Abstract
Significance: Healing of epidermal wounds is a fundamentally conserved process found in essentially all multicellular organisms. Studies of anatomically simple and genetically tractable model invertebrates can illuminate the roles of key genes and mechanisms in wound healing.
Recent Advances: The nematode skin is composed of a simple epithelium, the epidermis (also known as hypodermis), and an associated extracellular cuticle. Nematodes likely have a robust capacity for epidermal repair; yet until recently, relatively few studies have directly analyzed wound healing. Here we review epidermal wound responses and repair in the model nematode Caenorhabditis elegans.
Critical Issues: Wounding the epidermis triggers a cutaneous innate immune response and wound closure. The innate immune response involves upregulation of a suite of antimicrobial peptides. Wound closure involves a Ca2+-triggered rearrangement of the actin cytoskeleton. These processes appear to be initiated independently, yet, their coordinated activity allows the animal to survive otherwise fatal skin wounds.
Future Directions: Unanswered questions include the nature of the damage-associated molecular patterns sensed by the epidermis, the signaling pathways relaying Ca2+ to the cytoskeleton, and the mechanisms of permeability barrier repair.

Andrew D. Chisholm, PhD
Scope and Significance
This review focuses on epidermal wound repair in nematodes such as Caenorhabditis elegans. Two major branches of the wound repair process are discussed: cutaneous innate immune responses to damage and mechanisms that close the wound. Analysis of the genetic basis of these responses has revealed potentially conserved pathways involved in epidermal wound repair, including a G-protein–coupled receptor (GPCR)/mitogen-activated protein kinase (MAPK) cascade in innate immunity and a transient receptor potential M class (TRPM) channel/Ca2+ pathway that regulates the actin cytoskeleton.
Translational Relevance
Despite the major differences in morphology and molecular composition of skin layers between different animal groups, increasing evidence points to the conservation of underlying molecular pathways in wound repair. Well-known examples are the Grainyhead/Grhl transcription factors involved in barrier repair in insects and in mammals. Studies of wound responses in C. elegans have pointed to conserved roles for plasma membrane TRPM channels in epithelial Ca2+ signaling. Reactive oxygen species (ROS) may also play ancient and conserved roles in promoting tissue repair. Additional wound healing pathways discovered in simple models could provide new leads for therapies targeting wound healing pathologies.
Clinical Relevance
Clinically, important wound healing pathologies range from excessive wound healing (keloid and hypertrophic scarring) to nonhealing chronic wounds and diabetic ulcers. The latter constitute a growing public health problem, costing an estimated $5–$10 billion annually, in the United States. Improved understanding of epidermal repair mechanisms in simple models will enhance our understanding of wound healing biology and should contribute to better approaches to wound care.
Background and Overview of Wound Healing Processes and Models
Wound healing is required for organismal integrity and survival and an essential precursor to skin regeneration.1 Wound healing in the mammalian skin involves a large number of tissues, cellular processes, and signaling pathways. In overview, three main branches of wound repair are the epidermal innate immune response, cell migration and wound closure, and barrier repair.2,3 Epidermal barrier epithelia of different animals display many differences in the structure and molecular composition, yet are increasingly recognized as being built on a common genetic ground plan. For example, transcription factors of the Grainyhead family play conserved roles in barrier epithelium formation and wound healing.4,5
The complexity of mammalian skin wound repair has motivated examination of simpler models of wound healing. Studies of wound repair in the fruit fly Drosophila have identified signal transduction pathways, transcription factors, and cytoskeletal regulators involved in wound repair.6–9 Studies of repair in genetic model organisms such as Drosophila also allow the use of unbiased forward genetic screens to identify new and unexpected contributors to wound repair.8,10 Zebrafish are a tractable model for wound repair; studies in zebrafish revealed the role of ROS as wound-produced chemoattractants.11,12 Studies of scarless wound healing in embryos, mutants, or species that do not display scarring have highlighted the role of the inflammatory response in scar formation.13,14 Finally, single cell wound healing in models such as the Xenopus oocyte or the early Drosophila embryo reveals parallels between the single cell and epithelial repair processes.15,16 Here, we review wound healing in the nematode C. elegans, a comparatively recent addition to the pantheon of wound healing models.
Discussion
C. elegans epidermal biology
Nematode worms are a large and successful animal phylum with representatives in almost every ecological niche. C. elegans is a small free-living nematode well known for its tractability for molecular and genetic manipulation: animals are small (1 mm long) and easily propagated in the laboratory on bacterial food sources. The generation time of C. elegans is 3 days and the lifespan ∼3 weeks. C. elegans is also essentially transparent, facilitating imaging of epidermal cells and subcellular structures during wounding in vivo.
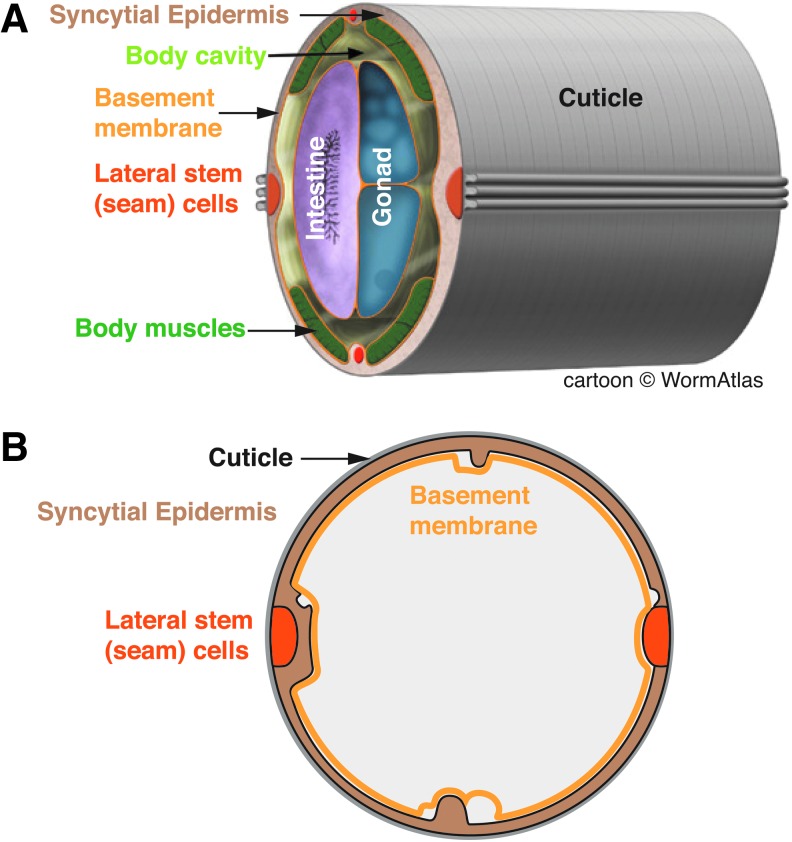
The skin of C. elegans like that of most nematodes is composed of a simple epithelium, the epidermis, or hypodermis, which secretes the outer extracellular layer or cuticle (Fig. 1); the biology of the nematode epidermis has recently been reviewed in depth.17,18 Nematodes lack a rigid exoskeleton; the organismal shape is maintained by internal pressure (the hydrostatic skeleton). The collagenous cuticle is flexible yet contributes to the overall rigidity to the animal. In addition to the cuticle, additional less well-characterized extracellular layers (epicuticle and surface coat) are present and may contribute to the permeability barrier function of the skin.
Figure 1.
Anatomy of the Caenorhabditis elegans epidermis. (A) Stereo view of the adult hermaphrodite midbody, adapted from WormAtlas (www.wormatlas.org, Z. Altun and D.H. Hall, 2012). Major tissues are color coded. (B) Cross section, highlighting the epidermis and associated extracellular matrices (cuticle and basement membrane). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The mature C. elegans epidermis consists of syncytial cells formed by cell–cell fusion as well as lateral stem cells (seam cells). In postembryonic development, the epidermis grows in size by addition of new cells from the seam; in adults, the epidermis is postmitotic and undergoes polyploidization to support continued growth. Thus, the adult epidermal syncytium combines the advantages of single-cell wound models (accessible imaging and cell biology) with the characteristics of a differentiated barrier epithelium.
Evidence for epidermal wound healing in nematodes
The literature on wound healing in nematodes is remarkably sparse. Abnormalities in tail morphology in nematodes collected from the wild were interpreted as resulting from imperfect wound healing or regeneration by Allgén.19 However, wound healing was not directly assayed, and there appears to have been little follow-up to these early observations. In 1988, Poinar concluded that “although the paucity of the information on the subject leaves one unable to draw any definite conclusions on wound healing and regeneration, the observations of Allgén and others indicate that some type of wound repair may exist in nematodes and that further investigation into this subject is warranted”.20 Indeed, it has long been known that C. elegans survives epidermal puncture wounds caused by microinjection needles, initially used for electrophysiological recording21 and subsequently for introduction of macromolecules such as nucleic acids.22 Wounding by stabs with a microinjection needle is a simple and robust method to elicit epidermal wound responses, but may also damage internal tissues. More precise wounding of the epidermis using femtosecond laser irradiation induces a subset of wound responses (see next section).
Cutaneous innate immune responses to wounding and infection
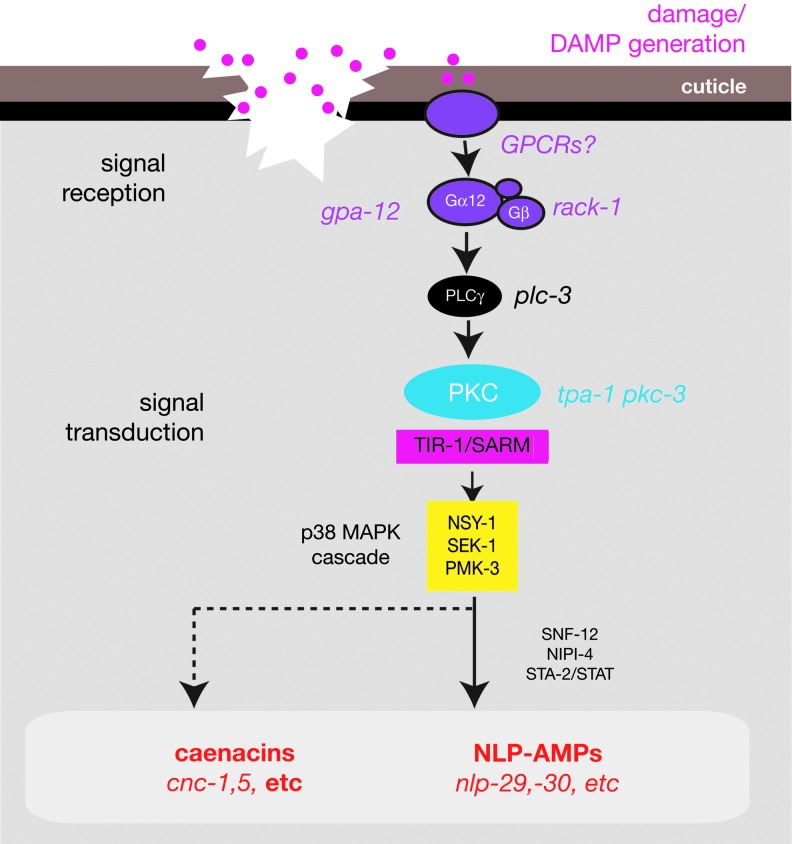
The first wound response to be characterized in detail in C. elegans is the epidermal innate immune response. Analysis of the epidermal innate immune response to damage began with pioneering studies of skin-penetrating pathogens.23 Many nematophagous (nematode-eating) fungi attack their hosts through the skin.24 Fungi such as Drechmeria coniospora generate spores that stick to the cuticle and extend hyphae through the underlying epidermis to eventually colonize the animal. Fungal infection specifically induces epidermal expression of a large set of antimicrobial peptides (AMPs) and other proteins.25,26 The signal transduction pathways responsible for induction of AMP expression in response to infection have been extensively characterized and reviewed recently.27,28 In overview, at least two major pathways regulate epidermal AMP induction: a MAPK cascade required for induction of the neuropeptide-like (nlp) genes23 (Fig. 2), and a TGFβ cascade involved in induction of caenacin (cnc) peptide expression.29 As the role of TGFβ signaling in the response to wounding is not yet clear, we focus here on the pathway involved in nlp AMP induction.
Figure 2.
Signal transduction cascade inducing antimicrobial peptide expression after epidermal wounding, based on Engelmann and Pujol.27 For details, see section titled “Cutaneous innate immune responses to wounding and infection.” Not shown are genes such as nipi-3 or hsp-3, which are preferentially involved in the response to fungal infection. Induction of caenacins, such as cnc-1 or cnc-5 after wounding is partly dependent on the PMK-1 pathway. The role of TGFβ signaling in wound responses is not yet known. SNF-12 and STA-2/STAT may act downstream of the p38 MAPK cascade; NIPI-4 acts downstream of GPA-12 but has not been further positioned in the pathway. MAPK, mitogen-activated protein kinase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The process of skin penetration by fungal hyphae resembles wounding, leading to the question of whether responses to infection are specific to the pathogen or are more general responses to skin damage. Using needles or lasers to wound the skin, Pujol et al. showed that physical damage was sufficient to induce some of the epidermal AMPs that are induced by infection, through the same MAPK cascade involved in AMP induction after infection.30 The Tribbles-like kinase NIPI-3 is preferentially required for AMP induction after infection but not wounding, suggesting infection and wounding act through different upstream sensors that converge on common outputs to regulate AMP expression.30 The chaperone HSP-3 is also specifically required for infection-induced but not for wound-induced AMP expression.31 Because C. elegans is constantly associated with its bacterial food source (E. coli in the laboratory), complete sterile wounding has not been performed. However, these experiments suggest that the innate immune response to infection overlaps with a transcriptional response to epidermal or cuticle damage. Although nematode AMPs do not resemble mammalian AMPs in primary sequence, sterile injury rather than infection or inflammation is a major inducer of the mammalian cutaneous innate immune response.32
Upstream of the TIR-1/MAPK pathway, AMP induction by wounding is known to require a signaling cascade involving PKCδ/TPA-1, phospholipase Cγ/PLC-3, the Gα protein GPA-12, and the Gβ-like protein RACK-1.33 The involvement of G protein signaling in the innate immune response to wounding suggests that one or more GPCRs sense tissue damage or a ligand generated by damage. Such host-derived damage-associated molecular patterns (DAMPs)34 have been identified in some paradigms of injury35 but have not yet been characterized in C. elegans.
Fungal infection and wounding also induce production of ROS through the Ca2+-activated enzyme Duox/BLI-3.36 ROS appear to act through the DAF-16/FOXO pathway to promote survival after infection or wounding; the transcriptional targets of DAF-16 in the epidermal innate immune response have not yet been elucidated. Interestingly, FOXO transcription factors have recently been implicated in mammalian wound healing.37
Wound closure: epidermal calcium and cytoskeletal rearrangement
How does the epidermis physically close wounds? Recent findings indicate that wounding triggers a rapid and sustained elevation of epidermal Ca2+ that is required for actin to polymerize into rings surrounding the wound site. Closure of these actin rings is required for survival of wounding (Fig. 3).
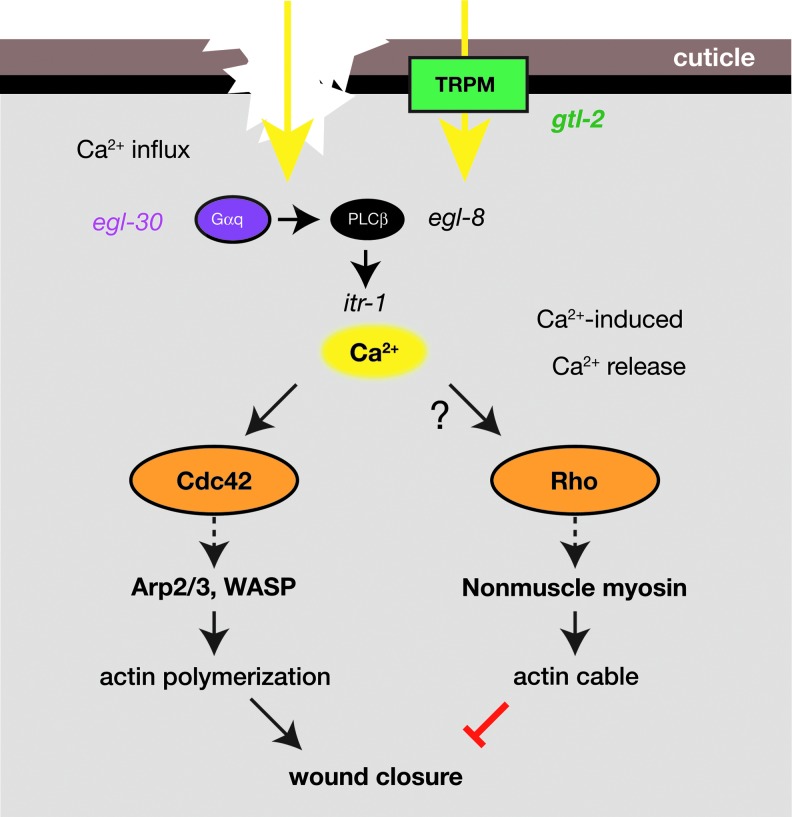
Figure 3.
Signals and processes involved in epidermal wound closure, based on Xu and Chisholm.42 Wounding triggers a sustained rise in epidermal cytosolic Ca2+, initially due to influx from extracellular pools and subsequently from calcium-induced Ca2+ release. The TRPM channel GTL-2 is involved in the initial Ca2+ influx. Ca2+ is required for formation of actin rings that close wounds and may act through the antagonistic small GTPases CDC-42, which is required for actin ring formation, and RHO-1, which inhibits ring formation. TRPM, transient receptor potential, M class. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Calcium signals have long been known to be central to epidermal homeostasis and wound repair.38–40 Elevation of intracellular Ca2+ is seen in numerous models of single cell and tissue damage and appears to be a near-universal response to cellular injury. The advent of genetically encoded Ca2+ sensors such as the GCaMPs41 has greatly simplified imaging of Ca2+ dynamics in vivo. In C. elegans, wounding triggers elevation of Ca2+ at the wound site within less than a second42; the elevated intracellular Ca2+ spreads out in a wave-like manner through the epidermal syncytium, eventually extending several hundred microns. The elevation in epidermal Ca2+ persists for 1–2 h after injury before returning to baseline levels.
The elevated epidermal Ca2+ appears to be derived from multiple sources. Ca2+ influx through the breach in the plasma membrane might account for some of the initial increase in cytosolic Ca2+; the external Ca2+ reservoir could reside in the cuticle or pseudocoelom. A plasma membrane TRPM channel GTL-2 is also required for Ca2+ influx in C. elegans wounding and might mediate Ca2+ influx directly.42 Interestingly, a TRPM channel is also required in Drosophila wound healing, acting upstream of the actin cytoskeleton.43 In zebrafish, TRPV1 functions in keratinocyte migration, mechanistically relevant to wound healing.44 Numerous TRP channels are expressed in the mammalian skin,45 and some are implicated in epidermal barrier repair.46 Whereas the exact role of TRP channels in epidermal Ca2+ homeostasis is likely to differ between species, these results suggest an important conserved role for Ca2+ channels in wound repair.
Downstream of GTL-2, Gαq/EGL-30 and PLCβ/EGL-8 act within the epidermis to control Ca2+ levels through the inositol trisphosphate receptor ITR-1.42 The sustained rise in epidermal Ca2+ involves ITR-1–dependent Ca2+-induced calcium release from internal stores, as Ca2+ levels are reduced by expression of dominant-negative ITR-1. Wound-triggered Ca2+ signaling is not required for AMP induction,42 although as mentioned above it may play a role in ROS production. The epidermal Ca2+ signal appears to be specifically involved in the formation of actin rings at the wound site. After wounding, F-actin polymerization (visualized using GFP-moesin) begins within minutes, forming a complex ring structure that diminishes in radius over the next 1–2 h, corresponding to closure of the wound. Actin ring formation is blocked by Ca2+ chelation and in gtl-2 mutants; the gtl-2 defect is partly suppressed by incubation in buffers with high external Ca2+.
Exactly how Ca2+ triggers local actin polymerization at wound sites remains to be deciphered. Actin ring formation is dependent on the small GTPase CDC-42 and actin nucleation factors such as WASP and the Arp2/3 complex. Unexpectedly, the loss of function in nonmuscle myosin leads to accelerated closure of the actin ring, suggesting that ring closure is driven by actin polymerization rather than by a purse-string mechanism and that nonmuscle myosin-based contractility restrains wound closure.
Negative regulation of wound responses by DAPK-1
Wound repair pathways appear to be under negative control in many systems. Such negative regulation may allow rapid modulation of repair processes, such that they are only invoked after damage above a certain threshold and then are repressed as soon as the damage has been repaired. Some insights into the mechanisms of negative control of wound repair have come from analysis of the C. elegans death-associated protein kinase, DAPK-1. DAPK-1 is the C. elegans member of a conserved family of serine–threonine kinases that include human DAPK and mouse Dapk1.47 Mutations in dapk-1 were isolated as displaying late-onset hypertrophic cuticle growth and were subsequently found to display constitutively elevated levels of epidermal AMPs.48 Furthermore, dapk-1 mutants display accelerated wound closure compared with the wild type and suppress the wound closure defects of mutants such as gtl-2.42 Thus, in the absence of wounding, reduction in DAPK-1 activity results in inappropriate activation of wound responses (cuticle secretion and AMP expression); after wounding, lack of DAPK-1 accelerates wound closure. DAPK-1 therefore appears to act as a coordinate negative regulator of the multiple facets of the wound response. Although the DAPK family has been linked to apoptosis or autophagy in mammals,49 DAPK-1 does not appear to regulate wound responses through known cell death pathways. Mammalian DAPK has not yet been tested for roles in wound healing, but is a negative regulator of inflammatory responses.50 Identification of DAPK-1 interactors could shed light on how DAPK-1 regulates diverse wound response pathways.47
Special aspects of the C. elegans wound model
Whereas C. elegans exemplifies many general features of wound repair, the nematode skin also exhibits some distinctive biological traits. These serve to illustrate the diversity of contexts in which wound healing can occur.
Most notably, the adult C. elegans epidermis is a postmitotic epithelium composed almost entirely of syncytia formed by cell–cell fusions. Stem cell (seam cell) divisions are completed in the fourth larval stage. Epidermal nuclei undergo polyploidization in the course of adult growth. As the adult epidermis is composed of postmitotic syncytia, wounding does not (apparently) induce a proliferative response as in other models. In Drosophila, wounding can induce epidermal polyploidization51; this has not yet been investigated in C. elegans.
In many animals, injury activates coagulation systems leading to clotting of the blood (vertebrates) or hemolymph (insects, other invertebrates). Although vertebrate and invertebrate clotting factors are generally divergent, in both cases they are produced by circulating blood cells or hemocytes or are present as inactive precursors in the circulating hemolymph. As part of the inflammatory response to injury, neutrophils or macrophages (or their equivalents) are recruited to wound sites. In adult Drosophila and zebrafish, circulating blood cells are attracted to sites of injury52,53; in Drosophila embryos, hemocytes migrate toward wounds even in the absence of a developed circulatory system.54 In contrast, C. elegans lacks a defined circulatory system or migratory blood cells; a fluid-filled body cavity or pseudocoelom distributes nutrients and other molecules within the animal. Induction of the AMP cnc-2 in the epidermis after fungal infection involves neuronal expression of a TGFβ signal29; it is not yet known if wounding triggers neuroimmune or systemic wound responses analogous to those described in other organisms.
C. elegans lacks orthologs of known invertebrate coagulation or melanization factors such as transglutaminase or phenoloxidase.55 However, C. elegans encodes several tyrosinases capable of generating melanin, and melanin has been detected in the C. elegans cuticle.56 Melanization has been observed as a reaction to UV damage in the parasitic nematode Teladorsagia circumcincta.57 It would be interesting to explore whether wounding triggers a melanization reaction in C. elegans.
Summary and Remaining Questions
C. elegans has a robust and sophisticated set of responses that repair epidermal damage and defend against pathogenic attack at wounds. It seems likely that other nematodes would exhibit similar wound healing responses. At present, the innate immune response and wound closure are the best characterized of the C. elegans wound healing processes, yet many areas remain poorly understood, foremost of which are the identities of the initiating triggers (DAMPs) of the innate immune response. The involvement of distinct G protein subunits in the innate immune response and in wound closure argues that GPCRs may mediate initial damage sensing. Although the large number of GPCRs in the C. elegans genome (∼1500) makes searching for such receptors challenging, identification of wound-triggered GPCRs could greatly elucidate the mechanisms by which epithelia sense damage.
Actin rings form locally at wounds yet require a Ca2+ signal that is delocalized throughout the syncytial epidermis, raising the question of how such a widespread rise in epidermal Ca2+ can have a local effect at the wound site. Additional triggers such as compartment mixing might locally regulate actin polymerization. Alternatively, cytosolic Ca2+ may not be the relevant Ca2+ pool. The TRPM channel GTL-2 is critical for the wound-induced rise in epidermal Ca2+, yet it is not understood whether or how GTL-2 is gated by tissue damage.
Many other aspects of nematode wound healing remain to be explored. The mechanisms responsible for plasma membrane and cuticle resealing immediately after damage are not yet known nor is the precise mechanism leading to cuticle scarring after wounding.30 The restoration of the epidermal permeability barrier after wounding likely involves the synthesis of new cuticle and other extracellular layers,30 but mechanisms have not been characterized. Current studies have focused on relatively small needle or laser wounds that are efficiently repaired by wild-type animals; there has been little analysis of more drastic wounds such as severing of the tail, despite indications that animals may be capable of repairing such wounds. Finally, wound healing has mostly been studied in young adult animals; the effects of adult age on wound repair remain to be examined.
Take-Home Messages.
• The nematode C. elegans is a genetically tractable model organism that is able to heal and survive puncture or laser wounds to the skin.
• Skin wounding induces a cutaneous innate immune response involving transcriptional upregulation of AMPs.
• Independent of the innate immune response, a wound-triggered Ca2+ transient is required for actin cytoskeleton-mediated wound closure.
• Unresolved questions concern the nature of the DAMPs sensed by the epidermis.
Abbreviations and Acronyms
- AMP
antimicrobial peptide
- DAMP
damage-associated molecular pattern
- GPCR
G-protein–coupled receptor
- MAPK
mitogen-activated protein kinase
- ROS
reactive oxygen species
- TRPM
transient receptor potential, M class
- WASP
Wiskott-Aldrich syndrome protein
Acknowledgments and Funding Sources
The authors thank N. Pujol and J. Ewbank (CIRM Marseille) for many discussions and collaborations on C. elegans epidermal wound responses. Work in the Chisholm laboratory on C. elegans epidermal wound repair has been supported by the NSF, the France Berkeley Fund, and the NIH (R01 GM054657).
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author. No ghostwriters were used to write this article.
About the Author
Andrew Chisholm, PhD, is a Professor in the Division of Biological Sciences at the University of California, San Diego (Section of Cell and Developmental Biology and Section of Neurobiology). His laboratory studies epidermal morphogenesis, wound healing, and axon regeneration in C. elegans. He performed his PhD thesis work with Jonathan Hodgkin (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK) and his postdoctoral work with H. Robert Horvitz (MIT).
References
- 1.Martin P. Wound healing—aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 2.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 3.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci 2009;122:3209–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science 2005;308:381–385 [DOI] [PubMed] [Google Scholar]
- 5.Ting SB, Caddy J, Hislop N, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science 2005;308:411–413 [DOI] [PubMed] [Google Scholar]
- 6.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol 2004;2:E239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juarez MT, Patterson RA, Sandoval-Guillen E, McGinnis W. Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila. PLoS Genet 2011;7:e1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesch C, Jo J, Wu Y, Fish GS, Galko MJ. A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics 2010;186:943–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Tsarouhas V, Xylourgidis N, et al. The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat Cell Biol 2009;11:890–895 [DOI] [PubMed] [Google Scholar]
- 10.Campos I, Geiger JA, Santos AC, Carlos V, Jacinto A. Genetic screen in Drosophila melanogaster uncovers a novel set of genes required for embryonic epithelial repair. Genetics 2010;184:129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009;459:996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo SK, Freisinger CM, LeBert DC, Huttenlocher A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J Cell Biol 2012;199:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper L, Johnson C, Burslem F, Martin P. Wound healing and inflammation genes revealed by array analysis of ‘macrophageless’ PU.1 null mice. Genome Biol 2005;6:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denis JF, Levesque M, Tran SD, Camarda AJ, Roy S. Axolotl as a model to study scarless wound healing in vertebrates: role of the transforming growth factor beta signaling pathway. Adv Wound Care (New Rochelle) 2013;2:250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Single cell wound repair: dealing with life's little traumas. Bioarchitecture 2011;1:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnemann KJ, Bement WM. Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annu Rev Cell Dev Biol 2011;27:237–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisholm AD, Hsiao TI. The Caenorhabditis elegans epidermis as a model skin I: development, patterning, and growth. Wiley Interdiscip Rev Dev Biol 2012;1:861–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chisholm AD, Xu S. The Caenorhabditis elegans epidermis as a model skin II: differentiation and physiological roles. Wiley Interdiscip Rev Dev Biol 2012;1:879–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allgén CA. Freeliving marine nematodes. In: Odhner NH, eds. Further Zool Results Swed Antarct Exp 1901-03 under the Direction of Dr. Otto Nordenskjöld. Stockholm: Kungl. Botryckeriet P.A. Norstedt & Söner, 1959:1–293 [Google Scholar]
- 20.Poinar GO. Immune responses and wound repair. In: Poinar GO, Jr., Jansson H-B, eds. Diseases of Nematodes. Boca Raton, FL: CRC Press, 1988:133–140 [Google Scholar]
- 21.Tattar TA, Stack JP, Zuckerman BM. Apparent nondestructive penetration of Caenorhabditis elegans by microelectrodes. Nematologica 1977;23:267–269 [Google Scholar]
- 22.Kimble J, Hodgkin J, Smith T, Smith J. Suppression of an amber mutation by microinjection of suppressor tRNA in C. elegans. Nature 1982;299:456–458 [DOI] [PubMed] [Google Scholar]
- 23.Couillault C, Pujol N, Reboul J, et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol 2004;5:488–494 [DOI] [PubMed] [Google Scholar]
- 24.Gray NF. Nematophagous fungi with particular reference to their ecology. Biol Rev 1988;62:245–304 [Google Scholar]
- 25.Engelmann I, Griffon A, Tichit L, et al. A comprehensive analysis of gene expression changes provoked by bacterial and fungal infection in C. elegans. PLoS One 2011;6:e19055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ewbank JJ, Zugasti O. C. elegans: model host and tool for antimicrobial drug discovery. Dis Model Mech 2011;4:300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelmann I, Pujol N. Innate immunity in C. elegans. Adv Exp Med Biol 2010;708:105–121 [DOI] [PubMed] [Google Scholar]
- 28.Ewbank JJ. Signaling in the immune response. WormBook, ed. The C. elegans Research Community, WormBook. London, UK: Biomed Central. doi/ 10.1895/wormbook.1.83.1, http://www.wormbook.org [DOI] [Google Scholar]
- 29.Zugasti O, Ewbank JJ. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 2009;10:249–256 [DOI] [PubMed] [Google Scholar]
- 30.Pujol N, Cypowyj S, Ziegler K, et al. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr Biol 2008;18:481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Couillault C, Fourquet P, Pophillat M, Ewbank JJ. A UPR-independent infection-specific role for a BiP/GRP78 protein in the control of antimicrobial peptide expression in C. elegans epidermis. Virulence 2012;3:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roupe KM, Nybo M, Sjobring U, Alberius P, Schmidtchen A, Sorensen OE. Injury is a major inducer of epidermal innate immune responses during wound healing. J Invest Dermatol 2010;130:1167–1177 [DOI] [PubMed] [Google Scholar]
- 33.Ziegler K, Kurz CL, Cypowyj S, et al. Antifungal innate immunity in C. elegans: PKCδ links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe 2009;5:341–352 [DOI] [PubMed] [Google Scholar]
- 34.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004;4:469–478 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010;464:104–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou CG, Tu Q, Niu J, Ji XL, Zhang KQ. The DAF-16/FOXO transcription factor functions as a regulator of epidermal innate immunity. PLoS Pathog 2013;9:e1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roupe KM, Veerla S, Olson J, et al. Transcription factor binding site analysis identifies FOXO transcription factors as regulators of the cutaneous wound healing process. PLoS One 2014;9:e89274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol 2013;14:249–262 [DOI] [PubMed] [Google Scholar]
- 39.Lansdown AB. Calcium: a potential central regulator in wound healing in the skin. Wound Rep Regen 2002;10:271–285 [DOI] [PubMed] [Google Scholar]
- 40.Stanisstreet M. Calcium and wound healing in Xenopus early embryos. J Embryol Exp Morphol 1982;67:195–205 [PubMed] [Google Scholar]
- 41.Tian L, Hires SA, Mao T, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 2009;6:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu S, Chisholm AD. A Gαq-Ca(2+) signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr Biol 2011;21:1960–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antunes M, Pereira T, Cordeiro JV, Almeida L, Jacinto A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J Cell Biol 2013;202:365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham DM, Huang L, Robinson KR, Messerli MA. Epidermal keratinocyte polarity and motility require Ca(2)(+) influx through TRPV1. J Cell Sci 2013;126:4602–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toth BI, Olah A, Szollosi AG, Biro T. TRP channels in the skin. Br J Pharm 2014;171:2568–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denda M, Tsutsumi M, Denda S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp Dermatol 2010;19:791–795 [DOI] [PubMed] [Google Scholar]
- 47.Chuang M, Chisholm AD. Insights into the functions of the death associated protein kinases from C. elegans and other invertebrates. Apoptosis 2014;19:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tong A, Lynn G, Ngo V, et al. Negative regulation of Caenorhabditis elegans epidermal damage responses by death-associated protein kinase. Proc Natl Acad Sci U S A 2009;106:1457–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bialik S, Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem 2006;75:189–210 [DOI] [PubMed] [Google Scholar]
- 50.Lai MZ, Chen RH. Regulation of inflammation by DAPK. Apoptosis 2014;19:357–363 [DOI] [PubMed] [Google Scholar]
- 51.Losick VP, Fox DT, Spradling AC. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol 2013;23:2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babcock DT, Brock AR, Fish GS, et al. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc Natl Acad Sci U S A 2008;105:10017–10022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 2001;98:3087–3096 [DOI] [PubMed] [Google Scholar]
- 54.Stramer B, Wood W, Galko MJ, et al. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol 2005;168:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulenburg H, Kurz CL, Ewbank JJ. Evolution of the innate immune system: the worm perspective. Immunol Rev 2004;198:36–58 [DOI] [PubMed] [Google Scholar]
- 56.Calvo AC, Pey AL, Ying M, Loer CM, Martinez A. Anabolic function of phenylalanine hydroxylase in Caenorhabditis elegans. FASEB J 2008;22:3046–3058 [DOI] [PubMed] [Google Scholar]
- 57.Baker RH, Britton C, Roberts B, Loer CM, Matthews JB, Nisbet AJ. Melanisation of Teladorsagia circumcincta larvae exposed to sunlight: a role for GTP-cyclohydrolase in nematode survival. Int J Parasitol 2012;42:887–891 [DOI] [PubMed] [Google Scholar]