hood T, Sackellares JC, Gilman S, McKeever PE, Troncoso JC, Johnston MV. Altered excitatory and inhibitory amino acid receptor binding in hippocampus of patients with temporal lobe epilepsy. Ann. Neurol. 1991;29:529–541. doi: 10.1002/ana.410290513. [DOI] [PubMed] [Google Scholar]
- Mori T, Mori K, Fujii E, Toda Y, Miyazaki M, Harada M, Hashimoto T, Kagami S. Evaluation of the GABAergic nervous system in autistic brain, 123I-iomazenil SPECT study. Brain Dev. 2012;34:648–654. doi: 10.1016/j.braindev.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Ostrach LH, Crabtree JW, Campbell BG, Chow KL. Effects of bicuculline-induced epileptiform activity on development of receptive field properties in striate cortex and lateral geniculate nucleus of the rabbit. Brain Res. 1984;317:113–123. doi: 10.1016/0165-3806(84)90146-9. [DOI] [PubMed] [Google Scholar]
- Praline J, Hommet C, Barthez M-A, Brault F, Perrier D, Passage GD, Lucas B, Bonnard J, Billard C, Toffol BD, Autret A. Outcome at adulthood of the continuous spike-waves during slow sleep and Landau–Kleffner syndromes. Epilepsia. 2003;44:1434–1440. doi: 10.1046/j.1528-1157.2003.08403.x. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Risbrough V, Bontempi B, Menzaghi F. Selective immunolesioning of the basal forebrain cholinergic neurons in rats: effect on attention using the 5-choice serial reaction time task. Psychopharmacology. 2002;164:71–81. doi: 10.1007/s00213-002-1170-7. [DOI] [PubMed] [Google Scholar]
- Rotman Z, Deng P-Y, Klyachko VA. Short-term plasticity optimizes synaptic information transmission. J. Neurosci. 2011;31:14800–14809. doi: 10.1523/JNEUROSCI.3231-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum. Mol. Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]