Abstract
Despite longstanding reliance upon monolayer culture for studying cancer cells, and numerous advantages from both a practical and experimental standpoint, a growing body of evidence suggests more complex three-dimensional (3D) models are necessary to properly mimic many of the critical hallmarks associated with the oncogenesis, maintenance and spread of Ewing sarcoma (ES), the second most common pediatric bone tumor. And as clinicians increasingly turn to biologically-targeted therapies that exert their effects not only on the tumor cells themselves, but also on the surrounding extracellular matrix, it is especially important that preclinical models evolve in parallel to reliably measure antineoplastic effects and possible mechanisms of de novo and acquired drug resistance. Herein, we highlight a number of innovative methods used to fabricate biomimetic ES tumors, encompassing both the surrounding cellular milieu and extracellular matrix (ECM), and suggest potential applications to advance our understanding of ES biology, preclinical drug testing, and personalized medicine.
Keywords: Ewing sarcoma, MCTS, 3D, tissue-engineering, scaffolds, ECM, tumor model, chemotherapy, biological therapy, preclinical testing
1. Introduction
The Ewing's sarcoma family of tumors (ESFT) is an aggressive form of childhood cancer that has historically included classic Ewing sarcoma (ES), Askin tumors, and peripheral primitive neuroectodermal tumors (PNET) [1-6]. Though previously considered to be distinct clinical entities, given subtle variation in their presenting sites and immunophenotype, the World Health Organization now advocates a simplified nomenclature using ES to represent the aforementioned tumors, as they consistently have a round cell morphology, ubiquitously express CD99, and harbor a near-universal pathognomonic chromosomal translocation—affixing the N-terminal EWSR1 gene to a C-terminal ETS gene [7-9]. Although rare in comparison to carcinomas, ES is the second most common pediatric bone tumor, presenting in three cases per million [10]. Racial and gender disparities exist; ES is nine times more common in Caucasians than in African Americans and slightly more prevalent in males than females with a 6:5 ratio [11, 12].
Clinically, ES is an aggressive, rapidly fatal malignancy that can develop in osseous and extraskeletal sites and naturally spreads to lung, bones, and bone marrow if not rapidly treated [11, 13, 14]. In fact, even in the 60-70% of cases where a solitary site is visualized radiographically, micrometastatic disease is presumed to exist within the lung [15]. Fortunately, significant strides in multimodality treatment have enhanced the 5-year survival rate for those with localized tumors from 10% in the pre-chemotherapy era (prior to 1962) to about 75% today [16]. Yet, for inexplicable reasons, this progress in curing patients suffering from localized disease has not extended to those with metastatic or rapidly recurrent disease, and less than 30% of these patients will survive.
In an effort to change that dismal outcome, extensive research has defined key oncogenic events responsible for the growth and maintenance of ES. And not withstanding the lack of a conclusive cell of origin, experimental evidence suggests that ES emanates from a single pluripotent bone marrow-derived mesenchymal stem cell (MSC) that has neuroendocrine features and acquires a specific cancer-causing chromosomal translocation of the EWSR1 gene [17-21]. EWSR1-FLI1, which occurs in 85% of ES, results from the apposition of the N-terminal portion of the EWS gene (located at 22q12) with the C-terminal FLI1 gene of the ETS transcription factor family [22-24]. Less common translocations include EWS-ERG (5-8% of cases) [25] and EWSR1-ETV1 [26], EWSR1-EIAF [27], and EWSR1-FEV [28], which each occur in less than one percent of reported cases [24, 29]. On rare occasions, FUS (one of three TET genes, also known as TLS) can occasionally substitute for EWSR1 to produce a FUS-ERG positive ES [30] and non-ETS pairings with ZSG [31] or NFATc2 [32] have also been reported—the biological and prognostic significance of these exceedingly rare chromosomal aberrations is unknown.
While a number of innovative drugs in preclinical development are aimed squarely at the tumorigenic EWS-FLI1 fusion protein, it has been historically quite challenging to counteract this transcription factor, given its inaccessibility within the cell nucleus. Thus, in addition to conventional chemotherapies that clearly have a prominent role in treatment, most biologically targeted therapies used in the treatment of ES either target downstream signaling cascades induced by EWS-FLI1 activity (e.g., IGF1/IGF-1R, TGF-β, Hedgehog/GLI, Wnt/β-catenin, and Notch/p53) or the surrounding tumor matrix upon which ES cells rely [33-41]. Of particular relevance to the field of tissue-engineering, it is these latter targets and/or processes, which include the extracellular matrix (ECM), nascent blood vessel formation [42, 43], and cell migration or metastasis that will be of greatest relevance since they are poorly modeled by traditional monolayer culture techniques [44].
2D monolayer culture has, of course, been the mainstay for culturing cancer cells for at least five decades, and continues to be the predominant method for testing the antineoplastic drug candidates in the preclinical setting given its many advantages over more complex systems; 2D culture is readily performed using standardized methods, promotes rapid cell growth, uses translucent material amenable to monitoring cells in real-time using light microscopy, requires no special equipment, and is cost effective. Yet, as the cancer biology community can attest, cells placed upon chemically inert flat polystyrene tissue-culture plates under conditions of high oxygen tension and abundant glucose and nutrients poorly mimic how the cells would otherwise behave within their native in vivo host [45]. As a direct result of this iatrogenic effect on cancer cells grown in the laboratory, it is not surprising that the precise protein targets and/or signaling cascades being interrogated in high-throughput anti-cancer drug screens are profoundly different from what occurs in patients and this, in part, explains why the majority of biologically targeted therapies may succeed in the lab but ultimately fail in the clinic. This holds true for traditional cytotoxic chemotherapies as well, since most work by indiscriminately damaging DNA in rapidly dividing cells that grow in monolayers at unparalleled rates unheard of in even their fastest clinical counterparts. Thus, for all the advantages of 2D cell culture for cancer research, if the information gained is unreliable—or even worse, leads to expensive clinical trials that provide false hope to patients—one must reassess whether this preclinical approach is still appropriate when better options exist [46-49].
In recognition of the previously described shortfalls inherent in monolayer culture, and ease with which ES cells/explants can be cultivated in immunocompromised mice, there has been a recent resurgence in the use of mouse models [50, 51]. In part, this gradual pendulum swing back towards tumor xenografts, which had favored in vitro testing over the last several decades, is occurring in an attempt to better mimic elements of the tumor microenvironment (e.g., tumor associated stroma, growth factors, and abundant heterotypic cells). Further, the use of low-passage number explants obtained directly from patients—rather than from long-established cell lines—appears to reduce phenotypic drift from corresponding human tumors and helps to preserve surrounding tumor-associated architecture [52].
Despite the advantages of these so-called patient-derived tumor explants (PDX), they are not without their own challenges: (i) xenografts can still behave differently when placed into a murine host, especially in subsequent generations of engraftment where mouse tissue has replaced the human tumor-associated stroma; (ii) monitoring can be difficult, particularly for orthotopic locations; (iii) specialized surgical skills are required; and (iv) engraftment rates are usually less than 75%. Additionally, because xenografts are placed into immunocompromised mice devoid of a functioning human immune system, it is all but impossible to use them to evaluate the growing list of immunomodulatory drugs, such as checkpoint inhibitors, gaining increased utility in the clinic. Last, PDX models are costly to generate since they require expensive core-needle or open biopsies of human tumors and, subsequently, incur substantial labor costs to perform drug testing.
Overall, challenges associated with current 2D and xenograft tumor models have motivated researchers to develop innovative ex vivo 3D cell culture systems that provide a more physiologically relevant cellular environment that meets the needs of the basic science and clinical research community (Table 1) [53, 54]. Prominent examples include the culture of cells in relatively simple spheroids [55], pellet cultures [56], or cell-matrix interactions (using protein gels or synthetic polymer scaffolds) [57-59]. More complex 3D cancer models that rely upon co-culture or hybrid culture systems have included encapsulated protein gels seeded with multiple cell types [60], hybrid methods with 3D scaffolds layered upon 2D monolayers [61], and heterotypic cell populations grown as tumor spheroids [62].
Table 1.
Methods used for ex vivo culture of ES cells
|

|

|
|

|
|
|---|---|---|---|---|---|
| Patient | PDX | 3D | 2D | ||
| Bioengineered 3D Scaffolds | MCTS | ||||
| Complexity | Organism | Xenograft | Organ/Tissue | Multicellular aggregate | Cellular |
| Substrate | NA | (Orthotopic/Heterothopic) | Synthetic Fibers (e.g. PCL) | Biological Gels (e.g. Matrigel, Collagen, Laminin rich gels) | Plastic or Glass |
| Cell Type | NA | Homotypic/Heterotypic | Homotypic/Heterotypic | Homotypic/Heterotypic | Homotypic |
| Tumor Niche | Physiologic | Chimeric: Animal/Human | Yes | Limited | No |
| Biology Studies | Limited | Yes (Costly) | Yes (Specialized techniques) | Limited | No |
| Preclinical Drug Testing (correlation with human trials) | NA | High (Low throughput) | Intermediate (Intermediate throughput) | Intermediate (High throughput) | Low (High throughput) |
| Biomarker development | Yes | Yes | Unknown | No | No |
| Personalized Medicine (CLIA-certified) | Yes | Yes | No | No | No |
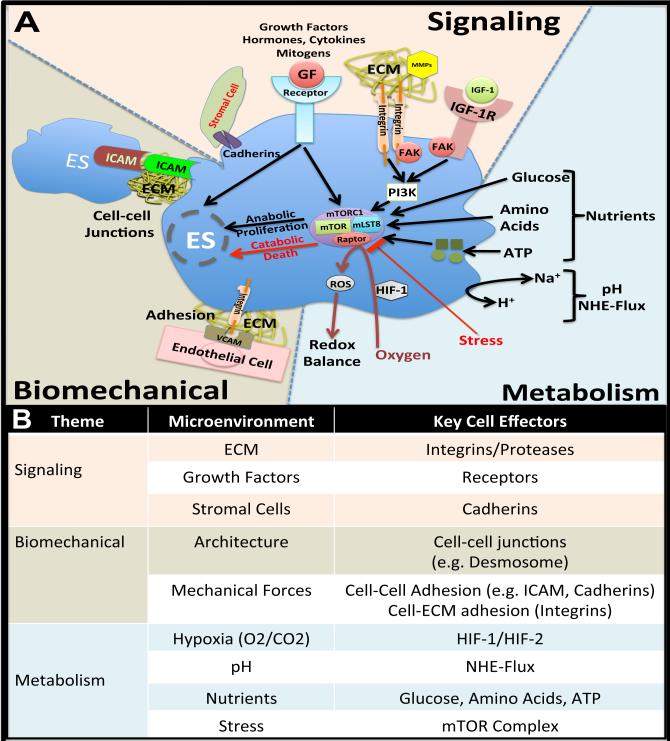
Many of the strategies developed originally for tissue-regenerative purposes have recently been adapted for the purpose of modeling human tumors in the preclinical setting, and the increased control of tumor microenvironment has revealed significant advantages over 2D and xenograft culture. In fact, it is this ability for tissue-engineered systems to devolve complex microenvironmental themes (e.g., signaling molecules, biomechanical forces, and metabolic factors) from poorly controlled native/self-organizing cell aggregates that makes their use so appealing. As shown in Figure 1, each component of a tumor's microenvironmental niche can be varied experimentally to determine the relative impact on the cancer cell.
Figure 1.
(A) Principal components of the tumor microenvironment that affect cell behavior. Signaling factors include ECM, GFs, etc. Biomechanical forces include 3D architecture and mechanical loading. Hypoxia, pH, nutrients and stress are affected affecting malignant cell phenotype. (B) Key cell effectors in tumor microenvironment. Abbreviations: GF, growth factor; ECM, extracellular matrix; MMPs, metalloproteinases; IGF-1, insulin growth factor 1; IGF-1R, insulin growth factor 1-receptor; FAD, focal adhesion kinase; PI3K, phosphatidylinositide 3-kinases; ATP, Adenosine triphosphate; mTOR, mammalian target of rapamycin; NHE-Flux, sodium-proton exchanger; HIF-1, hypoxia inducible actor-1; ROS, reactive oxygen species; and ICAM, intercellular adhesion molecule 1.
3D culture, for example, can be designed to resemble the in vivo malignancy's shape and environment, which in turn, can influence the behavior and gene expression of the cell, as has been demonstrated in a colorectal cancer model [63]. Further, by manipulating not only the 3D architecture itself, but also the heterotypic cell composition, one can selectively reintroduce key interactions between tumor cells and surrounding stromal cells within an in vivo-like human tumor microenvironment. By creating a more biomimetic 3D environment of cancer, these 3D in vitro models provide important alternatives to both 2D culture and in vivo models by, (i) delivering the applicable matrix constituents in a 3D configuration found clinically, (ii) co-culturing cancer cells, endothelial cells and other stromal associated cells in spatially adequate manner, (iii) examining and adjusting hypoxia to mimic levels found in native in vivo tumor environment and (iv) censoring the release of angiogenic factors by cancer cells in response to hypoxia.
As we delve more deeply into the steady rise of tissue-engineered tumors, and highlight both current applications and future directions they may take in helping to advance our collective understanding of tumor behavior, we take care to distinguish a cancer cell's innate behavior to self-organize (i.e., to form spheroids and other cell aggregates) from their capacity to form complex 3D relationships upon exogenous non-biologically derived tissue-engineered substratum. Though this review highlights the role of tissue-engineered models of ES, we acknowledge the relatively rarity of this bone cancer and, therefore, suspect the concepts presented herein will find more broad spread utility as they relate to other sarcoma subtypes or carcinomas that metastasize to bone or lung.
2. Tumor structures and ECM generation intrinsic to the cancer cells
2.1 Tumor Spheroids
Multicellular tumor spheroids (MCTS) are spherical aggregates of tumor cells that autonomously form when cultured in non-adherent substrates that are devoid of requisite ECM or growth factors. Since their discovery, MCTS have been demonstrated to more closely resemble the phenotypic behavior of human tumor tissues and, for that reason, have been used extensively to model key elements of malignant tumor behavior [64]. Among numerous examples, they have proven useful to study avascular tumor growth, intracellular tumor hypoxia, and the effects of cell-ECM communication upon drug sensitivity. Within this subsection, we discuss common methods of spheroid formation, highlight their phenotypic resemblance to human tumors, and describe real-world applications that are helping to decipher the complex biology of ES and cancer more broadly.
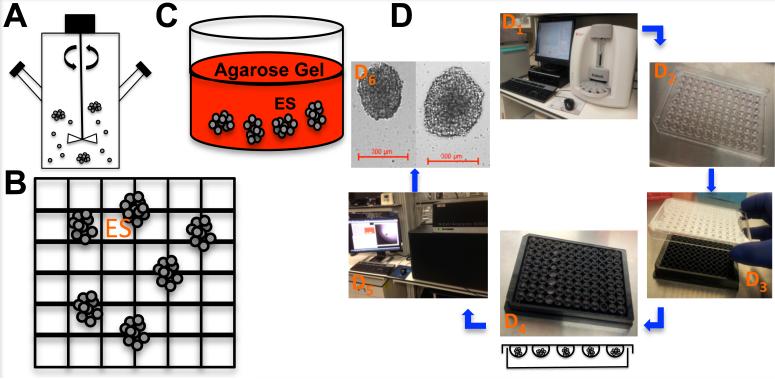
Since Inch et al. first described the formation of nodular carcinoma MCTS in 1970 using the spinner flask method, the number of methods capable of yielding MCTS has grown significantly and generally fall into two broad categories [65, 66]. The first group relies upon equipment that induces turbulence into the cell culture medium and, thereby, prevents cells from adhering to solid surfaces. In contrast, the second group achieves a similar effect simply by altering the extracellular environment to make it incompatible with cell-ECM adhesion. Prominent examples of the former ‘mechanical’ group include the original spinner flask and rotary wall vessel reactors (Figure 2A), which suspend cells between rotating cylindrical walls to that mimic some elements microgravity [53, 67]. Whereas the latter ‘intrinsically non-adherent group’ places cell suspensions onto non-adherent micro-etched nano-culture plates (NCP) (Figure 2B) or agarose coated Petri dishes (Figure 2C) that prevent cell binding. Newer production techniques include hanging drops (Figure 2D) [68, 69] and microfluidic chips [70], which can produce more uniform spheroid size at the risk of being more specialized and difficult to master.
Figure 2. Multicellular tumor spheroids (MCTS) in vitro production techniques of ES.
(A) Spinner flask spheroid cultures. (B) Micro-etched Nano-cultures. (C) Biologically (e.g. Collagen gel) derived 3D matrices cultures. (D) ES Hanging-drop cultures. (D1) ES cell line counting using Beckman Coulter Vi-Cell XR Cell Viability Analyzer. (D2) 20μL of cell suspension (100 ES cells) plated on the lid of a Greiner 96-well plate. (D3) The lid was placed back on the 96-well plate containing 100 mL of RPMI (cell culture complete medium) and carefully placed in the incubator for 72 hours. (D4) The lids were removed and 300 μL of RPMI was added to a Nano-Culture® Plate (Scivax NCP-LS) to allow the drop to come in contact with the media, re-incubate for one hour and remove 100 μL. (D5) Spheroids were imaged using the GE InCell Analyzer 6000. (D6) Images of ES spheroids cells at 2 × 104 cells / mL and 5 × 104 cells /mL.
Regardless of the chosen method to produce them, MCTS evolve from disorganized cell aggregates in the first week into highly symmetric spherical structures by two weeks that have distinct zones: (i) a central core of necrotic cells or ones undergoing apoptosis, (ii) an inner layer of non-proliferating quiescent cells, and (iii) an outer nutrient-rich layer of proliferating cells capable of interacting, albeit in a limited fashion, with surrounding ECM [55, 64]. Though subtle cell-type dependent differences in morphology exist, ranging from simple spheroids uniformly coated in ECM to more intricate structures that have glandular structures resembling ductal tissues, as a class MCTS appear to better mimic human tumors than 2D monolayers with respect to proteomic and genomic expression profiles [71-79]. Lawlor et al., for example, have noted that growth rates, cell morphology, cell-cell junctions, and kinase activation of ES spheroids closely mimic those of primary Ewing tumors [80]. Further, given their contrasts in pH, oxygen tension, and proliferative rates that exist between the inner and outer layers, MCTS can be used to determine layer-dependent antineoplastic effects that couldn't otherwise be observed using traditional monolayers [55, 81-85].
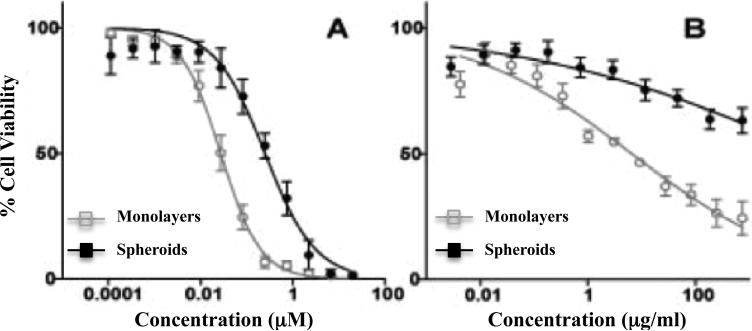
As such, ES spheroids have been used extensively to judge the effectiveness of chemotherapeutic and biologically targeted drugs (Figure 3) [53, 67, 80, 86, 87], to study the impact of cell signaling pathways that regulate ES cell proliferation [53, 80, 88], to investigate the effects of tumor architecture upon immune cell function, and to identify suitable antigens for immunotherapeutic strategies [89, 90]. ES MCTS have also proven useful for modeling micrometastatic disease, contributed to our understanding of anoikis (a form of cell death that results from lost ECM contact) [53, 88], and served as a platform to evaluate heterotypic interactions between tumor cells and vascular progenitor cells responsible for angiogenesis [77].
Figure 3. Chemotherapeutic and biologically targeted drug sensitivity testing ES monolayers and spheroids.
ES were cultured as both monolayers and spheroids in the presence of chemotherapeutic and biologically targeted drugs. Cell viability data are shown. (A) Response to doxorubicin (CID: 31703) (B) Response to fully humanized monoclonal antibody R1507 anti-human IGF-1R (Roche).
2.2 Tumor organoids
Though MCTS grown in nonadherent conditions can reproduce some features of human tissues and/or tumors (e.g. oxygen and drug diffusional gradients, cell-cell contact, etc.) their spherical self-organized structure cannot be said to truly mimic the more complex patterns observed in vivo. Surprisingly, a flurry of research reports published since 2013 have revealed an enormous untapped potential for noncancerous human embryonic pluripotent stem cells (PSC) and/or induced pluripotent stem cells (iPSC) to self-organize into lab-grown organ-like structures (i.e. organoids) when coaxed to do so by external spatiotemporal perturbation using nutrients, growth factors, or rarely heterotopic cells. Prominent examples include ex vivo models of embryonic human brain [91], functional liver buds that resemble human liver [92], and ureteric buds that differentiated towards the renal collecting system (Table 2) [93]. Additionally, a lung organoid has been described that forms beneath the renal capsule of mice in vivo [94]. That human PSCs intrinsically retain the capacity to self-assemble into spatially-complex higher-ordered organ-like structures ex vivo is truly amazing and suggests the genomic or epigenetic information contained within the PSCs is enough to drive organ-level differentiation if augmented by the ‘correct’ microenvironmental cues.
Table 2.
Organoids used to study normal physiology and diseases
| Organoid Type (Organ) | Disease Modeling | Cell Type | Scaffold Type | Bioreactor | Growth Factor/Nutrients | Reference/Journal |
|---|---|---|---|---|---|---|
| Brain | Microcephaly | hESC, hPSC, | Matrigel | Spinning | hbFGF, RA | Nature1 |
| Liver | Cirrhosis | iPSC-LB, iPSC-Hes, HUVECs, hMSC | Matrigel/Collagen IV | NA | hbFGF, hBMP4, HGF, oncostatin-M | Nature2 |
| Kidney | Polycystic kidney | hESC, iPSC | Matrigel coated plate | NA | BMP4, FGF2, RA, Activin A, BMP2 | Nature Cell Biology3 |
| Eye (Retinal) | Degenerative diseases | hESC, hiPSC | Laminin coated dishes | NA | BMP4, WNT3a | PNAS4 |
| Intestinal | Gut Defects, IBD, Transplantation, | hESC, iPSC, | Matrigel | NA | FGF4, WNT3a, Activin A, R-Spondin-1, Noggin, EGF | Nature5 |
| colon rectal cancer | Colorectal tumor cells | Collagen coated dishes | NA | EGF, bFGF | Gastroenterology6 | |
| Lung | Lung cancer | AEC, E14.5 lung single-cell suspension | In vivo Kidney capsule | NA | NA | JCI7 |
Abbreviations: IBD, inflammatory bowel disease; hESC, human embryonic stem cells; hPSC, human pluripotent stem cells; iPSC-LB, induced pluripotent stem cells- liver buds; iPSC-Hes, induced pluripotent stem cells-hepatic endoderm cells, HUVECs, human umbilical vein endothelial cells; hMSCs, human mesenchymal stem cells; hbFGF, human basic fibroblast growth factor, hBMP4, human bone morphogenetic protein 4; HGF, human growth factor; FGF2, fibroblast growth factor 2; RA, retinoic acid; BMP2, Bone morphogenetic protein 2; Wnt3a, wingless-type MMTV integration site family member 3A; FGF4, fibroblast growth factor 4; EGF, endothelial growth factor; AEC, alveolar epithelial cells.
References:
Lancaster MA & al. Nature. 501, 373-379 (2013)
Takebe T & al. Nature. 499, 481-499 (2013)
Xia Y & al. Nature Cell biology. 15, 1507-1516 (2013)
Meyer JS & al. PNAS. 106, 16698-16703 (2009)
Spence JR et al. Nature. 470, 105-108 (2011)
Sato T & al. Gastroenterology. 141, 1762-1772 (2011)
Chapman et al. JCI. 121, 2855-2862 (2011).
Whilst the referenced organoid models could revolutionize the tissue engineering and regenerative medicine fields by restoring or replacing diseased organs, we foresee novel opportunities to use organoids to study bidirectional regulatory feedback that exists between tumors and their supporting microenvironmental ECM. Among just a few of the examples, one could imagine an ex vivo study of organ-specific malignancies (i.e. brain cancer, colon cancer, etc.) using surrogate organoids that have been corrupted by site directed mutagenesis or less-specific radiation-induced genomic damage to produce tumors. Resulting 3D tissue/tumor hybrids could then be used to screen promising drug candidates, to study metastasis to and from the organoid, and to determine stromal growth factors necessary for tumor maintenance.
2.3 Biologically derived 3D substrates
Despite the advantage spheroids and organoids have over their 2D counterparts, it can be challenging to model their interaction with surrounding ECM, as most are devoid of supporting cells (endothelial cells, fibroblasts, etc.) or soluble growth factors. In an attempt to overcome those limitations, 3D models of ES have integrated naturally occurring substrates derived from human tumors, elements of the basement membrane, and/or gels rich in collagen or laminin [21, 95-98]. By adding back key elements of the surrounding ECM, one can also invoke membrane-bound integrin signaling and, thereby, activate a diverse array of downstream signaling cascades including those responsible for angiogenesis [95, 96, 99], cancer cell motility [97, 100], and drug sensitivity [101, 102].
Of those mentioned above, collagen-supplemented 3D matrices predominate and have been used extensively in several models of metastasis and cancer cell migration that require interaction between cancer cells and physiological cross-linked networks of collagen [103-107]. Collagen 3D matrices can be reproducibly manufactured and modified by a number of factors including their source, crosslinking chemistry, pH, temperature, and monomer concentration without affecting their microstructure. However, minor changes in these factors can significantly alter the resulting matrices and lead to inconsistent results from one laboratory to the next. To minimize these environmental variations and reduce one's reliance upon ill-defined biological derivatives that can lead to irreproducible results, an attractive alternative is to fabricate the tumor niche directly [108].
3. Engineering the tumor niche
The tumor niche, i.e. local microenvironment composed of stromal cells and ECM, plays a well-recognized role in cancer development [109-111]. And just as the niche can influence malignant cells, tumor cells can alter the physical, biochemical, and biomechanical properties of their surroundings to reinforce their malignant phenotype [112-117]. The resulting dysregulated ECM can promote cancer progression by facilitating malignant transformation, local tissue invasion, and subsequent metastasis [118]. Additionally, tumor-induced anomalies in the ECM can alter the behavior of stromal cells and lead to angiogenesis and inflammation that generates a tumorigenic microenvironment [119-135]. An in-depth understanding of the dynamic interplay between a tumor and the niche in which it's found will be critical in the effort to develop innovative antineoplastic therapies that act upon the tumor niche and deprive tumors of oncogenic stimuli.
As a method to delineate the most important interactions between a cancer and its tumor-associated microenvironment, particularly ones that profoundly influence a cancer cell's response to biologically targeted therapy, our laboratory has adapted methods normally employed in the field of regenerative medicine [54]. Surprisingly, the simple transfer of ES cells from a monolayer to 3D scaffold resulted in striking changes in ES cell morphology, behavior, growth kinetics, and sensitivity to antineoplastic drugs. Though we have taken just the first few steps to understand how the tissue-engineered scaffolds achieved those profound effects, we anticipate a tissue-engineered bone tumor niche will better mimic human ES tumors [136-139] and retain many of the advantages inherent in ex vivo preclinical cancer models.
3.1 Tissue-engineered 3D scaffolds
As applied to oncology research, the main purpose of tissue-engineered 3D scaffolds is to recapitulate essential architectural, mechanical, and biochemical elements of the tumor microenvironment in a way that promotes tumor cells to behave as they naturally would if present within a human tumor [140-144]. Also, the tissue-engineered 3D scaffolds should support physiological exchange of nutrients, oxygen, and metabolic waste byproducts, and ideally be compatible with standard experimental techniques (e.g. microscopy, immunohistochemistry, cell proliferation assays). While no scaffold material achieve all of those traits, several have proven useful for oncology research [145].
Defined generically as three-dimensional solid or porous biomaterials conducive for cell growth, the scaffolds can be native or synthetic, permanent or biodegradable, and can vary by other traits, such as porosity or surface functionality that influence their appropriateness for specific preclinical models (Table 3). Moreover, the methods used for fibrous scaffold fabrication can also determine the level of control scientists have over scaffold architecture, with more disordered weaving, knitting, braiding, and electrospinning techniques on one end of the spectrum (defined in Table 4), and well-ordered computer controlled 3D-printed scaffolds on the other. Though less common, scaffolds intended for implantation into animal models must also be safe to the host and biocompatible with the site in which it is inserted. Though a comprehensive discussion of the myriad scaffolds types is beyond the scope of this publication, several reviews cover this topic extensively [146, 147].
Table 3.
Methods, factors and biomedical application of scaffolds
| Method | Examples | Polymers | Properties | Application |
|---|---|---|---|---|
| Biodegradable porous scaffold: polymeric porous scaffolds with homogenous network | Casting, leaching and foaming methods | PLLA, PLGA, PDLLA, collagen, etc. | Controlled structure & production | Drug delivery, bone & cartilage tissue engineering |
| Fibrous scaffolds: mimicking the architecture of natural human tissue at the nanometer scale (Nano, micro & nonwoven fiber) | Electrospinning, self-assembly, & phase separation | PCL, PGA, PLA, PLGA | Biomechanical and biocompatible high surface area | Tissue engineering, drug delivery & wound healing |
| Hydrogel scaffold: shape-retentive polymeric network swollen with a high percentage of water | Microfluidics, micromolding, photolithography, & emulsification | PGS, PEG, PDMS, & Silicon PMMA, HA, PEG, Alginate PMMA, PAA, Fibronectin, chitosan Collagen, gelatin & HA | Biological, mechanical, & physical complexity of structure, shape & size | Microdevices, biochips, cell-based microreactors, etc. |
| Microsphere scaffold: prepared from a large variety of biodegradable materials, enabling easy control of porosity and pore interconnection | Thermal induction Particle aggregation Solvent evaporation Freezing & drying | PEG, PLLA Chitosan, HAP PLGA & PLAGA Collagen, PLGA, Chitosan | Highly porous for cell transplant Mechanical stability High cellular density Durable & flexible structure | Bone tissue engineering |
| Ceramic scaffold: useful due to their similarity to bone mineral & their osteo-conductivity and biocompatibility | Sponge replication Calcium phosphate coating | TCP, BCP, PU sponge, calcium phosphate, PLGA, PS, PP, collagens, silk and hair fibers, etc. | Enhanced biocompatibility and bioreactivity | Bone tissue engineering & orthopedic application |
| Functional scaffold: delivering of substances inducing cell growth | Growth factors, hormones and ligands release | Alginate, gelatin, collagens, fibrin, PLGA, PLA, etc. | Variable structure: hydrogels, membranes, microspheres, foams & membranes | Endothelium interaction, tumor vascular interactions, bone regeneration & wound healing |
| Acellular scaffold: elimination of the cellular composition without affecting the composition, mechanical integrity and biological activities of the remained ECM | Decellularisation | Biological organs (e.g. lung) | Retain biomechanical properties, anatomical structure & native ECM | Tissue engineering |
| “Tissue scaffold”: assist in the production of ECM, and possible integration with in vivo tissue growth | Robotic & automated deposition of cells in 3D space | Tubular collagen gel Sodium alginate | Layers deposition of ECM or cells Multicellular composition reconstituting tissues | Printing 3D organs, acellular polymeric scaffolds & biochips development |
Abbreviations: PLLA, polylactide; PLGA, poly lactic-co-glycolic acid; PDLLA, poly-D,L-lactide; PCL, Polycaprolactone; PGA, polyglycolide; PLA, polylactide; PGS, poly glycerol-sebacate; PEG, Poly ethylene glycol; PDMS, Polydimethylsiloxane; PMMA, polymethyl methacrylate; HA, hyaluronic acid; PAA, Poly acrylic acid; PLAGA, poly-lactide-co-glycolide; TCP, Tricalcium phosphate; BCP, Biphasic Calcium Phosphate; PU, Polyurethane; PS, Polystyrene; PP, Polypropylene
Table 4.
Fibrous scaffold fabrication methods
| Method | Definition |
|---|---|

|
Fibers are drawn under an applied electric field and deposited on a surface forming a fibrous scaffold |

|
Fibers are connected in series of loops |

|
Fibers are braided by intertwining three or more fiber strands |

|
Fibers are weaved by interlacing warp and weft fibers in perpendicular directions |
Naturally, given this wide assortment of scaffold options, several have proven value in culturing malignant tumors. This includes poly(ε-caprolactone) (PCL), polylactide (PLA), polyglycolide (PGA), and co-polymers (PLGA: poly lactic-co-glycolic acid), which are biodegradable and able to form various structures such as fibers, mesh, sponges (Table 5) [148, 149]. Cancer cell lines have been shown to adhere and grow on these synthetic scaffolds and appear capable of forming 3D structures that are morphologically and histologically similar to in vivo tumors [54, 150-152]. Our own laboratory has employed electrospun PCL as the preferred biomaterial to model the bone niche and found it useful to study ES cell growth kinetics, drug sensitivity, and mechanisms of acquired drug resistance [54]. In our hands, cells attached to the PCL fibers and proliferated throughout the uppermost portion of the scaffolds, reaching a maximum cell number of about 300,000 cells/scaffold and depth of 150μm.
Table 5.
Properties of polymers used for bioengineered substrates
| Polymer | Biological Activity | Biodegradation Rate (Months) | Biomechanical properties | Area of application |
|---|---|---|---|---|
| PGA | Inert: anticoagulant, antiviral & plasma cleaning | 6 to12 | Compressive and flexible Tensile strength 339-394 MPa | Tissue engineering in orthopedics |
| PLLA | Inert: improvement of tensile & suture, injectable form | >24 | Tensile modulus 1.2-16 GPa | Orthopedic & HIV infection |
| PLDLA | Inert: stimulate regeneration of the whole meniscus | 12 to 16 | Tensile modulus 1.9-2.4 GPa | Orthopedic |
| PCL | Inert: bone & cartilage repair | >24 | Compressive & modulable Tensile modulus up to 340-360 MPa | Suture coating, dental & orthopedic implants |
| PEU | Inert: bio-and blood-compatible materials | 1-2 | Tunable: 5-40 MPa | Cardiovascular devices, artificial organs, tissue replacement, & in vivo restoration of body joints |
| HA | Inert: angiogenesis, vehicle for osteogenesis, osteoconductive | 1-2 | Tensile strength 40-100 MPa Bend strength 20-80 MPa Compressive strength 100-900 MPa | Bone graft |
| PLGA | Inert: delivery of small molecule drugs, proteins and other macromolecules in commercial and research applications | 1-12 | Compressive & strength Highly tunable | Drug delivery carrier As scaffolds for tissue engineering |
| PLGA-Collagen | Active: transdermal delivery | 1-12 | Compressive & strength | Skin tissue engineering, bone substitutes, & artificial blood vessels and valves |
| Collagen | Active: nanoparticles for gene delivery and basic matrices for cell culture systems | Hours | Elastic modulus of native fibrils around 9 GPa | Gel formulation with liposomes for sustained drug delivery. |
Abbreviations: PGA, polyglycolide; PLLA, polylactide; PLDLA, Poly-L/D-lactide; PCL, Polycaprolactone; PEU, Polyesterurethanes; HA, hyaluronic acid; PLGA, poly lactic-co-glycolic acid
Although the parameters we selected to culture ES within PCL scaffolds would almost certainly need to be adapted if used to model other sarcoma subtypes, this study provides proof of concept that ES cells can attach and grow on unmodified microfiber scaffolds that lack ancillary bioactive moieties (Figure 4). It also brings to light several common findings we have encountered when growing osteosarcoma and ES cells within 3D PCL scaffolds. First, when grown in static culture ES cells eventually reach a maximal cell number and depth of penetration, with preferential growth in the uppermost section that is well oxygenated and more accessible to nutrients [153-158]. Under static conditions, oxygen and nutrient levels drop dramatically as distance from the scaffold surface increases, and viable cell growth is often limited to only about 200 μm [159, 160]. After micropipetting ES cells onto 3D PCL scaffolds, they start to proliferate and eventually migrate throughout this oxygenated zone until a maximum density is reached. More deeply seeded cells within the scaffold will be under metabolic stress and exhibit slower proliferation rates.
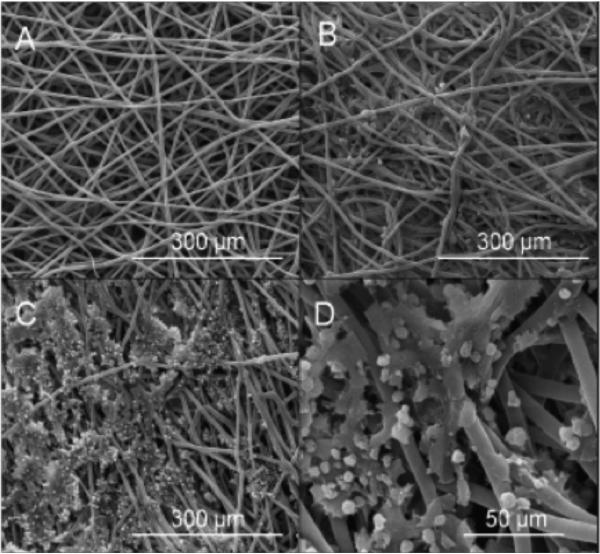
Figure 4. Scanning electron micrographs of PCL microfiber scaffolds.
(A) before cell seeding, (B) after 2 days in static ES cell culture, (C&D) after 24 days in static ES cell culture. After 24 days of static culture, ES exhibit significant 3D cell-cell and cell-matrix contacts.
As cell number and ECM deposition increases, uneven distribution of nutrients, oxygen, and metabolic waste by-products can emerge even in the uppermost 200 μm of the scaffolds. This, in turn, promotes cell apoptosis/death within unfavorable pockets within the scaffold – a problem exacerbated by the high metabolic demands of cancer cells [161-163]. As previously reported, diffusion gradients within tumor spheroids have been shown to produce this type of varied cell structure and are widely used to study the growth kinetics and hypoxic effects of tumor cells present within avascular tumor micro-regions within irregular tumor vasculature [72, 164, 165]. When grown under static growth conditions, ES impregnated PCL scaffolds could, therefore, equally serve to mimic the varied stress and oxygenation gradients experienced by ES tumors.
A second notable finding in our scaffold-based model of ES was that cell proliferation within PCL scaffolds more closely approximated the growth kinetics of human tumor xenografts, which were both significantly less than monolayer cultures [54]. As expected, since the majority of cytotoxic chemotherapies act in common upon cells undergoing DNA synthesis or cell division (i.e., actively progressing through the cell cycle), the faster mean doubling time of ES cells cultured as monolayers (24 hours) tends to overstate the true clinical activity later determined if/when a drug reaches the clinic [166]. Thus, if for no other reason than accurately modeling antineoplastic activity in the preclinical setting, scaffold-based cell culture appears to be advantageous over monolayer-based options [167].
In actuality, however, scaffold culture not only affects the proliferative rate but also profoundly impacts the ES morphology, internal signaling patterns, and oftentimes the response to biologically targeted therapies that have become increasingly common for the treatment of all cancer types. Illustrating this fact, our laboratory confirmed that ES cells acquire an in vivo-like round cell morphology when they are placed within PCL scaffolds and continue to express immunohistochemical biomarkers normally expressed by human ES tumors (CD99+, IGF-1R+, keratin−, and SMA−) [54]. Though more extensive testing is required in other pathways, the proteomic expression profiles along the IGF-1R/PI3K/mTOR signaling pathway, as measured by Western blotting and flow cytometry, would suggest that 3D PCL scaffolds and murine xenografts can reliably mimic critical signaling cascades of known importance in human ES tumors, a finding also observed in breast cancer cells grown in 3D cultures [168]. Taken together, an engineered ES tumor model reliant upon electrospun PCL fibers scaffolds appears to adequately mimic the morphology, growth kinetics, and protein expression profile of human tumors. Ultimately, one of the most important questions to be resolved is whether the 3D model shares enough fidelity with its human counterpart to advocate its use in a preclinical high-throughput drug-testing platform.
3.2 Exogenously derived ECM that supplements tissue-engineered scaffolds
Though the aforementioned experience culturing ES cells as spheroids or 3D scaffolds was limited to growth upon unmodified surfaces, they serve to lay the groundwork in our understanding of how ES and other cancerous cell types intrinsically interact with a spatially complex biologically inert microenvironment. As these models fail to adequately represent the richer complexity of human biology of proven importance for cancer growth, invasion, and metastasis the next challenge would be to add back key components of the ECM to inert scaffolds and/or employ decellularized biological tissues devoid of cells to isolate the specific effects induced by the residual ECM [136, 169-175].
With respect to the former approach, in which biological ECM supplements the tissue-engineered microenvironment, both Matrigel (a poorly defined gelatinous complex of proteins derived from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells) and peptides rich in Arginine-Glycine-Aspartic acid (RGD) motifs have been widely reported to affect cell behavior and alter cell self-organization. Matrigel, for example, can induce interconnected capillary-like networks of endothelial cells that would not otherwise form in monolayer culture and, interestingly, ES-secreted VEGF165 enhances this process. As a specific recognition site of integrins for their respective ligands, RGD is a ubiquitous biomimetic peptide present within plasma and ECM that is capable of promoting cell motility and stimulating adhesion between tumor cells and their supporting stromal cells and/or ECM. A number of excellent publications exist that expand on the important roles Matrigel and RGD motifs play in the tumor microenvironment [176-178].
With respect to ES and the other sarcoma subtypes, little is known about the ex vivo cell-derived ECM and this inquiry remains a burgeoning avenue of investigation. Several reports have described the composition of fibronectin, laminin, and collagen in ES cell lines and/or clinical samples [179-181]. Vijayakumar et al., for example, demonstrated low levels of beta-catenin (Wnt-pathway) in monolayer ES cultures [182]. Others have reported ECM/scaffold-related effects of the E-cadherin expression, with high expression by ES cells grown within scaffolds, intermediate expression in spheroids, and low expression in monolayer cultures [53]. Since E-cadherin-dependent co-expression of ErbB4 in ES spheroids appears to up regulate the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, and secondarily resistance to anoikis and cytotoxic chemotherapy, it is intriguing whether a similar phenomenon is occurring in our 3D PCL-based scaffold architecture [183]. ECM deposition by human tumors can clearly induce an adverse autocrine effect that promotes malignant cell invasion and metastasis. Thus, the ability to mimic this tumorigenic regulatory loop in ex vivo models of the tumor microenvironment may advance our understanding of dynamic interplay that occurs between tumors and their surrounding ECM, and potentially aid in the development of drugs that target the tumor microenvironment [184, 185].
Accurately isolating the effects of tumor-associated ECM from the effects of surrounding stromal cells would be nearly impossible using conventional 2D or xenograft preclinical models but can readily be accomplished in tissue-engineered systems. Decellularized connective tissues, usually obtained after perfusion with detergents, provide an example of this approach and have recently gained in popularity for cancer research given their unique ability to evaluate the oncogenic effects of naturally derived ECM. The effects of bone-specific ECM has been reported using decellularized PCL scaffolds that had originally supported osteogenic cells [186], and direct chemical conjugation of specific ECM proteins or proteoglycans to the surface of PCL fibers can allow controlled release of high-affinity ligands (e.g., heparin sulfate binding growth factors or cytokines) from the 3D scaffolds [187, 188].
4. Applications for cancer biology and preclinical drug testing
Though a longstanding goal of tissue engineering has been to fabricate artificial tissues and organs capable of replacing diseased human tissues, as of yet, there are still no FDA approved substitutes for major organ systems. Nevertheless, considerable progress has been made and many of the principles used to further that aim have been adapted to model the human tumor niche. As discussed previously, our laboratory has had the greatest success using electrospun scaffolds composed of PCL, a resorbable aliphatic synthetic polymer that has been extensively used by the tissue engineering and the biomedical community. It offers a number of highly desirable properties, including the following: (a) lacks intrinsic biological or biochemical activity, (b) is inexpensive (c) is readily manipulated to produce fibers of precise diameter, (d) has superior rheological and viscoelastic attributes, (d) is considered safe by the FDA, and (e) exhibits very slow in vivo degradation, which can be finely tuned using copolymer blending to achieve the intended biological effect. Both normal and cancerous cells adhere to PCL, and when electrospun, non-woven mats of 10 μm diameter PCL fibers can be made to resemble the native ECM [189], including physiological bone trabeculae and the bony ES tumor niche [54]. Perhaps most important for our applications, micrometer size electrospun PCL fibers provide the requisite porosity to enable cell infiltration and are compatible with flow perfusion bioreactors [190-195]. As specifically used to model ES, the 3D pattern of electrospun fibers appear to induce pronounced changes in ES cell morphology, growth kinetics, and expression of cancer-related pathways, which in turn, promotes chemosensitivity patterns more closely observed in xenografts and human tumors. Excellent reviews exist that highlight the broad applicability of PCL for tissue engineering, drug delivery, and medical devices [196, 197].
4.1 Morphologic characterization of 3D tumor models
Despite the aforementioned benefits, PCL 3D scaffolds also have their drawbacks. Cells grown within 3D scaffolds can be difficult to dislodge; isolation of RNA, DNA, and protein can be difficult; and low melting temperatures (60°C for PCL) preclude standard tissue embedding with paraffin. And as a class, polymeric 3D scaffolds are usually opaque and poorly amenable to routine light microscopy even when polystyrene or other clear substrates are used. Naturally, this poses a major challenge to monitoring cell morphology and proliferation in real time, which in turn, impairs ones ability to continuously monitor the health and viability of cultured cells. This disadvantage can be mitigated to some extent by simply culturing ES cells on multiple scaffolds concurrently then sectioning some at various time intervals. Once sectioned, they can be evaluated by scanning electron microscopy (SEM) or immunohistochemistry (IHC). Flow cytometry (FC) can be used to complement SEM and IHC analyses but, as with those techniques, interrupts the experiment in the course of completing the analysis (Figure 5). Since the cells are removed from the scaffolds prior to analysis, one cannot evaluate the spatial interrelationship of the cell-cell interaction. Theoretically, the cells extracted from scaffolds for FC analysis could be handled aseptically and replaced within a new scaffold but, in practice, this is uncommon and only performed in the rare case where a distinct subset of cancer cells is required.
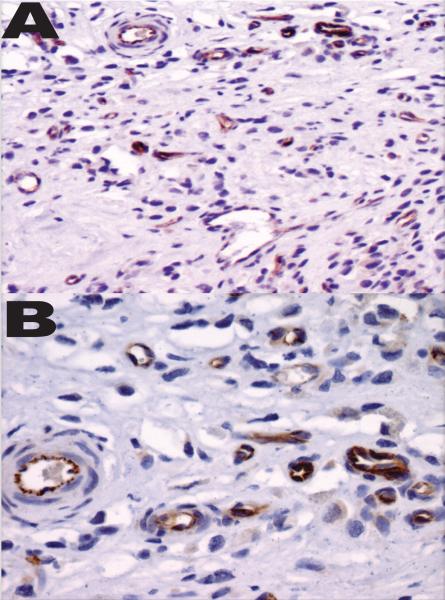
Figure 5.
IHC staining of human ES tumor for VE-Cadherin under low-(A) and high- power (B) magnification confirming the coexistence of EC.
4.2 3D models used to assess chemosensitivity
Though cell culture monolayers will continue to offer value to the cancer research community, particularly when used in high-throughput drug screening programs, the data resulting from their use can prove unreliable, or worse, contradictory to what one eventually observes when tested in xenografts or human tumors. Emblematic of this limitation, the NCI-60 (a well characterized set of 60 diverse cancer cell lines) has been extensively used to identify novel antineoplastic agents but poorly predicts whether any single drug will be effective in treating specific cancer type. While this incongruence may, to some extent, stem from artificially high cell division rates that overestimate the benefit of cytotoxic chemotherapies, marked phenotypic variation between monolayer and 3D culture systems (and human tumors) provides another explanation [53, 54]. In support of this hypothesis, the proteomic signature of human tumor spheroids and 3D scaffold models can be readily distinguished from equivalent cells maintained in monolayer culture. This appears to hold true for ES as well, as cells grown within spheroids or 3D scaffolds (natural and synthetic) more closely approximate the protein expression pattern of human tumors than do cell monolayers cells [53, 54, 80, 86, 90].
Drug companies have taken note and many have added 3D cell culture models to their preclinical testing pipeline while continuing to rely extensively upon xenograft testing to determine a drug candidate's activity, toxicity, and pharmacokinetic profile (i.e., absorption, distribution, metabolism, and elimination). As biologically targeted cancer therapies continue to garner a large share of the drug marketplace, one can anticipate an even greater need for preclinical culture models that more accurately replicate the signaling pathways responsible for cancer progression.
4.3 3D models amenable to high throughput drug screening
Toward that end, a number of techniques—such as hanging drops, micro-etched nano-cultures using NCP, and liquid overlay methods—are among just a handful capable of producing uniform ES spheroids amenable to high throughput drug screens (HTS). Although there is no consensus on what qualifies as a HTS, robotic automation of plate handling and drug administration has made it easier than ever to screen hundreds, if not thousands, of compounds per week in commercial scale laboratories. Such mechanization has also permitted complementary methods, such as RNA interference, that can be used to identify putative mechanisms of resistance. Our laboratory, as an example, has performed spheroid-based HTS in 384-well micro-etched NCP to identify several likely mechanisms by which ES evade mTOR inhibitor activity.
As expected, the use of HTS is critically dependent biometric measurement of drug effects, and at least four main types of assays have emerged for that purpose: (i) assays that measure morphological changes in spheroid volume using automated or semi-automated imaging, (ii) cytotoxicity and cell viability assays that measure cell membrane integrity or intracellular metabolic activity under robust detection readouts (e.g., absorption, luminescence, and fluorescence), (iii) apoptosis assays that reliably assess drug-induced apoptosis and cell death, and (iv) gene expression assays that evaluate specific phenomena like metastatic potential.
5. Future directions
As discussed in the introduction, 3D models ES come in two major varieties: cell aggregates capable of intrinsic self-organization (i.e., tumor spheroids) and multidimensional cell aggregates that grow within artificial substrates. The latter implementation should be especially well suited to answer scientific questions that relate heterotypic cell-cell interactions and provide a unique opportunity to explore autocrine or paracrine effects upon oncogenesis, angiogenesis, metastasis, and immunomodulation. Advances in biomaterials, such as scaffolds and gels, have provided the essential building blocks necessary to recreate selected aspects of the tumor microenvironment.
5.1 Heterotypic co-cultures
Though the ES cells grow as monotonous sheets that lack features of intrinsic self-organization (ductal structures in breast cancer, for example), microscopic review of ES tumors finds other connective tissue cells (fibroblasts and adipocytes), inflammatory cells (lymphocytes, macrophages, natural killer cells), and vascular cells, including pericytes and endothelial cells (Figure 5). Together with the associated ECM, the tumor microenvironment has been identified as a major factor influencing tumor survival, growth, invasion, metastasis, and response to chemotherapy [50, 198-202]. Because these heterotypic cell interactions, and surrounding ECM, can be selectively introduced into the tissue-engineered 3D scaffolds they serve as ideal platform to study the dynamic effects of cell-cell and cell-ECM contact.
5.1.1 ES-MSC co-culture for modeling Ewing sarcoma
As reported earlier, a wealth of information suggests ES originates from a single primitive MSC that acquired a tumorigenic EWSR1/FLI1 translocation, and as such, this cell type has been extensively used to elucidate the genetic perturbations that promotes malignant transformation [17, 20, 21, 198-201, 203-206]. Equally well-documented, ectopic expression of the resulting EWS/FLI1 fusion protein in permissive MSCs is necessary but insufficient of inducing malignant transformation without co-expression of other dysregulated proteins [207].
Given these facts, and taking into account the profound effects that the cell culture microenvironment has upon the collection, purification, and differentiation of human MSC, confusing or frankly incorrect scientific conclusions could be reached by studying EWSR1-FLI1-transfected hMSCs in monolayer cultures devoid of the relevant 3D architecture, ECM, growth factors, and support cells [208]. Our own experience, albeit starting from pre-established ES cell lines, demonstrated the simple transition from monolayer culture to biologically inert PCL scaffolds induced salient changes in the very same proteins (IGF-1, for example) that are required for malignant transformation. This would suggest that, at the very least, one should consider a more physiological bone tumor niche, whether natural or processed, in prospectively deciphering the critical interchange that exists between EWS-FLI and its coconspirators.
5.1.2 ES-EC co-culture and angiogenesis modeling
Endothelial cells are indispensible to the healthy formation of rich vascular networks, which occurs through a process of angiogenesis, vasculogenesis, and/or tumor cell vascular mimicry [209]. To mimic those processes within a synthetic tissue grown in the laboratory, and thereby improve tissue viability and function, ECs have been used in a wide range of tissue-engineering applications. Unexpectedly, even before a mature vascular network is formed, ECs can affect co-cultured cells and influence the surrounding microenvironment. When mixed with MSCs, for example, ECs enhance osteogenic matrix production within 3D PCL scaffolds [210, 211].
As expected, EC-mediated angiogenesis/vasculogenesis also plays an incredibly important role in tumorigenesis. As occurs for other tumors, ES tumors have been shown to possess the capacity to regulate their own survival and appear to do so by secreting soluble vascular endothelial growth factor (VEGF) that secondarily recruits bone marrow derived cells, pericytes, and endothelial cells into ES tumors [198-201]. A similar phenomenon occurs in preclinical models of ES, as co-culture of human EC and ES cells within an ECM gel can induce vascularized endothelial tubes [50]. Indirectly tied to VEGF expression, the insulin like growth factor-1 receptor (IGF-1R) has also been implicated in the modulation of angiogenesis and vasculogenesis [212] and dual targeting of IGF-1R and its ligand elicits anti-angiogenic effects [50].
To our knowledge, ECs have not been used in co-culture within ES cells within 3D scaffolds but this system would be expected to provide a unique opportunity to decipher the two-way feedback that exits between these cell types. A step in this direction, human MSCs, which could serve as a precursor for ES, and human umbilical vein endothelial cells (HUVECs) were co-cultured in 3D PCL-scaffolds under conditions of flow perfusion. Superior proliferation of both cell lines was achieved and the spatial distribution was more uniform throughout 3D-PCL scaffolds [213]. Since VEGF secretion by human ES tumors has been shown to encourage CD38- primitive MSCs to migrate from the bone marrow to the tumor, where they differentiate into ECs and/or pericytes capable of angiogenesis and vasculogenesis [198-201], it would be intriguing to know whether a similar effect could be replicated within a tissue-engineered ES model. If achievable, one could directly measure the effect of ES-produced IGF-1 and VEGF ligands upon ex vivo vasculogenesis and possibly use this novel model to develop and test antineoplastic agents that intercede with the ES's ability to self-regulate their microenvironment.
5.1.3 Use of the 3D ES model to study immune-mediated therapy
Immune cells, including tumor-infiltrating lymphocytes, macrophages, dendritic cells and NK cells, can either maintain or suppress ES tumor growth depending upon the context in which they interact [214-219]. And though beyond the scope of this review, a handful of biologically targeted therapies designed to stimulate the immune system—either directly or by down-regulating immune checkpoints such as programmed cell death 1 (PD1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4)—have generated incredible acclaim for their remarkable antineoplastic activity in appropriately selected patients. While it remains to be determined if those promising results would hold up when eventually applied to patients suffering from ES, this has become a robust area of research.
NK cells, expanded in vitro, have been shown to provoke significant anti-tumor effects in mice engrafted with ES cells, and in rare instances completely eradicated the disease [216]. Activated NK cells, more so than resting ones, have also shown immunoreactivity in ES tumors [217]. Yet, the regulation of NK cell cytolytic activity is complex and induces both activating and inhibitory receptors as well as induced tolerance to specific self-receptors. A refined understanding of these signals could empower novel therapies for ES.
As a platform for investigating the role of immune cells in suppressing ES tumor growth, one could envision co-culturing ES cells with the specific immune cell of interest (e.g. NK cells) within a PCL based tissue-engineered scaffold. Though the full complexity of the human and/or mouse immune system can't be modeled in its entirety ex vivo, the exceptional flexibility of the system and greater control of the ES-immune cell interactions may outweigh some of its limitations. There are a number of approaches one might take: (i) utilization of allogenic killer immunoglobulin-like receptor (KIR) incompatible NK cells, (ii) blockade of KIR with mAb, (iii) transfection of NK cells to express tumor reactive T-cell receptors (TCRs) or chimeric antigen receptors (CARs), and (iv) sensitization of ES tumor (or cells) to NK cell killing.
The successful activation of Cytotoxic T-cells (CTL) from healthy human peripheral blood mononuclear cells (PBMC), using a modified EWS-FLI1 peptide, and potent killing of cells bearing the EWS-FLI1 translocation provides renewed hope in developing T-cell mediated immunotherapy for ES treatment [220].
5.2 Multi-organ tissue-engineered systems to study metastasis
Most ES-related deaths occur when malignant cells have metastasized to the lung, grown within that location, and developed de novo or acquired resistance to chemotherapy. Although primary thoracic ES tumors (i.e. Askin tumors) do occur and can spread to adjacent lung tissue by direct extension, the vast majority of ES cells can more reasonably be expected transit to the lung after first leaving the primary site (e.g. bone tissue or less common extraskeletal tissues), entering the circulatory system, and adhering to pulmonary endothelium (Figure 6). Despite the deterministic nature of these steps, little is known about the cellular, biomolecular and environmental factors that explain the predictable nature in which ES cells appear in the lung, at least microscopically, very early in the disease course. For this reason, all patients diagnosed with ES are universally provided chemotherapy with the dual goals of shrinking the macroscopic, clinically detectable tumors and eradicating micrometastatic deposits that are assumed to exist within the lung even when small tumors are effectively treated by surgery or radiation.
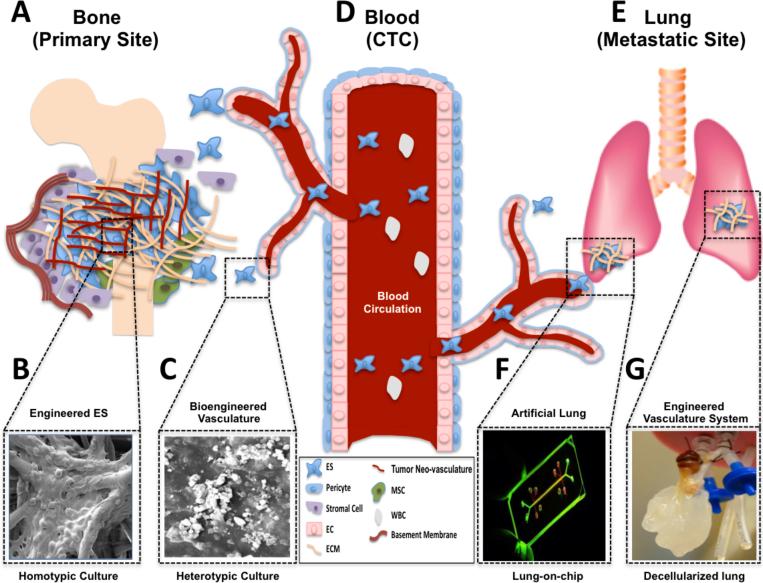
Figure 6.
Bioengineered models that mimic ES microenvironment at the primary, hematogenous and secondary sites. (A) Bioengineered preclinical models of ES interacting with osseous-like 3D scaffold (B) or synthetic vasculature (C). (D) Vascular system by which ES disseminate to lung. (E) Lung metastases modeled lung. (F) Lung-on-chip or (G) Decellularized lung. Abbreviations: CTC, circulating tumor cells; EC, endothelial cell; ECM, extracellular matrix; MSC, mesenchymal stem cell; and WBC, white blood cell.
Though ES cells intrinsically harbor the capacity to metastasize and can gain even greater metastatic potential by acquiring genetic aberrations, the surrounding stromal cells and ECM are critical partners that influence this continuum from bone to lung. In teasing apart the metastatic triggers intrinsic to the cancer cell from extrinsic ones ascribed to microenvironmental effects highlighted in Figure 1, tissue-engineered tumor models of the bone and pulmonary sites afford scientists previously unimaginable experimental control to adjust biomechanical forces, the signaling milieu, and metabolic stresses. Though not previously used to study ES metastasis, a number of tissue-engineered and/or ex vivo regenerated lung tissue models have been shown to mimic functional lung, and one could envision a closed system that uses microfluidic channels link tissue-engineered bone and lung tissues together in a way that mimics the complete pattern of hematogenous spread. As illustrated in Figure 6, the 3D scaffolds our group and others have reported upon can mimic the bone tumor niche, and both lung-on-a-chip [221] and decellularized lung models [222-227] appear to be conducive to the adhesion and growth of cancer cells. Thus far, only non-small cell lung cancers (NSCLC) have been implanted within these tissue-engineered lung models, however unpublished reports by Dr. Min Kim et al. (personal communication) indicate ES cells readily form distinct pulmonary nodules when placed within decellularized rat lungs that are maintained under conditions of flow perfusion previously used to grow NSCLC [228]. As a final piece of the puzzle, Ingber et al. demonstrated that miniaturized tissue-engineered organs grown ex vivo can be linked in series to mimic the physiological vascular communications that exist in the human body, so it stands to reason that similar methods could be adapted to join synthesized bone and lung together as an innovative system to study ES metastasis.
Though considerable effort will surely be required to move beyond theoretical possibilities, and recognizing that even the best ex vivo systems lack important factors inherent in living organisms, multi-organ tissue-engineered systems present an enticing chance to interrogate, and hopefully thwart, the critical factors that make the lung microenvironment such a hospitable place for ES and other sarcoma subtypes to grow.
5.3 Personalized Therapy
Given the relative rarity of ES and further molecular sub-classification of patients by their tumor's translocation type and proteomic signature [22-31, 229], it is impossible to adequately test all drugs or rational drug combinations in each patient subset [230]. A partial solution to this problem has been to extensively profile each patient's tumor to identify dysregulated proteomic pathways, genomic mutations, or other ‘-omic’ aberrations responsible for tumorigenesis and to isolate mechanisms of de novo and acquired drug resistance [231-233]. This approach, however, must eventually be validated using drug candidates in xenografts or cell lines that truthfully mimic the human tumors from which they derive. Though simple in theory, this latter step has remained a challenge in practice, as pre-established xenografts and/or cell lines devolve over time and eventually lose the phenotypic traits originally present within the respective tumors from which they'd been taken.
In an effort to maintain the intimate link between clinical tumor samples and the derived xenografts and/or cell lines, academic laboratories and pharmaceutical companies alike have made major investments establishing primary cell lines and PDX from patients that have carefully annotated clinical response data available. To ensure PDX maintain a high correlation with the source tumor, early generation explants are used before they lose the surrounding human-derived ECM, considered essential for maintaining fidelity with their human counterparts. Similarly, 3D primary cell culture models are being developed to mirror the native ECM and architectural structures present within human ES tumors in the hopes those elements will preserve, or at least prolong, a differentiated phenotype that is truly representative of the original ES tumor. Admittedly, the scientific community has much less experience growing primary cell lines as spheroids and very few laboratories have the specialized expertise necessary to successfully culture ES cells within tissue-engineered 3D tumor microenvironments.
The limited 3D tissue-engineered tumor models that do exist lack standardization and may need to incorporate subtle changes in the fabricated scaffolds to enable primary cell culture of different cancer types. Thus, a one-size-fits-all tissue-engineered approach for all cancer types is unlikely and wouldn't necessarily be expected given the vast differences sarcomas and carcinomas have in their proclivity for certain metastatic sites. Sarcomas (including ES), for example, spread more commonly to the lungs and bone, whereas carcinomas usually migrate first to lymph nodes before metastasizing elsewhere. Given this affinity for one tissue type over another, one would contend that a principal advantage tissue-engineered 3D scaffolds have over spheroids and monolayer cultures is their capacity for customization to meet the unique microenvironmental needs of the cancer type of interest. Our laboratory has just begun the time-intensive task of culturing primary ES cells within 3D PCL scaffolds and continues to optimize the techniques required to maintain cell viability within an ex vivo tissue-engineered tumor niche (Supplemental Figure 1). The next step will be to correlate the expression profiles of clinical samples with their paired PDX and cell-embedded 3D scaffolds to ensure they successfully recapitulate the human ES tumors. Subsequently, we anticipate using biomimetic ES tumor models to methodically evaluate biologically targeted therapies in advance of early phase human clinical trials of the most promising drug candidates. Simultaneously, one expects the preclinical ES models will shed new light of drug resistance mechanisms and promote the use of innovative drug combinations that would not have been apparent from more primitive monolayer culture models that lack in vivo-like signaling cascades.
6. Conclusion and perspectives
Complex 3D models of human cancer (including ES) are just emerging in academic labs throughout the country and are anticipated to revolutionize the study of the tumor microenvironment. By providing new tools to manipulate the ex vivo tumor niche of both the primary and metastatic sites in ways not currently possible using murine models, tissue-engineered cancer models could serve as an ideal platform to test new cancer therapeutics. Challenges remain, particularly in scaling up these systems for HTS and adapting them for ubiquitous use by the cancer research community, but these hurdles are not insurmountable.
Supplementary Material
Acknowledgements
We are thankful to the Wells Alliance for their philanthropic support of this research. This work is supported in part by an RO1 CA151533 grant from the National Cancer Institute (NCI). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NCI or the National Institute of Health.
Abbreviations
- ESFT
Ewing's sarcoma family tumors
- ES
Ewing sarcoma
- MCTS
multicellular tumor spheroids
- 3D
three-dimensional
- PCL
poly(ε-caprolactone)
- ECM
Extracellular Matrix
- PDX
patient-derived tumor explants
- MSC
mesenchymal stem cells
- EC
endothelial cells
- NCP
Nano-culture plate
- HTS
high throughput screening
- NK cell
Natural killer cell
- IGF-1R
Insulin like growth factor-1 receptor
- mTOR
mammalian target of rapamycin
References
- 1.Balamuth NJ, Womer RB. Ewing's sarcoma. The lancet oncology. 2010;11:184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Ewing J. Classics in oncology. Diffuse endothelioma of bone. James Ewing. Proceedings of the New York Pathological Society, 1921. CA Cancer J Clin. 1972;22:95–98. doi: 10.3322/canjclin.22.2.95. [DOI] [PubMed] [Google Scholar]
- 3.Angervall L, Enzinger FM. Extraskeletal neoplasm resembling Ewing's sarcoma. Cancer. 1975;36:240–251. doi: 10.1002/1097-0142(197507)36:1<240::aid-cncr2820360127>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Askin FB, Rosai J, Sibley RK, Dehner LP, McAlister WH. Malignant small cell tumor of the thoracopulmonary region in childhood: a distinctive clinicopathologic entity of uncertain histogenesis. Cancer. 1979;43:2438–2451. doi: 10.1002/1097-0142(197906)43:6<2438::aid-cncr2820430640>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe R, Santamaria M, Yunis EJ, Tannery NH, Agostini RM, Jr., Medina J, Goodman M. The neuroectodermal tumor of bone. Am J Surg Pathol. 1984;8:885–898. doi: 10.1097/00000478-198412000-00001. [DOI] [PubMed] [Google Scholar]
- 6.de Alava E, Gerald WL. Molecular biology of the Ewing's sarcoma/primitive neuroectodermal tumor family. J Clin Oncol. 2000;18:204–213. doi: 10.1200/JCO.2000.18.1.204. [DOI] [PubMed] [Google Scholar]
- 7.Ordonez JL, Osuna D, Herrero D, de Alava E, Madoz-Gurpide J. Advances in Ewing's sarcoma research: where are we now and what lies ahead? Cancer research. 2009;69:7140–7150. doi: 10.1158/0008-5472.CAN-08-4041. [DOI] [PubMed] [Google Scholar]
- 8.Kauer M, Ban J, Kofler R, Walker B, Davis S, Meltzer P, Kovar H. A molecular function map of Ewing's sarcoma. PloS one. 2009;4:e5415. doi: 10.1371/journal.pone.0005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher CDM, World Health Organization. International Agency for Research on Cancer . WHO classification of tumours of soft tissue and bone. 4th ed. IARC Press; Lyon: 2013. [Google Scholar]
- 10.Esiashvili N, Goodman M, Marcus RB., Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. Journal of pediatric hematology/oncology. 2008;30:425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 11.Lin PP, Wang Y, Lozano G. Mesenchymal Stem Cells and the Origin of Ewing's Sarcoma. Sarcoma. 2011;2011 doi: 10.1155/2011/276463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkin DM, Stiller CA, Nectoux J. International variations in the incidence of childhood bone tumours. International journal of cancer Journal international du cancer. 1993;53:371–376. doi: 10.1002/ijc.2910530305. [DOI] [PubMed] [Google Scholar]
- 13.Riggi N, Stamenkovic I. The Biology of Ewing sarcoma. Cancer Lett. 2007;254:1–10. doi: 10.1016/j.canlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Parkin DM, Stiller CA, Nectoux J. International variations in the incidence of childhood bone tumours. Int J Cancer. 1993;53:371–376. doi: 10.1002/ijc.2910530305. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig JA. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Current opinion in oncology. 2008;20:412–418. doi: 10.1097/CCO.0b013e328303ba1d. [DOI] [PubMed] [Google Scholar]
- 16.Skubitz KM, D'Adamo DR. Sarcoma. Mayo Clinic proceedings Mayo Clinic. 2007;82:1409–1432. doi: 10.4065/82.11.1409. [DOI] [PubMed] [Google Scholar]
- 17.Riggi N, Cironi L, Provero P, Suva ML, Kaloulis K, Garcia-Echeverria C, Hoffmann F, Trumpp A, Stamenkovic I. Development of Ewing's sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–11468. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 18.Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer cell. 2007;11:421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Meltzer PS. Is Ewing's sarcoma a stem cell tumor? Cell stem cell. 2007;1:13–15. doi: 10.1016/j.stem.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Riggi N, Suva ML, Suva D, Cironi L, Provero P, Tercier S, Joseph JM, Stehle JC, Baumer K, Kindler V, Stamenkovic I. EWS-FLI-1 expression triggers a Ewing's sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res. 2008;68:2176–2185. doi: 10.1158/0008-5472.CAN-07-1761. [DOI] [PubMed] [Google Scholar]
- 21.Miyagawa Y, Okita H, Nakaijima H, Horiuchi Y, Sato B, Taguchi T, Toyoda M, Katagiri YU, Fujimoto J, Hata J, Umezawa A, Kiyokawa N. Inducible expression of chimeric EWS/ETS proteins confers Ewing's family tumor-like phenotypes to human mesenchymal progenitor cells. Molecular and cellular biology. 2008;28:2125–2137. doi: 10.1128/MCB.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desmaze C, Zucman J, Delattre O, Thomas G, Aurias A. Unicolor and bicolor in situ hybridization in the diagnosis of peripheral neuroepithelioma and related tumors. Genes Chromosomes Cancer. 1992;5:30–34. doi: 10.1002/gcc.2870050105. [DOI] [PubMed] [Google Scholar]
- 23.Zucman J, Delattre O, Desmaze C, Plougastel B, Joubert I, Melot T, Peter M, De Jong P, Rouleau G, Aurias A, et al. Cloning and characterization of the Ewing's sarcoma and peripheral neuroepithelioma t(11;22) translocation breakpoints. Genes Chromosomes Cancer. 1992;5:271–277. doi: 10.1002/gcc.2870050402. [DOI] [PubMed] [Google Scholar]
- 24.Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen PH, Lessnick SL, Lopez-Terrada D, Liu XF, Triche TJ, Denny CT. A second Ewing's sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nature genetics. 1994;6:146–151. doi: 10.1038/ng0294-146. [DOI] [PubMed] [Google Scholar]
- 26.Jeon IS, Davis JN, Braun BS, Sublett JE, Roussel MF, Denny CT, Shapiro DN. A variant Ewing's sarcoma translocation (7;22) fuses the EWS gene to the ETS gene ETV1. Oncogene. 1995;10:1229–1234. [PubMed] [Google Scholar]
- 27.Urano F, Umezawa A, Yabe H, Hong W, Yoshida K, Fujinaga K, Hata J. Molecular analysis of Ewing's sarcoma: another fusion gene, EWS-E1AF, available for diagnosis. Japanese journal of cancer research : Gann. 1998;89:703–711. doi: 10.1111/j.1349-7006.1998.tb03274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter M, Couturier J, Pacquement H, Michon J, Thomas G, Magdelenat H, Delattre O. A new member of the ETS family fused to EWS in Ewing tumors. Oncogene. 1997;14:1159–1164. doi: 10.1038/sj.onc.1200933. [DOI] [PubMed] [Google Scholar]
- 29.Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, Ambros PF, Sheer D, Turc-Carel C, Triche TJ, et al. The Ewing family of tumors--a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 30.Shing DC, McMullan DJ, Roberts P, Smith K, Chin SF, Nicholson J, Tillman RM, Ramani P, Cullinane C, Coleman N. FUS/ERG gene fusions in Ewing's tumors. Cancer research. 2003;63:4568–4576. [PubMed] [Google Scholar]
- 31.Mastrangelo T, Modena P, Tornielli S, Bullrich F, Testi MA, Mezzelani A, Radice P, Azzarelli A, Pilotti S, Croce CM, Pierotti MA, Sozzi G. A novel zinc finger gene is fused to EWS in small round cell tumor. Oncogene. 2000;19:3799–3804. doi: 10.1038/sj.onc.1203762. [DOI] [PubMed] [Google Scholar]
- 32.Szuhai K, Ijszenga M, de Jong D, Karseladze A, Tanke HJ, Hogendoorn PCW. The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:2259–2268. doi: 10.1158/1078-0432.CCR-08-2184. [DOI] [PubMed] [Google Scholar]
- 33.Toretsky JA, Erkizan V, Levenson A, Abaan OD, Parvin JD, Cripe TP, Rice AM, Lee SB, Uren A. Oncoprotein EWS-FLI1 activity is enhanced by RNA helicase A. Cancer research. 2006;66:5574–5581. doi: 10.1158/0008-5472.CAN-05-3293. [DOI] [PubMed] [Google Scholar]
- 34.Owen LA, Kowalewski AA, Lessnick SL. EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing's sarcoma. PloS one. 2008;3:e1965. doi: 10.1371/journal.pone.0001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nature reviews. Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 36.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 37.Herrero-Martin D, Osuna D, Ordonez JL, Sevillano V, Martins AS, Mackintosh C, Campos M, Madoz-Gurpide J, Otero-Motta AP, Caballero G, Amaral AT, Wai DH, Braun Y, Eisenacher M, Schaefer KL, Poremba C, de Alava E. Stable interference of EWS-FLI1 in an Ewing sarcoma cell line impairs IGF-1/IGF-1R signalling and reveals TOPK as a new target. British journal of cancer. 2009;101:80–90. doi: 10.1038/sj.bjc.6605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahm KB, Cho K, Lee C, Im YH, Chang J, Choi SG, Sorensen PH, Thiele CJ, Kim SJ. Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet. 1999;23:222–227. doi: 10.1038/13854. [DOI] [PubMed] [Google Scholar]
- 39.Ban J, Bennani-Baiti IM, Kauer M, Schaefer KL, Poremba C, Jug G, Schwentner R, Smrzka O, Muehlbacher K, Aryee DN, Kovar H. EWS-FLI1 suppresses NOTCH-activated p53 in Ewing's sarcoma. Cancer Res. 2008;68:7100–7109. doi: 10.1158/0008-5472.CAN-07-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beauchamp E, Bulut G, Abaan O, Chen K, Merchant A, Matsui W, Endo Y, Rubin JS, Toretsky J, Uren A. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–9082. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarro D, Agra N, Pestana A, Alonso J, Gonzalez-Sancho JM. The EWS/FLI1 oncogenic protein inhibits expression of the Wnt inhibitor DICKKOPF-1 gene and antagonizes beta-catenin/TCF-mediated transcription. Carcinogenesis. 2010;31:394–401. doi: 10.1093/carcin/bgp317. [DOI] [PubMed] [Google Scholar]
- 42.Fuchs B, Inwards CY, Janknecht R. Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing's sarcoma. Clin Cancer Res. 2004;10:1344–1353. doi: 10.1158/1078-0432.ccr-03-0038. [DOI] [PubMed] [Google Scholar]
- 43.Dalal S, Berry AM, Cullinane CJ, Mangham DC, Grimer R, Lewis IJ, Johnston C, Laurence V, Burchill SA. Vascular endothelial growth factor: a therapeutic target for tumors of the Ewing's sarcoma family. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:2364–2378. doi: 10.1158/1078-0432.CCR-04-1201. [DOI] [PubMed] [Google Scholar]
- 44.Chaturvedi A, Hoffman LM, Welm AL, Lessnick SL, Beckerle MC. The EWS/FLI Oncogene Drives Changes in Cellular Morphology, Adhesion, and Migration in Ewing Sarcoma. Genes & cancer. 2012;3:102–116. doi: 10.1177/1947601912457024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nature reviews. Molecular cell biology. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 46.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 47.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 49.Bhogal N. Immunotoxicity and immunogenicity of biopharmaceuticals: design concepts and safety assessment. Current drug safety. 2010;5:293–307. doi: 10.2174/157488610792246037. [DOI] [PubMed] [Google Scholar]
- 50.Bid HK, London CA, Gao J, Zhong H, Hollingsworth RE, Fernandez S, Mo X, Houghton PJ. Dual targeting of the type 1 insulin-like growth factor receptor and its ligands as an effective antiangiogenic strategy. Clin Cancer Res. 2013;19:2984–2994. doi: 10.1158/1078-0432.CCR-12-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolb EA, Gorlick R, Maris JM, Keir ST, Morton CL, Wu J, Wozniak AW, Smith MA, Houghton PJ. Combination testing (Stage 2) of the Anti-IGF-1 receptor antibody IMC-A12 with rapamycin by the pediatric preclinical testing program. Pediatric blood & cancer. 2012;58:729–735. doi: 10.1002/pbc.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013;73:5315–5319. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PH. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer research. 2007;67:3094–3105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fong EL, Lamhamedi-Cherradi SE, Burdett E, Ramamoorthy V, Lazar AJ, Kasper FK, Farach-Carson MC, Vishwamitra D, Demicco EG, Menegaz BA, Amin HM, Mikos AG, Ludwig JA. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6500–6505. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. Journal of biotechnology. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Bernstein P, Dong M, Corbeil D, Gelinsky M, Gunther KP, Fickert S. Pellet culture elicits superior chondrogenic redifferentiation than alginate-based systems. Biotechnology progress. 2009;25:1146–1152. doi: 10.1002/btpr.186. [DOI] [PubMed] [Google Scholar]
- 57.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nirmalanandhan VS, Duren A, Hendricks P, Vielhauer G, Sittampalam GS. Activity of anticancer agents in a three-dimensional cell culture model. Assay and drug development technologies. 2010;8:581–590. doi: 10.1089/adt.2010.0276. [DOI] [PubMed] [Google Scholar]
- 59.Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer. 2010;116:1739–1750. doi: 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue engineering. 2006;12:905–915. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 61.Van Hoof D, Braam SR, Dormeyer W, Ward-van Oostwaard D, Heck AJ, Krijgsveld J, Mummery CL. Feeder-free monolayer cultures of human embryonic stem cells express an epithelial plasma membrane protein profile. Stem Cells. 2008;26:2777–2781. doi: 10.1634/stemcells.2008-0365. [DOI] [PubMed] [Google Scholar]
- 62.Chambers KF, Pearson JF, Aziz N, O'Toole P, Garrod D, Lang SH. Stroma regulates increased epithelial lateral cell adhesion in 3D culture: a role for actin/cadherin dynamics. PloS one. 2011;6:e18796. doi: 10.1371/journal.pone.0018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rak J, Mitsuhashi Y, Erdos V, Huang SN, Filmus J, Kerbel RS. Massive programmed cell death in intestinal epithelial cells induced by three-dimensional growth conditions: suppression by mutant c-H-ras oncogene expression. The Journal of cell biology. 1995;131:1587–1598. doi: 10.1083/jcb.131.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamilton G. Multicellular spheroids as an in vitro tumor model. Cancer Lett. 1998;131:29–34. doi: 10.1016/s0304-3835(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 65.Inch WR, McCredie JA, Sutherland RM. Growth of nodular carcinomas in rodents compared with multi-cell spheroids in tissue culture. Growth. 1970;34:271–282. [PubMed] [Google Scholar]
- 66.Sutherland RM, McCredie JA, Inch WR. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 1971;46:113–120. [PubMed] [Google Scholar]
- 67.Myatt SS, Redfern CP, Burchill SA. p38MAPK-Dependent sensitivity of Ewing's sarcoma family of tumors to fenretinide-induced cell death. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:3136–3148. doi: 10.1158/1078-0432.CCR-04-2050. [DOI] [PubMed] [Google Scholar]
- 68.Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med. 2007;140:141–151. doi: 10.1007/978-1-59745-443-8_8. [DOI] [PubMed] [Google Scholar]
- 69.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnology and bioengineering. 2003;83:173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 70.Wu LY, Di Carlo D, Lee LP. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdevices. 2008;10:197–202. doi: 10.1007/s10544-007-9125-8. [DOI] [PubMed] [Google Scholar]
- 71.Kunz-Schughart LA. Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell biology international. 1999;23:157–161. doi: 10.1006/cbir.1999.0384. [DOI] [PubMed] [Google Scholar]
- 72.Friedrich J, Ebner R, Kunz-Schughart LA. Experimental anti-tumor therapy in 3-D: spheroids--old hat or new challenge? Int J Radiat Biol. 2007;83:849–871. doi: 10.1080/09553000701727531. [DOI] [PubMed] [Google Scholar]
- 73.Chang TT, Hughes-Fulford M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue engineering. Part A. 2009;15:559–567. doi: 10.1089/ten.tea.2007.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cody NA, Zietarska M, Filali-Mouhim A, Provencher DM, Mes-Masson AM, Tonin PN. Influence of monolayer, spheroid, and tumor growth conditions on chromosome 3 gene expression in tumorigenic epithelial ovarian cancer cell lines. BMC medical genomics. 2008;1:34. doi: 10.1186/1755-8794-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaedtke L, Thoenes L, Culmsee C, Mayer B, Wagner E. Proteomic analysis reveals differences in protein expression in spheroid versus monolayer cultures of low-passage colon carcinoma cells. Journal of proteome research. 2007;6:4111–4118. doi: 10.1021/pr0700596. [DOI] [PubMed] [Google Scholar]
- 76.Shimada M, Yamashita Y, Tanaka S, Shirabe K, Nakazawa K, Ijima H, Sakiyama R, Fukuda J, Funatsu K, Sugimachi K. Characteristic gene expression induced by polyurethane foam/spheroid culture of hepatoma cell line, Hep G2 as a promising cell source for bioartificial liver. Hepatogastroenterology. 2007;54:814–820. [PubMed] [Google Scholar]
- 77.Timmins NE, Dietmair S, Nielsen LK. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis. 2004;7:97–103. doi: 10.1007/s10456-004-8911-7. [DOI] [PubMed] [Google Scholar]
- 78.Yamashita Y, Shimada M, Harimoto N, Tanaka S, Shirabe K, Ijima H, Nakazawa K, Fukuda J, Funatsu K, Maehara Y. cDNA microarray analysis in hepatocyte differentiation in Huh 7 cells. Cell transplantation. 2004;13:793–799. doi: 10.3727/000000004783983396. [DOI] [PubMed] [Google Scholar]
- 79.Zietarska M, Maugard CM, Filali-Mouhim A, Alam-Fahmy M, Tonin PN, Provencher DM, Mes-Masson AM. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC) Molecular carcinogenesis. 2007;46:872–885. doi: 10.1002/mc.20315. [DOI] [PubMed] [Google Scholar]
- 80.Lawlor ER, Scheel C, Irving J, Sorensen PH. Anchorage-independent multi-cellular spheroids as an in vitro model of growth signaling in Ewing tumors. Oncogene. 2002;21:307–318. doi: 10.1038/sj.onc.1205053. [DOI] [PubMed] [Google Scholar]
- 81.Dufau I, Frongia C, Sicard F, Dedieu L, Cordelier P, Ausseil F, Ducommun B, Valette A. Multicellular tumor spheroid model to evaluate spatio-temporal dynamics effect of chemotherapeutics: application to the gemcitabine/CHK1 inhibitor combination in pancreatic cancer. BMC Cancer. 2012;12:15. doi: 10.1186/1471-2407-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. Journal of biomolecular screening. 2006;11:922–932. doi: 10.1177/1087057106292763. [DOI] [PubMed] [Google Scholar]
- 83.Rodriguez-Enriquez S, Gallardo-Perez JC, Aviles-Salas A, Marin-Hernandez A, Carreno-Fuentes L, Maldonado-Lagunas V, Moreno-Sanchez R. Energy metabolism transition in multicellular human tumor spheroids. J Cell Physiol. 2008;216:189–197. doi: 10.1002/jcp.21392. [DOI] [PubMed] [Google Scholar]
- 84.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 85.Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Critical reviews in oncology/hematology. 2000;36:193–207. doi: 10.1016/s1040-8428(00)00086-x. [DOI] [PubMed] [Google Scholar]
- 86.Villaverde MS, Gil-Cardeza ML, Glikin GC, Finocchiaro LM. Interferon-beta lipofection I. Increased efficacy of chemotherapeutic drugs on human tumor cells derived monolayers and spheroids. Cancer gene therapy. 2012;19:508–516. doi: 10.1038/cgt.2012.27. [DOI] [PubMed] [Google Scholar]
- 87.Awad O, Yustein JT, Shah P, Gul N, Katuri V, O'Neill A, Kong Y, Brown ML, Toretsky JA, Loeb DM. High ALDH activity identifies chemotherapy-resistant Ewing's sarcoma stem cells that retain sensitivity to EWS-FLI1 inhibition. PLoS One. 2010;5:e13943. doi: 10.1371/journal.pone.0013943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strauss SJ, Ng T, Mendoza-Naranjo A, Whelan J, Sorensen PH. Understanding micrometastatic disease and Anoikis resistance in ewing family of tumors and osteosarcoma. The oncologist. 2010;15:627–635. doi: 10.1634/theoncologist.2010-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holmes TD, El-Sherbiny YM, Davison A, Clough SL, Blair GE, Cook GP. A human NK cell activation/inhibition threshold allows small changes in the target cell surface phenotype to dramatically alter susceptibility to NK cells. J Immunol. 2011;186:1538–1545. doi: 10.4049/jimmunol.1000951. [DOI] [PubMed] [Google Scholar]
- 90.Kailayangiri S, Altvater B, Meltzer J, Pscherer S, Luecke A, Dierkes C, Titze U, Leuchte K, Landmeier S, Hotfilder M, Dirksen U, Hardes J, Gosheger G, Juergens H, Rossig C. The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. British journal of cancer. 2012;106:1123–1133. doi: 10.1038/bjc.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 93.Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu MZ, Dubova I, Esteban CR, Montserrat N, Campistol JM, Izpisua Belmonte JC. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nature cell biology. 2013;15:1507–1515. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 94.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reddy K, Zhou Z, Jia SF, Lee TH, Morales-Arias J, Cao Y, Kleinerman ES. Stromal cell-derived factor-1 stimulates vasculogenesis and enhances Ewing's sarcoma tumor growth in the absence of vascular endothelial growth factor. Int J Cancer. 2008;123:831–837. doi: 10.1002/ijc.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee TH, Bolontrade MF, Worth LL, Guan H, Ellis LM, Kleinerman ES. Production of VEGF165 by Ewing's sarcoma cells induces vasculogenesis and the incorporation of CD34+ stem cells into the expanding tumor vasculature. International journal of cancer. Journal international du cancer. 2006;119:839–846. doi: 10.1002/ijc.21916. [DOI] [PubMed] [Google Scholar]
- 97.Benini S, Perbal B, Zambelli D, Colombo MP, Manara MC, Serra M, Parenza M, Martinez V, Picci P, Scotlandi K. In Ewing's sarcoma CCN3(NOV) inhibits proliferation while promoting migration and invasion of the same cell type. Oncogene. 2005;24:4349–4361. doi: 10.1038/sj.onc.1208620. [DOI] [PubMed] [Google Scholar]
- 98.Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Antitumor effect of nutrient synergy on human osteosarcoma cells U-2OS, MNNG-HOS and Ewing's sarcoma SK-ES.1. Oncology reports. 2005;13:253–257. [PubMed] [Google Scholar]
- 99.Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, Liu B, Cao Y, Tryggvason K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer research. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- 100.Carpenter PM, Dao AV, Arain ZS, Chang MK, Nguyen HP, Arain S, Wang-Rodriguez J, Kwon SY, Wilczynski SP. Motility induction in breast carcinoma by mammary epithelial laminin 332 (laminin 5) Molecular cancer research : MCR. 2009;7:462–475. doi: 10.1158/1541-7786.MCR-08-0148. [DOI] [PubMed] [Google Scholar]
- 101.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue engineering. Part B, Reviews. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 102.Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 103.Jedeszko C, Sameni M, Olive MB, Moin K, Sloane BF. Visualizing protease activity in living cells: from two dimensions to four dimensions, Current protocols in cell biology / editorial board. Juan S. Bonifacino ... [et al.] 2008 doi: 10.1002/0471143030.cb0420s39. Chapter 4 Unit 4 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moss NM, Liu Y, Johnson JJ, Debiase P, Jones J, Hudson LG, Munshi HG, Stack MS. Epidermal growth factor receptor-mediated membrane type 1 matrix metalloproteinase endocytosis regulates the transition between invasive versus expansive growth of ovarian carcinoma cells in three-dimensional collagen. Molecular cancer research : MCR. 2009;7:809–820. doi: 10.1158/1541-7786.MCR-08-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. The Journal of cell biology. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 107.Cavallo-Medved D, Rudy D, Blum G, Bogyo M, Caglic D, Sloane BF. Live-cell imaging demonstrates extracellular matrix degradation in association with active cathepsin B in caveolae of endothelial cells during tube formation. Experimental cell research. 2009;315:1234–1246. doi: 10.1016/j.yexcr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Accounts of chemical research. 2008;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 109.Bissell MJ, Radisky D. Putting tumours in context. Nature reviews. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 115.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 116.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nature reviews. Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Current opinion in cell biology. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Genis L, Gonzalo P, Tutor AS, Galvez BG, Martinez-Ruiz A, Zaragoza C, Lamas S, Tryggvason K, Apte SS, Arroyo AG. Functional interplay between endothelial nitric oxide synthase and membrane type 1 matrix metalloproteinase in migrating endothelial cells. Blood. 2007;110:2916–2923. doi: 10.1182/blood-2007-01-068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovascular research. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- 122.Bignon M, Pichol-Thievend C, Hardouin J, Malbouyres M, Brechot N, Nasciutti L, Barret A, Teillon J, Guillon E, Etienne E, Caron M, Joubert-Caron R, Monnot C, Ruggiero F, Muller L, Germain S. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood. 2011;118:3979–3989. doi: 10.1182/blood-2010-10-313296. [DOI] [PubMed] [Google Scholar]
- 123.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circulation research. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 124.Myers KA, Applegate KT, Danuser G, Fischer RS, Waterman CM. Distinct ECM mechanosensing pathways regulate microtubule dynamics to control endothelial cell branching morphogenesis. The Journal of cell biology. 2011;192:321–334. doi: 10.1083/jcb.201006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. The international journal of biochemistry & cell biology. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sorokin L. The impact of the extracellular matrix on inflammation. Nature reviews. Immunology. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 127.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. The Journal of clinical investigation. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nature medicine. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 129.Franco C, Britto K, Wong E, Hou G, Zhu SN, Chen M, Cybulsky MI, Bendeck MP. Discoidin domain receptor 1 on bone marrow-derived cells promotes macrophage accumulation during atherogenesis. Circulation research. 2009;105:1141–1148. doi: 10.1161/CIRCRESAHA.109.207357. [DOI] [PubMed] [Google Scholar]
- 130.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. The Journal of experimental medicine. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adler B, Ashkar S, Cantor H, Weber GF. Costimulation by extracellular matrix proteins determines the response to TCR ligation. Cellular immunology. 2001;210:30–40. doi: 10.1006/cimm.2001.1800. [DOI] [PubMed] [Google Scholar]
- 132.Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 133.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 134.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 135.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nature immunology. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 136.Gill BJ, West JL. Modeling the tumor extracellular matrix: Tissue engineering tools repurposed towards new frontiers in cancer biology. Journal of biomechanics. 2013 doi: 10.1016/j.jbiomech.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 137.Moll C, Reboredo J, Schwarz T, Appelt A, Schurlein S, Walles H, Nietzer S. Tissue engineering of a human 3D in vitro tumor test system. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/50460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Phan-Lai V, Florczyk SJ, Kievit FM, Wang K, Gad E, Disis ML, Zhang M. Three-dimensional scaffolds to evaluate tumor associated fibroblast-mediated suppression of breast tumor specific T cells. Biomacromolecules. 2013;14:1330–1337. doi: 10.1021/bm301928u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mitra M, Mohanty C, Harilal A, Maheswari UK, Sahoo SK, Krishnakumar S. A novel in vitro three-dimensional retinoblastoma model for evaluating chemotherapeutic drugs. Molecular vision. 2012;18:1361–1378. [PMC free article] [PubMed] [Google Scholar]
- 140.Hutmacher DW, Horch RE, Loessner D, Rizzi S, Sieh S, Reichert JC, Clements JA, Beier JP, Arkudas A, Bleiziffer O, Kneser U. Translating tissue engineering technology platforms into cancer research. Journal of cellular and molecular medicine. 2009;13:1417–1427. doi: 10.1111/j.1582-4934.2009.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim YJ, Bae HI, Kwon OK, Choi MS. Three-dimensional gastric cancer cell culture using nanofiber scaffold for chemosensitivity test. International journal of biological macromolecules. 2009;45:65–71. doi: 10.1016/j.ijbiomac.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 142.Poh PS, Hutmacher DW, Stevens MM, Woodruff MA. Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication. 2013;5:045005. doi: 10.1088/1758-5082/5/4/045005. [DOI] [PubMed] [Google Scholar]
- 143.Mohan N, Gupta V, Sridharan B, Sutherland A, Detamore MS. The potential of encapsulating 'raw materials' in 3D osteochondral gradient scaffolds. Biotechnology and bioengineering. 2013 doi: 10.1002/bit.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cirillo V, Guarino V, Ambrosio L. Design of bioactive electrospun scaffolds for bone tissue engineering. Journal of applied biomaterials & functional materials. 2012;10:223–228. doi: 10.5301/JABFM.2012.10343. [DOI] [PubMed] [Google Scholar]
- 145.Infanger DW, Lynch ME, Fischbach C. Engineered culture models for studies of tumor-microenvironment interactions. Annual review of biomedical engineering. 2013;15:29–53. doi: 10.1146/annurev-bioeng-071811-150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int J Polym Sci. 2011 [Google Scholar]
- 147.Coutu DL, Yousefi AM, Galipeau J. Three-dimensional porous scaffolds at the crossroads of tissue engineering and cell-based gene therapy. J Cell Biochem. 2009;108:537–546. doi: 10.1002/jcb.22296. [DOI] [PubMed] [Google Scholar]
- 148.Sahoo SK, Panda AK, Labhasetwar V. Characterization of porous PLGA/PLA microparticles as a scaffold for three dimensional growth of breast cancer cells. Biomacromolecules. 2005;6:1132–1139. doi: 10.1021/bm0492632. [DOI] [PubMed] [Google Scholar]
- 149.Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, Jain TK, Panda AK, Labhasetwar V. 3-D tumor model for in vitro evaluation of anticancer drugs. Molecular pharmaceutics. 2008;5:849–862. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- 150.Haroun AA, Abo-Zeid MA, Youssef AM, Gamal-Eldeen A. In vitro biological study of gelatin/PLG nanocomposite using MCF-7 breast cancer cells. Journal of biomedical materials research. Part A. 2013;101:1388–1396. doi: 10.1002/jbm.a.34441. [DOI] [PubMed] [Google Scholar]
- 151.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 152.Zhang M, Boughton P, Rose B, Lee CS, Hong AM. The use of porous scaffold as a tumor model. International journal of biomaterials. 2013;2013:396056. doi: 10.1155/2013/396056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Karande TS, Ong JL, Agrawal CM. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Annals of biomedical engineering. 2004;32:1728–1743. doi: 10.1007/s10439-004-7825-2. [DOI] [PubMed] [Google Scholar]
- 154.Malda J, Woodfield TB, van der Vloodt F, Kooy FK, Martens DE, Tramper J, van Blitterswijk CA, Riesle J. The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials. 2004;25:5773–5780. doi: 10.1016/j.biomaterials.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 155.Zhao F, Pathi P, Grayson W, Xing Q, Locke BR, Ma T. Effects of oxygen transport on 3-d human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnology progress. 2005;21:1269–1280. doi: 10.1021/bp0500664. [DOI] [PubMed] [Google Scholar]
- 156.Kellner K, Liebsch G, Klimant I, Wolfbeis OS, Blunk T, Schulz MB, Gopferich A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnology and bioengineering. 2002;80:73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 157.Lewis MC, Macarthur BD, Malda J, Pettet G, Please CP. Heterogeneous proliferation within engineered cartilaginous tissue: the role of oxygen tension. Biotechnology and bioengineering. 2005;91:607–615. doi: 10.1002/bit.20508. [DOI] [PubMed] [Google Scholar]
- 158.Volkmer E, Drosse I, Otto S, Stangelmayer A, Stengele M, Kallukalam BC, Mutschler W, Schieker M. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue engineering. Part A. 2008;14:1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- 159.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Effects of oxygen on engineered cardiac muscle. Biotechnology and bioengineering. 2002;78:617–625. doi: 10.1002/bit.10245. [DOI] [PubMed] [Google Scholar]
- 160.Dunn JC, Chan WY, Cristini V, Kim JS, Lowengrub J, Singh S, Wu BM. Analysis of cell growth in three-dimensional scaffolds. Tissue engineering. 2006;12:705–716. doi: 10.1089/ten.2006.12.705. [DOI] [PubMed] [Google Scholar]
- 161.Park J, Berthiaume F, Toner M, Yarmush ML, Tilles AW. Microfabricated grooved substrates as platforms for bioartificial liver reactors. Biotechnology and bioengineering. 2005;90:632–644. doi: 10.1002/bit.20463. [DOI] [PubMed] [Google Scholar]
- 162.Nishikawa M, Yamamoto T, Kojima N, Kikuo K, Fujii T, Sakai Y. Stable immobilization of rat hepatocytes as hemispheroids onto collagen-conjugated poly-dimethylsiloxane (PDMS) surfaces: importance of direct oxygenation through PDMS for both formation and function. Biotechnology and bioengineering. 2008;99:1472–1481. doi: 10.1002/bit.21690. [DOI] [PubMed] [Google Scholar]
- 163.Nahmias Y, Kramvis Y, Barbe L, Casali M, Berthiaume F, Yarmush ML. A novel formulation of oxygen-carrying matrix enhances liver-specific function of cultured hepatocytes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:2531–2533. doi: 10.1096/fj.06-6192fje. [DOI] [PubMed] [Google Scholar]
- 164.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 165.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;240:177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 166.Davey P, Tudhope GR. Anticancer chemotherapy. Br Med J (Clin Res Ed) 1983;287:110–113. doi: 10.1136/bmj.287.6385.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. Journal of surgical oncology. 1997;65:284–297. doi: 10.1002/(sici)1096-9098(199708)65:4<284::aid-jso11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 168.dit Faute MA, Laurent L, Ploton D, Poupon MF, Jardillier JC, Bobichon H. Distinctive alterations of invasiveness, drug resistance and cell-cell organization in 3D-cultures of MCF-7, a human breast cancer cell line, and its multidrug resistant variant. Clinical & experimental metastasis. 2002;19:161–168. doi: 10.1023/a:1014594825502. [DOI] [PubMed] [Google Scholar]
- 169.Abaza M, Luqmani YA. The influence of pH and hypoxia on tumor metastasis. Expert review of anticancer therapy. 2013;13:1229–1242. doi: 10.1586/14737140.2013.843455. [DOI] [PubMed] [Google Scholar]
- 170.Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its microenvironment: A synergistic interplay. Seminars in cancer biology. 2013;23:522–532. doi: 10.1016/j.semcancer.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 171.Willis AL, Sabeh F, Li XY, Weiss SJ. Extracellular matrix determinants and the regulation of cancer cell invasion stratagems. Journal of microscopy. 2013;251:250–260. doi: 10.1111/jmi.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer research. 2013;73:4965–4977. doi: 10.1158/0008-5472.CAN-13-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. The Journal of cell biology. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jinka R, Kapoor R, Sistla PG, Raj TA, Pande G. Alterations in Cell-Extracellular Matrix Interactions during Progression of Cancers. International journal of cell biology. 2012;2012:219196. doi: 10.1155/2012/219196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix biology : journal of the International Society for Matrix Biology. 2011;30:363–368. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Santos E, Garate A, Pedraz JL, Orive G, Hernandez RM. The synergistic effects of the RGD density and the microenvironment on the behavior of encapsulated cells: In vitro and in vivo direct comparative study. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.35073. [DOI] [PubMed] [Google Scholar]
- 177.Tome Y, Uehara F, Mii S, Yano S, Zhang L, Sugimoto N, Maehara H, Bouvet M, Tsuchiya H, Kanaya F, Hoffman RM. 3-Dimensional Tissue Is Formed From Cancer Cells In Vitro on Gelfoam(R), But Not on Matrigel. J Cell Biochem. 2014 doi: 10.1002/jcb.24780. [DOI] [PubMed] [Google Scholar]
- 178.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 179.Scarpa S, D'Orazi G, Modesti M, Modesti A. Ewing's sarcoma lines synthesize laminin and fibronectin, Virchows Archiv. A. Pathological anatomy and histopathology. 1987;410:375–381. doi: 10.1007/BF00712756. [DOI] [PubMed] [Google Scholar]
- 180.Scarpa S, Modesti A, Triche TJ. Extracellular matrix synthesis by undifferentiated childhood tumor cell lines. Am J Pathol. 1987;129:74–85. [PMC free article] [PubMed] [Google Scholar]
- 181.Stracca-Pansa V, Dickman PS, Zamboni G, Bevilacqua PA, Ninfo V. Extracellular matrix of small round cell tumors of childhood: an immunohistochemical study of 67 cases. Pediatric pathology / affiliated with the International Paediatric Pathology Association. 1994;14:111–125. doi: 10.3109/15513819409022031. [DOI] [PubMed] [Google Scholar]
- 182.Vijayakumar S, Liu G, Rus IA, Yao S, Chen Y, Akiri G, Grumolato L, Aaronson SA. High-frequency canonical Wnt activation in multiple sarcoma subtypes drives proliferation through a TCF/beta-catenin target gene, CDC25A. Cancer Cell. 2011;19:601–612. doi: 10.1016/j.ccr.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mendoza-Naranjo A, El-Naggar A, Wai DH, Mistry P, Lazic N, Ayala FR, da Cunha IW, Rodriguez-Viciana P, Cheng H, Tavares Guerreiro Fregnani JH, Reynolds P, Arceci RJ, Nicholson A, Triche TJ, Soares FA, Flanagan AM, Wang YZ, Strauss SJ, Sorensen PH. ERBB4 confers metastatic capacity in Ewing sarcoma. EMBO molecular medicine. 2013;5:1087–1102. doi: 10.1002/emmm.201202343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, Haslett C. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nature medicine. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 185.Zhang Y, Lu H, Dazin P, Kapila Y. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: fibronectin and integrin alphav mediate survival signals through focal adhesion kinase. The Journal of biological chemistry. 2004;279:48342–48349. doi: 10.1074/jbc.M407953200. [DOI] [PubMed] [Google Scholar]
- 186.Thibault RA, Mikos AG, Kasper FK. Protein and mineral composition of osteogenic extracellular matrix constructs generated with a flow perfusion bioreactor. Biomacromolecules. 2011;12:4204–4212. doi: 10.1021/bm200975a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hartman O, Zhang C, Adams EL, Farach-Carson MC, Petrelli NJ, Chase BD, Rabolt JF. Biofunctionalization of electrospun PCL-based scaffolds with perlecan domain IV peptide to create a 3-D pharmacokinetic cancer model. Biomaterials. 2010;31:5700–5718. doi: 10.1016/j.biomaterials.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee J, Yoo JJ, Atala A, Lee SJ. The effect of controlled release of PDGF-BB from heparinconjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials. 2012;33:6709–6720. doi: 10.1016/j.biomaterials.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sreerekha PR, Menon D, Nair SV, Chennazhi KP. Fabrication of fibrin based electrospun multiscale composite scaffold for tissue engineering applications. Journal of biomedical nanotechnology. 2013;9:790–800. doi: 10.1166/jbn.2013.1585. [DOI] [PubMed] [Google Scholar]
- 190.Levorson EJ, Hu O, Mountziaris PM, Kasper FK, Mikos AG. Cell-Derived Polymer/Extracellular Matrix Composite Scaffolds for Cartilage Regeneration, Part 2: Construct Devitalization and Determination of Chondroinductive Capacity. Tissue engineering. Part C, Methods. 2013 doi: 10.1089/ten.tec.2013.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Levorson EJ, Santoro M, Kurtis Kasper F, Mikos AG. Direct and Indirect Co-culture of Chondrocytes and Mesenchymal Stem Cells for the Generation of Polymer/Extracellular Matrix Hybrid Constructs. Acta biomaterialia. 2013 doi: 10.1016/j.actbio.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liao J, Guo X, Grande-Allen KJ, Kasper FK, Mikos AG. Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials. 2010;31:8911–8920. doi: 10.1016/j.biomaterials.2010.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liao J, Guo X, Nelson D, Kasper FK, Mikos AG. Modulation of osteogenic properties of biodegradable polymer/extracellular matrix scaffolds generated with a flow perfusion bioreactor. Acta biomaterialia. 2010;6:2386–2393. doi: 10.1016/j.actbio.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-alpha on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010;31:1666–1675. doi: 10.1016/j.biomaterials.2009.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saraf A, Lozier G, Haesslein A, Kasper FK, Raphael RM, Baggett LS, Mikos AG. Fabrication of nonwoven coaxial fiber meshes by electrospinning. Tissue engineering. Part C, Methods. 2009;15:333–344. doi: 10.1089/ten.tec.2008.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Woodruff MA, Hutmacher DW. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog Polym Sci. 2010;35:1217–1256. [Google Scholar]
- 197.Brown TD, Dalton PD, Hutmacher DW. Direct writing by way of melt electrospinning. Adv Mater. 2011;23:5651–5657. doi: 10.1002/adma.201103482. [DOI] [PubMed] [Google Scholar]
- 198.Reddy K, Cao Y, Zhou Z, Yu L, Jia SF, Kleinerman ES. VEGF165 expression in the tumor microenvironment influences the differentiation of bone marrow-derived pericytes that contribute to the Ewing's sarcoma vasculature. Angiogenesis. 2008;11:257–267. doi: 10.1007/s10456-008-9109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reddy K, Zhou Z, Schadler K, Jia SF, Kleinerman ES. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing's tumor vessels. Molecular cancer research : MCR. 2008;6:929–936. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhou Z, Stewart KS, Yu L, Kleinerman ES. Bone marrow cells participate in tumor vessel formation that supports the growth of Ewing's sarcoma in the lung. Angiogenesis. 2011;14:125–133. doi: 10.1007/s10456-010-9196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yu L, Su B, Hollomon M, Deng Y, Facchinetti V, Kleinerman ES. Vasculogenesis driven by bone marrow-derived cells is essential for growth of Ewing's sarcomas. Cancer research. 2010;70:1334–1343. doi: 10.1158/0008-5472.CAN-09-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Stewart KS, Kleinerman ES. Tumor Vessel Development and Expansion in Ewing's Sarcoma: A Review of the Vasculogenesis Process and Clinical Trials with Vascular-Targeting Agents. Sarcoma. 2011;2011:165837. doi: 10.1155/2011/165837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Castillero-Trejo Y, Eliazer S, Xiang L, Richardson JA, Ilaria RL., Jr. Expression of the EWS/FLI-1 oncogene in murine primary bone-derived cells Results in EWS/FLI-1-dependent, ewing sarcoma-like tumors. Cancer research. 2005;65:8698–8705. doi: 10.1158/0008-5472.CAN-05-1704. [DOI] [PubMed] [Google Scholar]
- 204.Kovar H, Bernard A. CD99-positive “Ewing's sarcoma” from mouse-bone marrow-derived mesenchymal progenitor cells? Cancer research. 2006;66:9786. doi: 10.1158/0008-5472.CAN-06-0205. author reply 9786. [DOI] [PubMed] [Google Scholar]
- 205.Riggi N, Suva ML, De Vito C, Provero P, Stehle JC, Baumer K, Cironi L, Janiszewska M, Petricevic T, Suva D, Tercier S, Joseph JM, Guillou L, Stamenkovic I. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes & development. 2010;24:916–932. doi: 10.1101/gad.1899710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Torchia EC, Jaishankar S, Baker SJ. Ewing tumor fusion proteins block the differentiation of pluripotent marrow stromal cells. Cancer Res. 2003;63:3464–3468. [PubMed] [Google Scholar]
- 207.Toretsky JA, Steinberg SM, Thakar M, Counts D, Pironis B, Parente C, Eskenazi A, Helman L, Wexler LH. Insulin-like growth factor type 1 (IGF-1) and IGF binding protein-3 in patients with Ewing sarcoma family of tumors. Cancer. 2001;92:2941–2947. doi: 10.1002/1097-0142(20011201)92:11<2941::aid-cncr10072>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 208.Monument MJ, Bernthal NM, Randall RL. Salient features of mesenchymal stem cells-implications for Ewing sarcoma modeling. Frontiers in oncology. 2013;3:24. doi: 10.3389/fonc.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.DuBois SG, Marina N, Glade-Bender J. Angiogenesis and vascular targeting in Ewing sarcoma: a review of preclinical and clinical data. Cancer. 2010;116:749–757. doi: 10.1002/cncr.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dahlin RL, Gershovich JG, Kasper FK, Mikos AG. Flow Perfusion Co-culture of Human Mesenchymal Stem Cells and Endothelial Cells on Biodegradable Polymer Scaffolds. Annals of biomedical engineering. 2013 doi: 10.1007/s10439-013-0862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gershovich JG, Dahlin RL, Kasper FK, Mikos AG. Enhanced osteogenesis in cocultures with human mesenchymal stem cells and endothelial cells on polymeric microfiber scaffolds. Tissue engineering. Part A. 2013;19:2565–2576. doi: 10.1089/ten.tea.2013.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Strammiello R, Benini S, Manara MC, Perdichizzi S, Serra M, Spisni E, Picci P, Scotlandi K. Impact of IGF-I/IGF-IR circuit on the angiogenetic properties of Ewing's sarcoma cells. Horm Metab Res. 2003;35:675–684. doi: 10.1055/s-2004-814149. [DOI] [PubMed] [Google Scholar]
- 213.Gershovich JG, Dahlin RL, Kasper FK, Mikos AG. Enhanced Osteogenesis in Cocultures with Human Mesenchymal Stem Cells and Endothelial Cells on Polymeric Microfiber Scaffolds. Tissue engineering. Part A. 2013 doi: 10.1089/ten.tea.2013.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lehner M, Gotz G, Proff J, Schaft N, Dorrie J, Full F, Ensser A, Muller YA, Cerwenka A, Abken H, Parolini O, Ambros PF, Kovar H, Holter W. Redirecting T cells to Ewing's sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PloS one. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Suminoe A, Matsuzaki A, Hattori H, Koga Y, Hara T. Immunotherapy with autologous dendritic cells and tumor antigens for children with refractory malignant solid tumors. Pediatric transplantation. 2009;13:746–753. doi: 10.1111/j.1399-3046.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- 216.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3901–3909. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Verhoeven DH, de Hooge AS, Mooiman EC, Santos SJ, ten Dam MM, Gelderblom H, Melief CJ, Hogendoorn PC, Egeler RM, van Tol MJ, Schilham MW, Lankester AC. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Molecular immunology. 2008;45:3917–3925. doi: 10.1016/j.molimm.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 218.Ahn YO, Weigel B, Verneris MR. Killing the killer: natural killer cells to treat Ewing's sarcoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3819–3821. doi: 10.1158/1078-0432.CCR-10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Guo W, Guo Y, Tang S, Qu H, Zhao H. Dendritic cell-Ewing's sarcoma cell hybrids enhance antitumor immunity. Clinical orthopaedics and related research. 2008;466:2176–2183. doi: 10.1007/s11999-008-0348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Evans CH, Liu F, Porter RM, O'Sullivan RP, Merghoub T, Lunsford EP, Robichaud K, Van Valen F, Lessnick SL, Gebhardt MC, Wells JW. EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5341–5351. doi: 10.1158/1078-0432.CCR-12-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nichols JE, Cortiella J. Engineering of a complex organ: progress toward development of a tissue-engineered lung. Proceedings of the American Thoracic Society. 2008;5:723–730. doi: 10.1513/pats.200802-022AW. [DOI] [PubMed] [Google Scholar]
- 223.Nichols JE, Niles JA, Cortiella J. Design and development of tissue engineered lung: Progress and challenges. Organogenesis. 2009;5:57–61. doi: 10.4161/org.5.2.8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 225.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nature medicine. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 226.Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, La Francesca S, Sakamoto J, Vega S, Ogadegbe M, Mlcak R, Deyo D, Woodson L, McQuitty C, Lick S, Beckles D, Melo E, Cortiella J. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A. 2013;19:2045–2062. doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nichols JE, Niles JA, Vega SP, Cortiella J. Novel in vitro respiratory models to study lung development, physiology, pathology and toxicology. Stem cell research & therapy. 2013;4(Suppl 1):S7. doi: 10.1186/scrt368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mishra DK, Thrall MJ, Baird BN, Ott HC, Blackmon SH, Kurie JM, Kim MP. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. The Annals of thoracic surgery. 2012;93:1075–1081. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Szuhai K, Ijszenga M, de Jong D, Karseladze A, Tanke HJ, Hogendoorn PC. The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:2259–2268. doi: 10.1158/1078-0432.CCR-08-2184. [DOI] [PubMed] [Google Scholar]
- 230.Burchill SA. Ewing's sarcoma: diagnostic, prognostic, and therapeutic implications of molecular abnormalities. Journal of clinical pathology. 2003;56:96–102. doi: 10.1136/jcp.56.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kelleher FC, Thomas DM. Molecular pathogenesis and targeted therapeutics in Ewing sarcoma/primitive neuroectodermal tumours. Clinical sarcoma research. 2012;2:6. doi: 10.1186/2045-3329-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Subbiah V, Anderson P. Targeted Therapy of Ewing's Sarcoma. Sarcoma. 2011;2011:686985. doi: 10.1155/2011/686985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Owens C, Abbott LS, Gupta AA. Optimal management of ewing sarcoma family of tumors: recent developments in systemic therapy. Paediatr Drugs. 2013;15:473–492. doi: 10.1007/s40272-013-0037-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.