Abstract
In the midst of global biodiversity loss and rising disease incidence in wildlife, there has been growing interest in the role of infectious disease in species extinction. At local scales infectious disease is a common driver of population declines but globally it is an infrequent driver of species extinction and endangerment. For those unfortunate species threatened by disease questions remain, including when, along the pathway to extinction, do pathogens become a threat? We used the 2011 IUCN Red List, focusing on amphibians, birds, and mammals to test the null hypothesis that the proportion of species threatened by disease is the same in each status category (least concern to extinct). Overall, we found that pathogens appear to increase in importance as species move towards extinction though this varies with host taxonomy. We compare this finding to other threats (e.g. land-use change and invasive species) and discuss the role of potential ecological and artifactual drivers. Furthermore, we identify what other threats most frequently co-occur with infectious disease to examine the specific role of disease in driving extinction. We determined that infectious disease is rarely the sole driver of extinction and that being affected by other threats increases the odds of infectious disease co-occurring as a driver of extinction. Ultimately, our conclusions echo previous calls for baseline data on the presence of pathogens in species when they show the first signs of extinction risk and arguably before.
Keywords: Endangered, Extinction, Infectious Disease, IUCN, Parasite, Pathogen, Threatened Species
Introduction
Despite imperfect and relatively little data on the diversity and abundance of pathogens in wild populations, recent strides have been made to quantify the role of infectious disease in species extinction. At local scales infectious disease is a common driver of temporary or permanent population declines (de Castro & Bolker 2005; Pedersen et al. 2007; Smith et al. 2009a; Smith et al. 2009b). Globally, however, infectious disease (disease hereafter) appears to be an infrequent driver of species extinction or endangerment (Smith et al. 2006). Analysis of the 2004 International Union for Conservation of Nature (IUCN) Red List revealed that disease was a contributing threat in <4% of known species extinctions since 1500 and <8% of those critically endangered (Smith et al. 2006). The minor role of disease as a driver of species extinction is in sharp contrast to invasive species, habitat destruction, and overexploitation, each of which are cited (alone and in combination with other threats) as causing 45-54% of well documented animal extinctions (Clavero & Garcia-Berthou 2005; Hoffmann et al. 2010; IUCN 2012). Nevertheless, some diseases pose uncontested threats to certain taxa: i.e. chytridiomycosis in hundreds of amphibian species worldwide, avian pox and malaria in the majority of Hawaii's native avifauna, facial tumor disease in the Tasmanian Devil (Sarcophilus harrisii), fibropapillomatosis in green sea turtles (Chelonia mydas) and white-nose syndrome in six species of North American bats (Atkinson et al. 1995; Daszak et al. 1999; Schloegel et al. 2006; Jones et al. 2007; Blehert 2009; Van Houtan et al. 2010; Foley et al. 2011; Hof et al. 2011).
Basic theory predicts that diseases should drive species to extinction when pre-epidemic population size is small, transmission is frequency-dependent, reservoir hosts are available, or when the pathogen can survive in the abiotic environment for long periods or under myriad conditions (de Castro & Bolker 2005; Smith et al. 2009a). The latter is an especially concerning characteristic of a growing number of pathogenic fungi threatening wildlife and domesticated species worldwide (Fisher et al. 2012). For many pathogens, transmission rates decline with decreasing host populations to the extent that directly transmitted pathogens may be lost if host populations drop below a particular threshold density (Anderson et al. 1986; McCallum & Dobson 1995). Following this logic, species on the verge of extinction should harbor relatively few pathogenic species, an outcome supported by a study of Red List primates where total parasite species richness was significantly lower among threatened compared to non-threatened species (Altizer et al. 2007). While this study did not distinguish between parasites that are known threats to primate species and those simply identified as present in a population, the findings, in combination with those described above, suggest that the impact of single pathogenic species may be all that is required to tip a host toward extinction. A remaining question is therefore, when does disease become a threat to species on the road to extinction and does it co-occur, predictably, with other threats?
On one hand, we might expect diseases to more commonly threaten host species early in the extinction process (i.e. least concern or near threatened species) – when populations are still large enough to sustain transmission and encounter a greater pool of potential pathogens (Anderson et al. 1986; McCallum & Dobson 1995). On the other hand, disease may be more common threat among endangered species, whose populations are reduced (both in terms of genetic diversity and absolute abundance) and likely stressed from other threats that predispose them to infection (O'Brien & Evermann 1988; Aguirre & Tabor 2008). For example, amphibians impacted by land-use change and climate change may be more likely to face threats from disease (Hof et al. 2011). Disease may also become a significant threat to a species following the onset of other specific threats. For example, invasive species are predators on a large number of Red List species, but also have the potential to harbor parasites and pathogens that may spillover and establish in native populations (Gurevitch & Padilla 2004; de Castro & Bolker 2005; Smith & Carpenter 2006).
It is difficult to determine the sequence of the onset of threats to species on the road to extinction. In part, this is due to a lack of long-term data on the changing conditions surrounding species in their native ranges and the pool of parasites and pathogens they harbor (Plowright et al. 2008). Preliminary insight may be gained, however, by examining the relative role, and cooccurrence, of threats associated with groups of species at varying levels of extinction risk (e.g. vulnerable vs. endangered vs. critically endangered species). We attempt this using the Red List of threatened mammals, birds, and amphibians, with the goal of gaining new insight into global trends in disease threats to wildlife. First, we examined whether there is variation in the proportion of species between Red List status categories that are threatened by disease in order to assess when along the road to extinction disease becomes a contributing threat. We then determined what other threats most frequently co-occur with disease and whether being affected by these other threats increases the likelihood that of having disease as a threat.
Methods
The Red List does not provide a simple means of assessing temporal trends in disease, or other threats, to individual species over time. However, data in the Red List can be used to calculate variation in the relative occurrence of a given threat between status categories for groups of species. Here we used the Red List to examine whether there was variation in the relative occurrence of disease between Red List status categories. We searched the Red List for amphibian, bird, and mammal species listed in the following status categories: Least Concern, Near Threatened, Vulnerable, Endangered, Critically Endangered, Extinct in the Wild, and Extinct for assessment years 1996-2011 (n= 19,378 species). Following established methods (Smith et al. 2006; see Appendix S1), each species account was read in full to identify those for which disease was a listed threat, totaling 917 species (554 amphibians, 190 birds, and 173 mammals) (Table S1).
Because the Red List does not categorize threats based on the strength of supporting evidence (Smith et al. 2006; Heard et al. 2011) each species account must be read in full to determine which threats are based on evidence or are hypothesized to be current or future threats (after Smith et al. 2006). For each of the 917 species threatened by disease, we classified into the two following groups: 1) Evidence-based threat - the species for which disease has occurred historically, is ongoing, or present (see Appendix S1), or 2) Hypothesized threat - the species for which disease is only a hypothesis (no evidence currently exists to support the claim, ongoing research is attempting to discern the threat of disease, or disease has been suggested to pose a threat in the future). Of the 19,378 Red Listed species we examined, there were only 1.2% (240 species including 83 amphibians, 43 birds, and 114 mammals) for which the threat of disease was grounded in evidence and 3.5% (677 species including 471 amphibians, 147 birds, and 49 mammals) for which the threat of disease was hypothesized. Analyses were initially conducted for all species (evidence + hypothesized threat of disease) and those with just evidence of a disease threat. However, results for both groups were quantitatively and qualitatively similar and so we only present reults from the evidence-based group (240 species) in the manuscript.
We tested the null hypothesis that the proportion of species threatened by disease is the same in each Red List status category. We used contingency table analysis (CTA) for species in each taxonomic class (amphibians, birds, and mammals) separately and combined (‘all species’). We calculated expected values using the number of species threatened and not threatened by disease in each Red List status category to determine if the propotion of species affected by disease varies as species move towards extinction. For this analysis, we excluded extinct in the wild and extinct species from the CTA because these categories do not represent specific stages in the pathway towards extinction and all threats that ever affected the species are included without distinguishing the role they played. As a result of these inclusions, extinct and extinct in the wild status categories are fundamentally different from other Red List status categories. Additionally, there were ultimately too few species with actual evidence of disease being a threat to conduct statistical analyses (Appendix S1). Because CTA tests only for variation among groups, and not directional trends, we graphically depicted our findings to qualitatively assess differences between Red List status categories. To contextualize our findings for disease, we also examined whether the proportion of species affected by other drivers of extinction varied between Red List status categories. Currently, there are more than 12 potential drivers of extinction listed in the Red List, each having at minimum 3 additional sub-categories. Therefore, for ease of investigation, we created six general categories of threats: 1) land-use change, 2) overexploitation, 3) problematic/invasive species, 4) pollution, 5) geological events, and 6) climate change/severe weather (see Appendix S1).
We also identified which of these six other threats most frequently co-occur with disease. To identify these co-occuring threats, we read the full account Red List account for each of the species affected by disease and identified which other threats were also present. We then calculated the proportion of the 240 evidence-based disease species affected by each of the six co-occurring threats and depicted this graphically for amphibians, birds, mammals, and all species. While the Red List does not determine the order of the onset of threats, it is possible to determine the correlative strength of co-occurrence. We examined whether being affected by any of the six other threats increased the probability that a species was also likely to be threatened by disease by calculating the odds ratio for each other threat. We used odds ratios, commonly used in epidemiology, to estimate the strength of association between a categorical outcome, such as occurrence of a disease, and factors suspected of contributing to the odds of the outcome event (Woodward 2005; Merrill 2010). This methodology has been adopted recently in conservation biology and ecology studies to evaluate the impacts of multiple stressors on threatened species (Davidson & Knapp 2007; Johnson et al. 2008; Witte et al. 2008). Here, we used the odds ratio to determine whether being threatened by any of the six major threats in the Red List increased the likelihood of also being threatened by disease. Statistical evaluations of odds ratios incorporated Fisher's Exact Test. Analyses were conducted separately for amphibians, birds, mammals, and all species.
Results
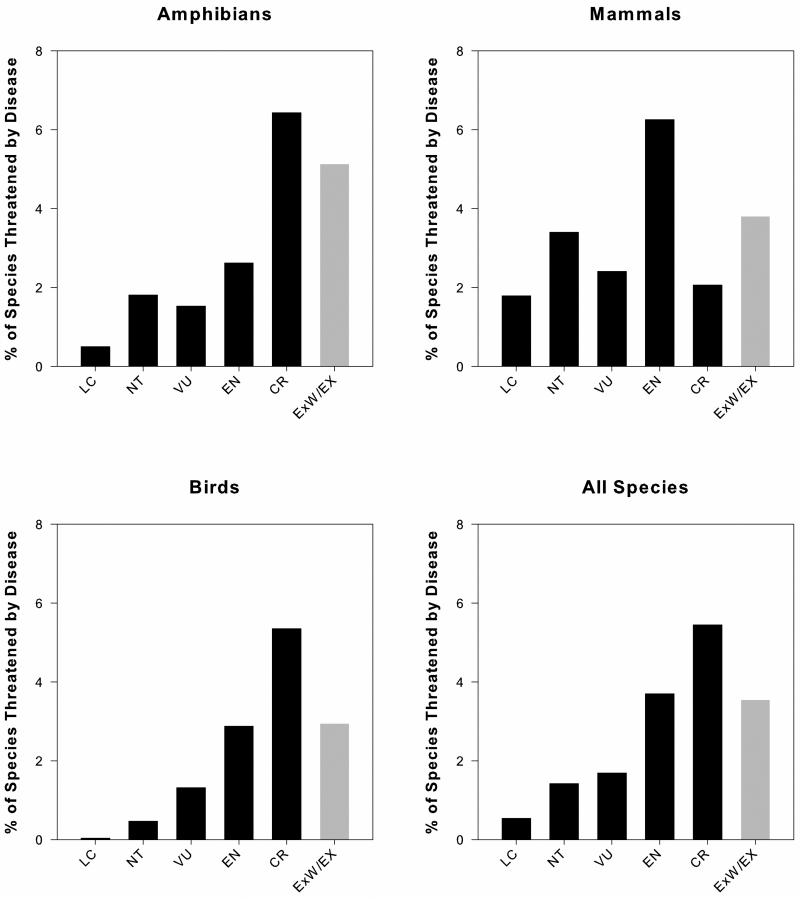
The proportion of species affected by disease varied significantly between Red List status categories for amphibians (χ2=90.8, p<0.0001), birds (χ2=269.7, p<0.0001), mammals (χ2=45.4, p<0.0001), and all species (χ2= 264.4, p<0.0001) (Fig. 1). A qualitative trend showing an increase in the proportion of species threatened by disease as species move towards extinction was evident for amphibians, birds and all species (Fig. 1). In contrast, mammals show no clear directional trend between Red List status categories, with the highest proportion of mammals affected by disease is in the endangered status category (Fig. 1).
Figure 1.
The proportion of species with evidence of a disease threat: LC=Least Concern, NT=Near Threatened, VU=Vulnerable, EN=Endangered, CR=Critically Endangered, ExW/Ex=Extinct in the Wild and Extinct. Proportions were calculated using the number of species threatened by disease divided by the total number of species in each corresponding Red List status category. Extinct species are shaded because they are excluded from statistical analyses and because they are categorically different from other Red List status categories (see Methods). CTA showed that proportions significantly varied between Red List status categories for amphibians (χ2=90.8, p<0.0001), birds (χ2=269.7, p<0.0001), mammals (χ2=45.4, p<0.0001), and all species (χ2= 264.4, p<0.0001).
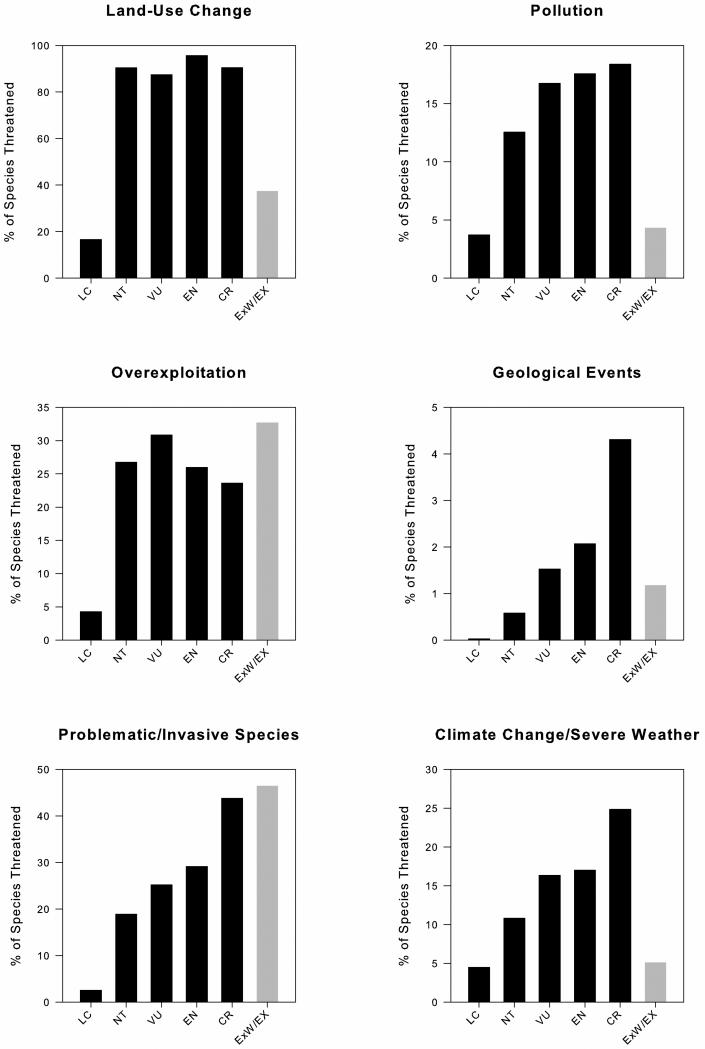
The proportion of species affected by the six other major threats varied between Red List status categories for all species: land-use change (χ2= 9425.8, p<0.0001), overexploitation (χ2=2193.6, p<0.0001), problematic/invasive species (χ2= 3151.61, p<0.0001), pollution (χ2=938.4, p<0.0001), geological events (χ2= 368.9, p<0.0001), and climate change/severe weather (χ2= 917.68 p<0.0001) (Fig. 2). Problematic/invasive species, pollution, geological events, and climate change, showed similar directional trends to disease, as the proportion of species affected by each of these threats increased as species moved up in status category (Fig. 2). However, this was not true for land-use change or overexploitation, which showed no clear directional trends (Fig. 2).
Figure 2.
The proportion of species affected by six major threats in IUCN Red List: LC=Least Concern, NT=Near Threatened, VU=Vulnerable, EN=Endangered, CR=Critically Endangered, ExW/Ex=Extinct in the Wild and Extinct. Proportions were calculated using the number of species threatened divided by the total number of species in each corresponding status category. Extinct species are shaded because they are excluded from statistical analyses and because they are categorically different from other Red List status categories (see Methods). CTA showed tha proportions significantly varied between Red List status categories for each of the six drivers of extinction: land-use change (χ2= 9425.8, p<0.0001), overexploitation (χ2=2193.6, p<0.0001), invasive species (χ2= 3151.61, p<0.0001), pollution (χ2= 938.4, p<0.0001), geological events (χ2= 368.9, p<0.0001), and climate change/severe weather (χ2= 917.68 p<0.0001).
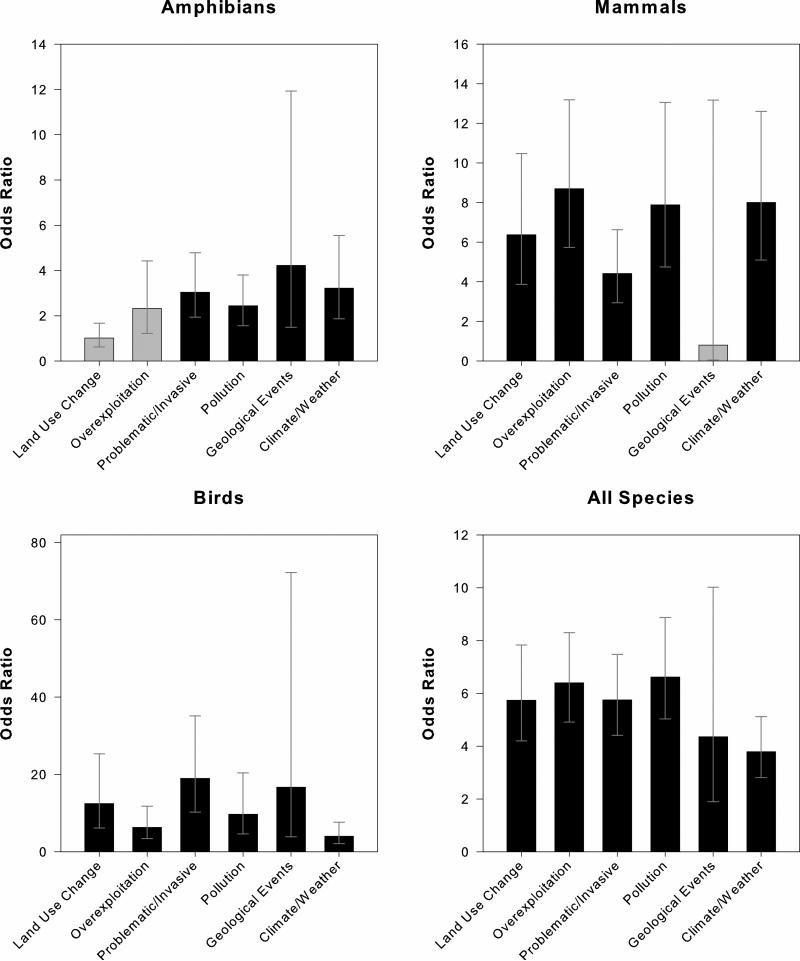
Disease was infrequently the sole threat to Red Listed species (1.3% of all species with disease; Table 1). Land-use change threatned amphibians, birds, and mammals nearly equally, while the second most common co-occurring threat varied was different for each taxonomic group (Table 1). Odds ratios calculations showed that being threatened by any of the six major threats increased the likelihood of also having disease co-occur as a listed threat for amphibians, birds, mammals, and all species (Fig. 3) However, there were no consistent trends between our three taxonomic groups in terms of which threats most significantly increased the likelihood of a species also being threatened by disease (Fig. 3).
Table 1.
Co-occuring threats for the 240 Red Listed species analyzed that have evidence of a disease threat.
| THREATS | AMPHIBIANS | BIRDS | MAMMALS | ALL SPECIES |
|---|---|---|---|---|
| No Other Extinction Drivers | 0.0% | 0.0% | 2.7% | 1.3% |
| Land-Use Change | 73.5% | 76.7% | 82.9% | 78.5% |
| Overexploitation | 13.3% | 37.2% | 71.2% | 44.7% |
| Invasive/Problematic Species | 37.4% | 60.5% | 33.3% | 39.7% |
| Pollution | 39.8% | 20.9% | 18.9% | 26.6% |
| Geological Events | 4.8% | 4.7% | 0.0% | 2.5% |
| Climate Change/Severe Weather | 20.5% | 30.2% | 25.2% | 24.5% |
Figure 3.
Odds ratios and confidence intervals showing the increase in likelihood of having disease co-occur with each of the six other major threats. Odds ratios greater than one indicate that there is an increased likelihood of having disease also occur as a threat than compared to when the threat is absent. Odds ratios were calculated for each of the six threats using the following example formula for land-use change: (# of species threatened by disease and land-use change divided by # of species not threatened by disease, but threatened by land-use change) divided by (# of species threatened by disease, but not by land-use change divided by # of species not threatened by disease or land-use change). Black bars correspond to statistically significant odds ratios, whose confidence intervals exclude 1.0, (p<0.05) as determined by Fisher's Exact Test, while grey bars represent non-significant results.
Discussion
Despite recent updates to the Red List, evidence for the role of disease as a major threat to species at risk of extinction remains scant. Smith et al. 2006 reported supporting evidence in less than half of critically endangered species with disease listed as a threat in the 2004 Red List. Our analysis suggests little progress has been made to acquire evidence for many hypothesized disease-induced species declines, as only 26% (240 species) of the 917 species with disease listed as a driver of extinction had evidence of an actual threat (Table S1). The listing of hypothesized, ongoing, and future predicted threats to Red List species is the result of a precautionary approach adopted by assessment teams. This is certainly justified, but the sheer number of non-evidence backed threats to species approaching extinction implies a major lack of on-the-ground surveillance systems providing data critical to designing effective conservation strategies (Smith et al. 2006; Heard et al. 2011).
Examination of species with an evidence-based disease threat suggests that the proportion of species threatened by disease varies between IUCN status categories and significantly increases for amphibians, birds, and all of our species combined as these taxa travel down the road toward extinction (Fig. 1). This finding may be the result of 1) ecological factors associated with increasing threat levels that predispose species to infection with harmful parasites and pathogens, or 2) discovery bias—an artifact of increasing resources and accumulation of knowledge about the threats to species as they move closer to extinction. The latter may be especially plausible for disease given the difficulties associated with studying parasites and pathogens in wild populations (Plowright et al. 2008). A case in point is the recently extinct Polynesian tree snail (Partula turgida), the final five individuals of which were diagnosed with a parasitic infection only after they were pulled into captivity in hopes of curtailing extinction (Cunningham & Daszak, 1998). Disease was considered the final threat that drove the species to extinction, but the causal parasites may never have been discovered if the few remaining individuals were not studied so thoroughly. If discovery bias is the sole driving mechanism behind the accumulation of threats to species on the path to extinction then the same pattern should hold for other threats, many of which also become increasingly apparent when extinction risk increases and species are more thoroughly studied. However, a preliminary look at the other six threats in the Red List show that this trend occurs some, but not all, of the time (Fig. 2)
Collectively, land-use change, overexploitation, problematic/invasive species, pollution, geological events, and climate change/severe weather exhibit a wide range of variation in relative importance among status categories. Given that threats in the Red List are status-specific (i.e. they may be deleted when species move between categories), this variation implies the role of both ecological processes and scientific effort as drivers behind the timing of threats to species as they become increasingly threatened with extinction (Fig. 2). For example, it is perhaps no surprise that almost all near threatened and threatened species are impacted by land-use change, which is the most pervasive human impact worldwide (Vitousek et al. 1997; Wilcove et al. 1998; Hoffmann et al. 2010), while climate change/severe weather, a frequently hypothesized future threat in the Red List, may be more likely to be documented in species that closer to extinction (Hoegh-Guldberg et al. 2008; Araujo et al. 2011) (Fig. 2).
The potential ecological reasons for why disease would be a more common threat to species on the verge of extinction may also be explained by variation in the characteristics of the parasites and pathogens associated with species in different status categories. Theory predicts an increasing representation of frequency dependent, host generalist pathogens that utilize reservoir hosts or which can survive in the abiotic environment in status categories closer to extinction (Smith et al. 2006; Pedersen et al. 2007). However, a paucity of parasite and pathogen diversity associated with threatened species prevents us from assessing such a trend (Table S1). By far the most common diseases found in threatened birds are avian influenza, botulism, and malaria, which are all host generalists. Not surprisingly, chytridiomycosis is the most common disease threat to amphibians. The causal fungus, Batrachochytrium dendrobatidis, has a large host range, can persist for months in the soil, and is asymptomatic in some species (e.g. North American Bullfrogs, Rana catesbeiana) (Dobson & Foufopoulos 2001; Johnson & Speare 2005). Mammals did not exhibit an increasing trend in disease as species become more threatened, with the highest proportion of disease found in endangered species (Fig. 1). Many of the diseases associated with threatened mammals are density dependent pathogens originating in domestic/feral animals, including rinderpest, canine and phocine distemper viruses (IUCN 2011). Pathogen spillover events from domestic to wild mammals may occur at intermediate status levels where other threats like invasive species and land-use change have already occurred, facilitating contact events and setting the stage for pathogen spread before populations fall below the necessary threshold density required to maintain them (Pedersen et al. 2007). Broader analyses that include non-threatened species may provide enough resolution to allow in depth examination of meaningful shifts in parasite and pathogen characteristics as species move toward extinction.
While it is beyond the scope of this paper to address the causal factors driving the timing of individual threats in the extinction process, our findings indicate that disease is infrequently a single contributing threat (Table 1) and that being affected by any of the other six major threats increases the likelihood of a species being simultaneously threatened by disease (Fig. 3). Because there is limited statistical variation among the odds ratios of each of our six major threats for amphibians, birds, and mammals (Fig. 3), we also cannot infer which other threats most significantly increase the likelihood of species also being threatened by disease. Therefore, we suggest that examination of the literature detailing the timing and onset of these threats to Red List species is required to assess whether disease follows other threats or vice versa.
Our findings suggest that a combination of discovery bias, interactions with other threats, and the basic ecology of pathogens collectively influence the timing of disease as a threat to species on the road to extinction. They also provide the first overview of what threats most commonly co-occur with disease and suggest that disease is unlikely to be the sole contributing threat on the pathway to extinction. We recognize that our findings may be influenced by the relative lack of evidence for disease-threatened animals in the Red List and the paucity of pathogens associated with threatened species. Nevertheless, we hope they incite more in depth examination of the onset of disease and other Red List threats to species on the road to extinction and the exemplify the need for the compilation of baseline data on the presence of pathogens in species populations when they show the first signs of extinction risk. Ultimately, striving to collect such data will provide a clear benefit to wildlife conservation in the future.
Supplementary Material
Acknowledgements
M.J. Heard was supported by a Voss Postdoctoral Fellowship from the Environmental Change Initiative at Brown University. This project was also supported by a Brown University Environmental Change Initiative Conservation Medicine Working Group Grant (PI K.F. Smith, 2010-2011). We thank all the members of the working group for discussion that helped to shape the project and initial conversation from Dov Sax.
Footnotes
Supporting Information
Supporting Information (Appendix S1) is available online. The authors are solely responsible for the content and functionality of these materials. Queries (other than absence of the material) should be directed to the corresponding author.
Literature Cited
- Aguirre AA, Tabor GM. Global Factors Driving Emerging Infectious Diseases Impact on Wildlife Populations. Animal Biodiversity and Emerging Diseases: Prediction and Prevention. 2008;1149:1–3. doi: 10.1196/annals.1428.052. [DOI] [PubMed] [Google Scholar]
- Altizer S, Nunn CL, Lindenfors P. Do threatened hosts have fewer parasites? A comparative study in primates. Journal of Animal Ecology. 2007;76:304–314. doi: 10.1111/j.1365-2656.2007.01214.x. [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM. The Invasion, Persistence and Spread of Infectious-Diseases within Animal and Plant-Communities. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1986;314:533–570. doi: 10.1098/rstb.1986.0072. [DOI] [PubMed] [Google Scholar]
- Araujo MB, Alagador D, Cabeza M, Nogues-Bravo D, Thuiller W. Climate change threatens European conservation areas. Ecology Letters. 2011;14:484–492. doi: 10.1111/j.1461-0248.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson CT, Woods KL, Dusek RJ, Sileo LS, Iko WM. Wildlife disease and conservation in Hawaii: Pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected Iiwi (Vestiaria coccinea). Parasitology. 1995;111:S59–S69. doi: 10.1017/s003118200007582x. [DOI] [PubMed] [Google Scholar]
- Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, Coleman JTH, Darling SR, Gargas A, Niver R, Okoniewski JC, Rudd RJ, Stone WB. Bat White-Nose Syndrome: An Emerging Fungal Pathogen? Science. 2009;323:227–227. doi: 10.1126/science.1163874. [DOI] [PubMed] [Google Scholar]
- Brook BW, Sodhi NS, Bradshaw CJA. Synergies among extinction drivers under global change. Trends in Ecology & Evolution. 2008;23:453–460. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Clavero M, Garcia-Berthou E. Invasive species are a leading cause of animal extinctions. Trends in Ecology & Evolution. 2005;20:110–110. doi: 10.1016/j.tree.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cunningham AA, Daszak P. Extinction of a species of land snail due to infection with a microsporidian parasite. Conservation Biology. 1998;12:1139–1141. [Google Scholar]
- Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R. Emerging infectious diseases and amphibian population declines. Emerging Infectious Diseases. 1999;5:735–748. doi: 10.3201/eid0506.990601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Knapp RA. Multiple stressors and amphibian declines: Dual impacts of pesticides and fish on yellow-legged frogs. Ecological Applications. 2007;17:587–597. doi: 10.1890/06-0181. [DOI] [PubMed] [Google Scholar]
- de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecology Letters. 2005;8:117–126. [Google Scholar]
- Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2001;356:1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J, Clifford D, Castle K, Cryan P, Ostfeld RS. Investigating and managing the rapid emergence of white-nose syndrome, a novel, fatal, infectious disease of hibernating bats. Conservation Biology. 2011;25:223–231. doi: 10.1111/j.1523-1739.2010.01638.x. [DOI] [PubMed] [Google Scholar]
- Gurevitch J, Padilla DK. Are invasive species a major cause of extinctions? Trends in Ecology & Evolution. 2004;19:470–474. doi: 10.1016/j.tree.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Heard M, Smith KF, Ripp K. Examining the Evidence for Chytridiomycosis in Threatened Amphibian Species. Plos One. 2011;6:e23150. doi: 10.1371/journal.pone.0023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh-Guldbert, Hughes OL, McIntyre S, Lindenmayer DB, Parmesan C, Possingham HP, Thomas CD. Assisted colonization and rapid climate change. Science. 2008;321:345–346. doi: 10.1126/science.1157897. [DOI] [PubMed] [Google Scholar]
- Hof C, Araujo MB, Jetz W, Rahbek C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature. 2011;480:516–U137. doi: 10.1038/nature10650. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Hilton-Taylor C, Angulo A, Bohm M, Brooks TM, Butchart SHM, Carpenter KE, Chanson J, Collen B, Cox NA, W. R., et al. The Impact of Conservation on the Status of the World's Vertebrates. Science. 2010;330:1503–1509. doi: 10.1126/science.1194442. [DOI] [PubMed] [Google Scholar]
- IUCN The IUCN Red List of Threatened Species. Version 2011.2. 2011 http://www.iucnredlist.org. Downloaded on 2 February 2012.
- Johnson ML, Speare R. Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Diseases of Aquatic Organisms. 2005;65:181–186. doi: 10.3354/dao065181. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Olden JD, vander Zanden MJ. Dam invaders: impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment. 2008;6:359–365. [Google Scholar]
- Jones ME, Jarman PJ, Lees CM, Hesterman H, Hamede RK, Mooney NJ, Mann D, Pukk CE, Bergfeld J, McCallum H. Conservation management of tasmanian devils in the context of an emerging, extinction-threatening disease: Devil facial tumor disease. Ecohealth. 2007;4:326–337. [Google Scholar]
- Lafferty KD, Porter JW, Ford SE. Are diseases increasing in the ocean? Annual Review of Ecology Evolution and Systematics. 2004;35:31–54. [Google Scholar]
- Lambin EF, Geist HJ, Lepers E. Dynamics of land-use and land-cover change in tropical regions. Annual Review of Environment and Resources. 2003;28:205–241. [Google Scholar]
- Marcogliese DJ, Pietrock M. Combined effects of parasites and contaminants on animal health: parasites do matter. Trends in Parasitology. 2011;27:123–130. doi: 10.1016/j.pt.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Mccallum H, Dobson A. Detecting Disease and Parasite Threats to Endangered Species and Ecosystems. Trends in Ecology & Evolution. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- Merrill RM. Introduction to Epidemiology. 5th edition. Jones and Bartlett Publishers; Sudbary, MA: 2010. [Google Scholar]
- Obrien SJ, Evermann JF. Interactive Influence of Infectious-Disease and Genetic Diversity in Natural-Populations. Trends in Ecology & Evolution. 1988;3:254–259. doi: 10.1016/0169-5347(88)90058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AB, Jones KE, Nunn CL, Altizer S. Infectious diseases and extinction risk in wild mammals. Conservation Biology. 2007;21:1269–1279. doi: 10.1111/j.1523-1739.2007.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Sokolow SH, Gorman ME, Daszak P, Foley JE. Causal inference in disease ecology: investigating ecological drivers of disease emergence. Frontiers in Ecology and the Environment. 2008;6:420–429. [Google Scholar]
- Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, Daszak P. The decline of the sharp-snouted day frog (Taudactylus acutirostris): The first documented case of extinction by infection in a free-ranging wildlife species? Ecohealth. 2006;3:35–40. [Google Scholar]
- Smith KF, Acevedo-Whitehouse K, Pedersen AB. The role of infectious diseases in biological conservation. Animal Conservation. 2009;12:1–12. [Google Scholar]
- Smith KF, Behrens MD, Sax DF. Local Scale Effects of Disease on Biodiversity. Ecohealth. 2009;6:287–295. doi: 10.1007/s10393-009-0254-9. [DOI] [PubMed] [Google Scholar]
- Smith KF, Carpenter SM. Potential spread of introduced black rat (Rattus rattus) parasites to endemic deer mice (Peromyscus maniculatus) on the California Channel Islands. Diversity and Distributions. 2006;12:742–748. [Google Scholar]
- Smith KF, Sax DF, Lafferty KD. Evidence for the role of infectious disease in species extinction and endangerment. Conservation Biology. 2006;20:1349–1357. doi: 10.1111/j.1523-1739.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- Van Houtan KS, Hargrove SK, Balazs GH. Land Use, Macroalgae, and a Tumor-Forming Disease in Marine Turtles. PLoS ONE. 2010;5:e12900. doi: 10.1371/journal.pone.0012900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of the earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Ward JR, Lafferty KD. The elusive baseline of marine disease: Are diseases in ocean ecosystems increasing? Plos Biology. 2004;2:542–547. doi: 10.1371/journal.pbio.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E. Quantifying threats to imperiled species in the United States. BioScience. 1998;48:607–615. [Google Scholar]
- Witte CL, Sredl MJ, Kane AS, Hungerford LL. Epidemiologioc analysis of factors associated with local disappearances of native Ranid frogs in Arizona. Conservation Biology. 2008;22:375–383. doi: 10.1111/j.1523-1739.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- Woodward M. Epidemiology: Study design and data analysis. 2nd edition. CRC Press; Boca Raton, Florida.: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.