Abstract
Objective: Cell-free fetal DNA (cffDNA) testing has opened new options in prenatal screening for trisomy 21. Due to the higher costs of cffDNA testing there is an ongoing debate on how to combine different screening strategies. Methods: For this study, a model-based approach was used to evaluate all births in Germany in 2012 together with the percentage of euploid and trisomic pregnancies. Detection rates (DR), false positive rates (FPR), the costs of different screening strategies for trisomy 21 and combinations of these strategies were compared. The number of fetuses with trisomy 21 at 12 + 0 weeks of gestation was estimated based on maternal age distribution. We examined the screening performance of a screening strategy based on maternal age, first trimester screening (FTS) and cffDNA testing as well as the combinations “maternal age and cffDNA” and “FTS and cffDNA”. Results: In 2012 673 544 children were born. Median maternal age at delivery was 30.2 years (25th–75th quartile: 27.0–34.0). Based on maternal age distribution the expected number of fetuses with trisomy 21 at 12 weeksʼ gestation was 1788. Our study population therefore consisted of 675 332 pregnancies. Screening based only on maternal age or FTS or cffDNA resulted in detection rates of 63.3 %, 92.2 % and 99.0 % and false positive rates of 21.8 %, 8.0 % and 0.1 %, respectively. When maternal age was combined with cffDNA, cffDNA testing was only offered to women over a certain age; if a cut-off of 30 years was used, this resulted in a DR of 85.2 % and a FPR of 1.7 %. If primary screening consisted of FTS with cffDNA testing only done when the risk was between 1 : 10 and 1 : 1000, the detection rate was 96.7 % and the false positive rate was 1.2 %. Conclusion: In this model-based study we showed that prenatal screening for trisomy 21 can be improved even more by combining FTS and cffDNA. Further studies are necessary to examine whether these results can be reproduced in reality.
Key words: first trimester screening, cell-free fetal DNA, trisomy, screening
Abstract
Zusammenfassung
Zielsetzung: Durch die Einführung der zellfreien fetalen DNA (cffDNA)-Analyse eröffnen sich derzeit neue Möglichkeiten im pränatalen Screening auf Trisomie 21. Der höheren Testgüte stehen jedoch höhere Kosten gegenüber, sodass durch eine Kombination der verschiedenen Screening-Verfahren die Vorteile der verschiedenen Ansätze nutzbar gemacht werden sollen. In dieser Arbeit sollen die Testgüte und die Kosten der verschiedenen Screening-Ansätze untersucht werden. Methoden: In dieser Arbeit wurden in einer Modellrechnung die Detektions- und Falschpositivrate (DR und FPR) sowie die Kosten unterschiedlicher Ansätze im Screening auf Trisomie 21 und deren Kombinationen verglichen. Das Modell basierte auf den Geburten in Deutschland 2012, die den euploiden Anteil repräsentierten. Der Anteil der Feten mit Trisomie 21 bei 12 + 0 SSW wurde auf der Basis der Altersstruktur des mütterlichen Alters und deren Häufigkeit in der Geburtenkohorte 2012 geschätzt. Berechnet wurde die Testgüte für das Screening anhand des mütterlichen Alters, des Ersttrimester-Screenings, der cffDNA-Analyse sowie den Kombinationen aus mütterlichem Alter und cffDNA und ETS und cffDNA. Ergebnisse: 2012 wurden 673 544 Kinder geboren. Das mediane Alter der Mütter bei Entbindung lag bei 30,2 (25.–75. Quartil 27,0–34,0) Jahren. Entsprechend der mütterlichen Altersstruktur sind daher in der 12 + 0 SSW 1788 Feten mit Trisomie 21 zu erwarten. In Summe beinhaltet die Studienpopulation somit 675 332 Schwangerschaften. Das Screening anhand des mütterlichen Alters und des ETS resultiert in einer DR von 63,3 und 92,2 % bei einer FPR von 21,8 und 8,0 %. Für die cffDNA-Analyse werden als DR und FPR 99,0 und 0,1 % angenommen. Bei der Kombination des mütterlichen Alters und der cffDNA-Analyse wird ab einem mütterlichen Alter eine cffDNA-Analyse durchgeführt. Bei einem Schwellenwert von 30 Jahren liegen die DR und FPR bei 85,2 und 1,7 %. Bei Verwendung des ETS und im Intermediär-Risikokollektiv cffDNA sind bei Schwellenwerten von 1 : 10 und 1 : 1000 eine DR und eine FPR von 96,7 und 1,2 % zu erwarten. Schlussfolgerung: In dieser Modellrechnung konnte gezeigt werden, dass vor allem durch die Kombination aus ETS und cffDNA-Analyse das Screening auf Trisomie 21 optimiert werden kann. Nachfolgende Studien sollten untersuchen, ob die positiven Aspekte dieses Kombinationsansatzes auch in der Realität zu beobachten sind.
Schlüsselwörter: Ersttrimester-Screenings, zellfreie fetale DNA, Trisomie, Screening
Introduction
Prenatal screening for trisomy 21 has continuously improved in the past decades. Combined first trimester screening (FTS) has played an important role in this, and in many countries it is now a standard part of prenatal care. Over the past few years it has been expanded by the addition of risk stratification for numerous complications of pregnancy 1, 2.
The introduction of FTS has resulted in a significant decrease in the rates of invasive diagnostic procedures (chorionic villus sampling [CVS] and amniocentesis), despite the continued increase in median maternal age. The impact of FTS was particularly pronounced in England and Denmark. Morgan et al. showed that after changes in screening policy, the overall screen-positive rate in England, which was previously based on biochemical screening done in the second trimester, decreased from 6 to 3.1 % 3. Similarly, Ekkelund et al. reported that after the nation-wide introduction of FTS in Denmark, the false positive rate dropped to 3.3 % 4. By comparison, outdated screening programs based only on maternal age were found to have a false positive rate of 20 % when a cut-off of 35 years was used.
The introduction of cell-free fetal DNA (cffDNA) testing has offered a new way of screening for trisomy 21 5, 6. The reported detection rate and the false positive rate are 99 and 0.1 %, respectively 7; this approach involves fewer potential sources of error for medical practitioners and, with the exception of being qualified to provide information and consultation in accordance with the German Gene Diagnostics Law, no additional training is needed. The drawbacks of cffDNA are that it focuses only on the most common chromosomal disorders; that the test results of some pregnancies will be inconclusive because of insufficient cffDNA in maternal blood; that dichorionic multiple pregnancies, particularly vanishing-twin syndrome, reduce the test quality; and finally, that this form of screening is considerably more expensive than other methods.
The question is therefore what would be the best combination of different screening methods for Germany which would not relinquish the benefits of previous strategies but would instead improve the quality of screening. The economic impact of introducing an updated screening algorithm must also be taken into account.
Methods
This study used a model-based calculation to compare detection rates, false positive rates and the costs of different screening methods for trisomy 21 as well as outcomes for combinations of these methods. For the model a study population was defined consisting of euploid and trisomy 21 pregnancies. The published data on screening performance for the different screening strategies was used to determine detection rates and false positive rates for the study population.
Study population
The study population consisted of all births recorded in Germany in 2012 together with maternal age 8. It was assumed that all pregnancies were euploid pregnancies. To estimate the number of fetuses with trisomy 21 in week 12+ of gestation, the number of expected pregnancies with trisomy 21 in week 12 + 0 of gestation was compiled from the sum calculated for each maternal age between the ages of 15 and 50, according to the maternal age distribution for the birth cohort of 2012 8, 9, 10.
The number of fetuses with trisomy 21 delivered at term was calculated for every maternal age by multiplying the maternal age risk by the number of live term births. Because of the increased rate of miscarriages for fetuses with trisomy 21, the expected number of fetuses with trisomy 21 born at term was multiplied by an adjustment factor to calculate the probable number of fetuses with trisomy 21 at 12 + 0 weeks of gestation. The total number of expected fetuses with trisomy 21 in week 12 + 0 of gestation was calculated by adding up the number of cases calculated for each maternal age.
The risk of trisomy 21 for a child delivered at term is:
Risk = 0.000627 + e− 16.2395 + 0.286 × (mat. age – 0.5) × 1.5
The adjustment factor for 12 + 0 week of gestation is:
adj = 100.9425−1.023 × LOG₁₀(12) + 0.2718 × LOG₁₀(12) × LOG₁₀(12)
and
Risk12 SSW = Risk × adj
Test quality and cost of individual screening methods
Test quality of screening based on maternal age was calculated based on age distribution in the study. A pregnancy was considered screen-positive when the maternal age was above a defined cut-off.
Assessment of screening using cffDNA was based on a detection rate and a false positive rate of 99 and 0.1 % 7. Abnormal cffDNA results were considered screen-positive. If the result of cffDNA testing was inconclusive, which was expected for 3 % of tests, the pregnancy was also categorized as screen-positive. It was assumed that the incidence of inconclusive tests was similarly distributed for the euploid and the trisomy 21 groups 5.
For combined FTS it was assumed that the distribution of risks in the study population corresponded to that of the reference population (Table 1) 1. If the risk was above a specific cut-off, the pregnancy was defined as screen-positive.
Table 1 Risk distribution in combined FTS for euploid pregnancy and fetuses with trisomy 21.
| Risk | Euploid pregnancy | Trisomy 21 |
|---|---|---|
| ≥ 1 : 10 | 0.6 % | 69.1 % |
| 1 : 11–1 : 50 | 1.6 % | 15.2 % |
| 1 : 51–1 : 100 | 1.7 % | 4.1 % |
| 1 : 100–1 : 250 | 4.1 % | 3.8 % |
| 1 : 251–1 : 1 000 | 13.1 % | 4.8 % |
| 1 : 1 001–1 : 5 000 | 29.6 % | 2.5 % |
| ≤ 1 : 5 001 | 49.4 % | 0.5 % |
| Total | 100 % | 100 % |
When a combination of screening methods was used, primary screening was done based either on maternal age or on FTS. A second screening using cffDNA was then carried out for the subgroup defined as at risk in the primary screening. The test quality of the individual screening methods has been described above.
It was posited that all screen-positive pregnancies would be investigated further using invasive diagnostics, and that the detection rate and false positive rate of invasive testing methods was 100 and 0 %, respectively.
The costs of FTS and of invasive diagnostic testing were estimated as €150 and €1000, respectively. Screening based on maternal age incurred no costs, and the cost of cffDNA testing was estimated as €500. In individual calculations, the cost of cffDNA testing was also calculated based on an estimated cost of €250.
Results
According to data from the German Federal Office of Statistics, 673 544 children were born in 2012. Mean maternal age at delivery was 30.2 years (25th–75th quartile: 27.0–34.0) (Fig. 1). Based on maternal age distribution, it was estimated that 1333 children with trisomy 21 would be born at term and the expected number of fetuses with trisomy 21 in week 12 + 0 of gestation would be 1788 (Fig. 2). The study population therefore consisted of 675 332 pregnancies.
Fig. 1.
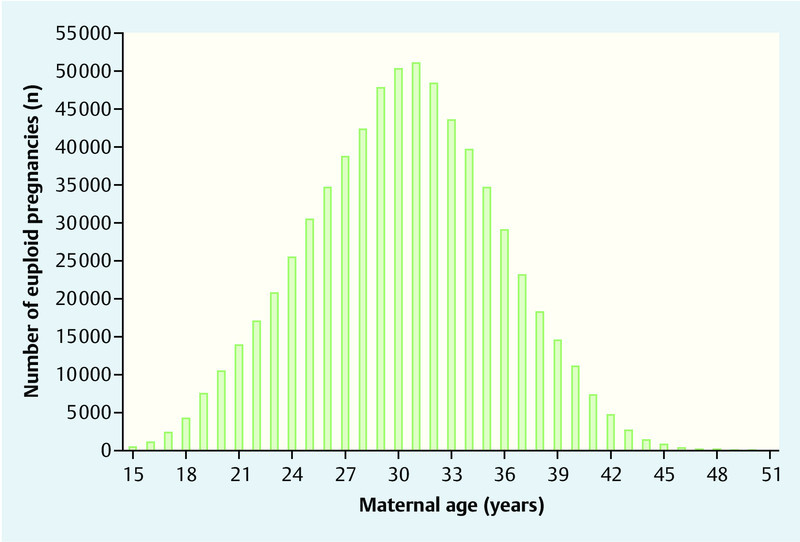
Maternal age distribution at delivery of euploid children in Germany in 2012.
Fig. 2.
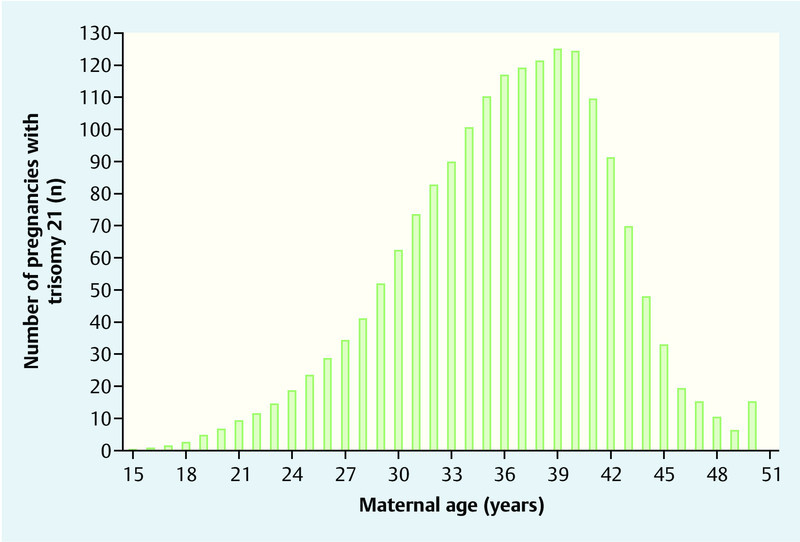
Estimated maternal age distribution at delivery of children with trisomy 21 in Germany in 2012.
Screening based on maternal age
Table 2 shows the detection rate, false positive rate and cost of screening for trisomy 21 based on maternal age using different cut-offs. The most commonly used cut-off of 35 years had a detection rate of 63.3 % and a false positive rate of 21.8 %. The cost of such a screening strategy, which would be limited to the costs of invasive diagnostic procedures, was calculated as €148 267 000 or €219.55 per pregnancy.
Table 2 Test quality and cost of screening for trisomy 21 based on maternal age.
| Cut-off (years) | Detection raten = 1 788 | False positive raten = 673 544 | Cost (€) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Costs are calculated based on screening for age plus invasive diagnostic procedures:screening for maternal age: 675 332 pregnancies × €0 plusinvasive diagnostics [number of screen-positive cases] × €1 000 | |||||
| 25 | 1 716 | 96.0 | 570 683 | 84.7 | 572 399 000 |
| 30 | 1 538 | 86.0 | 378 181 | 56.1 | 379 719 000 |
| 35 | 1 131 | 63.3 | 147 136 | 21.8 | 148 267 000 |
| 40 | 541 | 30.3 | 28 455 | 4.2 | 28 996 000 |
Screening based on FTS
When screening for trisomy 21 was done using FTS and a cut-off of 1 : 250, 92.2 % of cases with trisomy 21 were detected; the false positive rate was 8.0 %. For this cut-off, the costs were calculated as €156 832 800 or €232.23 per pregnancy. Table 3 summarizes the results when other cut-offs are used.
Table 3 Test quality and cost of screening for trisomy 21 using FTS.
| Cut-off (risk) | Detection raten = 1 788 | False positive raten = 673 544 | Cost (€) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Costs are based on the use of FTS plus invasive procedures:FTS: 675 332 pregnancies × €150 plusinvasive diagnostics [number of screen-positive cases] × €1 000 | |||||
| 1 : 50 | 1 507 | 84.3 | 14 818 | 2.2 | 117 624 800 |
| 1 : 100 | 1 581 | 88.4 | 26 268 | 3.9 | 129 148 800 |
| 1 : 250 | 1 649 | 92.2 | 53 884 | 8.0 | 156 832 800 |
| 1 : 1 000 | 1 779 | 99.5 | 341 487 | 50.7 | 444 565 800 |
Screening based on cffDNA
When screening was done using cffDNA, the detection rate and false positive rate were 99 and 0.1 %, respectively. If a 3 % fail rate requiring subsequent investigation with invasive diagnostics was postulated for the test, the total false positive rate was 3.1 % (n = 20 859) while the detection rate remained the same at 99.0 % (n = 1770). The cost of such a screening strategy was calculated as €360 295 000 or €533.51 per pregnancy if the estimated cost of cffDNA testing was set at €500; the cost of the same screening strategy was €191 462 000 or €283.51 per pregnancy if the cost of cffDNA testing was reduced to €250 (675 332 × €500 or €250 for cffDNA plus €1000 × 22 629 for invasive diagnostics).
Screening based on maternal age and cffDNA
Primary screening was based on maternal age. If the result was higher than a defined cut-off, a second screening was done using cffDNA. If the results were abnormal or inconclusive, the pregnancy was considered screen-positive and was investigated using invasive procedures.
If the cut-off was 30 years, 86.0 % (n = 1538) of fetuses with trisomy 21 and 56.1 % (n = 378 181) of euploid pregnancies were investigated further using cffDNA (Table 2). The overall detection rate was 85.2 % and the false positive rate was 1.7 %. The cost of this approach was €203 094 500 or €300.73 per pregnancy if the cost per cffDNA test was €500; overall costs were €108 164 750 or €160.17 per pregnancy if the cost of the cffDNA test was €250. Table 4 shows the test quality for other cut-off values.
Table 4 Test quality and cost of screening based on maternal age followed by cffDNA when a defined cut-off is exceeded.
| Cut-off (risk) | Detection raten = 1 788 | False positive raten = 673 544 | Cost (€) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Costs were calculated based on screening by maternal age followed by cffDNA plus invasive diagnostics:screening by maternal age: 675 332 pregnancies × €0 pluscffDNA [pregnancies of women above a specific maternal age] × €500 plusinvasive diagnostics [screen-positive cases] × €1 000 | |||||
| 25 | 1 699 | 95.0 | 17 674 | 2.6 | 305 572 500 |
| 30 | 1 523 | 85.2 | 11 712 | 1.7 | 203 094 500 |
| 35 | 1 120 | 62.6 | 4 557 | 0.7 | 79 810 500 |
| 40 | 536 | 30.0 | 882 | 0.1 | 15 916 000 |
Screening based on FTS and cffDNA
In this approach primary screening consisted of FTS with two threshold values used to define high-risk and low-risk groups. Additional testing consisting of cffDNA was done in the intermediate-risk group. Patients classified as high risk after FTS and with abnormal or inconclusive cffDNA test results were considered screen-positive and investigated further using invasive diagnostic procedures.
The frequency distribution for the respective risk classes is shown in Table 5. When the threshold values 1 : 10 and 1 : 1000 were used, then 0.6, 20.4 and 79.0 % of euploid pregnancies were classified respectively as high risk, intermediate risk or low risk. The corresponding distribution for fetuses with trisomy 21 was 69.1, 27.9 and 3.0 %, respectively. If these threshold values were used, the overall detection rate was 96.7 % (n = 1729) and the false positive rate was 1.2 % (n = 8296). Total costs were €180 275 800 and €266.94 per pregnancy or €145 800 300 and €215.89 per pregnancy, depending on whether the cost of cffDNA was set at €500 or at €250. Table 6 shows the test quality and costs for the different threshold values.
Table 5 Distribution of euploid and trisomy 21 pregnancies classified as high/intermediate or low risk after FTS.
| Frequency distribution for euploid pregnancies or trisomy 21 in FTS (%) | Threshold value used to defined the high-risk population | ||||||
|---|---|---|---|---|---|---|---|
| 1 : 10 | 1 : 50 | 1 : 100 | |||||
| Trisomy 21 | Euploid | Trisomy 21 | Euploid | Trisomy 21 | Euploid | ||
| Threshold value used to define the low-risk group | 1 : 1 000 | 69.1/27.9/3.0 | 0.6/20.4/79.0 | 84.3/12.7/3.0 | 2.2/18.8/79.0 | 88.4/8.6/3.0 | 3.9/17.1/79.0 |
| 1 : 5 000 | 69.1/30.4/0.5 | 0.6/50.0/49.4 | 84.3/15.2/0.5 | 2.2/48.4/49.4 | 88.4/11.1/0.5 | 3.9/46.7/49.4 | |
Table 6 Test quality and cost of screening for trisomy 21 using FTS followed by cffDNA in the intermediate-risk group.
| False positive rate (n,%)Detection rate (n,%)Cost (€) | Threshold value used to define the high risk group | |||
|---|---|---|---|---|
| 1 : 10 | 1 : 50 | 1 : 100 | ||
| Costs are calculated based on FTS, cffDNA and invasive diagnostics:FTS: 675 332 pregnancies × €150 pluscffDNA [number of pregnancies in the intermediate-risk group] × €500 plusinvasive diagnostics [number of screen-positive cases] × €1 000 | ||||
| Threshold value used to define the low-risk group | 1 : 1 000 | 8 296 (1.2)1 729 (96.7)180 275 800 | 18 740 (2.8)1 732 (96.9)185 198 300 | 29 835 (4.4)1 733 (96.9)190 532 800 |
| 1 : 5 000 | 14 471 (2.1)1 773 (99.2)286 201 800 | 24 914 (3.7)1 776 (99.3)291 123 300 | 36 009 (5.3)1 777 (99.4)296 457 300 | |
Discussion
This study used a model-based approach to show that use of cffDNA for primary screening or a combination of cffDNA with other screening methods could increase the detection rates for trisomy 21 to over 95 % use and reduce the false positive rate to under 3 %. If the focus is on only test quality, then a combination of FTS and cffDNA testing was the most effective, as the detection rate with this combination was 96.7 % and the false positive rate was 1.2 %. It was also more cost efficient than primary screening using cffDNA alone, as the false positive rate for the latter approach was 3.1 % due to inconclusive cffDNA test results. A combination of screening based on maternal age and cffDNA testing did not achieve a similarly high test quality. If the objective is to achieve a detection rate of more than 95 %, then cffDNA would have to be made available to test pregnant women with a maternal age of 25 and above. This would mean that an additional cffDNA test would need to be carried out in 85 % of the study population which would almost double the cost of screening compared to FTS or standard screening strategies based on maternal age.
If the focus is mainly on costs, then primary screening using cffDNA was the most expensive option with an average cost of around €530 per pregnancy if the cost per cffDNA test was €500. Combining cffDNA with FTS at threshold values of 1 : 10 and 1 : 1000 could reduce the cost by around half; the detection rate for this combination was over 96 %.
A further relevant reduction in costs could be achieved if the cost of cffDNA were to drop to around €250 per test. The cost of a combined strategy using FTS and cffDNA would then be around €215; primary screening based on cffDNA would then cost €280 per pregnancy.
In addition to the actual cost of the cffDNA test, the percentage of inconclusive test results is crucially important for the overall cost, as inconclusive test results will generally be followed by invasive diagnostic procedures which can be up to four times more expensive than cffDNA testing. The false positive rate together with the number of inconclusive test results means that the rate of invasive investigations is more than 3 %, which reduces the expected benefits of cffDNA again. However, taking repeat blood samples and re-analysis of samples can reduce the failure rate to around 1–2 %, which would result in a further significant reduction in overall costs 11.
Our results were comparable to those reported in other previous studies. Cuckle et al. also used a model-based approach to estimate the costs of different screening strategies (FTS, cffDNA and a combination of both methods) 12. They based their model on an estimated cost of $150 for FTS and $1000 for invasive diagnostic procedures. Calculations were then done using different unit costs for cffDNA testing ($500, $1000, $1500 and $2000). They concluded that the lionʼs share of screening costs for trisomy 21 would consist of the cost of cffDNA testing, and that cffDNA would only become interesting for public health purchasers if the cost of testing could be significantly reduced. Until that happens, the authors recommended using a 2-stage approach consisting of FTS followed by cffDNA testing in a subpopulation.
Morris et al. used a similar approach to investigate the cost of FTS, cffDNA, combinations of both methods and invasive diagnostics in a model-based calculation for 10 000 patients. In contrast to the 2-stage model we used in our study where cffDNA testing was proposed for the intermediate-risk group, the authors assumed that cffDNA would be used in groups with an FTS risk of 1 : 150 and above. They did not define a high-risk group for which direct invasive diagnostics would be recommended. They concluded – just as Cuckle et al. did – that the use of cffDNA would become interesting for public healthcare systems if there were a further decline in the price of the test, as increased cffDNA testing would reduce the number of invasive diagnostic procedures required. Up until that point they also recommended using a 2-stage strategy 13.
Beulen et al. discussed whether cffDNA testing could become the standard method for screening high-risk populations and whether it should become part of the overall screening strategy for trisomy 21 in the Netherlands and concluded that in view of the current cost structure, cffDNA was only suitable as an optional secondary screening test 14.
Song et al. used a model-based approach to compare FTS with integrated screening and cffDNA in a high-risk population previously identified using other screening tests and to test women aged 35 years and above. The authors concluded that the improved test quality of cffDNA meant that it was preferable to the other forms of screening 15.
Our study was based on specific assumptions which must be taken into consideration:
Around 10 % of patients in Germany have private healthcare. Higher payments for medical costs are expected in this patient cohort.
The model-based calculation assumed that all pregnant women would avail themselves of the opportunity to screen for trisomies. In fact, some pregnant women reject all forms of genetic screening during pregnancy. However there is currently no robust study data on the percentage of women rejecting genetic screening. In principle, it must be assumed that the uptake rate for cffDNA testing will be higher than for FTS or screening based on maternal age. This is due to the simplicity of the test (taking a blood sample), the excellent test quality and the easier interpretation of test results.
The combination of FTS and cffDNA can only achieve the calculated test quality if primary screening – in other words, FTS – has a detection rate of 90 % and a false positive rate of 5 % as described in previous studies. In a study by Lüthgens et al. in more than 38 000 pregnancies, it was found that the detection rate in Germany was around 10 % lower than expected, which would mean that the overall test quality falls short of expectations 16.
This study only considered the actual costs of screening which were calculated based on the sum of the screening costs plus the costs of invasive diagnostic procedures. To comprehensively assess the costs involved, it would also be necessary to include the additional costs of early or late termination of pregnancy, additional direct medical costs incurred over the course of a lifetime of a person with trisomy 21, and the indirect costs arising in consequence of the potentially reduced capacity to work of one of the parents due to the higher level of care required by a child with trisomy 21. Such an evaluation would be equivalent to weighing up the value of a life, and ethical and moral reasons prevented this line of investigation from being pursued further.
In principle, it could be asked why only the intermediate-risk group should benefit from cffDNA testing when FTS is combined with cffDNA testing and why it should not be done in all pregnancies above a certain threshold value. It is therefore important to emphasize that trisomy 21 only constitutes around 50 % of possible chromosomal defects and that other chromosomal disorders are often also associated with low PAPP-A levels and high nuchal translucency. But these markers are also indications of a higher risk for trisomy 21 17. Given the potential for other chromosomal abnormalities which may not be recognized using cffDNA, invasive diagnostic procedures should be carried out in high-risk populations in preference to cffDNA testing. In a study involving FTS performed in more than 21 000 FTS pregnancies it was found that out of 212 fetuses with chromosomal abnormalities 23 (10.9 %) had chromosomal abnormalities which could not have been detected using cffDNA. Around 70 % of these cases had an FTS risk of 1 : 50 18. In a high-risk group with an FTS risk of more than 1 : 10, which corresponds to around 1 % of the population, around half will have trisomy 21; for reasons of cost-efficiency and time constraints direct karyotyping should be done in this group 1.
Conclusion
In this model-based study we showed that a combination of FTS and cffDNA testing could optimize screening for chromosomal disorders and reduce costs to reasonable levels compared to primary screening done using only cffDNA. Further studies will be necessary to show whether the positive findings of this combined approach can be reproduced in reality.
Finally, it should be noted that state-of-the-art prenatal care in the first trimester should not only focus on the detection of fetal aneuploidies but must also include screening for other fetal abnormalities and serious complications of pregnancy, some of them treatable, as part of individualized optimal care 2.
Acknowledgement
This study was carried out with the support of the Working Group for Materno-fetal Medicine.
Footnotes
Conflict of Interest Maximilian Schmid is a consultant to Ariosa Diagnostics, manufacturer of the Harmony® prenatal test.
Supporting Information
German supporting information for this article
References
- 1.Kagan K O, Wright D, Baker A. et al. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2008;31:618–624. doi: 10.1002/uog.5331. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaides K H. A model for a new pyramid of prenatal care based on the 11 to 13 weeksʼ assessment. Prenat Diagn. 2011;31:3–6. doi: 10.1002/pd.2685. [DOI] [PubMed] [Google Scholar]
- 3.Morgan S, Delbarre A, Ward P. Impact of introducing a national policy for prenatal Down syndrome screening on the diagnostic invasive procedure rate in England. Ultrasound Obstet Gynecol. 2013;41:526–529. doi: 10.1002/uog.12384. [DOI] [PubMed] [Google Scholar]
- 4.Ekelund C K, Jørgensen F S, Petersen O B. et al. Danish Fetal Medicine Research Group . Impact of a new national screening policy for Downʼs syndrome in Denmark: population based cohort study. BMJ. 2008;337:a2547. doi: 10.1136/bmj.a2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagan K O, Eiben B, Kozlowski P. Kombiniertes Ersttrimesterscreening und zellfreie fetale DNA – „Next Generation Screening“. Ultraschall Med. 2014;35:229–236. doi: 10.1055/s-0034-1366353. [DOI] [PubMed] [Google Scholar]
- 6.Kagan K O, Hoopmann M, Kozlowski P. Assessment of foetal DNA in maternal blood – a useful tool in the hands of prenatal specialists. Geburtsh Frauenheilk. 2012;72:998–1003. doi: 10.1055/s-0032-1327960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil M M, Akolekar R, Quezada M S. et al. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: meta-analysis. Fetal Diagn Ther. 2014;35:156–173. doi: 10.1159/000358326. [DOI] [PubMed] [Google Scholar]
- 8.Statistisches Bundesamt GeburtenMutterBiologischesAlter.html;Online:https://www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/Bevoelkerung/Geburten/Tabellen/GeburtenMutterBiologischesAlter.htmlStand: 25.11.2013
- 9.Cuckle H S, Wald N J, Thompson S G. Estimating a womanʼs risk of having a pregnancy associated with Downʼs syndrome using her age and serum alpha-fetoprotein level. Br J Obstet Gynaecol. 1987;94:387–402. doi: 10.1111/j.1471-0528.1987.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 10.Snijders R J, Sundberg K, Holzgreve W. et al. Maternal age- and gestation-specific risk for trisomy 21. Ultrasound Obstet Gynecol. 1999;13:167–170. doi: 10.1046/j.1469-0705.1999.13030167.x. [DOI] [PubMed] [Google Scholar]
- 11.Willems P J, Dierickx H, Vandenakker E. et al. The first 3,000 Non-Invasive Prenatal Tests (NIPT) with the Harmony test in Belgium and the Netherlands. Facts Views Vis Obgyn. 2014;6:7–12. [PMC free article] [PubMed] [Google Scholar]
- 12.Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome – a cost sensitivity analysis. Prenat Diagn. 2013;33:636–642. doi: 10.1002/pd.4157. [DOI] [PubMed] [Google Scholar]
- 13.Morris S, Karlsen S, Chung N. et al. Model-based analysis of costs and outcomes of non-invasive prenatal testing for Downʼs syndrome using cell free fetal DNA in the UK National Health Service. PLoS ONE. 2014;9:e93559. doi: 10.1371/journal.pone.0093559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beulen L, Grutters J P, Faas B H. et al. The consequences of implementing non-invasive prenatal testing in Dutch national health care: a cost-effectiveness analysis. Eur J Obstet Gynecol. 2014;182:53–61. doi: 10.1016/j.ejogrb.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Song K, Musci T J, Caughey A B. Clinical utility and cost of non-invasive prenatal testing with cfDNA analysis in high-risk women based on a US population. J Matern Fetal Neonatal Med. 2013;26:1180–1185. doi: 10.3109/14767058.2013.770464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lüthgens K, Abele H, Alkier R. et al. [Cross-validation of the first trimester screening algorithm of the FMF London on 38,700 pregnancies in Germany] Ultraschall Med. 2011;32:367–372. doi: 10.1055/s-0031-1273348. [DOI] [PubMed] [Google Scholar]
- 17.Petersen O B, Vogel I, Ekelund C. et al. the Danish Fetal Medicine Study Group; the Danish Clinical Genetics Study Group . Potential diagnostic consequences of applying non-invasive prenatal testing: population-based study from a country with existing first-trimester screening. Ultrasound Obstet Gynecol. 2014;43:265–271. doi: 10.1002/uog.13270. [DOI] [PubMed] [Google Scholar]
- 18.Kagan K O, Hoopmann M, Hammer R. et al. Screening auf Chromosomenstörungen mittels Ersttrimester-Screening und non-invasive prenatal Testing. Ultraschall Med. 2015;36:40–46. doi: 10.1055/s-0034-1385059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
German supporting information for this article


