Abstract
Most cases of breast cancer are diagnosed in older adults. Compared with younger women, older women as a group are at increased risk for breast-cancer-specific mortality and at higher risk for treatment-associated morbidity as well. At the same time, older women are less likely to be offered preventive care or adjuvant therapy for this disease. There are major gaps in evidence regarding the optimal evaluation and treatment of older women with breast cancer due to significant under-representation in clinical trials. Chronologic age alone is an inadequate predictor of treatment tolerance and benefit in this heterogeneous population. Multiple issues uniquely associated with aging have an impact on cancer care, including functional impairment, comorbidity, social support, cognitive function, psychological state, and financial stress. Applying geriatric principles and assessment to this older adult population would inform decision-making, by providing estimates of life expectancy and identifying individuals most vulnerable to morbidity. Ongoing research is seeking to identify which assessment tools can best predict outcomes in this population, and thus guide us in tailoring treatments to maximize benefits in older adults with breast cancer.
Breast cancer is a disease associated with aging. The median age at breast cancer diagnosis is 61, and the median age for breast cancer mortality is 69.1 As the US population ages, the number of older adults with breast cancer and the number of breast cancer survivors is also rising. Among older adults, factors other than chronological age can influence treatment decisions and outcomes as independent predictors of morbidity and mortality. These factors (included in what is termed “a geriatric assessment”) include physical function, comorbid medical conditions, cognitive function, psychological state, social support, polypharmacy and geriatric syndromes, and financial considerations. While a geriatric assessment is routinely performed in daily geriatric practice, it is only starting to accompany oncology care.
In this review, we outline the essential components of a geriatric assessment, discuss screening tools for measuring the individual domains, and examine the utility of the assessment in a general geriatric population. In addition, we highlight studies that report on the use of a geriatric assessment among patients with breast cancer, examine gaps in our present evidence-based knowledge, and issue a call for additional research.
The Geriatric Assessment
Physical function
Assessment of physical function, a key element in geriatric assessment, provides information independent of comorbidity in older oncology patients.2 In the general geriatric population, impairment in physical function has been consistently associated with increased risk of future disability and mortality.3,4 Likewise, physical function may predict susceptibility to toxicity for older adults receiving cancer treatment.5
Oncologists report that treatment recommendations for older women with breast cancer are usually influenced by perceived functional status.6 There is no consensus regarding which tools to use in evaluating physical function in older patients with cancer or how to modify treatment plans accordingly.
Clinical questions to consider include: 1) Does physical function predict meaningful outcomes for older women with breast cancer? 2) Are oncology performance status scales adequate in assessing physical function for the older cancer patient? 3) How should treatment plans be altered based on individual’s physical function?
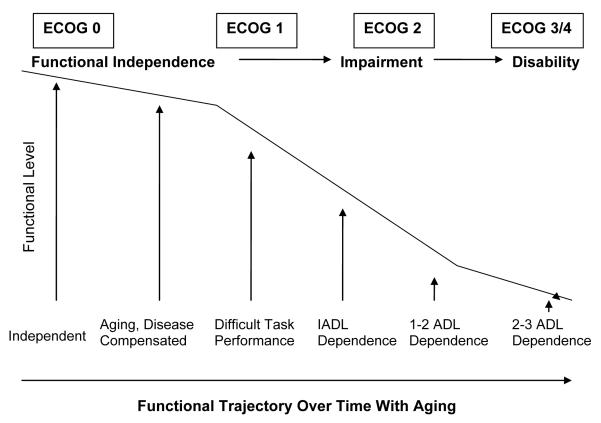
Traditionally, physical function has been assessed with oncology performance scales such as the Eastern Cooperative Oncology Group (ECOG) scale or Karnofsky performance status (KPS). Poor performance on the ECOG scale has been associated with decreased survival in older women being treated with palliative chemotherapy for metastatic breast cancer.7 Physical function assessment using the ECOG scale alone, however, is inadequate when determining risk for many older adults with cancer. For example, while scores of 3-4 on the ECOG scale correlate with overt disability, scores of 0-2 encompass a broad range of functions in older adults (Figure 1). Many older patients present with an ECOG score of <3 in clinical practice.8 This does not give oncologists information about subclinical disabilities that might predict tolerability and response to therapy. More sensitive measures are needed.
Figure 1. Conceptual Model for How ECOG Performance Score Can Relate to Activities of Daily Living.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ADL, activities of daily living; IADL, instrumental activities of daily living
In geriatrics, functional status is commonly assessed using Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) scales.9,10 ADLs cover basic self-care skills (ie, bathing, dressing) while IADLs address skills needed to maintain independence in the community (ie, transportation, taking medications). These task-specific scales have been proposed for use in a geriatric assessment for older patients with cancer (NCCN Guidelines), since they add vital information to the ECOG performance scale. Repetto et al evaluated 363 older cancer patients in a geriatric oncology clinic, and found 9% with ADL disability and 38% with IADL disability in patients with an ECOG performance score <2.8
In studies of a geriatric assessment of older women with breast cancer, a substantial number presented with ADL or IADL disabilities.11-13 For example, Girre et al evaluated 105 patients aged ≥ 70, and reported that 42% required assistance with ADLs and 54% required assistance with IADLs, despite the fact that only 7% of patients received an ECOG score >2. In a study of older adults enrolled in a phase II trial for metastatic breast cancer, Del Mastro and colleagues reported that 26% of the trial population required assistance with ADLs and 73% required assistance with IADLs.14
Further research is needed to understand the significance of ADL and IADL scores when weighing the risk and benefits of treatment of older women with breast cancer. Limited data suggest that ADL disability may be associated with inferior treatment response.4 In non-breast cancer studies (ie, non-small cell lung cancer, acute leukemia), IADL disability has been associated with decreased survival.15,16 Additional short survey measures, such as the Vulnerable Elders Survey 13 (VES-13), which includes self-reported items on health, activities of daily living, and mobility, are being investigated in older adults with cancer and are included for consideration in the NCCN Guidelines for Senior Adult Oncology.17,18
In addition to self-reported functional status, objective measures of physical performance can provide an assessment of physical function that is free of patient or physician bias. Physical performance measures provide a quantitative and reproducible assessment of specific functional tasks such as walking speed, lower extremity strength, or grip strength. These tests complement self-report functional assessment by detecting subclinical changes that may also predict morbidity and mortality.
Objective physical performance measures have been shown to predict hospitalizations, disability, and mortality in the ambulatory geriatric population.3,19 These measures include the short physical performance battery (SPPB), walking speed, standing balance, chair stands, the “Timed Get Up and Go” test, and isometric grip strength.3,19,20 The Timed Get Up and Go test has been previously evaluated as part of a geriatric assessment feasibility study in older cancer patients.21 This test measures how many seconds it takes an individual to stand from a seated position, walk a distance of 10 feet, turn, walk back to the chair and sit down again.20 The simplicity of this test makes it a practical choice for the clinical setting. Future research, including an ongoing multi-site geriatric assessment study, will help to determine the utility of this and other measures in predicting who is at increased risk of cancer treatment toxicity, thus facilitating its use in the clinical setting.
At present there is no consensus on which physical function measures better predict outcomes and life expectancy for older women with breast cancer. Consensus guidelines, including the NCCN, recommend ADL and IADL assessments in addition to oncology performance scales in making decisions about treatment.22,23 Ongoing and future prospective studies will help to validate these measures and provide clinical cutoff scores to be used to guide treatment decisions in older adults with cancer.
Comorbidity
The majority of older adults with breast cancer have comorbid medical illnesses that can affect breast cancer treatment. When making treatment decisions and weighing the impact of comorbid medical conditions, there are 2 main questions to consider:
1) Is the patient more likely to die of cancer or another comorbid condition?
2) Will the comorbid medical condition influence the patient’s ability to tolerate treatment, and if so, should treatment modifications be made?
Is the patient more likely to die of cancer or another comorbid condition?
In order to answer this question, an estimate of life expectancy must be weighed against the risk of cancer relapse during this projected life expectancy. Average life expectancy by age and gender is summarized in Table 1.24 Comorbid medical conditions can further influence life expectancy within each age cohort. There are several tools that project the risk of mortality within a specific time frame based on comorbid medical conditions. For example, Lee and colleagues developed a prognostic index of mortality risk over 4 years based on these variables: age, sex, comorbid medical conditions, and functional status.25 Another tool commonly used is the Charlson Comorbidity Index, which ranks and compares comorbid medical conditions that increase the 1-year risk of mortality.26 A Charlson Comorbidity Index that takes into account the impact of age, in addition to comorbid conditions, is also available.27
Table 1.
Life Expectancy by Age and Gender
| Life Expectancy |
|||
|---|---|---|---|
| Age | All | Female | Male |
| Birth | 77.4 | 80 | 74.7 |
| 65 | 18.4 | 19.7 | 16.8 |
| 70 | 14.8 | 15.9 | 13.4 |
| 75 | 11.7 | 12.5 | 10.5 |
| 80 | 8.9 | 9.5 | 7.9 |
| 85 | 6.6 | 7.0 | 5.9 |
| 90 | 4.8 | 5.0 | 4.3 |
After getting an estimate of life expectancy, the next step is to weigh the risk of breast cancer mortality against mortality from another cause or causes. Satariano and colleagues evaluated the risk of breast-cancer-specific mortality vs mortality from another cause among a cohort of patients with breast cancer. In this database, patients with breast cancer with ≥ 3 out of 7 selected comorbid conditions had a 20 times higher mortality rate from causes other than breast cancer.28 Similar findings have been seen in other studies of older adults with early stage hormone receptor positive breast cancer, in which the majority of deaths are due to causes other than breast cancer.29
On the other hand, among patients with high-risk breast cancer, the danger of death from breast cancer is likely to outweigh that from the other comorbid condition. This is best illustrated by a recent randomized trial of poly- vs single-agent adjuvant chemotherapy for older adults with breast cancer. The majority of participants had node-positive breast cancer or a tumor size >2 cm. With a relatively short follow-up of 2.4 years, receipt of polychemotherapy was associated with a decrease in the risk of relapse and mortality from breast cancer, highlighting the importance of considering adjuvant treatment among patients with high-risk disease where breast cancer might limit life expectancy.30 In this situation, the risk of breast cancer outweighed the risk of dying from another comorbid disease.
Tools such as Adjuvant! Online have been developed, which incorporate breast cancer characteristics, age, and a general estimate of comorbidity (ie, major medical problems, medical problems average for age, minor medical problems, and perfect health). Together these data provide an estimate of the risk of dying from cancer versus another medical problem.31 Since the impact or severity of specific medical problems is not considered, utilizing a comorbidity index in addition to Adjuvant! Online may be useful in daily practice.
Will the comorbid medical condition influence the patient’s ability to tolerate treatment?
Among older adults with breast cancer, comorbidity is a better predictor of the toxicity risk from adjuvant chemotherapy than age alone.32 For example, hypertension was the most prevalent comorbid medical condition in a study of 1800 postmenopausal woman age ≥ 55,33 and hypertension is a risk factor for anthracycline-induced cardiomyopathy. In addition, prior or concurrent use of antihypertensive medications is a risk factor for congestive heart failure among patients receiving anthracycline- and trastuzumab-based adjuvant therapy.34 Another example of a common comorbid medical condition that can have a serious impact on breast cancer treatment is diabetes. Patients with pre-existing diabetes or glucose intolerance will be at increased risk for hyperglycemia secondary to steroids, which are commonly used as antiemetics or to prevent or treat chemotherapy allergic reactions. Patients with diabetes are also at risk for neuropathy, which may be exacerbated by neurotoxic chemotherapy drugs such as taxanes or vinca alkaloids. In addition, age-related changes in physiology can affect the tolerance to cancer treatment. For example, age-related declines in renal function or renal insufficiency secondary to diabetes or another comorbid medical condition must be considered when dosing chemotherapy that is renally metabolized.35,36
In summary, comorbid medical conditions and age-related changes in physiology can influence the risk of toxicity, as well as the dosing and the side effect profile of treatment.
Cognition
Impaired cognitive function is a common complaint among older women presenting for medical treatment, and the differential diagnosis of type and extent of cognitive impairment is an important consideration in treatment planning and prognosis. The main question is: Does this patient have cognitive impairment, and if so, to what degree will this impairment affect decision-making capacity, compliance, and tolerance to treatment?
Cognitive disorders in older patients are underdiagnosed without screening. One-fifth of geriatric cancer patients have screened positively for cognitive disorders in an academic setting.37,38 It is estimated that 6%-10% of people age ≥ 65 years suffer from dementia. The prevalence increases to 25%-48% in samples of community-living populations >80 years of age.39 The prevalence of early or mild cognitive impairment is estimated to be even higher.39,40 Cognitive impairment is associated with an increased risk for progression to dementia, with progression rates of 10%-15% per year as compared with 1% to 2.5% in persons who are cognitively intact.40-42
Cognitive disorders such as dementia limit life expectancy.43 They have a major impact on cancer treatment.44-46 Cognitive disorders interfere with compliance to medications, consent to treatment, and increase caregiver burden; it has been shown that cognitively impaired persons receive less definitive cancer care than other patients.44,46,47
Over the last several years, investigators have prospectively studied the impact of breast cancer treatment on cognitive functioning, following up on complaints of memory changes and impaired concentration. Unfortunately, the data are still limited regarding the impact of adjuvant chemotherapy on an older person’s cognition. In one longitudinal prospective study of older patients with breast cancer, 51% of 45 evaluable patients perceived a decline in cognitive function from pre-chemotherapy to 6 months post-completion of chemotherapy.48 Other studies demonstrated no significant change in Mini-Mental Status Exam (MMSE) scores after chemotherapy or hormonal therapy over a short period of time.11,49 In one longitudinal study, 28 older women with breast cancer who received adjuvant chemotherapy, underwent neuropsychological testing and a comprehensive geriatric assessment (CGA) before therapy and 6 months after completion of chemotherapy.50 Thirty-nine percent of patients scored 2 standard deviations below normative data at 6 months compared to their baseline neuropsychological test scores,50 but exploratory analyses of longitudinal CGA results demonstrated no changes in functional status, comorbidity, or depression scores.51 At the same time, one population-based study suggests that women with breast cancer who receive chemotherapy have a higher likelihood of developing dementia after long-term follow-up.45 More prospective, long-term, larger studies are necessary to definitively assess the impact of breast cancer treatment on the cognitive function of older patients.
Clinical suspicion of dementia is not as sensitive as available screening tools.52 A CGA cognitive assessment tool for older breast cancer patients should be used to screen for baseline impairment and to follow effects of therapy on cognitive functioning. Cognitive screening tools include the Blessed Dementia Rating Scale,53 Mini-Mental State Examination,54 Mini-Cog,55 and Short Portable Mental Status Questionnaire.56 The purpose of screening is to assess cognitive capacity and to stratify risk; abnormal scores should trigger a comprehensive work-up with cognitive specialists. Unfortunately, these tools have not yet demonstrated the ability to detect changes in cognition due to treatment, and a more detailed neuropsychological evaluation is needed to accomplish this goal.48
Polypharmacy
Age-related changes in physiology can influence the pharmacodynamics and pharmacokinetics of cancer-related drugs, thus affecting the efficacy as well as toxicity.57,58 Predicting drug efficacy and tolerance is even more complicated because of the high prevalence of polypharmacy in this population.59,60 The key clinical conditions to consider: 1) Is the patient on any medications that have a high risk of adverse events in older adults? 2) Can this medication be substituted for a safer alternative or discontinued? 3) Will any of the medications that the patient is currently taking interact with the medications prescribed for cancer?
Studies of older adults with cancer report the average number of medications ranges from 4-9, depending on the population sampled.59-62 One study of 105 patients ≥70 years of age, including a large proportion of breast cancer patients, reported 74% taking ≥ 3 medications.63 This number likely increases in patients who receive chemotherapy.60
Polypharmacy is associated with adverse drug reactions, increased risk of drug-drug interactions, and decreased compliance with medications.59 These risks are particularly important considerations in older adults who are challenged with chemotherapy treatments. To date, there are no evidence-based guidelines for evaluation and management of polypharmacy in older adults receiving cancer therapy. Practical recommendations include a careful review of the medication list prior to initiation of cancer treatment. Unnecessary or potentially inappropriate medications should be discontinued if possible. Use of consensus guidelines for medications that carry a high risk of toxicity in older adults, such as the Beer’s list, may be helpful in identifying and discontinuing potentially harmful medications.64 Finally, a review of potential or existing drug-drug interactions should be performed. These simple measures may improve compliance, tolerance, and efficacy of treatments.
Nutritional Assessment
Nutritional status plays a major role in the overall prognosis for older adults, and in the general geriatric population, late-life weight loss has been associated with increased mortality.65 Under-nutrition in older adults at the time of cancer diagnosis influences tolerance to therapy and response to treatment. The key clinical questions to be addressed in an oncology consultation: 1) Does the patient have evidence of impaired nutritional status, and 2) Can a nutritional consult help find the etiology (ie, lack of access to food, poorly fitting dentures, cancer/chemotherapy-related symptoms, etc.) and implement interventions to prevent or reverse nutritional decline?
Weight loss prior to diagnosis or treatment has been associated with poor outcomes in multiple tumor types.66,67 An analysis of 3047 patients enrolled in ECOG chemotherapy trials demonstrated a negative association between weight loss prior to chemotherapy and survival.68 In the breast cancer subset, an association between weight loss and decreased chemotherapy response was also noted.
Data on the prognostic significance of nutritional status in the older patient with breast cancer is limited. However, small geriatric assessment studies have demonstrated that assessment of nutritional status can detect impairments in some older adults.11,12 In one study of older cancer patients, 60% with breast cancer, weight loss >10% three months prior to diagnosis was reported in 7% of patients.12 Approximately 14% had a body mass index (BMI) <18.5 kg/m2, suggestive of under-nutrition.
Various measures can be used to screen for impaired nutritional status, including self-reported weight loss prior to treatment, and calculation of BMI. The Mini-Nutritional Assessment (MNA) has been validated in elderly populations and piloted in older adults with breast cancer. This tool includes anthropometric measurements; questions related to lifestyle, mobility, and medications; a brief dietary questionnaire; and self-perception of health and nutrition.69 It takes approximately 10 minutes to perform, and has detected nutritional risk in 8 of 15 older breast cancer patients evaluated.11 Larger studies will be needed to determine if tools such as the MNA add prognostic information to practical markers of frailty, such as self-reported weight loss and low BMI.
Psychological State
Distress related to the disease of cancer is defined as “Multifactorial, unpleasant emotional experience of a psychological (cognitive, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment.”70 Older adults with cancer reportedly experience similar or less distress than younger adults.71 However, socially isolated patients are most vulnerable to the impact of distress, which frequently goes unrecognized.72 The key clinical questions include: 1) Are there screening tools that help identify older adults with cancer who are distressed? 2) What is the cause of the distress, and will it interfere with the patient’s ability to successfully complete treatment; and 3) How can we help the older adult with cancer to better handle distress?
Several screening tools can be used to evaluate for distress. The National Comprehensive Cancer Network (NCCN) guidelines endorse a simple distress “thermometer,” which consists of a single question asking the patient to characterize their level of distress on a scale of 0-10.73 A score of ≥ 4 on the distress thermometer correlates with scores on other standardized depression scales, and warrants further evaluation.74-76
Distress is a term that encompasses a variety of psychological states including depression. Depression is associated with several adverse clinical outcomes including functional decline, a need for informal caregiving, and increased utilization of healthcare resources. 77-79 Screening tools include the Geriatric Depression Scale80 and Hospital Anxiety and Depression Scale81; however, these scales have not been specifically developed for older adults with cancer, where the typical symptoms of depression may be confounded by tumor- and treatment-related symptoms. In a busy oncology practice where time is limited, a single question: “Do you often feel sad and depressed?” may be an adequate screen.82,83 Others have reported, however, that this simplified method may miss a significant proportion of patients who are depressed.84
Financial Considerations and Social Support
Evaluation of social support should address both economic and social barriers to treatment. The questions to consider are: 1) What are the financial and social barriers that could compromise cancer care in this older patient, and 2) Can these barriers be overcome or mediated prior to the initiation of treatment?
Cancer care is expensive.85 Older patients with Medicare face significant “out-of-pocket” treatment costs.86,87 There are significant payment gaps within Medicare coverage for health care including private nursing, physical therapy or rehabilitation, transportation, dental care, eyeglasses, and hearing aids. These services are often necessary for adequate cancer care, but can be prohibitively expensive for some older patients. Even with the creation of Medicare Part D, many older persons pay for medications out-of-pocket because prescription drug coverage, including for those drugs needed for supportive care such as pain management and nausea control, are not always fully covered. In addition, older adults with limited fixed incomes may forego medications such as antinausea or pain medications if faced with an either/or decision between an anticancer drug and supportive medications.85
The caretaker of an older person with cancer is also an important consideration,88,89 since the presence or absence of social support can influence the care an older person with breast cancer receives.90 For example, unmarried women with breast cancer are less likely to receive definitive treatment.90 Older persons who do not have an appropriate and easily accessed social support system may have more difficulty psychologically and emotionally with their cancer diagnosis.91 Several studies in older breast cancer patients have noted that poor social support correlates with adverse health outcomes. Low social support is independently associated with decreased satisfaction and poor psychosocial outcomes after breast cancer treatment as well.91,92 In the Nurses Health Study, socially isolated women had a 66% increased risk of all-cause mortality and a 2-fold increased risk of breast-cancer-specific mortality when adjusting for significant covariates, including stage of disease.93 Poor social support is also linked to medical adherence.94 Aspects of social function include social network (social relationships and contacts), social support (provision of assistance by network), subjective well-being, and social resources (income, assets, housing). These all independently affect complex cancer decisions and influence cancer care.95
A geriatric assessment should include a comprehensive assessment of the older breast cancer patient’s financial needs in order to recognize and address barriers to effective and safe treatment. Evaluation of social support and financial barriers is essential in devising strategies for appropriate care, early recognition of potential problems/needs, and initiation of preventative intervention.92 A social worker with a background in aging can be an invaluable resource in identifying community and financial resources to help meet the patient’s needs.
Geriatric Syndromes: Problems Common to Older Adults
Many of the most common conditions that geriatricians treat, including delirium, falls, frailty, dizziness, and urinary incontinence, are classified as geriatric syndromes. The term “geriatric syndrome” is used to capture those clinical conditions that do not fit into discrete disease categories.96 At the same time, the concept of geriatric syndromes remains poorly defined. These syndromes, prevalent among older and frailer individuals, have multifactorial etiologies and an adverse impact on health outcomes. Risk factors for the development of geriatric syndromes include older age, baseline cognitive impairment, baseline functional impairment, and impaired mobility.96 Koroukian et al evaluated 952 older breast cancer patients, and found that 35% had at least 1 geriatric syndrome at diagnosis.97 Geriatric syndromes were also found to be prevalent in hospitalized older patients with cancer.98 In older populations, the presence of geriatric syndromes predicts further functional decline, hospitalizations, and mortality.96,99 More research is needed to understand the impact of geriatric syndromes individually or in concert on the outcomes of older women with breast cancer.100,101
Conclusions
Most breast cancers occur in older adults. Factors other than chronological age, which are addressed in a geriatric assessment, independently predict morbidity and mortality and can alter the spectrum of cancer care from diagnosis to treatment to outcome.
In this article, we discuss the standard components of a geriatric assessment and highlight screening tools that can be used in daily practice to measure each of the domains. Further research is needed to identify which domains, as well as specific questions within each domain, most effectively predict morbidity and mortality among older adults with breast cancer, and to develop interventions that will help vulnerable older adults with breast cancer receive quality cancer care. Further study that integrates geriatric and oncology care principles should provide us with the guidelines we need.
Table 2.
Geriatric Assessment Domains and Measures
| Domain | Possible Measures | Predictive Value | Numbers of Items |
|---|---|---|---|
| Physical Function | Activities of Daily Living (ADL) |
Predicts institutionalization and mortality in geriatric populations |
6 |
| Instrumental Activities of Daily Living (IADL) |
Predicts survival in selected small studies of older cancer patients |
8 | |
| Vulnerable Elders Survey | Predicts mortality and functional decline in geriatric populations |
13 | |
| Get Up and Go Test | Correlates with ability to transfer independently |
1 exam maneuver | |
| Short Physical Performance Battery |
Predicts future disability and mortality in geriatric populations |
3 exam maneuvers | |
| Comorbidity | Charlson Comorbidity Scale |
Analyzes 1-year risk of mortality. Age- adjusted index is available |
19 |
| Lee Prognostic Index | Analyzes 4-year risk of mortality (includes age, sex, comorbid medical conditions, and functional status) |
12 | |
| ACE-27 | Analyzes the impact of comorbidity in pts with cancer |
27 | |
| Cognition | Mini-Mental State Examination54 |
All are screening tools for cognitive Impairment and have been associated with dementia and increased morbidity and mortality in community-dwelling samples. |
-MMSE-11 questions that review memory, orientation, and executive functioning |
| -Blessed Orientation- Memory-Concentration (OMC) Test102 |
Associated with impaired survival in cancer patients |
-Blessed OMC-6 questions |
|
| -Short Portable Mental Status Questionnaire (SPMC)56 |
Associated with mortality in community- dwelling older populations |
-SPMC-10 questions | |
| Nutrition | Self-reported weight loss | Associated with impaired survival in cancer patients. |
1 |
| Body Mass Index (BMI) | <18.5 kg/m2 cutoff per World Health Organization (WHO). |
1 | |
| Mini-Nutritional Assessment (MNA) |
Predictive of mortality in hospitalized elders. |
18 | |
| Psychological State | Distress Thermometer | High concordance between scores on the Distress Thermometer and the Hospital Anxiety and Depression Scale.74 |
1 |
| Cut-off score of 4 had the greatest sensitivity and specificity when compared with the Center for Epidemiological Studies Depression scale.75 |
|||
| Patients with cancer who scored ≥ 4 on the Distress Thermometer were more likely to also report physical, emotional, practical, and family problems.76 |
|||
| Social Support | MOS Social Support Survey: Emotional/Information and Tangible Subscales103 |
Tangible Subscale measures access to material aid or behavioral assistance and Emotional/Information subscales measure the expression of positive affect and empathetic understanding; the offering of advice, information, guidance, or feedback. |
20 |
| Geriatric Syndromes -Incontinence -Osteoporosis -Frailty -Dementia -Delirium -Falls -Pressure Sores -Neglect and abuse |
-No comprehensive tool exists but questions to ask include frequency and severity of syndromes that are not assessed within other measures of the CGA. |
||
Acknowledgment of Research Support
Dr. Hurria’s efforts are supported by K23 AG026749-01 (Paul Beeson Career Development Award in Aging Research) and American Society of Clinical Oncology-Association of Specialty Professors-Junior Development Award in Geriatric Oncology. Dr. Klepin’s efforts are supported in part by the Intramural Research Program of the NIH, National Institute on Aging (NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106), the Wake Forest University OAIC (P30 AG-021332) and the Association of Specialty Professors-American Society of Hematology Geriatric Hematology Research Award.
REFERENCES
- 1.Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2003. National Cancer Institute; Bethesda, MD: 2006. [Google Scholar]
- 2.Extermann MOJ, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 4.Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–93. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 5.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005;16:1795–800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 6.Hurria A, Naeim A, Elkin E, et al. Adjuvant treatment recommendations in older women with breast cancer: a survey of oncologists. Crit Rev Oncol Hematol. 2007;61:255–60. doi: 10.1016/j.critrevonc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Pentheroudakis G, Fountzilas G, Kalofonos HP, et al. Palliative chemotherapy in elderly patients with common metastatic malignancies: A Hellenic Cooperative Oncology Group registry analysis of management, outcome and clinical benefit predictors. Crit Rev Oncol Hematol. 2008;66:237–47. doi: 10.1016/j.critrevonc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 9.Katz S, Ford AB, Moskowitz RW, et al. Studies Of Illness In The Aged. The Index Of Adl: A Standardized Measure Of Biological And Psychosocial Function. Jama. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 10.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 11.Extermann M, Meyer J, McGinnis M, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol. 2004;49:69–75. doi: 10.1016/s1040-8428(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 12.Girre V, Falcou MC, Gisselbrecht M, et al. Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci. 2008;63:724–30. doi: 10.1093/gerona/63.7.724. [DOI] [PubMed] [Google Scholar]
- 13.Retornaz F, Monette J, Batist G, et al. Usefulness of frailty markers in the assessment of the health and functional status of older cancer patients referred for chemotherapy: a pilot study. J Gerontol A Biol Sci Med Sci. 2008;63:518–22. doi: 10.1093/gerona/63.5.518. [DOI] [PubMed] [Google Scholar]
- 14.Del Mastro L, Perrone F, Repetto L, et al. Weekly paclitaxel as first-line chemotherapy in elderly advanced breast cancer patients: a phase II study of the Gruppo Italiano di Oncologia Geriatrica (GIOGer) Ann Oncol. 2005;16:253–8. doi: 10.1093/annonc/mdi056. [DOI] [PubMed] [Google Scholar]
- 15.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865–72. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 16.Wedding U, Rohrig B, Klippstein A, et al. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol. 2006;132:665–71. doi: 10.1007/s00432-006-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balducci L, Cohen HJ, Engstrom PF, et al. Senior adult oncology clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:572–90. doi: 10.6004/jnccn.2005.0032. [DOI] [PubMed] [Google Scholar]
- 18.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–9. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 19.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 20.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 21.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 22.Carlson RW, Moench S, Hurria A, et al. NCCN Task Force Report: breast cancer in the older woman. J Natl Compr Canc Netw. 2008;6(Suppl 4):S1–S25. quiz S26-S27. [PubMed] [Google Scholar]
- 23.Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101–15. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 24.Arias E. United States Life Tables, 2003, National Vital Statistics Report. National Center for Health Statistics; Hyattsville, MD: 2006. [PubMed] [Google Scholar]
- 25.Lee SJ, Lindquist K, Segal MR, et al. Development and validation of a prognostic index for 4-year mortality in older adults. Jama. 2006;295:801–8. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 28.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–10. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 29.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–7. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 30.Muss H, Berry D, Cirrincione C, et al. CALGB/CTSU 49907: Standard Chemotherapy (CMF or AC) vs Capecitabine (X) in Early-Stage Breast Cancer Patients 65 and Older American Society of Clinical Oncology. Illinois; Chicago: 2008. [Google Scholar]
- 31.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–91. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 32.Zauderer M, Patil S, Hurria A. Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. Jama. 2001;285:885–92. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 34.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–8. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997;80:1273–83. [PubMed] [Google Scholar]
- 36.Dees EC, O’Reilly S, Goodman SN, et al. A prospective pharmacologic evaluation of age-related toxicity of adjuvant chemotherapy in women with breast cancer. Cancer Invest. 2000;18:521–9. doi: 10.3109/07357900009012191. [DOI] [PubMed] [Google Scholar]
- 37.Extermann M, Aapro M. Assessment of the older cancer patient. Hematol Oncol Clin North Am. 2000;14:63–77. viii–ix. doi: 10.1016/s0889-8588(05)70278-1. [DOI] [PubMed] [Google Scholar]
- 38.Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109:802–10. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 39.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–34. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelley BJ, Petersen RC. Alzheimer’s disease and mild cognitive impairment. Neurol Clin. 2007;25:577–609. v. doi: 10.1016/j.ncl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 43.Wolfson C, Wolfson DB, Asgharian M, et al. A reevaluation of the duration of survival after the onset of dementia. N Engl J Med. 2001;344:1111–6. doi: 10.1056/NEJM200104123441501. [DOI] [PubMed] [Google Scholar]
- 44.Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc. 2004;52:1681–7. doi: 10.1111/j.1532-5415.2004.52461.x. [DOI] [PubMed] [Google Scholar]
- 45.Heck JE, Albert SM, Franco R, et al. Patterns of Dementia Diagnosis in Surveillance, Epidemiology, and End Results Breast Cancer Survivors Who Use Chemotherapy. J Am Geriatr Soc. 2008 doi: 10.1111/j.1532-5415.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 46.Gorin SS, Heck JE, Albert S, et al. Treatment for breast cancer in patients with Alzheimer’s disease. J Am Geriatr Soc. 2005;53:1897–904. doi: 10.1111/j.1532-5415.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin JS, Samet JM, Hunt WC. Determinants of survival in older cancer patients. J Natl Cancer Inst. 1996;88:1031–8. doi: 10.1093/jnci/88.15.1031. [DOI] [PubMed] [Google Scholar]
- 48.Hurria A, Goldfarb S, Rosen C, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient’s perspective. Breast Cancer Res Treat. 2006;98:343–8. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Cantor A, Meyer J, et al. Can older cancer patients tolerate chemotherapy? A prospective pilot study. Cancer. 2003;97:1107–14. doi: 10.1002/cncr.11110. [DOI] [PubMed] [Google Scholar]
- 50.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54:925–31. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 51.Hurria A, Zuckerman E, Panageas KS, et al. A prospective, longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy. J Am Geriatr Soc. 2006;54:1119–24. doi: 10.1111/j.1532-5415.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 52.Chodosh J, Petitti DB, Elliott M, et al. Physician recognition of cognitive impairment: evaluating the need for improvement. J Am Geriatr Soc. 2004;52:1051–9. doi: 10.1111/j.1532-5415.2004.52301.x. [DOI] [PubMed] [Google Scholar]
- 53.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 54.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 55.Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451–4. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 56.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 57.Hamberg P, Verweij J, Seynaeve C. Cytotoxic therapy for the elderly with metastatic breast cancer: a review on safety, pharmacokinetics and efficacy. Eur J Cancer. 2007;43:1514–28. doi: 10.1016/j.ejca.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Hurria A, Lichtman SM. Clinical pharmacology of cancer therapies in older adults. Br J Cancer. 2008;3:517–522. doi: 10.1038/sj.bjc.6604201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corcoran M. Polypharmacy in the older patient with cancer. Cancer Control. 1997;4:419–428. [PubMed] [Google Scholar]
- 60.Sokol K, Knudsen JF, Li MM. Polypharmacy in older oncology patients and the need for an interdisciplinary approach to side-effect management. J Clin Pharm Ther. 2007;32:169–175. doi: 10.1111/j.1365-2710.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 61.Flood K, Carroll MB, Le CV, Ball L, Esker DA, Carr DB. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006;24:2298–2303. doi: 10.1200/JCO.2005.02.8514. [DOI] [PubMed] [Google Scholar]
- 62.Ingram S, Seo PH, Martell RE, Clipp EC, Doyle ME, Montana GS, et al. Comprehensive assessment of the elderly cancer patient: the feasibility of self-report methodology. J Clin Oncol. 2002;20:770–775. doi: 10.1200/JCO.2002.20.3.770. [DOI] [PubMed] [Google Scholar]
- 63.Girre V, Falcou MC, Gisselbrecht M, Gridel G, Mosseri V, Bouleuc C, et al. Does a geriatric oncology consultation modify the cancer treatement plan for elderly patients? J Gerontol A Biol Sci Med Sci. 2008;63:724–730. doi: 10.1093/gerona/63.7.724. [DOI] [PubMed] [Google Scholar]
- 64.Fick D, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication used in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 65.Newman ABYD, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. Jr Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 66.Andreyev HJNA, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J. Cancer. 1998;34:503–509. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 67.Buccheri GFD. Importance of weight loss definition in the prognostic evaluation of non-small-cell lung cancer. Lung Cancer. 2001;34:433–440. doi: 10.1016/s0169-5002(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 68.Dewys WDBC, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 69.Vellas BGY, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The mini nutritional assessemnt (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15:116–122. doi: 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 70.Holland JC, Bultz BD. The NCCN guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5:3–7. [PubMed] [Google Scholar]
- 71.Vinokur AD, Threatt BA, Vinokur-Kaplan D, et al. The process of recovery from breast cancer for younger and older patients. Changes during the first year. Cancer. 1990;65:1242–54. doi: 10.1002/1097-0142(19900301)65:5<1242::aid-cncr2820650535>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 72.Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counselling? Br J Cancer. 2001;84:179–85. doi: 10.1054/bjoc.2000.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffman BM, Zevon MA, D’Arrigo MC, et al. Screening for distress in cancer patients: the NCCN rapid-screening measure. Psychooncology. 2004;13:792–9. doi: 10.1002/pon.796. [DOI] [PubMed] [Google Scholar]
- 74.Roth AJ, Kornblith AB, Batel-Copel L, et al. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82:1904–8. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 75.Ransom S, Jacobsen PB, Booth-Jones M. Validation of the Distress Thermometer with bone marrow transplant patients. Psychooncology. 2006;15:604–12. doi: 10.1002/pon.993. [DOI] [PubMed] [Google Scholar]
- 76.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494–502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- 77.Kua J. The prevalence of psychological and psychiatric sequelae of cancer in the elderly - how much do we know? Ann Acad Med Singapore. 2005;34:250–6. [PubMed] [Google Scholar]
- 78.Penninx BW, Guralnik JM, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. Jama. 1998;279:1720–6. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 79.Langa KM, Valenstein MA, Fendrick AM, et al. Extent and cost of informal caregiving for older Americans with symptoms of depression. Am J Psychiatry. 2004;161:857–63. doi: 10.1176/appi.ajp.161.5.857. [DOI] [PubMed] [Google Scholar]
- 80.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 81.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 82.Chochinov HM, Wilson KG, Enns M, et al. “Are you depressed?” Screening for depression in the terminally ill. Am J Psychiatry. 1997;154:674–6. doi: 10.1176/ajp.154.5.674. [DOI] [PubMed] [Google Scholar]
- 83.Mahoney J, Drinka TJ, Abler R, et al. Screening for depression: single question versus GDS. J Am Geriatr Soc. 1994;42:1006–8. doi: 10.1111/j.1532-5415.1994.tb06597.x. [DOI] [PubMed] [Google Scholar]
- 84.Osborn DP, Fletcher AE, Smeeth L, et al. Performance of a single screening question for depression in a representative sample of 13 670 people aged 75 and over in the UK: results from the MRC trial of assessment and management of older people in the community. Fam Pract. 2003;20:682–4. doi: 10.1093/fampra/cmg610. [DOI] [PubMed] [Google Scholar]
- 85.Bried EM, Scheffler RM. The financial stages of cancer in the elderly. Oncology (Williston Park) 1992;6:153–60. [PubMed] [Google Scholar]
- 86.Warren JL, Brown ML, Fay MP, et al. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002;20:307–16. doi: 10.1200/JCO.2002.20.1.307. [DOI] [PubMed] [Google Scholar]
- 87.Penberthy L, Retchin SM, McDonald MK, et al. Predictors of Medicare costs in elderly beneficiaries with breast, colorectal, lung, or prostate cancer. Health Care Manag Sci. 1999;2:149–60. doi: 10.1023/a:1019096030306. [DOI] [PubMed] [Google Scholar]
- 88.Kim Y, Given BA. Quality of life of family caregivers of cancer survivors: across the trajectory of the illness. Cancer. 2008;112:2556–68. doi: 10.1002/cncr.23449. [DOI] [PubMed] [Google Scholar]
- 89.Dubenske LL, Wen KY, Gustafson DH, et al. Caregivers’ differing needs across key experiences of the advanced cancer disease trajectory. Palliat Support Care. 2008;6:265–72. doi: 10.1017/S1478951508000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Osborne C, Ostir GV, Du X, et al. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41–7. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 91.Silliman RA, Dukes KA, Sullivan LM, et al. Breast cancer care in older women: sources of information, social support, and emotional health outcomes. Cancer. 1998;83:706–11. [PubMed] [Google Scholar]
- 92.Mandelblatt JS, Edge SB, Meropol NJ, et al. Predictors of long-term outcomes in older breast cancer survivors: perceptions versus patterns of care. J Clin Oncol. 2003;21:855–63. doi: 10.1200/JCO.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Kroenke CH, Kubzansky LD, Schernhammer ES, et al. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24:1105–11. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 94.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23:207–18. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 95.Bernabei R, Venturiero V, Tarsitani P, et al. The comprehensive geriatric assessment: when, where, how. Crit Rev Oncol Hematol. 2000;33:45–56. doi: 10.1016/s1040-8428(99)00048-7. [DOI] [PubMed] [Google Scholar]
- 96.Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–91. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006;24:2304–10. doi: 10.1200/JCO.2005.03.1567. [DOI] [PubMed] [Google Scholar]
- 98.Flood KL, Carroll MB, Le CV, et al. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006;24:2298–303. doi: 10.1200/JCO.2005.02.8514. [DOI] [PubMed] [Google Scholar]
- 99.Anpalahan M, Gibson SJ. Geriatric syndromes as predictors of adverse outcomes of hospitalization. Intern Med J. 2008;38:16–23. doi: 10.1111/j.1445-5994.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 100.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–31. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 101.Wedding U, Hoffken K. Care of breast cancer in the elderly woman--what does comprehensive geriatric assessment (CGA) help? Support Care Cancer. 2003;11:769–74. doi: 10.1007/s00520-003-0537-6. [DOI] [PubMed] [Google Scholar]
- 102.Katzman R, Brown T, Fuld P, et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 103.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]