Abstract
Objective
We sought to describe the presentation of external head and neck lymphedema in patients treated for head and neck cancer and examine their initial response to complete decongestive therapy.
Study Design
Case series with chart review.
Setting
MD Anderson Cancer Center, Houston, TX.
Subjects and Methods
Patients evaluated for head and neck cancer at MD Anderson Cancer Center after treatment 01/2007-01/2013 were retrospectively reviewed. Response to complete decongestive therapy was evaluated per changes in lymphedema severity rating or surface tape measures. Predictors of therapy response were examined using regression models.
Results
1,202 patients were evaluated. Most patients (62%) had soft, reversible pitting edema (MDACC Stage 1b). Treatment response was evaluated in 733 patients after receiving therapy; 439 (60%) improved after complete decongestive therapy. Treatment adherence independently predicted complete decongestive therapy response (p<0.001).
Conclusions
These data support the effectiveness of a head and neck cancer-specific regimen of lymphedema therapy for cancer patients with external head and neck lymphedema. Our findings suggest that head and neck lymphedema is distinct from lymphedema that affects other sites, requiring adaptations in traditional methods of management and measurement.
Keywords: lymphedema, cancer, head and neck, treatment, manual lymphatic drainage
INTRODUCTION
Head and neck lymphedema (HNL) is a common side effect of head and neck cancer (HNC) treatment. It has been estimated that more than 50% of treated HNC patients will develop some degree of HNL.1,2 HNL is characterized by swelling resulting from the blockage of normal drainage pathways in the lymphatic system. Although the etiology of HNL varies, common causes include surgery that removes lymph nodes, or impairment of lymphatic vessel contractility (“lymphangiomotoricity”) associated with radiation therapy or surgery. Additionally, the tumor itself may cause vessel obstruction. In some cases, infection such as recurrent cellulitis may further impair lymphatic functioning.3,4 If left untreated, chronic edema coupled with permanent fibrosis may result in significant long-term cosmetic, functional, and psychosocial consequences that are often irreversible, including discomfort and problems associated with speech, respiration, voice, and swallowing.5,6
Although interest has increased in the last decade, information remains sparse regarding the evaluation and effectiveness of conservative treatments for HNL. Complete Decongestive Therapy (CDT) remains the gold-standard treatment for patients with extremity lymphedema.7 Unfortunately, current outcome measures lack sensitivity and generalizability to the head and neck.8 Herein, we report our 6-year experience managing more than 1,200 patients with HNL and propose a targeted method of evaluation and treatment to accommodate the unique characteristics of this patient population.9,10,11 In addition, we describe the presentation of external HNL in patients treated for head and neck cancer (HNC) and examine their initial response using CDT.
MATERIALS AND METHODS
Study Design and Inclusion/Exclusion Criteria
We examined a case series longitudinally with retrospective chart review. All patients referred for evaluation (1/2007-1/2013) of external HNL following surgery, radiation, or combined modality treatment for HNC at the University of Texas MD Anderson Cancer Center (MDACC) were eligible for inclusion. A total of 1,255 patients were evaluated. Fifty-three patients who did not have HNL or a diagnosis of HNC were excluded. Therefore, 1,202 patients were included in the study. The study was approved by the Institutional Review Board at MDACC. A waiver of informed consent was obtained.
MDACC Lymphedema Program
Evaluation
The diagnosis of external HNL was made based on clinical examination by the referring physician prior to referral for HNL management. Patients were evaluated by a certified lymphedema therapist (CLT) in the outpatient Head and Neck Center. Patient and chart review of medical history and contraindications confirmed candidacy for HNL treatment. Contraindications to treatment included hyperthyroidism, >50% internal carotid artery blockage, upper quadrant deep vein thrombosis, acute radiation dermatitis, acute renal failure, or a history of multiple cerebrovascular accidents or transient ischemic attacks.12
HNL severity was graded based on tissue characteristics, including firmness, pitting, and reversibility of swelling. Patients were evaluated using our adapted version of Földi's “Stages of Lymphedema”9,13 scale, the MD Anderson Cancer Center (MDACC) HNL rating scale in which lymphedema stage is associated with lymphedema presentation and patient complaint. Stage 0 represents lymphedema without visible edema but a complaint of tissue heaviness while stage 1a shows soft visible edema without pitting that is reversible. Stage 1b demonstrates soft pitting edema that still remains reversible while stage 2 displays firm pitting edema that is irreversible but lacks tissue changes. The most severe of stages, stage 3 lymphedema, shows irreversible tissue changes such as hyperkeratosis or papillomatosis. Neck and facial edema were also assessed using surface tape measurements. Measurements were summed to create a neck and a facial composite score for descriptive and comparative uniformity (Figure 1).5 Patient reported complaints regarding cosmesis, discomfort, breathing, or vision were also documented. Lymphedema evaluations were performed by two certified lymphedema therapists (B.S. and L.L.) who met criteria for clinical competency after equal training.
Figure 1. Facial Composite.
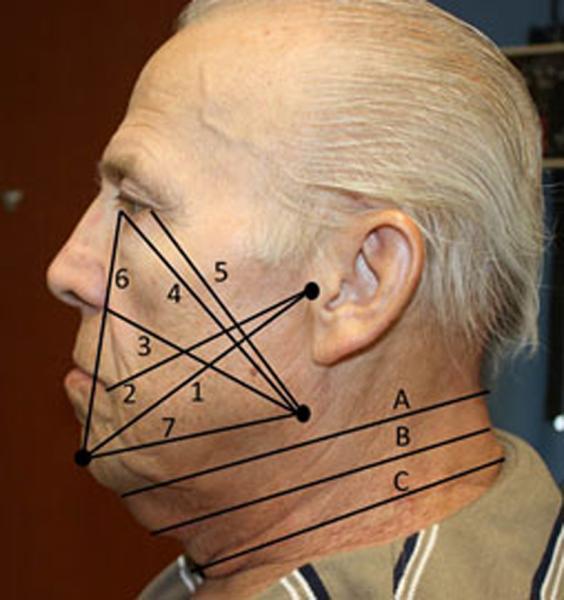
1) Tragus to chin, 2) Tragus to mouth corner, 3) Mandible to nasal wing, 4) Mandible to medial canthus, 5) Mandible to exocanthus, 6) Chin to medial canthus, 7) Mandible to chin
Neck Composite - circumferential measures: A) Superior neck, B) Middle neck, C) Inferior neck
Data were entered into the MDACC Lymphedema Tracking Database and the electronic medical record. Data were queried at two longitudinal time points: 1) baseline evaluation, and 2) first evaluation after CDT. Long-term data to determine durability of response were not available for this retrospective analysis. Baseline and initial follow-up data of patients were compared to determine change in tissue size (tape measures) and stage of HNL. Based on our initial experience with over 150 patients,5,6,14 the criteria we used to define clinically-detectable improvement in external HNL were: 1) a drop in lymphedema stage, or 2) a minimum threshold of 2% reduction in the composite measurement equating to at least 2 cm change in absolute values.
MDACC Lymphedema Treatment Regimen
All patients who received HNL treatment had at least one CDT training session for self-administration of techniques that included manual lymph drainage (MLD), use of compression garments and pads, skin care, and basic exercises for the face, neck, and oral cavity. Outpatient clinician-directed CDT was recommended for patients with severe edema or limited capacity for self-treatment. Outpatient CDT included 2-5 sessions per week by a CLT for 2-4 weeks accompanied by a home program that was performed once daily and continued up to3 months.
Patients who could not or declined to participate in the clinician administered outpatient treatment phase performed a self-administered home program of CDT only. Patients performed daily self-CDT at home up to3 months with subsequent follow up evaluation scheduled and coordinated with other medical appointments at that time to facilitate appointment attendance and avoid missed follow-up appointments. Modifications in treatment duration and frequency were made based on improvement in edema compared to baseline, over the course of treatment.
Complete Decongestive Therapy
Complete Decongestive Therapy (CDT) comprises 4 main components: 1) MLD, 2) tissue compression with bandaging and/or garments, 3) remedial exercises, and 4) skin care.7,12 All patients performed manual lymph drainage (MLD), tissue compression with bandaging and/or garments, and cervical and facial range of motion exercises during application of compression to maximize drainage. Published standards for CDT were always attempted prior to adapting therapy techniques 7,12 to accommodate anatomical differences in patients with head and neck lymphedema.5 Specific skin care regimens were directed by the patient's physician and reinforced by the CLT. MLD was performed to redirect fluid from the cervical region to the bilateral axillary lymph node beds based on scar patterns and severity. Anterior and lateral neck drainage channels were used if scarring was absent or insignificant, and posterior neck and trunk channels were utilized in cases with marked scarring. MLD was started per physician direction, a minimum of 4-6 weeks after surgery or the completion of XRT to allow adequate tissue healing.
Compression was performed using non-elastic, short-stretch bandaging, or a commercially available chinstrap for anterior neck and lower face lymphedema. Custom-fit compression masks were used for edema in the anterior cheeks, eyelids or lips. A garment and a custom-made foam softening pad were applied for 30 minutes before MLD in cases of pitting edema. All patients wore the compression garment and a custom-fit flattening pad for a minimum of 2-4 hours after completion of MLD to maintain even pressure.11
Study Variables
Study variables included patient demographics, cancer and treatment history, HNL characteristics, and lymphedema treatment outcomes. Patient demographics included age, sex, and race/ethnicity. Tumor site and staging according to TNM classification were collected. Patients were stratified by the primary HNC treatment modality (± systemic therapy) as RT, surgery, or combination surgery with RT. Surgeries were further classified as: primary (resection of primary H&N tumor), nodal (resection of regional lymph nodes), or combined (primary and nodal). Lymphedema characteristics included the MDACC HNL stage, composite surface tape measurements, and the location and laterality of HNL. Functional status at the time of HNL consultation and functional complaints were also summarized. Therapy type was coded as “outpatient” for clinician-administered therapy or “home” when treatment was self-administered. Full adherence was coded as accurate technique demonstration with self-reported daily performance of CDT >5 times per week. Partial adherence was coded as partially accurate technique demonstration and/or self-reported daily performance 3-4 times weekly, and non-adherence was coded when the patient performed therapy less than 3 times weekly or could not demonstrate accurate performance of the treatment regimen.
Statistical Methods
Descriptive statistics were calculated. Patient characteristics and features of HNL were summarized for 2 groups: 1) among all eligible patients who were evaluated for HNL in the study period (n=1,202) but may not have received treatment, and 2) in the treatment subgroup comprised of all patients who returned for re-assessment of HNL after evaluation and a course of lymphedema treatment (n=733). Response to treatment and predictors of treatment response were examined only in the treatment subgroup. Bivariate associations were examined using chi-square tests for categorical variables and t-tests for continuous variables. Multiple logistic regression models were fit using backward elimination methods to assess the independent effects of the CDT program (outpatient versus home, per intention-to-treat) and self-reported adherence (none, partial, or full) to lymphedema therapy.15 Adjustment variables considered in the multivariate models were: cancer site, cancer treatment group (surgery and/or radiation), surgery type, MDACC lymphedema stage, length of follow-up, and latency to lymphedema consult; a z-score test p<0.05 was used as the threshold for entry and removal of adjustment variables during model building. Adjusted odds-ratios and 95% confidence intervals were calculated based on the final model. Statistical significance was considered α-level 0.05.
RESULTS
Patient Characteristics
Twelve hundred two patients were evaluated and included in the description of HNL characteristics. A subgroup of 733 of these patients was evaluated for response to lymphedema therapy (See Table 1). The majority of the patients were Caucasian and male; median age was 61 years (range: 21-91). The majority of patients had a diagnosis of squamous cell carcinoma and the most common tumor sites were the oropharynx, oral cavity, and larynx, accounting for 73% of all cases. The “other” category included cancers involving the thyroid, salivary gland, nasopharynx, and nasal sinuses. T-classification was fairly evenly distributed between T1-T4 disease, whereas most patients (484, 40%) had N2 staging of neck disease and 22% had recurrent disease. Over half of the patients received combined modality treatment. The most common primary surgery preceding referral was total laryngectomy (179, 15%). Glossectomy (144, 12%) and mandibulectomy (108, 9%) were the next most common surgical procedures. More than half of all patients had lymph node dissections (653, 54%). Demographic and cancer histories did not significantly differ between those in the treatment subgroup (n=733) and those who did not get treatment (n=469, p>0.25), indicating a lack of selection bias in the subgroup analysis.
Table 1.
Patient Demographics, Tumor Characteristics and Treatment History
| Total cohort n=1,202 | Treatment subgroup n=733 | |
|---|---|---|
| Male | 937 (78%) | 580 (79%) |
| Female | 265 (22%) | 153 (21%) |
| Median Age in years | 61 (21-91) | 61 (24-91) |
| Race | ||
| Caucasian | 961 (80%) | 590 (81%) |
| African American | 69 (6%) | 39 (5%) |
| Hispanic | 131 (11%) | 81 (11%) |
| Other | 41 (3%) | 23 (3%) |
| Tumor Stage | ||
| T0 | 38 (3%) | 23 (3%) |
| T1 | 135 (11%) | 86 (12%) |
| T2 | 219 (18%) | 133 (18%) |
| T3 | 175 (15%) | 108 (15%) |
| T4 | 245 (20%) | 152 (21%) |
| Tx | 26(2%) | 14 (2%) |
| Unknown | 96 (8%) | 56 (8%) |
| Recurrent | 268 (22%) | 161 (22%) |
| Neck Disease | ||
| N0 | 191 (16%) | 110 (15%) |
| N1 | 114 (9%) | 67 (9%) |
| N2 | 484 (40%) | 309 (42%) |
| N3 | 29 (2%) | 16 (2%) |
| Nx | 19 (2%) | 14 (2%) |
| Unknown | 96 (8%) | 56 (8%) |
| Recurrent | 268 (22%) | 161 (22%) |
| Tumor Site | ||
| Oropharynx | 386 (32%) | 242 (33%) |
| Oral Cavity | 263 (22%) | 167 (23%) |
| Larynx/Hypopharynx | 246 (20%) | 160 (22%) |
| Other | 307 (26%) | 164 (22%) |
| Primary HNC Treatment | ||
| Radiation | 360 (30%) | 218 (30%) |
| Surgery | 145 (12%) | 91 (12%) |
| Surgery + radiation | 697 (58%) | 424 (58%) |
| Surgery Type | ||
| No surgery | 360 (30%) | 218 (30%) |
| Primary | 185 (15%) | 104 (14%) |
| Lymph node | 189 (16%) | 114 (16%) |
| Primary + lymph node | 468 (39%) | 297 (41%) |
Head & Neck Lymphedema Characteristics
HNL staging, sites of occurrence, and associated complaints are reported in Table 2. The two most common sites of edema were the neck and submental region, most with soft, reversible pitting edema (MDACC Stage 1b). Although the majority of patients reported cosmetic concerns and discomfort, more than a third (n=446) reported functional complaints of which difficulty swallowing was the most common (68%) followed by difficulty breathing (39%). The most common functional complaint among those patients who underwent total laryngectomy was difficulty breathing, associated with tracheostomal obstruction from submental edema often requiring cannulation of the stoma with a laryngectomy tube.
Table 2.
HNL Characteristics at Initial Presentation
| MDACC Stage | Evaluation cohort n=1, 202 | Treatment subgroup n=733 |
|---|---|---|
| 0 - no visible edema, heaviness" | 9 (1%) | 2 (1%) |
| 1a - soft visible edema, no pitting | 336 (28%) | 178 (24%) |
| 1b – reversible, pitting edema | 746 (62%) | 487 (66%) |
| 2 – non reversible, firm, pitting | 111 (9%) | 66 (9%) |
| HNL Site (not mutually exclusive) | ||
| Neck | 1,079 (90%) | 669 (91%) |
| Submental | 1,067 (89%) | 706 (96%) |
| Facial | 638 (53%) | 383 (53%) |
| Intra-oral | 156 (18%) | 100 (14%) |
| Associated Complaints | ||
| Cosmetic | 999 (83%) | 614 (84%) |
| Discomfort | 764 (64%) | 478 (65%) |
| Functional | 446 (37%) | 287 (39%) |
| Swallowing | 303(25%) | 192 (26%) |
| Breathing | 174 (15%) | 113 (16%) |
| Speaking | 128 (11%) | 76 (10%) |
| Vision | 27 (2%) | 14 (2%) |
Treatment Outcomes
Lymphedema therapy outcomes were examined in the treatment subgroup (733/1,202) who returned for follow-up evaluation after lymphedema therapy. The median time period from baseline to re-assessment was 69 days (range: 9-371 days). Among the 733 patients, 86 patients (12%) received outpatient CDT (mean=5.4 visits; range 1-14); the remaining 647 (88%) performed a daily self-administered home program of CDT only but did not receive outpatient therapy. A majority of patients had stage 1b or 2 HNL (76/86, 88%) (p<0.001).
At follow-up, 270 (36%) patients reported full home program adherence and 372 (51%) reported partial home program adherence. Overall, 60% (439/733) of patients demonstrated improvement in HNL at follow-up after CDT treatment. Table 3 shows the characteristics of lymphedema treatment and response. Figure 2 illustrates typical improvement after CDT in a patient with external HNL.
Table 3.
Treatment Response by HNL Stage and HNC Treatment
| n=733 | Improved | Odds ratio (unadjusted) | Odds ratio (adjusted)* | p-value | |
|---|---|---|---|---|---|
| Lymphedema program | |||||
| Home | 647 | 377 (58%) | 1.0 (reference) | 1.0 (reference) | |
| Outpatient | 86 | 62 (72%) | 1.9 (1.1-3.1) | 1.3 (0.7-2.1) | 0.383 |
| Self-reported adherence | |||||
| None | 91 | 29 (32%) | 1.0 (reference) | 1.0 (reference) | |
| Partial | 372 | 209 (56%) | 2.7 (1.7-4.5) | 3.3 (2.0-5.6) | |
| Full | 270 | 201 (74%) | 6.2 (3.7-10.4) | 8.1 (4.6-14.4) | <0.001 |
| HNL Stage | |||||
| 0 | 2 | 1 (50%) | 1.0 (reference) | ||
| 1a | 178 | 96 (54%) | 1.2 (0.1-19.0) | ||
| 1b | 487 | 294 (60%) | 1.5 (0.1-24.5) | ||
| 2 | 66 | 48 (73%) | 2.7 (0.2-44.9) | ||
| HNC treatment | |||||
| XRT | 218 | 115 (53%) | 1.0 (reference) | 1.0 (reference) | |
| Surgery | 91 | 65 (71%) | 2.2 (1.3-3.8) | 2.3 (1.3-4.0) | 0.004 |
| Surgery and XRT | 424 | 259 (61%) | 1.4 (1.0-2.0) | 1.3 (0.9-1.8) | 0.196 |
| Tumor site | |||||
| Larynx/hypopharynx | 160 | 92 (58%) | 1.0 (reference) | ||
| Oral cavity | 167 | 98 (59%) | 1.0 (0.7-1.6) | ||
| Oropharynx/nasopharynx | 242 | 146 (60%) | 1.1 (0.7-1.7) | ||
| Other | 164 | 103 (63%) | 1.3 (0.8-2.0) | ||
| Latency to HNL referral | |||||
| <6 weeks | 197 | 113 (57%) | 1.0 (reference) | ||
| 6 weeks to 2 months | 217 | 136 (63%) | 1.2 (0.8-1.9) | ||
| 3 to 5 months | 202 | 124 (61%) | 1.2 (0.8-1.8) | ||
| 6+ months | 17 | 66 (56%) | 1.0 (0.6-1.5) | 0.001 |
final multivariate logistic regression model assessing independent effect of lymphedema program and adherence retained the following adjustment variables: HNC treatment group and duration follow-up.
p-values from multivariate model shown
Figure 2.

Typical progression of response to CDT for HNL.
Predictors of Lymphedema Treatment Response
Overall, 439 of 733 patients (60%) demonstrated improvement in HNL after lymphedema therapy. Patients who received outpatient lymphedema therapy were more likely to demonstrate improvement at follow-up (62/86, 72%) compared with those who only received the self-administered home-based CDT program (377/647, 58%, p=0.014). Likewise, the level of treatment adherence significantly predicted treatment response (p<0.001). Multivariate models were fit to assess independent effects of therapy and adherence on lymphedema treatment response. In adjusted models, self-reported adherence significantly predicted response but the type of therapy program (self-administered home therapy ± outpatient CLT administered therapy) was no longer significant after adjustment. Those who reported partial adherence were 3.3-times (ORadjusted: 3.3, 95% CI: 2.0-5.6) more likely to improve and those who reported full adherence were 8.1-times (ORadjusted: 8.1, 95% CI: 4.6-14.4) more likely to improve than those reporting non-compliance with the program. In addition, patients who were referred for HNL evaluation between 6 weeks and 5 months were significantly more likely to respond to lymphedema therapy (p=0.001). Results of logistic regression models are shown in Table 3.
DISCUSSION
HNL is a common sequela of HNC treatment and is associated with symptom burden, functional deterioration, and poor QOL in HNC survivors.16,17 Unfortunately, HNL remains under-recognized and undertreated. Data describing presentation, management and treatment outcomes are sparse. To our knowledge, this study represents the first to examine characteristics and treatment outcomes in the largest reported single cohort comprising over 1,200 HNC patients with HNL. In our study, 60% of patients showed improvement regardless of initial stage or severity of HNL, cancer treatment history, or type of CDT therapy (outpatient vs self-administered home program).
Reversible, pitting edema (MDACC stage 1b) was the most common presentation of external HNL (62%) in our HNC patients, however, a unique finding in our patients with HNL compared with patients whose lymphedema affects other sites was the presentation of visible and palpable soft, non-pitting edema (MDACC stage 1a) in 28% of study group. The most common sites of HNL were in the neck (90%) and submental region (89%), whereas facial and intraoral edema presented in 53% and 18% of patients, respectively. Although facial and intraoral edema are less common compared with other affected areas of the head and neck, the functional effects to respiration, swallowing, speech, and vision can be significant. More than 1/3 of our patients reported functional complaints of which more than 2/3 comprised swallowing. Future studies will be important to examine and clarify the relationship between symptom burden and the stage and site of HNL.
Exercise and rehabilitation literature clearly document that treatment effectiveness is enhanced by consistent, accurate implementation.18-21 Our results support these data. Our findings showed that patient adherence was significantly associated with treatment outcomes (p<0.001). Patients who reported daily accurate performance of their lymphedema management routines were more likely to improve than those who were non-adherent. Moreover, 56% of patients who were only partially adherent still demonstrated improvement at follow-up. The high response rate (70%) among patients treated only with a home program suggests another potential advantage. That is, by reducing the need for outpatient therapy, the patient is able to perform therapy at home thereby decreasing cost and patient burden associated with this type of treatment regimen. The ability to provide effective programs of patient-administered treatment is appealing, particularly in a healthcare environment in which access to services may be limited and associated with a high financial encumbrance. Equally important for treatment benefit and success, especially in self-administered home programs, is the formal training sessions provided by a CLT who is knowledgeable in the management of patients with HNL that helps ensure accurate patient implementation. Future studies will need to help identify those patients best triaged to conventional clinician-directed therapy and those for whom self-administered treatment is sufficient.
We postulate that patients with HNL unlike patients with extremity lymphedema, may also benefit from the additional advantage of a gravitational effect that promotes drainage because of the upright positioning of the head.22-24 Anecdotally, most HNL patients, especially those without significant neck scars, report their worst edema when waking, followed by a reduction of swelling after becoming upright and physically active, likely an effect of enhanced lymphangiomotoricity. In contrast, patients with extremity edema experience worse edema while upright with improvement associated with elevation of the affected limb. Again, future studies will be important to substantiate this hypothesis.
An unanticipated finding from this study was that patients with more severe lymphedema (stage 1b & 2) showed greater likelihood of improvement compared with patients whose edema was mild (stage 0 and 1a, Table 3). We postulate that this finding may be an artifact of the limitations of current lymphedema rating scales to capture changes in patients with HNL. Current scales appear biased towards the ability to identify gross changes in patients with advanced stages of HNL but are less able to capture small treatment changes in patients with early stages of HNL. Current scales that are most often used to rate lymphedema in the extremities, such as Földi's “Stages of Lymphedema,” only distinguish gross tissue characteristics such as reversibility and firmness but do not capture or rate edema that is soft and non-pitting, a unique characteristic frequently found in patients with HNL.5,8 We adapted Földi's “Stages of Lymphedema” with the addition of stage 1A to our MDACC rating scale that we believe allows an improved ability to capture the visible, non-pitting edema that is typical of HNL but not commonly found in patients with extremity lymphedema. Our findings, along with those of other investigators, confirm the need for further studies to develop more sensitive tools to accurately rate HNL severity in patients with HNC.8
Our retrospective analysis showed that 84% of patients with external HNL reported cosmetic complaints and more than one-third also reported functional problems before lymphedema therapy. We acknowledge that a limitation of this analysis was the lack of patient-reported outcomes regarding satisfaction or improvement after lymphedema treatment. This is a critical measure of HNL treatment success that we are currently incorporating into prospective efforts.
Our study of over 1,200 consecutive patients presents a new and unique way to characterize lymphedema involving the head and neck region. In addition, the results of our investigation found that 60% of patients may benefit from CDT based on response assessment using existing measures of HNL. Despite the novel and important implications of this work, we recognize the limitations encountered in this observational study. Measurement of HNL remains challenging at best. Existing measures do not quantify the subtle changes in tissue firmness or composition, or the complex volumetric differences in HNL. Thus, the impact of CDT on these aspects of HNL remains poorly understood. Our reliance on existing measures that may not discriminate these subtle yet important changes may have resulted in overly conservative estimates of treatment effect in our analysis. Our data clearly support the efficacy of CDT for treatment of HNL. However, we further acknowledge the lack of a control group for comparison because of the cases series design of our outcomes assessment. Given the retrospective nature of our chart review, prospective randomization to CDT was not performed. Notably, the histories of patients who did and did not receive lymphedema therapy were not significantly different (p>0.25) and, in fact, the likelihood of improving after lymphedema therapy was higher among patients initially presenting with more severe staging of lymphedema. These outcomes may, in fact, have lessened the chance for a selection bias. Nevertheless, future prospective trials should include a control group for comparison to ensure the accuracy of treatment outcomes and to avoid selection bias. Finally, long-term longitudinal follow-up (in patients with or without CDT) was not available in this study. As such, the course of treatment response and the durability of early improvement could not be ascertained and therefore requires further study. Prospective trials will be necessary to validate the findings from this initial report.
CONCLUSIONS
This investigation provides the largest data set to date that supports the effectiveness of a head and neck specific regimen of lymphedema therapy for cancer patients with external HNL regardless of treatment setting or HNL severity. We believe that HNL is distinctly different from lymphedema in other sites, and thus, requires unique adaptations to traditional methods of description, measurement, and management. We are currently developing a validated rating scale to more accurately characterize HNL and assess the long term outcomes associated with our HNL treatment protocols. Future investigations must distinguish the patient characteristics and treatment variables associated with durable response as new techniques are developed to manage patients whose lymphedema is refractory to standard methods of treatment. We believe that our findings provide a foundation for future research efforts that seek to identify those patients at risk for the development of HNL as a debilitating consequence of HNC treatment.
Footnotes
Disclosures: none
REFERENCES
- 1.Buntzel J, Glatzel M, Mucke R, Micke O, Bruns F. Influence of amifostine on late radiation-toxicity in head and neck cancer--a follow-up study. Anticancer Res. 2007;27:1953–1956. [PubMed] [Google Scholar]
- 2.Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43:244–252. doi: 10.1016/j.jpainsymman.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Földi M, Földi E. Physiology and pathophysiology of the lymphatic system. In: Földi M, Földi E, editors. Földi's textbook of lymphology; for physicians and lymphedema therapists. 2nd ed. Urban and Fischer; Munich, Germany: 2006. pp. 180–222. [Google Scholar]
- 4.Zuther J. Anatomy. In: Zuther J, Norton S, editors. Lymphedema management: The comprehensive guide for practitioners. Thieme; New York: 2013. pp. 1–27. [Google Scholar]
- 5.Smith BG, Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18:153–158. doi: 10.1097/MOO.0b013e32833aac21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewin JS, Hutcheson KA, Smith BG, Barringer DA, Alvarez CP. Early experience with head and neck lymphedema after treatment for head and neck cancer.. Multidisciplinary Head and Neck Cancer Symposium; Chandler, Arizona. 2010. [Google Scholar]
- 7.Zuther J. Complete decongestive therapy. In: Zuther J, Norton S, editors. Lymphedema management: The comprehensive guide for practitioners. 3rd ed. Thieme; New York: 2013. pp. 128–163. [Google Scholar]
- 8.Deng J, Ridner SH, Dietrich MS, Wells N, Murphy BA. Assessment of external lymphedema in patients with head and neck cancer: a comparison of four scales. Oncol Nurs Forum. 2013;40(5):501–506. doi: 10.1188/13.ONF.501-506. [DOI] [PubMed] [Google Scholar]
- 9.Földi M, Földi E. Lymphostatic diseases. In: Földi M, Földi E, editors. Földi's textbook for physicians and lymphedema therapists. 2nd ed. Urban and Fischer; Munich, Germany: 2006. pp. 224–319. [Google Scholar]
- 10.Piso DU, Eckardt A, Liebermann A, et al. Early rehabilitation of head-neck edema after curative surgery for orofacial tumors. Am J Phys Med Rehabil. 2001;80:261–269. doi: 10.1097/00002060-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Smith BG. Head and neck lymphedema. In: Zuther J, Norton S, editors. Lymphedema management: The comprehensive guide for practitioners. 3rd ed. Thieme; New York: 2013. pp. 191–208. [Google Scholar]
- 12.Földi M, Földi E. Practical instructions for therapists-manual lymph drainage according to Dr. E. Vodder. In: Földi M, Földi E, editors. Földi’s textbook of lymphology for physicians and lymphedema therapists. 2nd ed. Urban and Fischer; Munich, Germany: 2006. pp. 526–546. [Google Scholar]
- 13.Zuther J. Pathology. In: Zuther J, Norton S, editors. Lymphedema management: The comprehensive guide for practitioners. Thieme; New York: 2013. pp. 45–71. [Google Scholar]
- 14.Smith BG, Hutcheson KA, Little LG, et al. Management of Lymphedema in Patients with Head and Neck Cancer.. American Head and Neck Society Annual Meeting; Orlando, FL. 2013. [Google Scholar]
- 15.Hosmer DW, Lemeshow SL. Applied Logistic Regression. 2nd ed. Wiley-Interscience; Hoboken, NJ: 2000. [Google Scholar]
- 16.Murphy BA, Gilbert J, Cmelak A, Ridner SH. Symptom control issues and supportive care of patients with head and neck cancers. Clin Adv Hematol Oncol. 2007;5:807–822. [PubMed] [Google Scholar]
- 17.Deng J, Murphy BA, Dietrich MS, et al. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck. 2013;35:1026–1035. doi: 10.1002/hed.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brewer BW, Cornelius AE, Van Raalte JL, Tennen H, Armeli S. Predictors of adherence to home rehabilitation exercises following anterior cruciate ligament reconstruction. Rehabil Psychol. 2013;58:64–72. doi: 10.1037/a0031297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mair V Breda AP, Nunes ME, de Matos LD. Evaluating compliance to a cardiac rehabilitation program in a private general hospital. Einstein (Sao Paulo) 2013;11:278–284. doi: 10.1590/S1679-45082013000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golightly YM, Allen KD, Caine DJ. A comprehensive review of the effectiveness of different exercise programs for patients with osteoarthritis. Phys Sportsmed. 2012;40:52–65. doi: 10.3810/psm.2012.11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boris M, Weindorf S, Lasinkski S. Persistence of lymphedema reduction after noninvasive complex lymphedema therapy. Oncology. 1997;11:99–109. [PubMed] [Google Scholar]
- 22.Hamilton DR, Sargsyan AE, Garcia K, et al. Cardiac and vascular responses to thigh cuffs and respiratory maneuvers on crewmembers of the International Space Station. J Appl Physiol. 2012;112:454–462. doi: 10.1152/japplphysiol.00557.2011. [DOI] [PubMed] [Google Scholar]
- 23.Diridollou S, Pavy-Le Traon A, Maillet A, et al. Characterisation of gravity-induced facial skin oedema using biophysical measurement techniques. Skin Res Technol. 2000;6:118–127. doi: 10.1034/j.1600-0846.2000.006003118.x. [DOI] [PubMed] [Google Scholar]
- 24.Conley J. Pendulosity in regional flaps about the head and neck. Ann Plast Surg. 1986;16:75–81. doi: 10.1097/00000637-198601000-00008. [DOI] [PubMed] [Google Scholar]


