Abstract
Post-translational modifications of collagen, such as non-enzymatic glycation (NEG), occur through the presence of extracellular sugars and cause the formation of advanced glycation end-products (AGEs). While AGEs have been shown to accumulate in a variety of collagenous human tissues and alter the tissues' functional behavior, the role of AGEs in modifying the mechanical properties of cancellous bone is not well understood.
In this study, an in vitro ribosylation model was used to examine the effect of NEG on the mechanical behavior of cancellous bone. Cancellous bone cores and individual trabeculae were harvested from the femoral heads of eight fresh human cadavers and paired for ribosylation and control treatments. The cores were subjected to either unconfined compression tests or were demineralized and subjected to stress relaxation tests. The trabeculae were loaded to fracture in four-point bending.
In vitro NEG significantly reduced the energy dissipation characteristics of the organic matrix as well as the post-yield properties including the stiffness loss of the individual trabeculae (p<0.05) and the damage fraction of cancellous bone (p<0.001). AGEs in cancellous bone cores from both treatment groups correlated with damage fraction (r2=0.36, p<0.05) and post-yield strain energy (r2=0.21, p<0.05); and with energy dissipation characteristics of the organic matrix (r2=0.35, p<0.05). In the control group, AGEs content increased up to six-fold with age (r2=0.95, p<0.008). This study shows that cancellous bone is susceptible to NEG that increases its propensity to fracture. Moreover, despite tissue turnover, cancellous bone may be susceptible to an age-related accumulation of AGEs.
Keywords: Bone quality, Collagen cross-linking, Cancellous bone, Biomechanics, Non-enzymatic glycation
Introduction
Over the course of aging, bone undergoes a myriad of biochemical changes that may be responsible for the declined mechanical performance of the tissue, leading to an increased incidence of fractures in the elderly [1]. Since bone is a composite material consisting of mineral and organic phases, alterations in its constituents will inevitably modify its biomechanical behavior. Collagen, which accounts for 90% of the organic phase in bone, is susceptible to post-translational modifications, including non-enzymatic glycation (NEG) [2]. Through the presence of reducing sugars in the extracellular matrix, NEG causes the formation of advanced glycation end-products (AGEs) that are present as intra- and inter-fibrillar cross-links between collagen fibers [2]. AGEs have been shown to accumulate with age and negatively impact biochemical and mechanical properties of the basement membrane, tendon, skin, articular cartilage, cardiovascular connective tissue, and cortical bone [3–8]. The alterations of the organic network in bone, caused by NEG, could be relevant to increased bone fragility with aging and in disease including diabetes [9–12].
NEG of bone has been shown to modify its post-yield properties [5,7]. These changes play an important role in the age-related fragility of cortical bone but are not well-understood in cancellous bone. Cancellous bone is of particular clinical interest because it shows the most obvious signs of osteoporotic bone loss through thinning of trabeculae and loss of connectivity [13]. However, the measures of bone mass and mineral density have been shown to be inconsistent predictors of bone strength [14,15]. Thus, other measures related to the bone quality may be necessary to fully understand and assess the risks associated with age-related fragility of cancellous bone. The goal of this study was to investigate the role of NEG in cancellous bone fragility by testing the following hypotheses: (1) NEG may modify cancellous bone fracture behavior at both the trabecular and apparent tissue levels, and (2) AGEs content may be correlated to the decline of specific mechanical properties relating to post-yield behavior in cancellous bone.
To test the above hypotheses, human cylindrical cancellous bone cores were extracted, incubated using a previously developed in vitro ribosylation procedure [7], and mechanically tested in unconfined compression. The energy dissipation characteristics of the cancellous bone organic matrix were evaluated using stress relaxation tests. In addition, individual trabeculae were excised from the same donors and incubated using the in vitro ribosylation procedure. Since it has been previously reported that the failure of a cancellous bone volume results from the buckling and bending of its structural elements [16,17], we tested individual trabeculae in four-point bending to examine the effect of NEG on the trabecular level properties of cancellous bone. Lastly, AGEs content was determined from the mechanically tested cancellous bone cores obtained from the respective donors and tested for correlation with changes in the mechanical properties at the trabecular and apparent tissue levels.
Methods
Specimen preparation
Eight fresh human femurs were selected from female donors of ages ranging from forty-two to ninety-seven (ages: 42, 46, 51, 62, 74, 81, 87, 97) to extract cylindrical cancellous bone cores and individual trabeculae. The donors were free of hepatitis B, HIV, and known metabolic bone diseases (NDRI, Philadelphia, PA).
A drill press with a modified jig was used to obtain multiple cores of cancellous bone, approximately 8 mm in diameter and 20 mm in length, from the femoral head of each donor. X-rays of each individual femur were taken to determine the principal trabeculae orientation, and the cores were taken longitudinally along these directions. The cancellous bone cores were cut into 8–10 mm segments using the ISOMET 11-1180 low-speed diamond blade saw (Buehler Corp., Lake Bluff, IL). The length and diameter of each core were measured and averaged using measurements taken at 30° rotations using a caliper.
Individual trabeculae were harvested along the longitudinal directions from 1-mm-thick frontal slices [18] taken from the lessor trochanteric region beneath the femoral neck of the same donors from which the cancellous bone cores were extracted. Longitudinal trabeculae were selected because they are aligned with the primary loading orientation, and as such they are larger and easier to handle than horizontal trabeculae. The trabeculae, found in the cancellous region, were removed using a surgical scalpel and had a length of at least 6 mm. Four to six trabeculae were extracted per donor, and any trabecula damaged during the course of extraction or handling was omitted from this study. All procedures were done in constant hydration.
Treatment groups
Thirty-two cylindrical cancellous bone cores and thirty-eight trabeculae were obtained (2–6 bone cores per donor; 4–8 trabeculae per donor). All specimens were paired by donor and anatomical location to undergo a sevenday in vitro incubation period in either ribosylation or control solutions. The 7-day in vitro ribosylation simulates an environment of increased and accelerated oxidative stress which is roughly equivalent to roughly 2–3 decades of aging [5] and has been shown to significantly increase the levels of fluorescent AGEs [7]. The ribosylation solution contains 0.6 M ribose, zwitterionic buffers (30 mM HEPES), and protease inhibitors to prevent enzymatic reactions (25 mM ε-amino-n-caproic acid, 5 mM benzamidine, 10 mM N-ethylmaleimide) in Hanks buffer. The control solution has the same composition as the ribosylation solution but was without ribose. During the incubation process, the pH of the solutions was monitored every 24 h and maintained between 7.3 and 7.6.
After the seven-day incubation period, the cancellous bone cores were divided into two sets: one to undergo unconfined compression, and the other to be demineralized and subjected to mechanical stress relaxation. Each set consists of pairs that contain one specimen that underwent control treatment and one specimen that underwent ribosylation treatment. The first set consisted of eleven pairs (22 cores) of mineralized cancellous bone cores taken from donors of ages 46, 51, 81, 87, and 97, and this set was designated for unconfined mechanical testing. The resulting data were used to compare the alterations in the mechanical behavior due to the in vitro ribosylation. A subset of 4 pairs (8 cores), including 1 control and 1 ribosylated core from donors of ages 46, 51, 87, and 97, were then analyzed for AGEs accumulation and used for regression analysis between unconfined compression mechanical behavior and the extent of AGEs accumulation.
The second set consisted of five pairs (10 cores) of cores taken from donors of ages 42, 46, 62, 74 and 81, and this set was demineralized and subjected to compressive stress relaxation. Following mechanical testing, this entire set was analyzed for AGEs accumulation and the results were used for regression analysis between stress relaxation mechanical behavior and the extent of AGEs accumulation.
The individual trabeculae were distributed such that half of samples from each donor underwent control treatment and the other half underwent ribosylation treatment. After the seven-day incubation period, all trabeculae from donors of ages 42, 46, 51, 62, 74, 81, 87, and 97 were loaded to failure in four-point bending (38 trabeculae). Representative loading curves for each type of mechanical testing can be seen in Fig. 1.
Fig. 1.
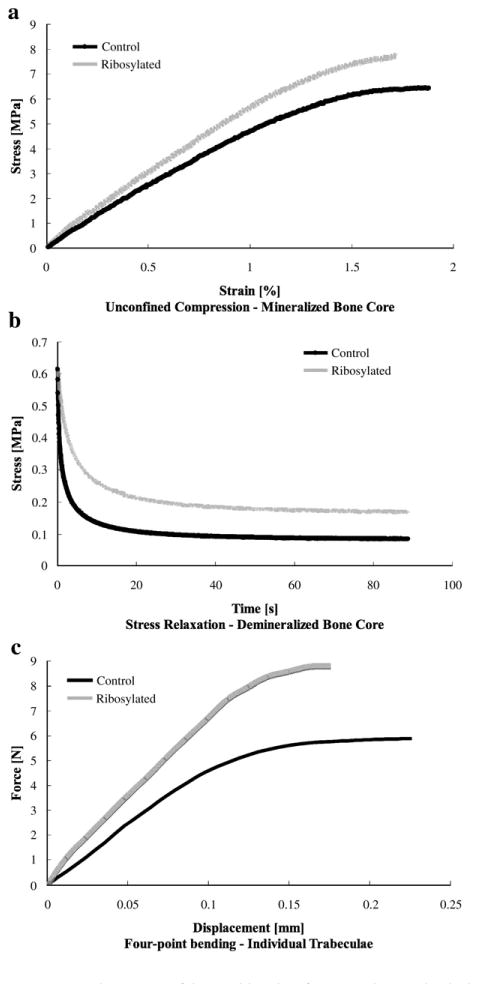
Representative curves of the resulting data for respective mechanical test performed at each level of tissue organization. (a) Top: loading of the mineralized cancellous bone cores to failure in unconfined compression. (b) Middle: stress relaxation of the demineralized cancellous bone matrix over a ninety-second period. (c) Bottom: loading of the individual trabeculae to failure in four-point bending.
Demineralization of cancellous bone cores was done by incubating the specimens in a 20% formic acid solution for a period of 7 days. The solutions were renewed every 24 h. Demineralization end-point determination assays (Polysciences, Warrington, PA) and digital X-rays (FujiFilm Medical Systems, CT, USA; 11 pixels/mm) were used to verify demineralization of each specimen.
Mechanical testing
Unconfined compression testing of control and ribosylated mineralized cancellous bone cores was conducted using the MTS Bionix 858 hydraulic servo mechanical testing station (MTS, Eden Prairie, MN). Each specimen was glued to brass endcaps using a cyanoacrylate glue and loaded to failure at 833 microstrains per second in constant flow of saline at 37 °C. The resulting gage length was at least 8 mm, and continuum assumptions were visually confirmed [19]. Displacement was obtained from an extensometer that measured the relative displacement of the endcaps. Load-displacement data were collected during the test, converted into stress and strain and used for calculating the following parameters: (1) elastic modulus (measured as the maximum value of slope based on an 11-part linear regression of the stress– strain curve between 0.1% and 0.8% apparent strains) [20,21]; (2) yield stress and strain (determined using the 0.2% offset method); (3) ultimate stress and strain (defined as the point at maximum load); (4) post-yield strain energy (defined as the area under the stress–strain curve between the yield and ultimate points); and (5) damage fraction (defined as the change between elastic and secant moduli divided by the elastic modulus, where secant modulus is defined as the stress–strain slope between the origin and the ultimate point).
Stress relaxation testing on the control and ribosylated demineralized bone cores was conducted using the Enduratec ELF 3200 micromechanical test system (Enduratec Inc, Eden Prairie, MN) and brass endcaps. Similar to the compression tests, the displacement was obtained from an extensometer that measured the relative displacement of the endcaps. The demineralized bone cores were loaded to 50% compressive strain and allowed to relax over 90 s. Preliminary studies in our laboratory on the loading of the cancellous bone organic matrix show that it will exhibit linear–elastic behavior up to 50% compressive strain. The resulting stress–strain curve was fitted to the one-dimensional stress relaxation equation of the following form (Eq. (1)):
| (1) |
where the coefficients σeq, Δσ0, and D in the equation describe stress at equilibrium, change in stress and the characteristics of the specimen undergoing stress relaxation, respectively. The amount of energy applied to the specimen can be approximated as a product of the changes in stress and strain. Thus, energy dissipation fraction for each specimen by the following linear–elastic relationship (Eq. (2)):
| (2) |
where Einit and Efinal describe the energy applied to the organic matrix and are defined as the respective equilibrium stress multiplied by the applied strain.
Four-point bending tests on individual trabeculae were done using custom design fixtures with the ELF 3200 micromechanical test system. Each trabecula was loaded to failure at a displacement rate of 0.05 mm/s, and the following parameters determined from the force–displacement curves: (1) ultimate force (maximum load); (2) kelastic (the slope of the force displacement curve in the linear region); (3) kfinal (the slope between the point of ultimate force and the origin); and (4) structural stiffness loss, as given by Eq. (3):
| (3) |
Extent of non-enzymatic glycation
Following mechanical testing, the cancellous bone cores were demineralized and digested with papain collagenase (0.4 mg/ml in 0.1 mM sodium acetate buffer, pH 6.0, 16 h, 65 °C). A total of 8 pairs (1 glycated and 1 control from each donor) of cancellous bone cores were measured for AGEs and used for examining aging trends and comparing treatment differences: 4 pairs that have undergone unconfined compression (ages: 46, 51, 87, and 97); and 4 pairs that have undergone stress relaxation (ages: 42, 62, 74, and 81). AGEs content was determined using fluorescence readings taken with a Synergy-HT Microplate reader at wavelengths of 370 nm excitation, and 440 nm emission (Biotek USA, Winooski, Vermont) and normalized to a quinine sulfate standard. The amount of collagen for each cancellous bone core was approximated based on the amount of hydroxyproline [22]. Hydroxyproline content was determined using a Dynatech MR-600 Microplate reader (Dynatech Inc. Alexandria, Virginia) that recorded the absorbance of the digested samples against a hydroxyproline standard at the wavelength of 570 nm.
Data were tested for normality, and the Student's t-tests were used to establish the causality between glycation and altered mechanical properties. Furthermore, a generalized linear model (GLM) containing two independent factors, namely age of the donor and glycation content (AGEs), was used to investigate the effect of glycation on mechanical properties; age was regarded as a covariate. Using sequential allocation of the sums of squares, the GLM accounts for the effects of glycation and then independently evaluates the effects of the age. Pearson correlation was used to determine the relationships between AGEs accumulation and damage fraction, post-yield strain energy, and energy dissipation fraction. All statistical analyses were performed on Minitab 13 (Minitab USA, State College, PA).
Results
The occurrence of NEG was visually observed in the ribosylated group for both individual trabeculae and cancellous bone cores through a change in the coloration towards a yellow tone in the ribosylated group compared to the white color of the control group (Fig. 2). Consistent with the color change, the AGEs content in cancellous bone cores, expressed in terms of nanograms quinine fluorescence per milligram of collagen, was significantly higher (p<0.022) in the ribosylated group (322.4±256.5) than the control group (169.9±120.7) (Fig. 3). The control cancellous bone core group also showed a significant increase in AGEs content with age (Fig. 4; p<0.008). In the cancellous bone cores, the correlations between damage fraction/post-yield strain energy and age of the donors were found to be non-significant and weak (p<0.53; r2 =0.07).
Fig. 2.
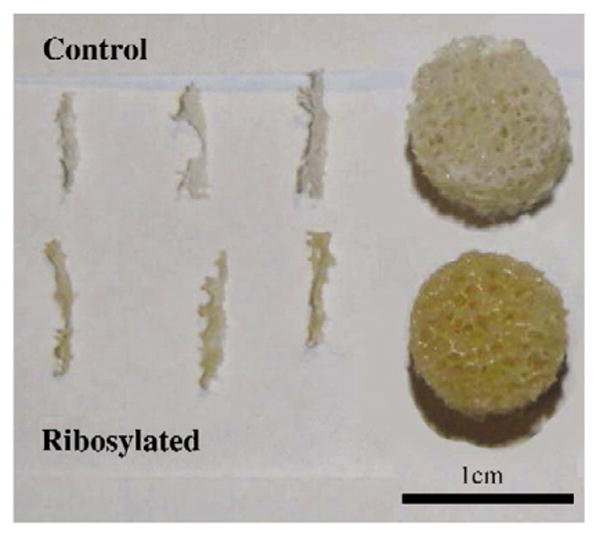
Cancellous bone cores and individual trabeculae show changes from a white (appears as a lighter shade) to a yellow color (a darker shade) after in vitro ribosylation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
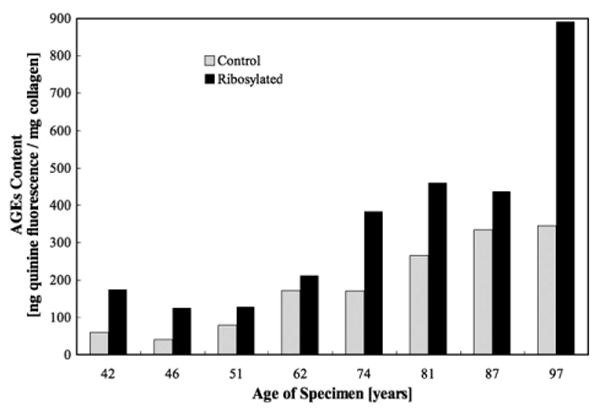
AGEs content was significantly higher in the ribosylated group than the control group in cancellous bone cores (Student's t-test, p<0.022).
Fig. 4.
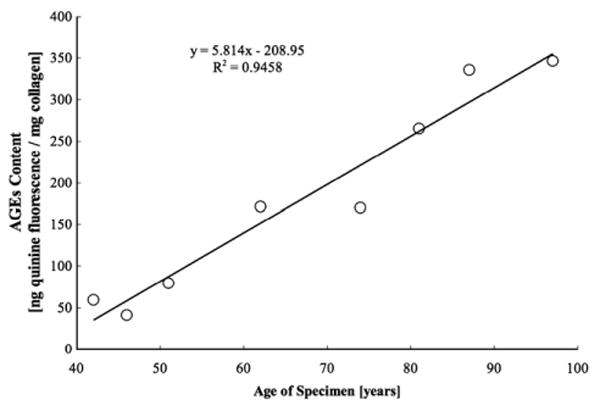
In vivo accumulation of AGEs content with age in the control group in cancellous bone cores (GLM, p<0.008).
Results from the unconfined compression tests of the cancellous bone cores showed that, while the ribysolated group did not demonstrate statistically significant differences from the control group in yield stress/strain, elastic modulus, and ultimate stress/strain (Table 1), significant differences were seen in key post-yield parameters including post-yield strain energy (p<0.05) and damage fraction (p<0.001) due to ribosylation. Structural properties from the 4-point bending of individual trabeculae (Table 2) showed that the reduction in stiffness loss at the level of individual trabeculae declined significantly (p<0.05) in the ribosylated group (0.191±0.12) than in the control group (0.344±0.13), and the reduction was similar in magnitude to the damage fraction reduction due to ribosylation at the apparent level in the mineralized cancellous bone cores (Fig. 5).
Table 1. Results from unconfined compression of the cancellous bone cores.
| Elastic modulus [MPa] |
Yield strain [%] |
Yield stress [MPa] |
Ultimate strain [%] |
Ultimate stress [MPa] |
Post-yield strain energy a [MJ/m3] |
Damage fractionb |
|
|---|---|---|---|---|---|---|---|
| Control | 708 ± 400 | 1.25 ± 0.44 | 7.29 ± 5.6 | 1.71 ± 0.56 | 8.05 ± 6.2 | 0.0000824 ± 0.000046 | 0.333 ± 0.12 |
| Ribosylated | 693 ± 430 | 1.19 ± 0.34 | 7.98 ± 6.4 | 1.48 ± 0.35 | 8.61 ± 6.7 | 0.0000429 ± 0.000030 | 0.207 ± 0.075 |
± values denote standard deviation.
Denotes significance (Student's t-test, p<0.05).
Denotes significance (Student's t-test, p<0.001).
Table 2. Results from four-point bending of the individual trabeculae.
| Ultimate force [N] | kelastic [N/mm] | kfinal [N/mm] | Stiffness loss a | |
|---|---|---|---|---|
| Control | 5.21 ± 3.4 | 53.6 ± 19 | 26.1 ± 12 | 0.344 ± 0.13 |
| Ribosylated | 5.83 ± 4.5 | 49.2 ± 17 | 31.7 ± 16 | 0.191 ± 0.12 |
± values denote standard deviation.
Denotes significance (Student's t-test, p<0.05).
Fig. 5.
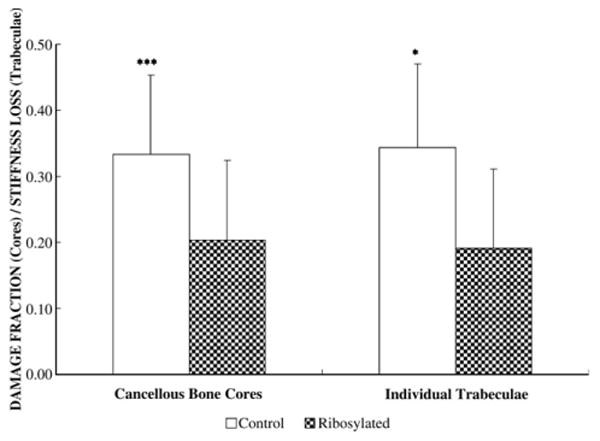
Damage fraction reduction due to in vitro ribosylation in cancellous bone cores (40% reduction) is similar to the extent of stiffness loss reduction seen in the trabeculae (45% reduction) that underwent the same treatment (p<0.001 for cancellous bone core; p<0.05 for individual trabeculae).
The results of the stress relaxation tests on demineralized cancellous bone cores show that the in vitro treatment of the organic matrix caused a two-fold reduction in the energy dissipation fraction of the ribosylated group than the control group (Table 3; p<0.001). The results also show significant negative correlations between AGEs content and damage fraction of cancellous bone cores (Fig. 6a; p<0.05); between AGEs content and post-yield strain energy of cancellous bone cores (Fig. 6b; p<0.05); and between AGEs content and energy dissipation fraction of the demineralized organic matrix (Fig. 7; p<0.05).
Table 3. Results from stress relaxation of the cancellous bone organic matrix.
| σeq [MPa] | Δσ0 [MPa] | D [s−1] | HA [GPa] | Energy dissipation fractiona | |
|---|---|---|---|---|---|
| Control | 0.0260 ± 0.029 | 0.0583 ± 0.045 | 0.154 ± 0.021 | 0.0511 ± 0.057 | 0.696 ± 0.077 |
| Ribosylated | 0.0500 ± 0.057 | 0.0510 ± 0.090 | 0.164 ± 0.036 | 0.0990 ± 0.11 | 0.351 ± 0.14 |
± values denote standard deviation.
Denotes significance (Student's t-test, p<0.001).
Fig. 6.
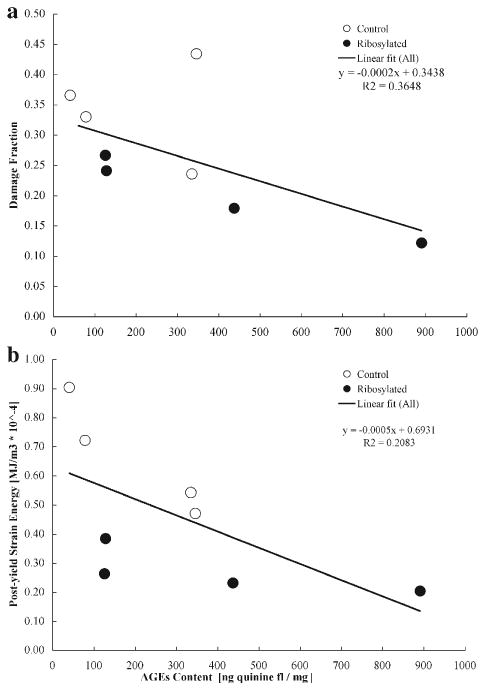
(a) Top: correlation between AGEs content and damage fraction of cancellous bone cores (Pearson correlation, p<0.05). (b) Below: correlation between AGEs content and post-yield strain energy of cancellous bone cores (Pearson correlation, p<0.05).
Fig. 7.
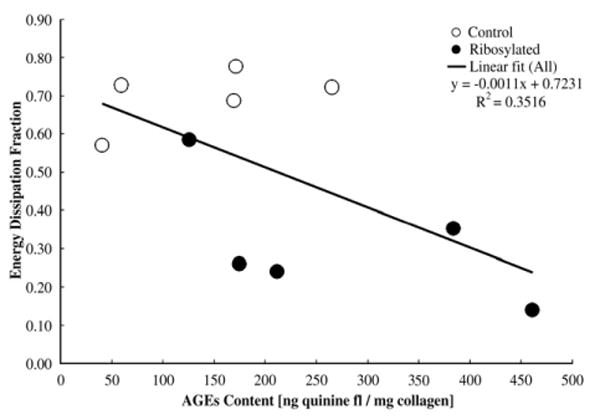
Correlation between AGEs content and energy dissipation fraction of the cancellous bone organic matrix (Pearson correlation, p<0.05).
Discussion
It is important to acknowledge several limitations when interpreting the results of this study. The first of which is the limited sample size and the sole use of female donors, which may not sufficiently address the age-related accumulation of AGEs. However, a much larger study consisting of over 100 donors conducted by Odetti et al. found that the concentration of pentosidine in human cancellous bone notably increased in elderly individuals of 65 years and older, and the accumulation of AGEs appears to be accelerated in osteoporotic individuals [23]. Furthermore, Odetti et al. also found that there were no statistical differences in AGEs accumulation between men and women. Thus, the results from this study suggest that, despite a more rapid rate of remodeling and tissue turnover compared to anatomically adjacent cortical bone tissue [24], cancellous bone may also be susceptible to NEG and the accumulation of AGEs. Secondly, local variations in apparent density within the same donor may have affected our cancellous bone mechanical measurements. Previous studies have shown that trabecular architecture exhibits heterogeneities between anatomical locations [25–27] and can be a source of error in testing cancellous bone. Our experimental design, however, allows us to account for some of the donor-specific heterogeneities by extracting the ribosylated and controls from the same anatomical location and using end-artifact free testing protocols [20,21]. Despite this, intra-site differences in apparent density may have contributed to some variations in the mechanical behavior. Lastly, AGEs can form among non-collagenous proteins and DNA adducts [28], and the use of tissue auto-fluorescence may include these cross-links. However, it is widely accepted that type I collagen constitutes approximately 90% of the organic phase in bone and is the primary load-bearing component of the organic phase. Thus, despite the formation of AGEs among other extracellular components, it has been suggested that the primary type of linkage would be among collagen molecules [28–30], which in turn play a significant role in the post-yield deformation of bone [8,31–34]. The use of specific biomarkers, such as pentosidine, as an indicator for the accumulation of AGEs is subject to the same assumptions [28].
Color change in long-lived proteins including collagen and lens crystalline has been considered as an evidence of the accumulation of AGEs [3,35]. The shift from white to yellow in our samples shows that cancellous bone, similar to cortical bone [7,8], is susceptible to alterations by NEG (Fig. 2). The occurrence of NEG and consequent accumulation of AGEs was also confirmed by the quantification of fluorescent biomarkers in these samples (Fig. 3). Consistent with our interpretation of previous studies [5,7], the results of this study show that 7 days of incubation causes accumulation of AGEs in bone and corresponds to 2–3 decades of aging. For example, the ribosylated sample from the 42-year-old donor exhibited a similar level of AGEs accumulation as the control sample from the 74-year-old donor. Hence, the extent of AGEs accumulation induced by in vitro NEG is clinically relevant since it remains in physiological ranges. This also allows us to correlate bone's post-yield properties with of AGEs accumulation ranging from normal to elevated pathological levels [2–6,23].
The fluorescence of the organic matrix due to modification by NEG has been well-characterized and used to show the accumulation of AGEs in various tissues and anatomical locations [36,37]. NEG produces a multitude of AGEs through covalent lysine and arginine modifications, and the use of background florescence provides an indicator for the NEG-mediated effects on collagen because multiple products will fluoresce at a given wavelength [38]. Others investigators have used specific markers including pentosidine as a marker of age-related cumulative damage in protein [5,8,18,28]. However, pentosidine is found to be in very low concentrations in tissues, and its individual significance to mechanical properties remains unknown [39,40]. This study does not address the role of enzymatic cross-links, such as pyridinolines and pyrroles. The presence of such cross-links has not been shown to correlate with age [8,39,40] and affect age-related mechanical properties [40]. However, since in vitro NEG only causes alterations in the form of non-enzymatic cross-links, the paired experimental design allows us to establish a causality between NEG modification and altered fracture behavior. Our study postulates that increased non-enzymatic cross-links in cancellous bone may account, in part, for the alteration in functional properties of cancellous bone tissue that occurs with age.
The comparison of elastic modulus between the ribosylated and control group of the mineralized cancellous bone cores showed no differences, suggesting that NEG did not affect the stiffness of cancellous bone. As a composite material, bone primarily derives its stiffness from its mineral phase and its post-yield properties from its organic phase [31]. Post-translational modifications to the organic matrix by NEG will therefore result in altered post-yield behavior. The results of this study are consistent with the above concept and previous work on cortical bone that showed no changes in tensile or compressive moduli despite the NEG-mediated changes in the organic matrix [7,32].
Mechanistically, bone material derives its resistance against fracture from microcracking-based damage mechanisms [41– 43] and from the stretching, deformation, and failure of the organic matrix [44,45]. Consistent with these concepts, the organic matrix extracted and tested from ribosylated group showed poor energy dissipation characteristics that manifested itself as altered damage fraction at the tissue and apparent levels. NEG of the organic matrix may therefore increase the propensity of cancellous bone to fracture by modifying toughening mechanisms. Because the fracture propensity of a brittle microcracking material including bone is more directly determined by its post-yield properties [7,41], we investigated the effect of NEG on post-yield strain energy and damage fraction of cancellous bone.
Compared to the control mineralized cancellous bone core group, post-yield strain energy and damage fraction were significantly lower in the ribosylated group. The observed difference in the post-yield energy, in which the ribosylated cancellous bone cores shows a two-fold reduction in magnitude, is a measure of the material's ability to dissipate energy by irreversible processes including but not limited to the formation of microcracks and diffuse microdamage [46]. Damage fraction, a well-established measure of ductility in the damage mechanics of bone and composite materials [46–48], is defined as the ratio of the change between the initial and the final modulus over the initial modulus. The reduction in damage fraction in the ribosylated group (Fig. 5) provides additional evidence of the decreased ability of NEG-altered cancellous bone to accumulate damage and dissipate energy. Riboslyated mineralized cancellous bone cores also deformed less before failure, but failed at higher loads (lower ultimate strain but higher ultimate stress). However, consistent with our previous cortical bone study [7], the changes in ultimate stress/strain failed to reach significance. In contrast to post-yield strain energy and damage fraction that represent the combined effects of the changes in both stress and strain, ultimate stress/strain provides a single parameter description of the fracture behavior and hence was not independently able to represent a transition to brittle bone behavior.
The reduction in structural stiffness loss in the ribosylated trabeculae is similar to the reduction in the damage fraction of ribosylated cancellous bone cores from the same anatomical location (Fig. 5). This finding is consistent with a recent study on the influence of NEG on trabecular microfracture [18] and demonstrates that the NEG affects trabecular tissue to a similar extent as the cancellous bone cores. Therefore, the brittle behavior of the cancellous bone cores can be traced back to the reduced ability of individual trabeculae to accumulate damage that consequently results in the catastrophic failure of a larger bone volume. The similarity between the ribosylation-induced reduction in the damage fraction of the mineralized cancellous bone cores and ribosylation-induced stiffness loss of the individual trabeculae suggests that the mechanism for the NEG-mediated alteration in the mechanical behavior may be similar at the apparent tissue and trabecular levels. NEG affects the lamellar level properties and propagates its adventitious effects to the apparent level properties. The inverse relationships between AGEs content and damage fraction, and between AGEs content and post-yield strain energy of cancellous bone, suggest that the increase of AGEs content contributes to the deterioration of specific aspects in mechanical behavior of cancellous bone as well as the organic matrix at the apparent level.
In summary, this study demonstrated that non-enzymatic glycation plays a significant role in modifying organic matrix properties and tissue-level and the apparent-level properties of cancellous bone. In addition, based on the results of this study and others, the extent of AGEs accumulation appears to increase with age and may play a significant role in the age-related fragility of cancellous bone.
Acknowledgments
This work was supported by NIH grant AG20618. The authors thank Glenn Berard, for assistance in sample preparation, and Terry Peters, for her assistance with statistical analysis. The human tissue samples were obtained from NDRI.
References
- 1.Singer BR, McLauchlan GJ, Robinson CM, Christe J. Epidemiology of fractures in 15,000 adults: the influence of age and gender. J Bone Joint Surg. 1998 Mar;80(2):243–8. doi: 10.1302/0301-620x.80b2.7762. [DOI] [PubMed] [Google Scholar]
- 2.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106(1998):1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 3.Monnier VM, Sell DR, Abdul-Karim FW, Emancipator SN. Collagen browing and cross-linking are increased in chronic experimental hyperglycemia: relevance to diabetes and ageing. Diabetes. 1988;37:867–72. doi: 10.2337/diab.37.7.867. [DOI] [PubMed] [Google Scholar]
- 4.Reiser KM. Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med. 1984;196(1):17–29. doi: 10.3181/00379727-196-43158c. [DOI] [PubMed] [Google Scholar]
- 5.Catanese J, III, Bank RA, TeKoppele JM, Keaveny TM. Increased cross-linking by non-enzymatic glycation reduces the ductility of bone and bone collagen. ASME Bioengr Conf. 1999;42:267–8. [Google Scholar]
- 6.Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thrope SR, Baynes JW, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350:381–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;22(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31(1):1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 9.Wolf SK. Diabetes mellitus and predisposition to athletic pedal fracture. J Foot Ankle Surg. 1998;37:16–22. doi: 10.1016/s1067-2516(98)80006-9. [DOI] [PubMed] [Google Scholar]
- 10.Meyer HE, Tverdal A, Falch JA. Risk factors for hip fractures in middle-aged Norwegian women and men. Am J Epidemiol. 1993;137:1203–11. doi: 10.1093/oxfordjournals.aje.a116622. [DOI] [PubMed] [Google Scholar]
- 11.Seeley DG, Kelsey J, Jergas M, Nevitt MC. Predictors of ankle and foot fractures in older women. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11:1347–55. doi: 10.1002/jbmr.5650110920. [DOI] [PubMed] [Google Scholar]
- 12.Hedlund LJ, Maki DD, Griffiths HJ. Calcaneal fractures in diabetic patients. J Diabetes its Complicat. 1998;12s:81–7. doi: 10.1016/s1056-8727(97)00052-4. [DOI] [PubMed] [Google Scholar]
- 13.Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Clin Orthop Relat Res. 1990;(252):163–6. [PubMed] [Google Scholar]
- 14.Lotz JD, Hayes WC. The use of computed tomography to estimate the risk of fracture of the hip from falls. J Bone Jt Surg. 1990;(71A):689–700. [PubMed] [Google Scholar]
- 15.Smith MD, Cody DD, Goldstein SA, Cooperman AM, Matthews LS, Flynn MJ. Proximal femur bone density and its correlation to fracture load and hip screw penetration load. Clin Orthop Relat Res. 1992;(283):244–51. [PubMed] [Google Scholar]
- 16.Fyhrie DP, Schaffler MB. Failure mechanisms in human vertebral cancellous bone. Bone. 1994;15(1):105–9. doi: 10.1016/8756-3282(94)90900-8. [DOI] [PubMed] [Google Scholar]
- 17.Müller R, Gerber SC, Hayes WC. Micro-compression: a novel technique for nondestructive assessment of local bone failure. Technol Heath Care. 1998;6(1998):433–44. [PubMed] [Google Scholar]
- 18.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37(6):825–32. doi: 10.1016/j.bone.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrigan TP, Jasty M, Mann RW, Harris WH. Limitations of the continuum assumption in cancellous bone. J Biomech. 1988;21(4):269–75. doi: 10.1016/0021-9290(88)90257-6. [DOI] [PubMed] [Google Scholar]
- 20.Hou FJ, Lang SM, Hoshaw SJ, Reimann DA, Fyhrie DP. Human vertebral body apparent and hard tissue stiffness. J Biomech. 1998 Nov;31(11):1009–15. doi: 10.1016/s0021-9290(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 21.Fyhrie DP, Vashishth D. Bone stiffness predicts strength similarly for human vertebral cancellous bone in compression and for cortical bone in tension. Bone. 2000;26(2):169–73. doi: 10.1016/s8756-3282(99)00246-x. [DOI] [PubMed] [Google Scholar]
- 22.Sell DR, Monnier VM. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19:77–92. doi: 10.3109/03008208909016816. [DOI] [PubMed] [Google Scholar]
- 23.Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro F, Federici M, et al. Advanced glycation end-products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710–7. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 24.Wronski TJ, Smith JM, Jee WSS. Variations in mineral apposition rate of trabecular bone within the beagle skeleton. Calcif Tissue Int. 1981;33:583. doi: 10.1007/BF02409495. [DOI] [PubMed] [Google Scholar]
- 25.Catanese J, III, Iverson EP, Ng RK, Keaveny TM. Heterogeneity of the mechanical properties of demineralized bone. J Biomech. 1999;32(12):1365–9. doi: 10.1016/s0021-9290(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 26.Morgan EF, Keaveny TM. Dependence of yield strain of human trabecular bone on anatomic site. J Biomech. 2001;34(5):569–77. doi: 10.1016/s0021-9290(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez CJ, Beaupre GS, Keller TS, Carter DR. The influence of bone volume fraction and ash fraction on bone strength and modulus. Bone. 2001;(29):74–8. doi: 10.1016/s8756-3282(01)00467-7. [DOI] [PubMed] [Google Scholar]
- 28.Sell DR, Nagaraj RH, Grandhee SK, Odetti P, Lapolla A, Fogarty J, et al. Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes/Metabol Rev. 1991;7(4):239–51. doi: 10.1002/dmr.5610070404. [DOI] [PubMed] [Google Scholar]
- 29.Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during non-enzymatic browning of proteins by glucose. J Biol Chem. 1991;266:11654–60. [PubMed] [Google Scholar]
- 30.Tessier F, Obrenovich M, Monnier VM. Structure and mechanism of formation of human lens fluorophore LM-1. J Biol Chem. 1999;274:20796–804. doi: 10.1074/jbc.274.30.20796. [DOI] [PubMed] [Google Scholar]
- 31.Burstein AH, Zika JM, Heiple KG, Klein L. Contribution of collagen and mineral to the elastic–plastic properties of bone. J Bone Joint Surg Am. 1975;57(7):956–61. [PubMed] [Google Scholar]
- 32.Wang X, Bank RA, TeKoppele JM, Hubbard GB, Athanasiou KA, Agrawal CM. Effect of collagen denaturation on the toughness of bone. Clin Orthop. 2000:228–39. doi: 10.1097/00003086-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45:108–16. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Bank RA, TeKoppele JM, Agrawal CM. The role of collagen in determining bone mechanical properties. J Orthop Res. 2001;19:1021–6. doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- 35.Bank RA, Bayliss MT, Lafeber PJG, Maroudas A, Tekoppele JM. Aging and zonal variation in post-translational modification of collagen in normal human articular cartilage. Biochem J. 1998;330:345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984;81:583–7. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, et al. Accumulation of Mallard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–9. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson GJ, Verner JJ, Nelson FR, Lin DL. Degradation of the cartilage collagen matrix associated with changes in chondrocytes in osteoarthrosis. Assessment by loss of background fluorescence and immunodetection of matrix components. J Orthop Res. 2001 Jan;19(1):33–42. doi: 10.1016/S0736-0266(00)00008-5. [DOI] [PubMed] [Google Scholar]
- 39.Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L. Age-related changes in the biochemical properties of human cancellous bone collagen:relationship to bone strength. Calcif Tissue Int. 1999;65:203–10. doi: 10.1007/s002239900683. [DOI] [PubMed] [Google Scholar]
- 40.Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res. 2002;17(9):1621–8. doi: 10.1359/jbmr.2002.17.9.1621. [DOI] [PubMed] [Google Scholar]
- 41.Vashishth D, Behiri JC, Bonfield W. Crack growth resistance in cortical bone: concept of microcrack toughening. J Biomech. 1997;30(8):763–769. doi: 10.1016/s0021-9290(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 42.Nalla RK, Stolken JS, Kinney JH, Ritchie RO. Fracture in human cortica bone: local fracture criteria and toughening mechanisms. J Biomech. 2005;38(7):1517–25. doi: 10.1016/j.jbiomech.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005;37(1):96–102. doi: 10.1016/j.bone.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK. Bone indentation recovery time correlates with bond reforming time. Nature. 2001;414(6865):773–6. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- 45.Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater. 2005;4:612–6. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 46.Vashishth D. Rising crack-growth-resistance behavior in cortical bone: implications for toughness measurements. J Biomech. 2004;37(6):943–6. doi: 10.1016/j.jbiomech.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Jepson KJ, Davy DT. Comparison of damage accumulation measures in human cortical bone. J Biomech. 1997;30(9):841–949. doi: 10.1016/s0021-9290(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 48.Zioupos P. Recent developments in the study of failure of solid biomaterials and bone: “fracture” and “pre-fracture” toughness. Mater Sci Engr. 1998;C6:33–40. [Google Scholar]


