Abstract
Background and Objective
Postpubertal women with inherited long-QT syndrome 2 (LQT2) are at increased risk for polymorphic ventricular tachycardia (pVT) and sudden cardiac death (SCD), particularly during the postpartum. We aimed at investigating whether sex hormones directly modulate the arrhythmogenic risk in LQTS.
Methods and Results
Prepubertal ovariectomized transgenic LQT2 rabbits were treated with estradiol (EST), progesterone (PROG), dihydrotestosterone (DHT), or placebo (OVX). During eight weeks of treatment, major cardiac events – spontaneous pVT or SCD – occurred in 5/7 EST rabbits, contrasting with 2/9 in OVX (p<0.05) and no events in 9 PROG and 6 DHT rabbits (p<0.01 vs. PROG, p<0.05 vs. DHT). Moreover, EST increased the incidence of pVT (p<0.05 vs. OVX), while PROG reduced PVCs, bigeminy, couplets, triplets, and pVT (p<0.01 vs. OVX, p<0.001 vs. EST). In vivo ECG monitoring, in vivo electrophysiological and ex vivo optical mapping studies revealed that EST promoted SCD by steepening the QT/RR slope (p<0.05), by prolonging cardiac refractoriness (p<0.05), and by altering the spatial pattern of APD dispersion. Isoproterenol-induced Ca2+ oscillations resulted in early afterdepolarisations (EAD) in EST-treated hearts (4/4), while PROG prevented SCD by eliminating this EAD formation in 4/7 hearts (p=0.058 vs. EST, p<0.05 vs. OVX). Analyses of ion currents demonstrated that EST increased the density of ICa,L compared to OVX (p<0.05), while PROG decreased it (p<0.05).
Conclusion
This study reveals the pro-arrhythmic effect of EST and the anti-arrhythmic effect of PROG in LQT2 in vivo, outlining a new potential anti-arrhythmic therapy for LQTS.
Keywords: long-QT syndrome, sex hormones, arrhythmogenesis, sudden cardiac death, transgenic rabbit model, ion channels, early afterdepolarisation, in vivo electrophysiological study
Introduction
The inherited long-QT syndrome (LQTS) is characterized by an impaired cardiac repolarization resulting in QT prolongation, polymorphic ventricular tachycardia (pVT), and sudden cardiac death (SCD)1. Importantly, patients with LQTS exhibit pronounced gender differences in cardiac repolarization and their arrhythmogenic risk. Data from the international LQTS registry show longer QT intervals, a steeper QT/RR ratio, and a higher risk for pVT and SCD in postpubertal women with LQTS type 2 (loss of IKr2)3. In contrast, before puberty, the arrhythmia incidence is higher in boys4. Moreover, both the menstrual cycle and the postpartum period are associated with changes in the incidence of pVT. LQT2 patients have a reduced risk during pregnancy and a markedly increased risk during the postpartum period5, 6. In addition, in the acquired drug-induced LQTS variant the risk for pVT is higher during menses and the follicular phase with high serum estradiol levels than during the luteal phase with high progesterone levels7. These observations strongly suggest a potential pro-arrhythmic role for estradiol and an anti-arrhythmic effect of progesterone. However, these postulated pro- and anti-arrhythmic sex hormone effects in LQTS have never been demonstrated in vivo and their underlying mechanisms have yet to be characterized.
We recently generated transgenic LQT2 rabbits over-expressing a loss-of-function pore mutation of the human HERG channel (HERG-G628S) in the heart, mimicking the human LQT2 phenotype with QT prolongation, steeper QT/RR ratio in females, spontaneous pVT, and SCD – with a particularly high incidence in the postpartum8, 9. Mechanisms underlying these arrhythmias include a pronounced spatial dispersion of action potential duration (APD) and dynamic APD changes that lead to discordant alternans8, 10, as observed in LQTS patients11. Here we demonstrate in prepubertal ovariectomized female LQT2 rabbits chronically treated with different sex hormones that estradiol and progesterone have direct and contrasting effects on arrhythmias and SCD by modulating the arrhythmogenic substrate and the generation of triggered activity.
Methods
(A detailed method description can be found in an accompanying online supplement.)
Ovariectomy, hormone treatment
Prepubertal LQT2 rabbits underwent ovariectomy or sham surgeries and 90-day release pellets (Innovative Research of America) containing 17β-estradiol, dihydrotestosterone, progesterone, or placebo were implanted subcutaneously resulting in similar estradiol levels as during follicular phase, progesterone levels comparable to pregnant rabbits, and dihydrotestosterone levels as in males12, 13 (Fig. 1 supplement).
Telemetric ECG monitoring: QT/RR ratio and arrhythmia screening
Using telemetric ECG devices (F70-EEE, DSI) QT/RR ratio and heart rate corrected QT indices were calculated8, 9. Arrhythmias and major cardiac events – pVT and SCD – within corresponding 2-hour intervals were analyzed and classified using Lown's classification14.
In vivo electrophysiological studies (EPS)
Catheter-based in vivo EPS were performed to assess ventricular effective refractory periods (VERP) in RVapex and base at baseline and during isoproterenol infusion (ISO, 0.10 - 0.25 μg/min)15.
Optical mapping
Dual voltage-calcium optical mapping (100x100 pixels, Ultima-L, Scimedia)16 was performed using fluorescence probes PGH1 for membrane potential (generously provided by Dr. Guy Salama, University of Pittsburgh) and rhod-2 for Cai (Invitrogen). Images were acquired from the LV anterior surface and the field of view was set to 1.5 × 1.5 cm2 with a spatial resolution of 150 × 150 μm2 8, 16. To investigate hormone effects on early afterdepolarisation (EAD) formation, hearts were exposed to an intra-coronary isoproterenol bolus (140 nM) after AV-ablation.
Patch clamp
Whole-cell recordings in cardiomyocytes isolated from LVapex were obtained with an Axopatch-200B amplifier (Axon Instruments) with standard patch-clamp techniques8.
Western Blot
Western blot experiments on crude membrane preparations of LV apex were performed8 using the following antibodies: anti-SERCA2a (Thermo Scientific, MA3-919), anti-PLN (Thermo Scientific, MA3-922), and anti-NCX (Thermo Scientific, MA3-926) as primary antibodies and HRP conjugated goat-anti-mouse (IgG polyclonal, Thermo Scientific) as secondary antibodies.
Statistical analysis
For normally distributed values, we used Student's t-test (paired and unpaired). Chi-square test was used for categorical variables. Analysis was performed with Prism 4.03 (Graphpad) and a p value ≤ 0.05 was considered significant.
Results
Sex hormone effects on arrhythmogenesis
To investigate hormone effects on arrhythmogenesis in LQTS, we treated prepubertal ovariectomized transgenic LQT2 rabbits with estradiol (EST), progesterone (PROG), dihydrotestosterone (DHT), or placebo (OVX) for 8 weeks. We first compared arrhythmia incidences within corresponding 2-hour intervals one week before and within 96 hours following EPS using telemetric ECG monitoring (Fig. 1A). In the week before EPS, no arrhythmias besides isolated sinus pauses occurred in either group. In the 96 hours after EPS, however, arrhythmia incidences were higher in all groups but varied significantly among groups. PROG significantly reduced the incidence of PVCs and couplets compared to OVX and EST rabbits, and importantly, bigeminy and triplets did not occur in any PROG rabbit (Fig. 1A), strongly indicating an anti-arrhythmic effect in PROG rabbits. Moreover, no single episode of non-sustained or sustained pVT occurred in any PROG rabbit further underlining an anti-arrhythmic PROG effect (Fig. 1A). The incidences of PVC and bigeminy were similar in EST and OVX rabbits. However, couplets tended to occur more often in EST rabbits (p=0.06) and nsVT occurred significantly more frequently in EST than in OVX rabbits, suggesting a pro-arrhythmic EST effect. Of note, all lethal pVTs were initiated by short-long-short sequences and an early PVC coinciding with the T wave (R-on-T phenomenon), as described in LQTS patients17, suggesting that EADs likely underlie arrhythmia initiation (Fig. 1B). Moreover, prior to the initiation of pVTs the heart rate slowed down, due to short-long-short sequences and AV block (in 5/8 rabbits) (Fig. 1B). The incidence of AV blocks, however, did not differ between groups.
Figure 1. Effect of Sex Hormones on Incidence of Arrhythmias.
A. Dot blots of differences in arrhythmia incidences (PVC, bigeminy, couplets, triplets, non-sustained VT (nsVT), all presented as beats/two hours; sustained VT (susVT), duration in seconds). Each dot represents a 2-hour interval of an individual rabbit (n=14 in EST, n=18 in OVX and PROG rabbits). * p<0.05, ** p<0.01, *** p<0.001. B. Telemetric ECG recordings of the initiation of lethal pVT in two EST rabbits, top two rows. Indicated are R-on-T (red square), short-long-short sequences, and P waves (P) during episodes of AV 2:1 block. Bottom row shows several episodes of nsVTs following couplets in an EST rabbit. C. Incidence of major cardiac events during 8 weeks of hormone-treatment. Incidences of SCD are indicated in brackets. * p<0.05, ** p<0.01.
We consecutively ranked the severity of arrhythmias using Lown's classification14: 9/9 PROG rabbits had either Lown 0 (no PVCs) or Lown 1 (less than 30 PVCs/hour) arrhythmias, while 5/9 OVX rabbits demonstrated at least Lown 3 arrhythmias (e.g., bigeminy) (p<0.01 vs. PROG), and 3/9 even had Lown 4 arrhythmias (e.g., couplets, triplets, or nsVT) (p=0.05 vs. PROG). In EST rabbits, the rate of Lown 4 arrhythmias (5/7) was even higher (p<0.01 vs. PROG).
Finally, we compared the incidence of major cardiac events – defined as spontaneous pVT and SCD – between hormone groups. In EST rabbits, the incidence of major cardiac events (5/7) was significantly higher than in OVX (2/9, p<0.05), PROG (0/9, p<0.01), and DHT (0/6, p<0.05) rabbits (Fig. 1C). Most lethal pVT occurred within 96-hours after EPS in rabbits that were fully awake similar to observations in previous studies9, 15. In a follow up study of rabbits that were not exposed to anesthesia, 3/13 EST rabbits died of pVT contrasting with no SCD in any other hormone group (0/9 DHT, 0/12 PROG, 0/12 OVX). All these observations strongly indicate a pro-arrhythmic effect of EST and an anti-arrhythmic effect of PROG in LQT2 rabbits.
Sex hormone-induced changes in cardiac repolarization
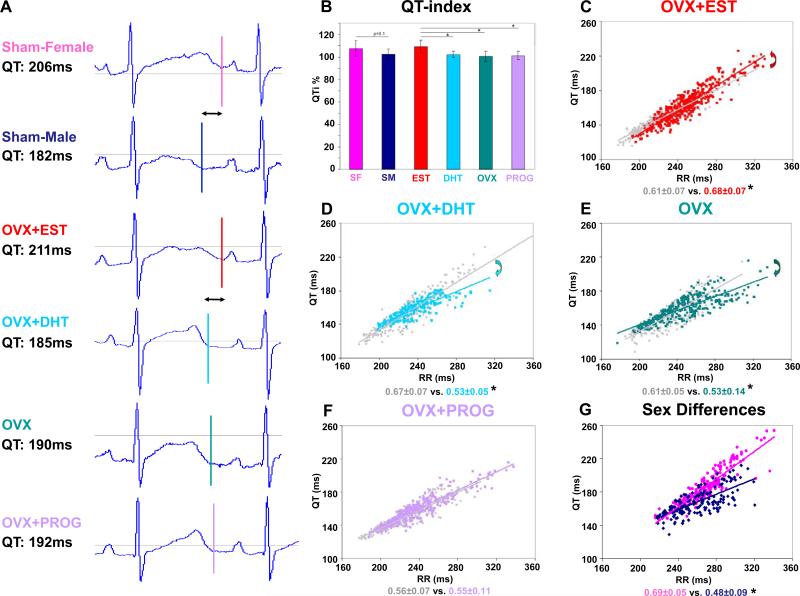
To investigate mechanisms that account for these pro- and anti-arrhythmic hormone effects, we first compared hormone effects on QT duration and QT/RR ratio in free-moving ECG monitored LQT2 rabbits. Heart-rate corrected QT indices were significantly longer in EST than in OVX, PROG or DHT rabbits, and tended to be longer in sham-operated females (SF) than in sham-operated males (SM) (p=0.1) (Fig. 2B). These differences in QT duration were particularly pronounced at slow heart rates. As demonstrated in representative ECG tracings in Figure 2A, at RR intervals of 300 ms, QT duration in EST rabbits was similar to SF, while in DHT rabbits the QT was shortened as observed in SM. In OVX and PROG rabbits QT duration was intermediate. Furthermore, EST steepened the QT/RR slope (Fig. 2C), mimicking the adult LQT2 female phenotype (Fig. 2G). In contrast, DHT decreased the steepness of the QT/RR slope (Fig. 2D), thus mimicking the male phenotype (Fig. 2G). Similarly, OVX decreased QT/RR steepness (Fig. 2E), while PROG did not alter QT/RR ratio (Fig. 2F).
Figure 2. Effect of Sex Hormones on QT Duration.
A. Exemplary, representative ECG traces of individual rabbits at 300 ms RR intervals. QT durations are indicated. B. QT indices in n=6 rabbits after 4 weeks of hormone-treatment calculated based on QT and RR intervals acquired over 24-hours of ECG monitoring. * p<0.05. C-F. QT/RR ratio in n=6 rabbits at baseline (grey) and after 4 weeks of treatment (color). Arrows indicate the direction of changes in QT/RR ratio. * p<0.05. G. QT/RR ratio in n=6 adult SF and SM. * p<0.05.
To further examine hormone effects on rate dependent changes of QT and VERP, we performed in vivo EPS. Figure 3 A-B depicts representative ECG recordings in EST and PROG rabbits during VERP determination in RVapex at a stimulation cycle length of 300 ms (S1 train). In the top panel, the coupled extra stimulus (S2) was captured, whereas in the lower panel the 10 ms shorter S2 stimulus failed to capture (at 240 ms in EST and 150 ms in PROG rabbit). In RVapex, VERPs were longer in EST than in OVX, PROG or DHT rabbits (Fig. 3C). In RVbase, however, hormone effects on the VERP were not as pronounced (Fig. 3C). Continuous infusion of ISO shortened the VERP in SF more than in SM. Similarly, the ISO-induced VERP-shortening was more pronounced in EST than in OVX, PROG, or DHT rabbits (Fig. 3D).
Figure 3. Effect of Sex Hormones on Cardiac Repolarization.
A. and B. Surface and intra-cardiac ECG in individual EST and PROG rabbit during VERP determination: (top to bottom) 12 surface ECG leads, right atrium (RA) (2 recordings), RVbase (2 recordings), RVmid (2 recordings), and RVapex. Top panel shows stimulation with 300 ms CL (S1) and coupled extrastimuli (S2) that are captured. Lower panel shows shorter S2 extrastimuli that fail to capture. C. VERP in RVapex (filled bars) and base (hatched bars) in n=6 rabbits per group. * p<0.05, ** p<0.01. All values are shown as mean ± SD. D. Delta-VERP baseline-isoproterenol in RVapex. ** p<0.01, *** p<0.001.
Sex hormones and APD dispersion
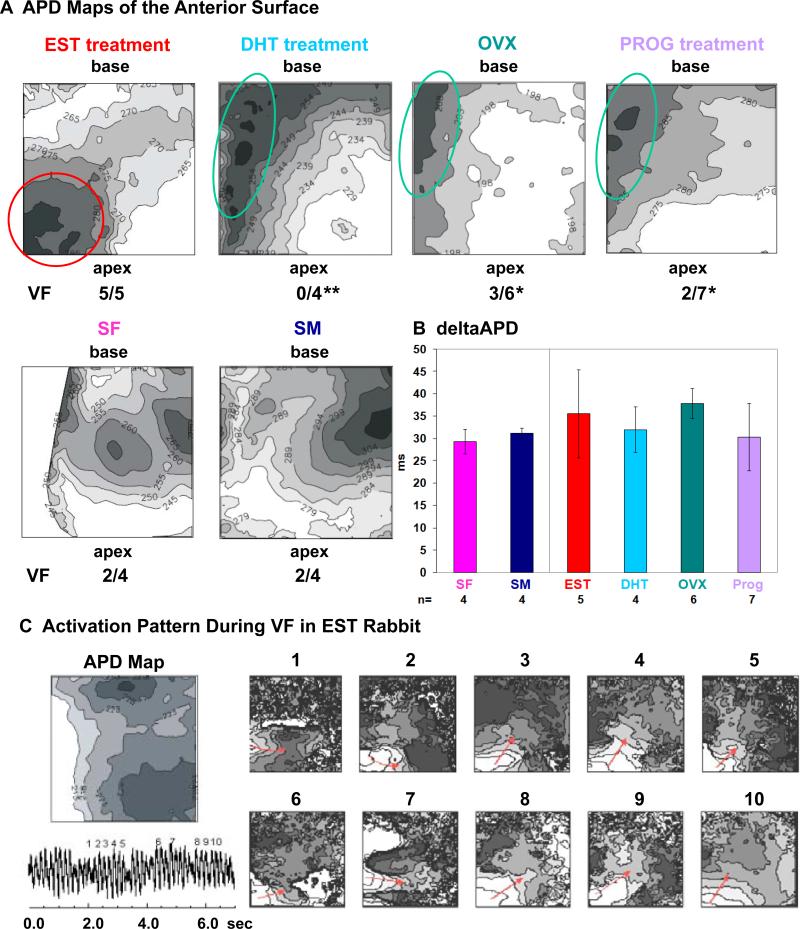
We next investigated whether sex hormones modulate spatial APD dispersion – a known mechanism underlying arrhythmogenesis in LQT2 syndrome11 – using optical mapping. Figure 4A shows representative APD maps of the LV anterior surface of hormone- treated LQT2 rabbit hearts with basal regions represented in the upper part of the image and apical regions in the lower part. Isolines of APD are drawn every 5 ms and dark regions represent long APD while bright regions represent short APD. In all OVX, PROG, and DHT rabbits (n=4-7 each) the region with the longest APD was found in the LV mid-septal region and the shortest APD in the apex. Yet, EST altered the pattern of APD dispersion, and the longest APD region was shifted towards the LVapex. However, we found no significant differences in mean APD and APD dispersion among groups (Fig. 4B). Programmed ventricular stimulation induced VF in all five EST rabbits, contrasting with 3/6 OVX (p<0.05), 2/7 PROG (p<0.05), and 0/4 DHT rabbits (p<0.01). In all EST rabbits, during VF, activation waves propagated around the apical region of prolonged APD. As illustrated in Figure 4C, activation waves encountered refractoriness in the apical region with prolonged APD, which caused unidirectional block and wave propagation around this region. Conduction velocity measured on the anterior surface of the left ventricle was similar in all groups (OVX, 0.55 ± 0.12 m/s; EST, 0.59 ± 0.2 m/s; PROG, 0.56 ± 0.1 m/s), indicating a lack of structural changes with hormone treatment.
Figure 4. Effect of Sex Hormones on APD Dispersion.
A. Representative APD maps of the anterior surface of the LV (field of view 1.5 x 1.5 cm2) of individual rabbits. Isolines of APD are drawn every 5 ms, darker regions represent longer APD. Indicated are regions of long APD in LV mid-base region (green circle) and LVapex (red circle). Rates of VF inducibility are listed. * p<0.05, ** p<0.01. B. ΔAPD defined as longest – shortest APD. All values are shown as mean ± SD. C. Activation pattern during VF in EST rabbit. Displayed are APD map, ECG trace of VF (bottom left), and consecutive maps (1-10) of the activation pattern during VF. Red arrows indicate the direction of activation waves rotating around the apical region of prolonged APD.
Sex hormones and EAD formation
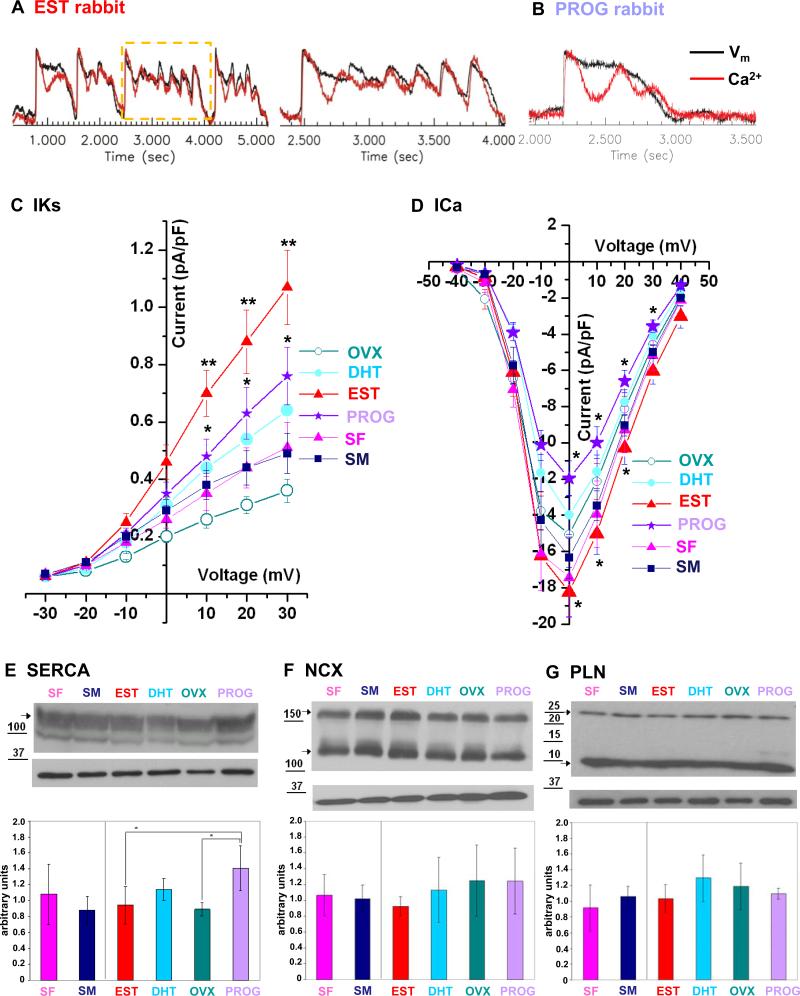
Since it is well known that pVT is initiated by EADs triggered by sympathetic surge18, we examined the response to a bolus of ISO using dual voltage-calcium optical mapping. In all EST (4/4), OVX (5/5), SF (4/4), and SM (4/4) rabbits, both membrane potentials and Ca2+ transients began to oscillate within 5-10 s after ISO bolus (Fig. 5A). Ca2+ started to rise prior to changes in membrane potential during the early phase of EAD formation, yet in later EADs with larger amplitude the membrane potential changes led to Ca2+ rise (Fig. 5A), suggesting that Ca2+ oscillations may be an important trigger for EAD formation in this LQT2 model. In contrast, in 4/7 PROG and in 2/4 DHT rabbits, Ca2+ transient oscillations failed to initiate EADs, resulting in a significantly reduced EAD formation rate in PROG rabbits (p=0.058 vs. EST, p<0.05 vs. OVX) (Fig. 5B).
Figure 5. Effect of Sex Hormones on Ca2+ Oscillations, EADs, Ion Currents, and Ca2+ Cycling Proteins.
A. Representative trace of Ca2+ oscillations and EADs in EST rabbit after ISO bolus. Black line indicates changes in voltage fluorescence signal (Vm); red line indicates changes in Ca2+ signal. The region shown in higher magnification in the right column is indicated by a yellow rectangle. B. Representative trace of Ca2+ oscillations and lack of EAD formation in PROG rabbit after ISO bolus. C.-D. Hormones effects on IKs and ICa,L current densities measured in cardiomyocytes harvested from LVapex of EST (n=15 cardiomyocytes), DHT (n=14), OVX (n=15), PROG (n=18), SF (n=6), and SM (n=6) rabbits. All values are shown as mean ± SEM. IKs: EST vs. OVX: p<0.01; EST vs. PROG, PROG and DHT vs. OVX p<0.05. ICa,L: EST vs. OVX, PROG vs. OVX: p<0.05; EST vs. PROG: p<0.01. E.-G. Representative western blots of SERCA2a, NCX, and PLN. Bar graphs indicate the expression levels of 3 independent experiments in 3 different rabbits per group in arbitrary units. All values are shown as mean ± SD. * p<0.05.
Sex hormone effects on ion currents
To elucidate underlying mechanisms on the cellular level, we performed patch clamp experiments with cells harvested from the LVapex, where we observed the most pronounced differences in APD maps and Ca2+ oscillations. IKs current density was significantly increased in all hormone groups, SF, and SM as compared to OVX rabbits. However, this increase was most pronounced in EST rabbits resulting in a significantly higher IKs current in EST than in PROG or DHT-treated cardiomyocytes (Fig. 5C). Importantly, EST increased the density of ICa,L currents while PROG decreased ICa,L as compared to OVX resulting in a significantly higher ICa,L in EST than in PROG rabbits (Fig. 5D). No differences were observed in Ito and IK1. Due to the dominant negative effect of the HERG pore mutant in LQT2 rabbits, IKr was absent in all groups8.
Sex hormone effects on Ca2+ cycling proteins
To test whether changes in Ca2+ cycling proteins may account for the differences in the propensity to develop EADs, we compared their expression in the LVapex. We observed a significantly increased expression of the sarcoplasmic reticulum calcium ATPase2a (SERCA2a) polypeptides in PROG as compared to EST and OVX rabbits (p<0.05; Fig. 5E) that may contribute to an increased Ca2+ reuptake into the SR thereby shortening Ca2+ transient duration. No hormone-induced differences were apparent in sodium-calcium exchanger (NCX) (Figure 5F), and phospholamban (PLN) (Figure 5G).
Discussion
Pro- and anti-arrhythmic effects of sex hormones in vivo
Sex differences in long-QT-related arrhythmias with a higher risk for polymorphic VT and SCD in women than in men and a particularly increased risk during the postpartum period have been well documented in the clinical setting3, 6. This study takes advantage of a transgenic LQT2 rabbit model that develops spontaneous pVTs and SCD8 to investigate the role of sex hormones in arrhythmogenesis in vivo.
Here we show for the first time a direct link between sex hormones and the incidence of arrhythmias and SCD. Our telemetry recordings of hormone-treated LQT2 rabbits demonstrate that PROG significantly reduces potential triggers for polymorphic VTs – such as bigeminy and couplets – and completely abolishes the occurrence of pVT. Moreover, we show that PROG is protective and prevents SCD, suggesting that high PROG levels during pregnancy likely account for the reduced risk in pregnant LQT2 patients5. Additionally, the marked reduction of PROG during the postpartum period likely promotes postpartal arrhythmias and SCD in LQT2 patients6. EST, in contrast, increases both, triggers and the sustainability of pVT and thereby promotes SCD, indicating that EST likely underlies the increased arrhythmogenic risk in postpubertal women with LQT23.
As in LQT2 patients3, SCD is a rare event in LQT2 rabbits. However, similar to observations in previous studies9, 15, lethal pVT occurred more frequently within 96-hours after isoflurane anesthesia. It may be possible that anesthesia-induced slowing of the heart rate15 or the IKs-blocking properties of isoflurane9 may contribute to the overall increased risk. However, all rabbits had recovered from anesthesia and were fully awake at the time of SCD. Since similar anesthetic dosages were used, it is likely that anesthesia increased the likelihood of events similarly in all groups and therefore enabled us to uncover the protective effect of PROG and the pro-arrhythmic effect of EST. Moreover, in a group of rabbits not exposed to anesthesia, we observed 3 SCD in 13 EST rabbits while no SCD was observed in any other group, further underlining the pro-arrhythmic effect of EST.
Hormone effects on the arrhythmogenic substrate
Enhanced APD dispersion due to a spatially heterogeneous prolongation of cardiac repolarization is considered a major contributor to LQT-related arrhythmias11. Moreover, we have previously identified an increased spatial APD dispersion across the anterior surface of the LV as a major mechanism underlying arrhythmias in transgenic LQT2 rabbits8, 10. We thus further explored whether sex hormones alter dispersion of repolarization in RV and LV. In EST rabbits, the longest APD region was shifted towards the LVapex, in line with the more pronounced VERP prolongation in the RVapex, suggesting that EST may exert its proarrhythmic effects, at least partly, by differentially changing APD in different regions of LV and RV thus modifying the arrhythmogenic substrate. Indeed, programmed ventricular stimulation induced VF in all five EST rabbits but only rarely and significantly less frequently in OVX, PROG, and DHT rabbits, thus indicating the pro-arrhythmic significance of these EST-induced changes. Moreover, during VF the activation waves propagated around the apical island of prolonged APD.
We further investigated how sex hormones differentially affect rate dependent repolarization (restitution). Our in vivo ECG monitoring studies are the first to demonstrate that EST steepens the QT/RR slope in LQT2 by prolonging QT duration at slow heart rates and shortening QT at fast heart rates, whereas DHT decreases the steepness of QT/RR slope. EST and DHT treatment, thus mimic sex differences in cardiac repolarization in LQT2 rabbits9, similar to observations in LQT2 patients3. ICa,L and IKs play an important role in conferring these hormone effects on QT/RR: EST rabbits have higher density of ICa,L than any other groups, resulting in prolongation of QT at slow heart rates, while the higher density of IKs in EST rabbits shortens QT at fast heart rates thus contributing to EST-induced steepening of QT/RR ratio.
Hormone effects on the susceptibility to pro-arrhythmic triggers
The initiation of pVTs in LQTS is known to be linked to EAD formation18. Moreover, previous animal studies demonstrated that hormones alter EAD formation in isolated cardiomyocytes13, 19. Here we demonstrate that sex hormones exert different effects on EAD formation in response to sympathetic stimuli at the organ level. In EST and OVX rabbits, ISO triggered both Ca2+ oscillations and EAD formation, while in PROG and DHT rabbits ISO triggered Ca2+ oscillations without EAD formation. Thus, PROG altered the response of the membrane potential to Ca2+ oscillations.
Experimental and simulation studies show that the reactivation of ICa,L plays an important role in EAD formation20, 21 and that reducing ICa,L by Ca2+ -blocking drugs such as verapamil is most effective in preventing pVT formation in animal models of LQTS22. Previous experimental and simulation data strongly suggest that ICa,L is a key player in the formation and propagation of EADs24, 25. Here we demonstrate that EST increased ICa,L while PROG and DHT decreased ICa,L as previously described23, 24. Thus, EST-induced increase in ICa,L likely contributes to the higher propensity to develop EADs, contributing to the proarrhythmic effects of EST. By contrast, the PROG-induced decrease in ICa,L reduces the likelihood of triggered activity. Our studies also demonstrate an increase of IKs in EST rabbits. Recent computer modeling studies show that both inward Ca2+ and outward K+ currents are essential for generating oscillatory behavior of the membrane potential during the plateau phase25 suggesting that the EST-induced increase of ICa,L and IKs facilitates triggered activity.
To further delineate molecular mechanisms underlying these pro- and anti-arrhythmic effects on the susceptibility to triggers, we investigated hormone effects on Ca2+ cycling proteins, which may contribute to arrhythmogenesis by altering cytoplasmic and SR Ca2+ concentrations, and the initiation of EADs. While previous studies in rats reported a DHT-and EST-induced increase in NCX26, 27, we observed no hormone effects on NCX or PLN expression in LQT2 rabbits. Yet, PROG increased SERCA2a expression in LQT2 rabbits, which may contribute to an increased Ca2+ reuptake into the SR thereby shortening Ca2+ transient duration. An increased expression of SERCA2a has previously been reported to reduce VT/VF in ischemia-reperfusion models28 and to reduce the susceptibility to alternans-mediated ventricular arrhythmias29. Consequently, this increase in SERCA2a and the decrease in ICa,L currents contribute to the anti-arrhythmic effect of PROG.
Limitations
Transgenic LQT2 rabbits over express the dominant-negative loss-of-function mutation HERG-G628S that is localized in the pore-region and leads to a complete loss of IKr30. Many missense mutations in HERG channels that are found in LQT2 patients, however, lead to a substantial decrease rather than a complete loss of IKr. The findings on hormone effects on arrhythmogenesis in this transgenic LQT2 rabbit model likely recapitulate the findings in human patients with pore mutations or other HERG mutations with a loss of functional IKr currents in the heart.
Conclusion
In this study, we demonstrate that EST promotes polymorphic VTs and SCD while PROG prevents arrhythmias and SCD in LQT2 in vivo. EST exerts this pro-arrhythmic effect by changing the arrhythmogenic substrate by steepening QT/RR ratio, prolonging cardiac refractoriness, and altering the spatial pattern of APD dispersion. The underlying mechanisms are an increased IKs current that contributes to the steepening of QT/RR by shortening QT at fast heart rates and notably a substantially increased ICa,L that contributes to both a longer refractoriness and a higher propensity to depolarize the membrane in response to Ca2+ oscillations. PROG, in contrast, exerts an anti-arrhythmic effect by preventing EAD formation in response to Ca2+ oscillations, likely due to an increase in SERCA2a and a decrease in the oscillatory ICa,L.
Clinical implications
To date, standard treatment of LQT2 patients consists of beta-blockade and implantation of ICDs1. Understanding the mechanisms that underlie sex hormones’ deleterious or protective effects could help to develop specific, hormone-based therapies. The experimental observation of an anti-arrhythmic PROG effect in transgenic LQT2 rabbits suggests a potential use of oral progestins as a new class of anti-arrhythmic treatment in LQTS. Further prospective studies are needed to provide evidence-based data to support this treatment option in LQTS.
Supplementary Material
Acknowledgements
The authors thank Louise Organ-Darling, Alfred E. Buxton, and Gary F. Mitchell for their valuable comments in reviewing the manuscript and Megan Shearer for her help with the ovariectomy surgeries.
Funding sources
G. Koren is the recipient of NIH grant RO1 HL046005-18 and HL093205; K.E. Odening was supported by grants from the German Cardiac Society (St. Jude Medical Stipendium), the German Research Foundation (DFG Forschungsstipendium OD 86/1-1), and by an AHA postdoctoral fellowship award (AHA 0826071D).
List of abbreviations
- LQT2
long-QT syndrome type 2
- pVT
polymorphic VT
- SCD
sudden cardiac death
- EST
estradiol
- OVX
ovariectomy
- PROG
progesterone
- DHT
dihydrotestosterone
- SF
sham-operated female
- SM
sham-operated male
- PVC
premature ventricular contraction
- RV
right ventricle
- LV
left ventricle
- EAD
early afterdepolarisation
- APD
action potential duration
- AV
atrioventricular
- EPS
electrophysiological study
- CL
cycle length
- AVWCL
AV Wenckebach cycle length
- AVNRP
AV nodal refractory period
- VERP
ventricular effective refractory periods
- ISO
isoproterenol
- VF
ventricular fibrillation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interests.
References
- 1.Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med. 2003;358:169–176. doi: 10.1056/NEJMcp0706513. [DOI] [PubMed] [Google Scholar]
- 2.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long-QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 3.Sauer AJ, Moss AJ, McNitt S, et al. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49:329–337. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg I, Moss AJ, Peterson DR, et al. Risk factors for aborted cardiac arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation. 2008;117:2184–2191. doi: 10.1161/CIRCULATIONAHA.107.701243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seth R, Moss AJ, McNitt S, et al. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007;49:1092–1098. doi: 10.1016/j.jacc.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 6.Khositseth A, Tester DJ, Will ML, Bell CM, Ackerman MJ. Identification of a common genetic substrate underlying postpartum cardiac events in congenital long QT syndrome. Heart Rhythm. 2004;1:60–64. doi: 10.1016/j.hrthm.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 8.Brunner M, Peng X, Liu GX, et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odening KE, Hyder O, Chaves L, et al. Pharmacogenomics of anesthetic drugs in transgenic LQT1 and LQT2 rabbits reveal genotype-specific differential effects on cardiac repolarization. Am J Physiol Heart Circ Physiol. 2008;295:H2264–2272. doi: 10.1152/ajpheart.00680.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziv O, Morales E, Song YK, et al. Origin of complex behaviour of spatially discordant alternans in transgenic rabbit model of type 2 long QT syndrome. J Physiol. 2009;587:4661–4680. doi: 10.1113/jphysiol.2009.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol. 2007;293:H2024–2038. doi: 10.1152/ajpheart.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–1474. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 13.Pham TV, Sosunov EA, Gainullin RZ, Danilo P, Jr, Rosen MR. Impact of Sex and Gonadal Steroids on Prolongation of Ventricular Repolarization and Arrhythmias Induced by IK-Blocking Drugs. Circulation. 2001;103:2207–2212. doi: 10.1161/01.cir.103.17.2207. [DOI] [PubMed] [Google Scholar]
- 14.Lown B, Calvert AF, Armington R, Ryan M. Monitoring for serious arrhythmias and high risk of sudden death. Circulation. 1975;52(6 Suppl):III189–198. [PubMed] [Google Scholar]
- 16.Odening KE, Kirk M, Brunner M, et al. Electrophysiological Studies of Transgenic Long QT Type 1 and Type 2 Rabbits Reveal Genotype-Specific Differences in Ventricular Refractoriness and His Conduction. Am J Physiol Heart Circ Physiol. 2010;299:H643–655. doi: 10.1152/ajpheart.00074.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi BR, Jang W, Salama G. Spatially discordant voltage alternans cause wavebreaks in ventricular fibrillation. Heart Rhythm. 2007;4:1057–1068. doi: 10.1016/j.hrthm.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viskin S, Viskin S, Alla SR, et al. Mode of onset of torsade de pointes in congenital long QT syndrome. J Am Coll Cardiol. 1996;28:1262–1268. doi: 10.1016/s0735-1097(96)00311-7. [DOI] [PubMed] [Google Scholar]
- 18.Choi BR, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physio. 2002;543:615–631. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara M, Danilo P, Jr, Rosen MR. Effects of gonadal steroids on ventricular repolarization and on the response to E4031. J Pharmacol Exp Ther. 1998;285:1068–1072. [PubMed] [Google Scholar]
- 20.January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 21.Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J. 1995;68:949–964. doi: 10.1016/S0006-3495(95)80271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farkas AS, Makra P, Csík N, et al. The role of the Na+/Ca2+ exchanger, INa and ICaL in the genesis of dofetilide-induced torsades de pointes in isolated, AV-blocked rabbit hearts. Br J Pharmacology. 2009;156:920–932. doi: 10.1111/j.1476-5381.2008.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci USA. 2008;105:15148–15153. doi: 10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scragg JL, Dallas ML, Peers C. Molecular requirements for L-type Ca2+ channel blockade by testosterone. Cell Calcium. 2007;47:11–15. doi: 10.1016/j.ceca.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Tran DX, Sato D, Yochelis A, Weiss JN, Garfinkel A, Qu Z. Bifurcation and chaos in a model of cardiac early afterdepolarizations. Phys Rev Lett. 2009;102:258103. doi: 10.1103/PhysRevLett.102.258103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden KL, Marsh JD, Jiang Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm Metab Res. 2004;36:197–202. doi: 10.1055/s-2004-814445. [DOI] [PubMed] [Google Scholar]
- 27.Chu SH, Goldspink P, Kowalski J, Beck J, Schwertz DW. Effect of estrogen on calcium-handling proteins, beta-adrenergic receptors, and function in rat heart. Life Sci. 2006;79:1257–1267. doi: 10.1016/j.lfs.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 28.Del Monte F, Lebeche D, Guerrero JL, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci USA. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a Gene Expression Identifies Molecular Mechanism and Therapeutic Target for Arrhythmogenic Cardiac Alternans. Circ Arrhythm Electrophysiol. 2009;2:686–694. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+-channel dysfunction in an inherited arrhythmia. Proc Natl Acad Sci USA. 1996;93:2208–2212. doi: 10.1073/pnas.93.5.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.