Abstract
Background: This study aimed to describe the development and psychometric evaluation of novel youth and parent measures of self-efficacy related to continuous glucose monitoring (CGM) in pediatric patients with type 1 diabetes. This evaluation also assessed the predictive validity of the CGM Self-Efficacy (CGM-SE) surveys on CGM use and hemoglobin A1c (HbA1c) levels.
Subjects and Methods: Study participants included 120 youth with type 1 diabetes for ≥1 year enrolled in a 2-year randomized clinical trial comparing CGM use with and without the addition of a family-focused CGM behavioral intervention. Youth and parents completed the CGM-SE surveys at randomization after a 1-week run-in to assess CGM tolerability. Analyses of predictive validity excluded the intervention group and included 61 youth in the control group in order to assess CGM use and HbA1c outcomes 3 and 6 months after randomization.
Results: At study entry, youth were 12.7±2.7 years old with a diabetes duration of 6.1±3.6 years and an HbA1c level of 8.0±0.8% (64±9 mmol/mol); blood glucose monitoring frequency was 6.8±2.4 times/day, and 84% received pump therapy. CGM-SE surveys had acceptable internal consistency (Cronbach's α=0.80 for youth and 0.82 for parents). Youth reporting higher baseline CGM self-efficacy (CGM-SE score of >80) had significantly greater CGM use and lower HbA1c level after 3 and 6 months compared with youth reporting lower baseline CGM self-efficacy (CGM-SE score of ≤80).
Conclusions: The CGM-SE surveys appear to have strong psychometric properties. CGM self-efficacy may offer an opportunity to assess the likelihood of CGM adherence and glycemic improvement in youth with type 1 diabetes in clinical and research settings.
Introduction
The current era of intensive insulin therapy places substantial demands on children and adolescents with type 1 diabetes along with their parents. Intensive insulin therapy requires numerous self-care behaviors, which are particularly evident in the use of continuous glucose monitoring (CGM) technologies, in order to maintain glycemic control. The JDRF CGM randomized clinical trial (RCT) yielded significantly improved glycemic outcomes without severe hypoglycemia in adults; however, children and adolescents only demonstrated improved glycemic control with consistent CGM use.1,2 Thus, there has been substantial interest in uncovering approaches to encourage consistent CGM use in the pediatric population. As such, we were interested in understanding perceived self-efficacy related to CGM in children and teens with type 1 diabetes and their parents.
Self-efficacy, a central part of the Social Cognitive Theory of Bandura,3 is an individual's perceived ability to carry out a certain behavior.4 Self-efficacy can augment one's motivation to perform certain tasks and can be an important indicator of health behavior change.5,6 Measuring self-efficacy is an integral part of the predictive evaluation of whether an individual will carry out a specific task and the level of perseverance he or she will exert when faced with challenges or barriers. Self-efficacy also influences outcome expectations or the result an individual anticipates that his or her actions will generate; higher levels of self-efficacy often parallel more positive outcome expectations.7
High self-efficacy, or strong perceived ability to make positive health behavior change despite challenges, may facilitate complex behaviors like CGM use in youth with type 1 diabetes. Given the challenges of consistent CGM use in youth with type 1 diabetes, it is important to determine whether self-efficacy specific to CGM is a factor in consistent CGM use and whether the level of self-efficacy predicts CGM use and, in turn, glycemic outcomes. Evaluating CGM self-efficacy in both youth and parents is particularly important considering the level of support and family involvement that are necessary for diabetes self-care tasks with intensive insulin therapy and advanced diabetes technologies. The ability to measure self-efficacy related to CGM use may help the pediatric multidisciplinary team to identify those youth and parents who may benefit from additional education and support. However, there is a paucity of published research in the area of assessing self-efficacy related to CGM in youth and adults with type 1 diabetes.
The first aim of this study was to design surveys to assess self-efficacy in children and adolescents with type 1 diabetes and their parents and to evaluate the psychometric properties of the new CGM Self-Efficacy (CGM-SE) surveys. The second aim of the study was to assess the predictive validity of the CGM-SE survey in a subset of participants in order to determine whether CGM self-efficacy predicts CGM use and subsequent glycemic control.
Research Design and Methods
Study population
Study participants were 120 youth with type 1 diabetes and their parents who participated in a 2-year RCT comparing CGM use with and without the addition of a family-focused CGM behavioral intervention. Eligibility criteria included youth ages 8–17 years old with type 1 diabetes for ≥1 year, established use of intensive insulin therapy (pump or multiple daily injections), insulin dose of ≥0.5 units/kg/day, blood glucose (BG) monitoring frequency of four or more times per day, hemoglobin A1c (HbA1c) level of 6.5–10% (48–86 mmol/mol), no consistent CGM use (defined as 6+ days/week) in the previous 6 months, and anticipation for ongoing care at the diabetes center. Parents/youth provided written informed consent/assent prior to initiating any study procedures; the Institutional Review Board approved the study protocol.
The current report utilizes the entire sample of 120 youth at baseline to evaluate the CGM-SE surveys (Aim 1) and the 61 participants randomized to the control group to assess predictive validity (Aim 2).
Data collection
The electronic medical record, parent–youth interviews, and BG meter downloads provided clinical and diabetes management data at baseline. Youth and parents completed multiple validated questionnaires (see below) at baseline. Immediately following the baseline study visit, youth wore the CGM device for a 1-week run-in period to assess CGM tolerability. At the end of the 1-week run-in period, participants were randomized, and youth and parents completed the CGM-SE survey developed for the 2-year RCT. CGM data were obtained at the 3- and 6-month visits by downloading the CGM device.
Measures
Development of the CGM-SE surveys
A pediatric multidisciplinary diabetes team, experienced in clinical research and CGM use, developed the self-efficacy survey items following a literature review. Item refinement occurred through pretesting and cognitive interviewing in youth and parents prior to the 2-year RCT. Survey items assess the confidence of youth and parents to manage the technical and behavioral aspects of CGM use. The stem of the survey items states, “I am sure that I can….” There are two versions of youth surveys based on age (8–12 years of age, 11 items; 13+ years of age, 15 items) and one version for parents (14 items). The additional four items on the youth version for 13+ years of age address performance of more complex tasks, such as downloading CGM data and making insulin adjustments based on CGM data. Response options are based on a 7-point Likert scale (from 0=strongly disagree to 6=strongly agree). Scores can range from 0 to 100, with higher scores reflecting higher self-efficacy. The total score is obtained by computing the mean of all items, multiplying the mean by 100, and then dividing by 6. The survey requires <5 min for completion.
Pediatric Quality of Life Inventory Generic Core Scales and Type 1 Diabetes Module.8,9
The Pediatric Quality of Life Inventory (PedsQL) Generic Core Scales (23 items) and the PedsQL Type 1 Diabetes Module (28 items) assess generic and diabetes-specific youth quality of life over the past month; higher scores reflect higher quality of life. The youth survey is a self-report of youth quality of life; the parent survey is a proxy report of youth quality of life.
Diabetes Management Questionnaire.10
The Diabetes Management Questionnaire (DMQ) (20 items) assesses adherence to daily diabetes self-management tasks; higher scores indicate greater adherence to diabetes management. Items are applicable to both injection-based therapy and insulin pump therapy. The youth survey is a self-report of adherence; the parent survey is a proxy report of adherence.
Problem Areas in Diabetes Survey—Parent Revised11 and —Pediatric12 versions
The Problem Areas in Diabetes Survey—Parent Revised (PAID-PR) (18 items) and — Pediatric (PAID-Peds) (20 items) assess diabetes burden over the past month; higher scores indicate more burden related to diabetes management. The PAID-PR is a self-report of parent burden; the PAID-Peds is a self-report of youth burden.
State-Trait Anxiety Inventory13 and State-Trait Anxiety Inventory for Children.14
The State-Trait Anxiety Inventory (STAI) (40 items) and the State-Trait Anxiety Inventory for Children (STAIC) (40 items) assess feelings of anxiety “right now” (state anxiety) and in general (trait anxiety); higher scores indicate more anxiety. The STAI is a self-report of parent anxiety; the STAIC is a self-report of youth anxiety.
Center for Epidemiologic Studies Depression Scale15 and Center for Epidemiologic Studies Depression Scale for Children.16
The Center for Epidemiologic Studies Depression Scale (CES-D) (20 items) and the Center for Epidemiologic Studies Depression Scale for Children (CES-DC) (20 items) assess symptoms of depression in the past week; higher scores reflect more depressive symptoms. The CES-D is a self-report of parent depressive symptoms; the CES-DC is a self-report of youth depressive symptoms.
Glycemic control
HbA1c was measured uniformly at baseline and 3 and 6 months after randomization using an assay standardized to the Diabetes Control and Complications Trial (reference range, 4.0–6.0% [20–42 mmol/mol]) (Roche Diagnostics, Indianapolis, IN).
CGM use
CGM data were downloaded at study visits using DexCom™ (San Diego, CA) proprietary software. We calculated the amount of weekly CGM use by averaging the total hours of wear during the 4 weeks preceding the 3-month and 6-month study visits. CGM use could range from 0 to 168 h/week. We also created a categorical variable of CGM use for the 3-month and 6-month visits with three categories: 0–2 days (≤48 h), 3–5 days (>48–120 h), and 6–7 days (>120–168 h), based on previously identified amounts of CGM use associated with glycemic outcomes.1,17
Statistical analysis
Data were analyzed using SAS version 9.2 for Windows software (SAS Institute, Cary, NC). Demographic and clinical characteristics are presented as mean±SD, median, or percentage.
Cronbach's α was used to assess internal consistency. Item-to-total correlations for each of the items in youth and parent instruments were calculated and analyzed to determine the interrelatedness of the items.18 To evaluate the psychometric properties of the CGM-SE survey across the sample age range and given the two different youth surveys based on age, we performed separate analyses by age. We did not assess test–retest reliability because we did not anticipate that self-efficacy would be a stable construct in youth initiating CGM. We assessed criterion validity of the CGM-SE surveys for all youth and parent participants at baseline. Pearson correlations with the PedsQL (general and diabetes-specific) and the DMQ assessed convergent validity, and correlations with the PAID-PR/PAID-Peds, STAI/STAIC, and CES-D/CES-DC assessed discriminant validity.
To assess predictive validity, we included the 61 participants in the control group as CGM self-efficacy was expected to change among intervention subjects. In analyses of the predictive validity of the CGM-SE surveys, we examined CGM use and HbA1c outcomes 3 and 6 months after randomization.
Paired t tests assessed differences between baseline, 3-month, and 6-month variables in the control group; unpaired t tests compared 3-month and 6-month CGM use and HbA1c outcomes according to baseline CGM-SE scores grouped in two categories. As there is no a priori dose of adequate CGM-SE to impact CGM use, we explored the distributions of youth and parent CGM-SE scores according to the three recognized categories of CGM use. Based on these observations, we identified a self-efficacy score of 80 as a threshold and then categorized youth and parent baseline self-efficacy scores in groups of ≤80 and >80. Analysis of variance assessed differences in CGM-SE scores at baseline and HbA1c levels at 3 and 6 months according to the categorical variable for CGM use (≤2 days, 3–5 days, 6+ days) at 3 and 6 months. Spearman's correlations determined predictive validity of the youth and parent baseline CGM-SE score with HbA1c level and CGM use at 3 and 6 months for the 61 control participants.
To confirm the predictive value of baseline CGM self-efficacy on both CGM use and HbA1c outcomes at 3 and 6 months, multiple regression analyses were used, controlling for youth age and diabetes duration. A P value of ≤0.05 defined significance.
Results
Participant characteristics
Overall, youth were 12.7±2.7 years of age, 49% were female, and 89% lived within two-parent households. Youth had a mean duration of diabetes of 6.1±3.6 years and checked BG levels 6.8±2.4 times/day, and the majority (84%) received insulin pump therapy. Eighty-three percent of participating parents were mothers. The younger group was composed of 68 youth <13 years old, and the older group was composed of 52 youth; HbA1c level was similar in both age groups, with a mean value of 7.9±0.8% (63±9 mmol/mol) for youth <13 years old and 8.0±0.8% (64±9 mmol/mmol) for youth ≥13 years old. Participant characteristics are presented in Table 1.
Table 1.
Baseline Participant Characteristics
| All youth (n=120) | Youth <13 years (n=68) | Youth ≥13 years (n=52) | |
|---|---|---|---|
| Age (years) | 12.7±2.7 | 10.8±1.4 | 15.3±1.5 |
| Sex (% female) | 49 | 51 | 46 |
| Race/ethnicity (% white) | 95 | 94 | 96 |
| Sex of participating parent (% female) | 83 | 87 | 79 |
| Family structure (% two-parent home) | 89 | 87 | 92 |
| Highest level parent education (%) | |||
| High school/GED | 11 | 13 | 8 |
| Junior/technical college or associate's degree | 26 | 24 | 29 |
| College degree | 34 | 34 | 35 |
| Graduate degree | 29 | 29 | 29 |
| Health insurance (%) | |||
| Private or military | 88 | 88 | 87 |
| Public | 13 | 12 | 13 |
| Age (years) at type 1 diabetes diagnosis | 6.6±3.6 | 5.9±2.8a | 7.5±4.3a |
| Type 1 diabetes duration (years) | 6.1±3.6 | 4.9±2.6b | 7.7±4.0b |
| Insulin dose (U/kg/day) | 0.9±0.3 | 0.9±0.3 | 0.9±0.2 |
| Blood glucose monitoring (times/day) | 6.8±2.4 | 7.5±2.1c | 5.9±2.5c |
| Insulin regimen (%) | |||
| Pump | 84 | 85 | 83 |
| Basal/bolus injections | 16 | 15 | 17 |
| HbA1c | |||
| % | 8.0±0.8 | 7.9±0.8 | 8.0±0.8 |
| mmol/mol | 64±9 | 63±9 | 64±9 |
Data are mean±SD (range) values or percentages.
Statistically significant differences: aP=0.02, bP<0.0001, cP=0.0002.
HbA1c, hemoglobin A1c.
CGM-SE survey scores
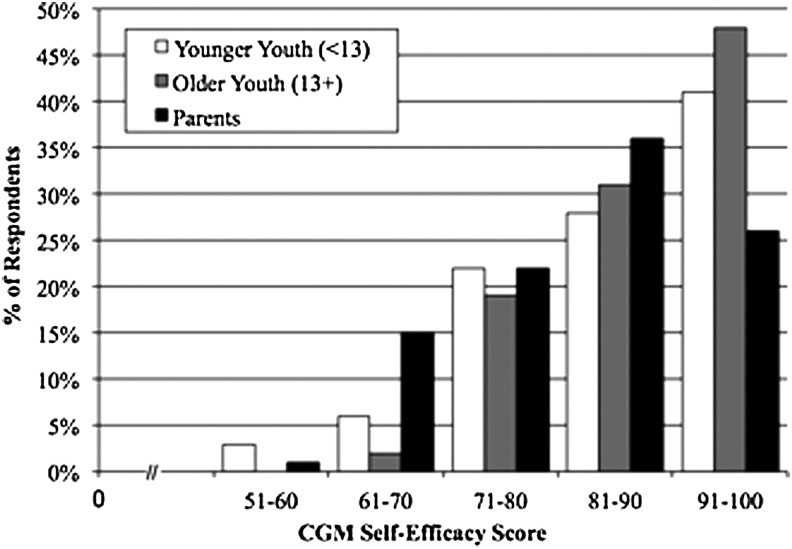
Mean baseline CGM-SE scores were 87±10 for youth (8–12 years of age, 87±11; ≥13 years of age, 88±9) and 84±10 for parents (Fig. 1). In additional analyses, youth scores were combined because of their similar distributions. As parental responses on the CGM-SE were essentially identical when comparing responses of parents of youth <13 years of age and responses of parents of youth ≥13 years of age by distribution, mean, and median, all parent scores are presented together. Although youth and parent CGM-SE scores were not correlated (r=0.13, P=0.15), youth and parent scores were each significantly correlated with several demographic and diabetes management variables. Youth CGM-SE scores were inversely correlated with the mean glucose level derived from 2 weeks of downloaded BG meter data (all youth, r=−0.30, P=0.0009; 8–12 years of age, r=−0.27, P=0.03; ≥13 years of age, r=−0.35, P=0.01). Parent CGM-SE scores were inversely correlated with age at diagnosis of diabetes in their children (r=−0.22, P=0.01), whereas parent CGM-SE scores were directly correlated with youth HbA1c levels (r=0.27, P=0.003). Neither youth or parent CGM-SE scores differed according to family structure or insulin regimen; scores were not related to youth age, diabetes duration, or frequency of BG monitoring.
FIG. 1.
Baseline continuous glucose monitoring (CGM) Self-Efficacy survey score distribution for youth and parents.
Psychometric properties of the CGM-SE survey (Aim 1)
The 11-item survey for youth <13 years of age, the 15-item survey for youth ≥13 years of age, and the 14-item parent survey demonstrated high internal consistency (Cronbach's α=0.80, 0.80, and 0.82, respectively). The surveys demonstrated good item-to-total correlations, with almost all items falling in the range of 0.40–0.80 for scale development (Table 2). Items <0.40 (two items for youth 8–12 years of age, four items for youth ≥13 years of age, and one item for parents) were retained because of their clinical relevance (Table 2).
Table 2.
Internal Consistency for Continuous Glucose Monitoring Self-Efficacy Survey: Item-to-Total Correlations and Cronbach's α
| Survey items: “I am sure I can …” | Youth <13 years of age | Youth ≥13 years of age | Parents |
|---|---|---|---|
| Insert sensor | 0.28 | 0.28 | 0.34 |
| Calibrate sensor | 0.58 | 0.62 | 0.51 |
| Keep the receiver | 0.32 | 0.41 | 0.58 |
| Look at the receiver | 0.48 | 0.59 | 0.53 |
| Respond to CGM alarms | 0.42 | 0.48 | 0.50 |
| Charge the receiver | 0.61 | 0.54 | 0.51 |
| Ask for help with CGM | 0.49 | 0.55 | — |
| Wear/work with child to wear CGM at least 6 days a week | 0.61 | 0.26 | 0.45 |
| Talk to my parents if having a hard time using CGM | 0.43 | 0.38 | — |
| Respond to CGM alarms at school | 0.55 | 0.55 | — |
| Respond to CGM alarms when with friends | 0.51 | 0.63 | — |
| Download CGM data | — | 0.17 | 0.53 |
| Problem-solve technical difficulties with device | — | 0.60 | 0.53 |
| Adjust insulin dose based on real-time data | — | 0.55 | 0.52 |
| Adjust insulin dose based on downloaded CGM data | — | 0.46 | 0.52 |
| Speak with medical team if needing help with CGM | — | — | 0.50 |
| Share CGM responsibilities with child | — | — | 0.42 |
| Be encouraging and supportive working with child on CGM | — | — | 0.41 |
| Cronbach's α | 0.80 | 0.80 | 0.82 |
Items are shortened for ease of presentation.
CGM, continuous glucose monitoring.
Convergent validity was established for the older youth CGM-SE survey by significant positive correlations with the PedsQL Generic Core Scales score (r=0.32, P=0.02). Similarly, convergent validity was established for the parent CGM-SE survey by positive correlations with the PedsQL Type 1 Diabetes Module (r=0.25, P=0.007) and the DMQ (r=0.19, P=0.04). As these correlations were weak, their clinical relevance requires further evaluation. There were no significant correlations between the youth and parent CGM-SE surveys with the PAID-PR/PAID-Peds, STAI/STAIC, or CES-D/CES-DC supporting discriminant validity.
Predictive validity of the CGM-SE surveys (Aim 2)
Participant characteristics
Control group participants (48% female) included in the predictive validity analyses consisted of 61 youth with type 1 diabetes. Their mean age was 12.7±2.9 years old, and they had a diabetes duration of 6.3±3.8 years, daily insulin dose of 0.9±0.3 units/kg, and BG monitoring frequency of 7.0±2.6 times daily. The majority (80%) received pump therapy. Characteristics of these control group participants were similar to the intervention participants.
CGM use
After 3 months, mean CGM use was 99.6±49.0 h/week (range, 0–157.3 h/week); after 6 months, mean CGM use was 82.5±55.6 h/week (range, 0–156.8 h/week). CGM use data were unavailable for only one participant because of multiple missed visits. It was not unexpected that the mean hours/week of CGM use decreased from 3 to 6 months (P=0.0013).
Glycemic control
At baseline, the mean HbA1c level for the 61 controls was 7.9±0.9% (range, 6.2–11.1%) (63±10 mmol/mol [range, 44–98 mmol/mmol]). After 3 months, the HbA1c level was 7.7±0.8% (range, 6.1–10.1%) (61±9 mmol/mol [range, 43–87 mmol/mmol]). After 6 months, the HbA1c level was 7.8±0.8% (range, 6.0–10.6%) (62±9 mmol/mol [range, 42–92 mmol/mmol]). There was one missing HbA1c value at 3 months because of missed visits. There was a significant difference between baseline versus 3-month and 6-month HbA1c (P=0.03 and P=0.01, respectively); the 3-month and 6-month HbA1c values did not differ.
CGM self-efficacy
At baseline, the mean CGM-SE score for the 61 control youth was 87±11 (range, 53–100); the mean CGM-SE score for the control parents was 84±10 (range, 62–100). There was a significant difference in youth CGM-SE scores according to CGM use at 3 and 6 months divided into three categories. At 3 months, youth who wore the CGM device 0–≤48 h/week had reported significantly lower CGM self-efficacy (80±3) compared with youth who wore the CGM device for >48–120 h/week (88±2) (P=0.04) or >120 h/week (88±2) (P=0.049). At 6 months, youth who wore the CGM device 0–≤48 h/week had reported significantly lower CGM self-efficacy (82±2) compared with youth who wore the CGM device for >48–120 h/week (90±2) (P=0.03) or >120 h/week (88±2) (P=0.08). It is interesting that parent CGM-SE scores did not differ according to youth CGM use at either 3 months or 6 months.
To assess the predictive validity of the CGM-SE surveys, we examined CGM use and HbA1c level after 3 and 6 months according to baseline CGM-SE scores. Youth who reported CGM-SE scores of >80 used CGM significantly more often at 3 and 6 months compared with youth with scores of ≤80: 3-month CGM use, 110.1±41.1 versus 70.8±58.2 h/week, respectively (P=0.005); 6-month CGM use, 94.4±50.7 versus 48.8±56.7 h/week, respectively (P=0.004). Additionally, youth reporting higher CGM self-efficacy had significantly lower HbA1c levels after 3 and 6 months compared with youth reporting lower CGM self-efficacy: 3-month HbA1c, 7.5±0.7% (58±8 mmol/mol) versus 8.3±0.9% (67±10 mmol/mol), respectively (P=0.0004); 6-month HbA1c, 7.6±0.7% (60±8 mmol/mol) versus 8.2±0.9% (66±10 mmol/mol) (P=0.02), respectively. Parent-reported CGM-SE did not predict youth CGM use or youth HbA1c level at 3 or 6 months.
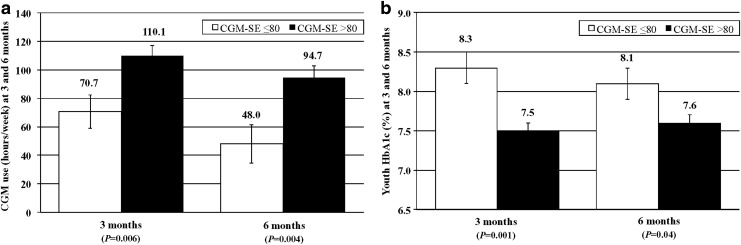
In a multivariate model (R2=0.13, P=0.045) controlling for youth age and diabetes duration, youth CGM-SE (P=0.006) significantly predicted 3-month CGM use (Fig. 2a). Youth reporting CGM-SE scores of >80 used CGM 110.1 h/week compared with 70.7 h/week after 3 months for youth reporting CGM-SE scores of ≤80 at baseline. A multivariate model (R2=0.14, P=0.04) also demonstrated that youth CGM-SE (P=0.004) significantly predicted 6-month CGM use. Youth reporting CGM-SE scores of >80 used CGM 94.7 h/week compared with 48.0 h/week after 6 months for youth reporting CGM-SE scores of ≤80 at baseline.
FIG. 2.
(a) Continuous glucose monitoring (CGM) use (in hours/week) and (b) hemoglobin A1c (HbA1c) level at 3 and 6 months according to level of baseline youth CGM Self-Efficacy (CGM-SE) survey score: youth CGM-SE score of ≤80 (white bars) and CGM-SE score of >80 (black bars). Data are mean±standard error values. (a) In multivariate models, baseline youth CGM-SE score significantly predicted CGM use (in hours/week) at 3 months (model yielded R2=0.13, P=0.045) and at 6 months (model yielded R2=0.14, P=0.04). (b) In multivariate models, baseline youth CGM-SE score significantly predicted HbA1c level at 3 months (model yielded R2=0.22, P=0.003) with a trend toward significance at 6 months (model yielded R2=0.11, P=0.08).
In a multivariate model (R2=0.22, P=0.003) controlling for youth age and diabetes duration, youth CGM-SE (P=0.001) also significantly predicted 3-month HbA1c level (Fig. 2b). Youth reporting CGM-SE scores of >80 had a 3-month HbA1c value of 7.5% compared with 8.3% for those reporting lower CGM-SE at baseline. A multivariate model (R2=0.11, P=0.08) also demonstrated that youth CGM-SE (P=0.04) significantly predicted 6-month HbA1c. Youth reporting CGM-SE scores of >80 had a 6-month HbA1c value of 7.6% compared with 8.1% for those reporting lower CGM-SE at baseline.
Discussion
CGM technologies remain an underutilized approach to improving glycemic control in children and adolescents with type 1 diabetes.19,20 Although youth may initiate CGM, many children and adolescents discontinue use because of the additional burdens associated with current CGM devices,21–24 where ongoing BG monitoring is required for CGM calibration as well as for confirmation of glucose levels prior to initiating treatments for either high or low glucose readings. Nonetheless, CGM technologies offer opportunities to improve glycemic control while avoiding severe hypoglycemia.1,25,26 Therefore, there remains a need to identify approaches to increase uptake and durability of CGM use, especially in the pediatric population. The current study identified the opportunity to utilize perceived self-efficacy related to CGM use in both children and adolescents with type 1 diabetes. The CGM-SE surveys demonstrated adequate psychometric properties for both the youth and parent versions, although only the youth versions, for those 8–12 years of age as well as for those 13 years of age and older, had significant predictive value for CGM use 3 and 6 months after initiation.
The psychometric evaluation provided support for the reliability and validity of the CGM-SE surveys. The surveys had good item-to-total correlations and high internal consistency for youth and parents, which established reliability.
Although baseline youth CGM-SE scores were not related to age, diabetes duration, HbA1c, or BG monitoring frequency, there was a significant inverse relationship between self-efficacy scores and mean BG levels, suggesting that youth with lower glucose levels seem to have greater confidence in their use of CGM. Although the literature has reported that greater general diabetes self-efficacy is associated with diabetes management adherence and BG monitoring frequency,6,27–29 we found no relationships between youth-reported CGM self-efficacy and diabetes adherence, depression symptoms, or anxiety. However, there was a significant positive association between self-efficacy reported by teens and general quality of life, suggesting a possible relationship between self-confidence in diabetes management and quality of life for teens with type 1 diabetes.
Parent CGM-SE scores had a significant direct relationship to youth HbA1c level and an inverse relationship to youth age at diagnosis. These findings suggest that parents of youth with higher HbA1c levels report greater confidence in their child's use of CGM, possibly reflecting parental hopefulness for improvements in their child's glycemic control with new advances such as CGM. It is not surprising that parent CGM-SE scores were also significantly related to parent report of diabetes management adherence and the parent proxy report of their child's diabetes-specific quality of life. Although these correlations were statistically significant, their clinical relevance requires ongoing exploration. It is notable that the youth and parent CGM-SE scores were not correlated.
In assessment of predictive validity of the CGM-SE surveys, only the youth-reported CGM-SE scores were significantly related to future CGM use and glycemic control. Youth who reported baseline CGM-SE scores of >80 had significantly greater CGM wear at 3 months and 6 months compared with those youth with lower scores. Similarly, youth with baseline CGM-SE scores of >80 had lower HbA1c levels at 3 months and 6 months compared with those youth with lower scores. The parent CGM-SE survey did not demonstrate the same predictive validity for pediatric CGM use, likely because the parent survey assesses the parents' confidence in helping their child use CGM as well as their confidence in their child's ability to use CGM.
It is important that this study revealed that youth CGM self-efficacy does predict CGM wear and improvement in glycemic control over a 6-month time period. This finding coincides with the theoretical model of Bandura3 that self-efficacy is an important determinant of behavior and that greater self-efficacy translates to effort expended by an individual over a duration of time in the face of challenging circumstances. This finding is pertinent given the known challenges that youth with type 1 diabetes face in wearing CGM technologies and achieving target glycemic control. Assessing self-efficacy at the onset of CGM use may be important for the multidisciplinary diabetes team to consider. In particular, youth who report low self-efficacy at the start of CGM, with CGM-SE scores of ≤80, likely warrant greater education and support for CGM implementation to succeed with durable CGM use over time. Identifying ways to decrease the burden of new technologies like CGM and the artificial pancreas may enhance patients' self-confidence in their use. As current artificial pancreas systems require substantial patient input, their use will likely also benefit from an understanding of self-efficacy.30
There are caveats to these analyses. First, the study included a relatively small sample of homogeneous young patients with type 1 diabetes. The majority of youth came from two-parent households with educated parents. Furthermore, all patients received intensive insulin therapy, and the overwhelming majority received insulin pump therapy. Although our sample of youth using CGM was similar to the pediatric patients using CGM in the Type 1 Diabetes Exchange,31 future assessments of the CGM-SE surveys should include more diverse populations, including those with lower literacy rates, in order to strengthen the generalizability of the instrument's utility.
Additionally, as the area of self-efficacy related to CGM use is novel, future analyses in larger sample sizes are needed to evaluate the sensitivity and specificity of the CGM-SE score threshold. Although we evaluated the self-efficacy score distributions according to previously established CGM use categories to determine the threshold of 80, further work in larger samples would help to verify the self-efficacy cut-point related to CGM use. Additional research in larger samples would also facilitate evaluation of the parent instrument.
As the parent study of this evaluation was aimed at implementing CGM in a pediatric sample, it is not surprising that the patients had to demonstrate acceptance and use of intensive insulin therapy prior to starting CGM. Nonetheless, although the patients were all intensively treated and desired to start CGM by virtue of their agreement to enter the 2-year RCT, several patients in the control group had already reduced their use of CGM after only 6 months of implementing the device. Additional follow-up will be needed to determine how CGM self-efficacy affects longer-term use. Future research will be needed to assess changes in self-efficacy over time in addition to determining if and how behavioral interventions to overcome barriers to CGM use impacts CGM self-efficacy.
Finally, evaluation of the performance of the parent CGM-SE survey only included youth 8–17 years of age; assessment in parents of patients under 8 years old is also needed, especially given the challenge associated with CGM use in this pediatric population.32,33
In summary, the CGM-SE surveys for youth and parents demonstrate adequate psychometric properties for assessing confidence in using CGM. In addition, the youth CGM-SE surveys demonstrate significant predictive validity for future CGM use and glycemic control, but the parent CGM-SE survey does not. Indeed, durable pediatric CGM use remains dependent on the child rather than on the parent. Thus, the youth CGM-SE surveys provide a method of assessing youth CGM self-efficacy in both research and clinical settings as efforts continue to address ways to increase uptake and sustained use of CGM in pediatric populations with type 1 diabetes. Additional assessment of the parent CGM-SE survey appears warranted.
Acknowledgments
We thank the families who participated in this investigation and the research staff for their efforts. We would also like to thank Carolyn Jenkins, DrPH, MSN, RD, LD, FAAN, Mathew Gregoski, PhD, and Martina Mueller, PhD, of the Medical University of South Carolina College of Nursing for their helpful comments and suggestions. This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers R01DK089349 and P30DK036836, the Katherine Adler Astrove Youth Education Fund, the Maria Griffin Drury Pediatric Fund, and the Eleanor Chesterman Beatson Fund.
Author Disclosure Statement
L.M.L. has received institutional grant support from Dexcom, Inc. for other research investigations and has served as a consultant for Dexcom, Inc. L.E.R., L.K.V., J.T.M., D.A.B., and M.L.K. declare no competing financial interests exist.
L.E.R. wrote the initial draft of the manuscript. L.E.R., L.K.V., and L.M.L. researched the data, conducted analyses, and revised the manuscript. J.T.M., D.A.B., and M.L.K. researched the data and reviewed/edited the manuscript. L.M.L. is the guarantor of this work and takes responsibility for the integrity of this work.
References
- 1.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 2.Chase HP, Beck RW, Xing D, Tamborlane WV, Coffey J, Fox LA, Ives B, Keady J, Kollman C, Laffel L, Ruedy KJ: Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther 2010;12:507–515 [DOI] [PubMed] [Google Scholar]
- 3.Bandura A: Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215 [DOI] [PubMed] [Google Scholar]
- 4.Cardwell MS: Improving medical adherence in women with gestational diabetes through self-efficacy. Clin Diabetes 2013;31:110–115 [Google Scholar]
- 5.Bandura A, Locke EA: Negative self-efficacy and goal effects revisited. J Appl Psychol 2003;88:87–99 [DOI] [PubMed] [Google Scholar]
- 6.Iannotti RJ, Schneider S, Nansel TR, Haynie DL, Plotnick LP, Clark LM, Sobel DO, Simons-Morton B: Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. J Dev Behav Pediatr 2006;27:98–105 [DOI] [PubMed] [Google Scholar]
- 7.Bandura A: Health promotion by social cognitive means. Health Educ Behav 2004;31:143–164 [DOI] [PubMed] [Google Scholar]
- 8.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL: The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care 2003;26:631–637 [DOI] [PubMed] [Google Scholar]
- 9.Varni JW, Seid M, Kurtin PS: PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 Generic Core Scales in healthy and patient populations. Med Care 2001;39:800–812 [DOI] [PubMed] [Google Scholar]
- 10.Mehta SN, Nansel TR, Volkening LK, Butler DA, Haynie DL, Laffel LM: Validation of a contemporary adherence measure for youth with type 1 diabetes: the Diabetes Management Questionnaire. Diabet Med 2015. (in press). doi: 10.1111/dme.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markowitz JT, Volkening LK, Butler DA, Antisdel-Lomaglio J, Anderson BJ, Laffel LM: Re-examining a measure of diabetes-related burden in parents of young people with Type 1 diabetes: the Problem Areas in Diabetes Survey—Parent Revised version (PAID-PR). Diabet Med 2012;29:526–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz JT, Butler DA, Volkening LK, Laffel LM: Youth-perceived burden of type 1 diabetes (T1D): Problem Areas in Diabetes—Pediatric Survey (PAID-Peds) [abstract]. Diabetes 2013;62:A203–A204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA: Test Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1983 [Google Scholar]
- 14.Spielberger CD: Manual for the State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press, 1973 [Google Scholar]
- 15.Radloff LS: The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 16.Fendrich M, Weissman MM, Warner V: Screening for depressive disorder in children and adolescents: validating the Center for Epidemiologic Studies Depression Scale for Children. Am J Epidemiol 1990;131:538–551 [DOI] [PubMed] [Google Scholar]
- 17.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA: Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther 2008;10:377–383 [DOI] [PubMed] [Google Scholar]
- 18.DiIorio CK: Measurement in Health Behavior. San Francisco: Jossey-Bass, 2005 [Google Scholar]
- 19.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, Dubose SN, Hall CA: The T1D Exchange Clinic Registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 20.Ludwig-Seibold CU, Holder M, Rami B, Raile K, Heidtmann B, Holl RW: Continuous glucose monitoring in children, adolescents, and adults with type 1 diabetes mellitus: analysis from the prospective DPV diabetes documentation and quality management system from Germany and Austria. Pediatr Diabetes 2012;13:12–14 [DOI] [PubMed] [Google Scholar]
- 21.Weinzimer S, Xing D, Tansey M, Fiallo-Scharer R, Mauras N, Wysocki T, Beck R, Tamborlane W, Ruedy K: Prolonged use of continuous glucose monitors in children with type 1 diabetes on continuous subcutaneous insulin infusion or intensive multiple-daily injection therapy. Pediatr Diabetes 2009;10:91–96 [DOI] [PubMed] [Google Scholar]
- 22.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care 2009;32:1947–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckingham B, Beck RW, Ruedy KJ, Cheng P, Kollman C, Weinzimer SA, DiMeglio LA, Bremer AA, Slover R, Tamborlane WV: Effectiveness of early intensive therapy on beta-cell preservation in type 1 diabetes. Diabetes Care 2013;36:4030–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tansey M, Laffel L, Cheng J, Beck R, Coffey J, Huang E, Kollman C, Lawrence J, Lee J, Ruedy K, Tamborlane W, Wysocki T, Xing D; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Satisfaction with continuous glucose monitoring in adults and youths with Type 1 diabetes. Diabet Med 2011;28:1118–1122 [DOI] [PubMed] [Google Scholar]
- 25.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slover RH, Welsh JB, Criego A, Weinzimer SA, Willi SM, Wood MA, Tamborlane WV: Effectiveness of sensor-augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes 2012;13:6–11 [DOI] [PubMed] [Google Scholar]
- 27.Kristensen LJ, Thastum M, Mose AH, Birkebaek NH: Psychometric evaluation of the adherence in diabetes questionnaire. Diabetes Care 2012;35:2161–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sander EP, Odell S, Hood KK: Diabetes-specific family conflict and blood glucose monitoring in adolescents with type 1 diabetes: mediational role of diabetes self-efficacy. Diabetes Spectr 2010;23:89–94 [Google Scholar]
- 29.Gillibrand R, Stevenson J: The extended health belief model applied to the experience of diabetes in young people. Br J Health Psychol 2006;11:155–169 [DOI] [PubMed] [Google Scholar]
- 30.Shah VN, Shoskes A, Tawfik B, Garg SK: Closed-loop system in the management of diabetes: past, present, and future. Diabetes Technol Ther 2014;16:477–490 [DOI] [PubMed] [Google Scholar]
- 31.Wong JC, Foster NC, Maahs DM, Raghinaru D, Bergenstal RM, Ahmann AJ, Peters AL, Bode BW, Aleppo G, Hirsch IB, Kleis L, Chase HP, Dubose SN, Miller KM, Beck RW, Adi S: Real-time continuous glucose monitoring among participants in the T1D Exchange Clinic Registry. Diabetes Care 2014;37:2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M, White NH, Weinzimer SA, Tamborlane W, Kollman C: A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care 2012;35:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsalikian E, Fox L, Weinzimer S, Buckingham B, White NH, Beck R, Kollman C, Xing D, Ruedy K: Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr Diabetes 2012;13:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]