Abstract
Background
Powerful computing capabilities in small, easy to use hand-held devices have made smart technologies such as smartphones and tablets ubiquitous in today’s society. The capabilities of these devices provide scientists with many tools that can be used to improve the scientific method.
Method
Here, we demonstrate how smartphones may be used to quantify the sensitivity of functional near-infrared spectroscopy (fNIRS) signal to head motion. By attaching a smartphone to participants’ heads during the fNIRS scan, we were able to capture data describing the degree of head motion.
Results
Our results demonstrate that data recorded from an off-the-shelf smartphone accelerometer may be used to identify correlations between head-movement and fNIRS signal change. Furthermore, our results identify correlations between the magnitudes of head-movement and signal artifact, as well as a relationship between the direction of head movement and the location of the resulting signal noise.
Conclusions
These data provide a valuable proof-of-concept for the use of off-the-shelf smart technologies in neuroimaging applications.
Keywords: Near-infrared spectroscopy, fNIRS, smartphone, technology, neuroimaging, accelerometer
1. Introduction
Smart technologies such as smartphones and tablets are ubiquitous in today’s society. These devices are equipped with computing power that rivals larger, less portable desktop computers, all while being small, portable, and easy to use. Experts predict that by the end of 2014, the number of smartphones in circulation will exceed the number of personal computers (IDC, 2013). Notably, these devices are highly configurable by downloadable applications that are unlimited in scope. As a result, novel uses of smart technologies in various aspects of science and medicine are highly sought-after.
Among the documented medical applications of smartphones is their use in remotely diagnosing strokes (Demaerschalk, 2012; Mitchell, 2011), presentation of radiographic evaluations (Ege et al., 2013), identification of concussion (Curaudeau, 2011; Kutcher, 2013), detection of irregular pulse (McManus, 2012), managing surgical implants (Fakhar, 2013), detecting and preventing falls in aging patients (Mellone, 2012; Sposaro, 2009), medical monitoring (Dunton, 2011; Isik, 2013; Lee, 2011; Maki, 2011; Van Wieringen, 2008), characterization of Parkinson’s disease tremors (LeMoyne, 2010), heart rate monitoring (Kwon, 2011), and Cobb angle measurement in patients with scoliosis (Shaw, 2012). Many of these and other smartphone applications rely on sensors that come standard in today’s smart devices. For instance, each device is equipped with an accelerometer, which measures the acceleration caused by movement and gravity. Importantly, numerous studies have demonstrated that such devices are highly accurate and reliable, rivaling the performance of stand-alone accelerometers (Balg et al., 2014; Demaerschalk et al., 2012; Ege et al., 2013; Izatt et al., 2012; Mellone et al., 2012; Nishiguchi et al., 2012; Ockendon & Gilbert, 2012). The precision of the accelerometers in modern smartphones allows for many of the findings reported above, such as reliable identification of sudden movements related to falling, and real-time measurement of the curve in a scoliosis patients’ spine.
Accelerometers may be used in cognitive neuroimaging as a tool to record a participants’ head movement during a scan. The ability to record the timing, magnitude, and direction of head motion provides researchers with a valuable tool that may be used to statistically remove artifacts within an imaging signal that is caused by head movement (Virtanen et al., 2011), or to help characterize particular behaviors such as nodding (Lee & Ha, 2001). While movement information can be calculated from functional images using rigid body transformation in fMRI (Friston, et al., 2011), other imaging devices such as functional near-infrared spectroscopy (fNIRS) and EEG are not equipped with such features. Moreover, stand-alone accelerometers designed for neuroimaging applications may be expensive, and depending on their size and components may not be conducive to particular experimental designs. The accelerometers within a smartphone may provide an ideal alternative to such stand-alone devices, and would provide researchers with a portable, convenient, and easy to use method to record patients head motion during experimental sessions.
Here, we provide the first evidence that smartphone accelerometers can be used to accurately record participants’ head movement during fNIRS neuroimaging. fNIRS uses light projected through a patient’s scalp to measure the oxygen levels of hemoglobin in the blood of the cerebral cortex. With an observation rate of 10Hz and a typical optode spacing of 3cm, fNIRS provides greater temporal resolution than fMRI while maintaining greater spatial resolution than EEG. While commonly thought of as more tolerant to movement than fMRI and EEG, head movement does affect fNIRS signal quality (Aslin & Mehler, 2005). However, the degree to which fNIRS signal amplitude fluctuates with head movement is unknown. To that end, the current project addresses this topic as well: We hypothesize that the magnitude and direction of head motion recorded by an off-the-shelf smartphone accelerometer will correlate with abrupt changes in fNIRS signal amplitude (i.e., spikes). This project is the first to identify and implement a direct use for smartphones during fNIRS neuroimaging and provides a much-needed platform for future applications of smart technologies to be adopted in cognitive neuroimaging.
2. Method
2.1 Participants
A total of six adult participants (n female= 3, age range 22-67, mean 37.5 years old) were recruited for participation. Each participant was recruited from the campus of Stanford University. Written informed consent was obtained from all participants, and the Stanford University Institutional Review Board approved the study protocol.
2.2 Experimental procedure
Prior to undergoing fNIRS imaging, the task requirements were given to each participant. Participants were sat comfortably at a desk and were told to keep their head upright and steady. Throughout the imaging session the participants performed a simple task in which they tapped the pointer finger on their right hand on a desk in 10-second blocks, followed by 20-second blocks of rest. Randomly throughout the tapping blocks, participants were instructed, by vocal command, to move their head in a slow and steady manner in one of four directions (left, right, forward, or backward), and to move either a short (e.g., ~10°) or long (e.g., ~30°) distance from their upright starting point (see Figure 1). Prior to beginning the experiment, each participant was allowed to practice performing both short and long head movements to. Each short direction was made six times, and each long direction was made seven times.
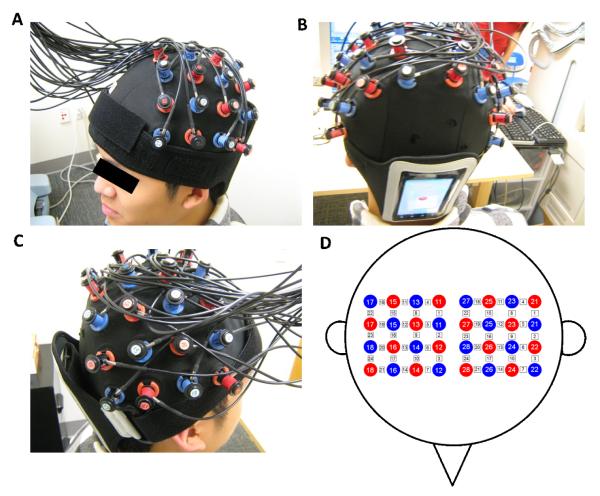
Figure 1.
Experimental setup. (A, B and C) A participant wearing the ETG 4000 cap and smartphone. (D) Cap configuration. Red circles indicate emitters; blue circles indicate detectors. White squares indicate measurement channels between emitters and detectors.
2.3 NIRS data acquisition
An ETG-4000 (Hitachi, Japan) Optical Topography system was used to measure the concentration changes in oxygenated (HbO) and deoxygenated (Hb) hemoglobin in the primary motor and somatosensory cortex of each participant. Two “4×4” measurement patches were attached to a regular swimming cap, which was positioned onto each participant’s head (see Figure 1). Specifically, the medial edges of both patches were aligned to the midline (i.e., the arc running from the nasion through Cz to the inion).
2.4 Accelerometer data acquisition and analysis
All accelerometer data were recorded on an unmodified Motorola DEFY XT water-resistant, dust-proof Android smartphone. The data were gathered from the smartphones’ on-board accelerometer by way of an in-house program. The smartphone was attached to the back of the participants’ head by way of a smartphone exercise armband with a large adjustable strap (see Figure 1abc).
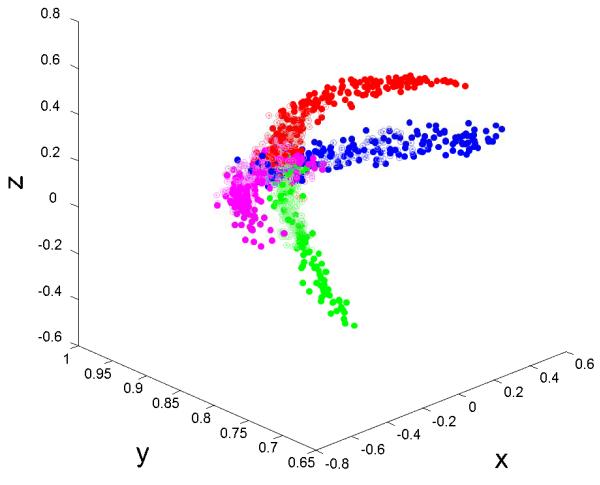
The accelerometer in the smartphone we used (and also in most smartphones such as the iPhone) continuously measures total acceleration, which is a vector sum of the force of gravity and linear acceleration. Since the head motion in our experiments is slow compared to gravity (i.e. the linear acceleration is negligible), the accelerometer data recorded is the x-, y-, and z-components of gravity in the reference frame of the smartphone (Figure 2). Thus, we can calculate the angle of the head at any time point based on the x, y, and z data. Each head motions’ amplitude was calculated as the maximal angle of the smartphone position during the motion period (typically less than 4s) from the origin (see Figure 3 for 3D trajectory of head motion).
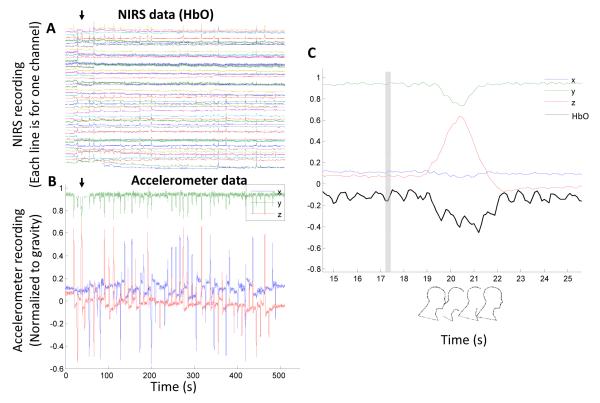
Figure 2.
Exemplary NIRS and accelerometer data. (A) A participant’s NIRS data (HbO from 48 channels). Rows from bottom to top are for channel 1 to 48. It’s evident that there are numerous motion artifacts (spikes). The black arrow indicates one of such spikes. (B) The same participant’s accelerometer data showing the subject’s head position. Because the head motion is slow compared to gravity (i.e. the linear acceleration is negligible), the accelerometer data recorded is the x-, y-, and z-components of gravity in the reference frame of the smartphone. The black arrow indicates one of the head motions and the timing is identical to the arrow in (A). (C) A zoom in of accelerometer data of the first head motion (“forward big”), and the HbO signal from channel 1. The vertical gray bar indicates the timing of motion instruction.
Figure 3.
A 3D plot of the trajectory of a participant’s head position measured by a smartphone accelerometer. Based on the orientation of the smartphone placement, the y-axis is primarily aligned to the vertical direction during rest. Color indicates the instructed motion direction. Red means forward, green means backward, blue means left and magenta means right. Open circles denotes short motion and solid circle denotes long motion. Based on the figure, it is evident that the smartphone accelerometer captures the expected head motion trajectory.
2.5 NIRS data analysis
All fNIRS data were initially treated with a 0.01 Hz high-pass filter to remove low frequency drift. Next, the filtered data were plotted along with the accelerometer data in order to visually identify artifacts in the fNIRS data that coincide with peaks along the x-, y-, or z-axis of the accelerometer data (Figure 2ab). The amplitude of a signal artifact is calculated as the peak amplitude of fNIRS signal normalized by the mean and standard deviation of the fNIRS data points in the entire session. The maximal absolute signal artifact among all channels is used in identifying the effect of a head movement. As opposed to neurobiological sources (Van Dijk et al., 2012; Zeng et al., 2014), abrupt motion-induced signal changes in fNIRS (i.e. spikes) are driven by displacement of the optodes on the head resulting in variations in the intensity of the near-IR light at the point of contact with the scalp (Aslin & Mehler, 2005). Because the degree of optode displacement corresponds with the degree of head movement, it is reasonable to hypothesize that signal amplitude change provides a suitable correlate of head motion. Based on this relationship, we plotted the amplitude of signal artifact against the degree of head motion amplitude to identify the sensitivity of fNIRS signal to head motion (Figure 4). To find the relationship between fNIRS artifact in different measurement regions and motion direction, we calculated the average of the signal artifact across repeats of the same motion type for each channel and plotted the heat maps in Figure 5.
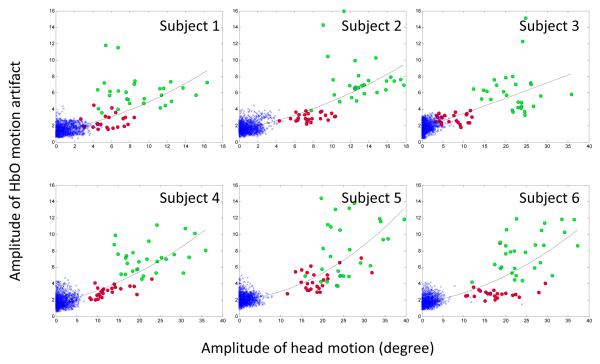
Figure 4.
Amplitude of HbO artifacts as a function of the amplitude of head motion. The x-axis gives the amplitude of the head motion (unit= degree), the y-axis gives the amplitude of the maximal HbO motion artifacts (normalized by the pooled standard deviation). Green circles denote “long” motion and red circles denote “short” motion. The blue cross (x) symbols are data from the “non-motion” period where the subjects were instructed to remain still. The trend line within each subplot highlights a very strong non-linear dependence on head motion. The fit of the quadratic (i.e., non-linear) term was significant for all participants except Subject 3. Bigger motion is clearly related to bigger HbO artifacts.
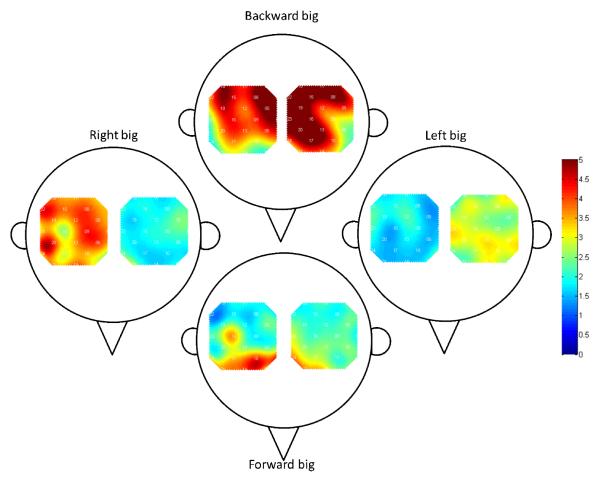
Figure 5.
Group-level amplitude change heat-maps for “long” head motions describing the location of HbO artifacts and direction of head motion. Color indicates the amplitude of the HbO motion artifact where red means larger artifact. The figures demonstrate that a motion in a direction (e.g. forward) will cause higher artifacts in that direction (e.g. frontal region). Identical visual analysis of the “short” head motions did not identify similar patterns of amplitude changes and were not displayed.
3. Results
3.1 Baseline movement
A cohesive clustering of data points near the plot origin is visually apparent within the fNIRS signal amplitude plots displayed in Figure 4. These points, marked by blue crosses, occurred when the participants were told to hold their heads upright (i.e., in between head movements). During these periods the participants had a baseline head motion of roughly 5°, and a noticeable variation in fNIRS amplitude is apparent. However, the observed range of variation in fNIRS amplitude (i.e., ~.5-3 standard deviations) is expected in a normal distribution.
3.2 Correlation between head movement and signal amplitude change
Our data highlight a positive correlation between the degree of head motion made by the participant and the amplitude of the HbO motion artifact. That is, the greater the degree of head motion in any direction, the greater amount of noise related to head motion within the fNIRS signal. In order to more precisely define this relationship, we employed a series of regression analyses. First, linear regression identified a significant linear relationship between the degree of head motion and fNIRS signal amplitude for all participants (R2 range= .35 - .52; p< .0001). However, as seen in Figure 4, fNIRS amplitude increases more abruptly (i.e., non-linearly) during long head movements compared to short movements. A second regression analysis, which included a quadratic term to capture this curvilinear relationship, identified significant beta values for the quadratic term (R2 range= .37 - .55; p< .0001) for all participants except number 3. These results suggest that fNIRS signal amplitudes increase with head motion, and that the rate of increase is greater during long compared to short head movements.
3.3 Correlation between direction of head motion and location of signal amplitude change
Figure 5 highlights a correlation between the directions that participant moved their head and the location of the resulting signal artifacts. Such artifacts were greatest in the locations of the brain that coincide with the direction of head movement. For example, moving the head forward resulted in a large artifact in anterior regions of the fNIRS optode patches, whereas moving the head to the right caused a large artifact in the channels within the right fNIRS patch. The signal amplitude change in the regions contralateral to the direction of head motion was comparatively small. Identical analysis of the “short” head motions did not reveal similar patterns of amplitude change.
4. Discussion
Here, we provide the first demonstration of the use of an off-the-shelf smartphone to measure head movement during fNIRS neuroimaging. By accessing the spatial position data automatically recorded by our smartphone’s accelerometer during an fNIRS scan, we were able to identify a relationship between head motion and fNIRS signal noise. Namely, signal noise increased non-linearly with increases in head motion. Moreover, the location of signal noise was related to the direction of head motion. Taken together, our data help characterize fNIRS signal noise caused by head motion, and provide a valuable proof-of-concept for smartphones’ use in modern neuroimaging research.
It is important to note that our experimental design, combined with functional regions of interest limited to the motor cortex, allowed for relatively easy placement of the smartphone onto the back of each participants head by way of a standard exercise armband (see Figure 1). However, because researchers are free to arrange fNIRS optodes in any configuration that is appropriate for their experimental design, generalization of our procedure may require alternative methods to affix the smartphone to the participants’ head. However, given the accuracy and reliability of smartphone accelerometers across a wide range of tasks, it is unlikely that measurement sensitivity and accuracy would vary given different placement locations on the head, or given different affixation methods.
Recent estimates suggest that there are currently more mobile devices in use, including smartphones and tablets, than there are people on the planet (VNI, 2014). Importantly, this figure is expected to rise 13-fold by 2017 to numbers exceeding 10 billion. As these devices become evermore ubiquitous the identification of specialized applications, such as that which we describe herein, has the potential to greatly improve the scientific method. For example, as demonstrated by Virtanen and colleagues (2011), accelerometer data may be used as a regressor to remove artifacts due to movement in an fNIRS signal. Importantly, multiple accelerometer apps are available at little to no cost for all smartphone systems by searching “accelerometer” in the application stores. Many of these apps collect and save accelerometer data locally and can be easily transferred to a computer via a USB port or wireless transmission, thus subserving the need for in-house programs. Together with our procedure, future researchers may employ smartphone accelerometers to record and wirelessly transmit head motion data into a repository where it may be combined with hemodynamic data recorded by fNIRS. In this manner researchers may seamlessly integrate smartphone-recorded head motion data into their data analysis pipeline.
One justifiable concern that arises when employing smartphones in neuroimaging is maintaining adequate timing. Notably, accelerometer reliability across smartphones has been shown to be high. In a study by Amick and colleagues (2013), the authors compared the accelerometers of five different smartphones and concluded “these devices demonstrated consistent sensitivity values across multiple devices as demonstrated by low coefficients of variability.” Nevertheless, in a study by Stopczynski and colleagues (2014), which employed a smartphone-based brain scanner application for EEG, maintaining consistent timing was notoriously difficult. However, it is worth noting that this study relied on a smartphone for data acquisition, processing (i.e., filtering, recording, 3D reconstruction), and feedback (e.g., 3D brain visualization, stimuli delivery, neurofeedback). Conversely, our method relies on a smartphone to record accelerometer data only, which requires much less processing power and does not affect the devices’ timing as severely. Moreover, because fNIRS measures hemodynamic fluctuations as opposed to electrical impulses, the need for perfect synchrony between an event (e.g., head movement) and fNIRS data is not needed.
For our study, simply aligning the fNIRS and accelerometer data based on abrupt “spikes” caused by head movements in both data streams was sufficient. Researchers that wish to adopt our method need only download an accelerometer app from an application store, then identify a feasible approach to affix a smartphone to their participants’ head (e.g., exercise arm band, see Figure 1) without disturbing the fNIRS optode arrangement. Next, they may instruct each participant to make a head movement that will be easily distinguishable within the accelerometer data (e.g., head nod) and the NIRS data immediately after the fNIRS scan begins. In this manner, a calibration landmark will be available to align both sets of data easily. Within applications wherein real time integration of accelerometer data is not necessary, researchers may export these data from the smartphone following the end of the scan and integrate the data during post hoc data processing.
Our project made use of standard hardware within a smartphone (i.e., accelerometer) that, despite its usefulness for our purposes, is restrictive in its range of possible applications. It is important to note that some experimental paradigms require accelerometer performance standards that may not be attainable or guaranteed within smartphones. However, as mentioned above, along with the accelerometer that comes standard in modern smartphones, the user-developed programs that employ smart technologies’ powerful computing capabilities are endlessly flexible, and may ultimately provide researchers with an unlimited number of uses that may supersede the need for specialized equipment. To that end, it will be important for future researchers to identify, develop, and share these applications so that other researchers may also benefit. Importantly, the ability to supplement specialized equipment with ubiquitous multi-use technology such as smartphones may have far reaching impacts on future scientific endeavors.
Highlights.
This study provides the first applied use of smartphones in fNIRS neuroimaging
Data from smartphone accelerometers correlate with motion-related noise in fNIRS
Longer head motion is more likely to induce signal noise in NIRS than shorter motions.
Acknowledgements
We would like to thank Manish Saggar for helpful discussion. Furthermore, we would like to thank Kristen Sheau for her support in collecting accelerometer data. This work is supported by S10 RR024657 (ALR PI) and the Stanford Institute for Neuro-Innovation and Translational Neurosciences (SINTN) fellowship (XC and ALR). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Amick RZ, Patterson JA, Jorgensen MJ. Sensitivity of tri-axial accelerometers within mobile consumer electronic devices: A pilot study. International Journal of Applied Science and Technology. 2013;3(2):97. [Google Scholar]
- Aslin RN, Mehler J. Near-infrared spectroscopy for functional studies of brain activity in human infants: promise, prospects, and challenges. Journal of Biomedical Optics. 2005;10(1):1–3. doi: 10.1117/1.1854672. [DOI] [PubMed] [Google Scholar]
- Balg F, Juteau M, Theoret C, Svotelis A, Grenier G. Validity and reliability of the iPhone to measure rib hump in scoliosis. Scoliosis. 2014;11:12. doi: 10.1097/BPO.0000000000000195. [DOI] [PubMed] [Google Scholar]
- Brigadoi S, Ceccherini L, Cutini S, Scarpa F, Scatturin P, Selb J, Gagnon L, Boas DA, Cooper RJ. Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. NeuroImage. 2014;85:181–191. doi: 10.1016/j.neuroimage.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curaudeau GA, Sharma N, Rovin RA. Development of an iPhone application for sideline concussion testing. Neurosurgical focus. 2011;31(5):E4. doi: 10.3171/2011.8.FOCUS11186. [DOI] [PubMed] [Google Scholar]
- Damaerschalk BM, Vargas JE, Channer DD, Noble BN, Kiernan TEJ, Gleason EA, Bobrow BJ. Smartphone teleradiology application is successfully incorporated into a telestroke network environment. Stroke. 2012;43(11):3098–3101. doi: 10.1161/STROKEAHA.112.669325. [DOI] [PubMed] [Google Scholar]
- Demaerschalk BM, Vegunta S, Vargas BB, Wu Q, Channer DD, Hentz JG. Reliability of real-time video smartphone for assessing National Institutes of Health Stroke Scale scores in acute stroke patients. Stroke. 2012;43(12):3271–3277. doi: 10.1161/STROKEAHA.112.669150. [DOI] [PubMed] [Google Scholar]
- Dunton GF, Liao Y, Intille SS, Spruijt-Metz D, Pentz M. Investigating children’s physical activity and sedentary behavior using ecological momentary assessment with mobile phones. Obesity. 2011;19(6):1205–1212. doi: 10.1038/oby.2010.302. [DOI] [PubMed] [Google Scholar]
- Ege T, Kose O, Koca K, Demiralp B, Basbozkurt M. Use of the iPhone for radiographic evaluation of hallux valgus. Skeletal radiology. 2013;42(2):269–273. doi: 10.1007/s00256-012-1455-9. [DOI] [PubMed] [Google Scholar]
- Fakhar K, Hastings E, Butson CR, Foote KD, Zeilman P, Okun MS. Management of deep brain stimulator battery failure: battery estimators, charge density, and importance of clinical symptoms. PloS one. 2013;8(3):e58665. doi: 10.1371/journal.pone.0058665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 2011;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gagnon L, Yücel MA, Boas DA, Cooper RJ. Further improvement in reducing superficial contamination in NIRS using double short separation measurements. NeuroImage. 2014;85:127–135. doi: 10.1016/j.neuroimage.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Data Corporation (IDC), IDC Media Center Tablet shipments forecast to top total PC shipments in the fourth quarter of 2013 and annually by 2015, according to IDC [Press release] 2013 Retrieved from http://www.idc.com/getdoc.jsp?containerId=prUS24-314413.
- Işik AH, Güler Ị, Şener MU. A Low-Cost Mobile Adaptive Tracking System for Chronic Pulmonary Patients in Home Environment. TELEMEDICINE and e-HEALTH. 2013;19(1):24–30. doi: 10.1089/tmj.2012.0056. [DOI] [PubMed] [Google Scholar]
- Izatt MT, Bateman GR, Adam CJ. Evaluation of the iPhone with an acrylic sleeve versus the Scoliometer for rib hump measurement in scoliosis. Scoliosis. 2012;7(1):1–7. doi: 10.1186/1748-7161-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutcher JS, McCrory P, Davis G, Ptito A, Meeuwisse WH, Broglio SP. What evidence exists for new strategies or technologies in the diagnosis of sports concussion and assessment of recovery? British journal of sports medicine. 2013;47(5):299–303. doi: 10.1136/bjsports-2013-092257. [DOI] [PubMed] [Google Scholar]
- Kwon S, Lee J, Chung GS, Park KS. Validation of heart rate extraction through an iPhone accelerometer. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE; IEEE; Aug, 2011. pp. 5260–5263. [DOI] [PubMed] [Google Scholar]
- Lee J, Ha I. Real-time motion capture for a human body using accelerometers. Robotica. 2001;19:601–610. [Google Scholar]
- Lee MH, Kim J, Jee SH, Yoo SK. Integrated solution for physical activity monitoring based on mobile phone and PC. Healthcare informatics research. 2011;17(1):76–86. doi: 10.4258/hir.2011.17.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoyne R, Mastroianni T, Cozza M, Coroian C, Grundfest W. Implementation of an iPhone as a wireless accelerometer for quantifying gait characteristics. Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE; IEEE; Aug, 2010. pp. 3847–3851. [DOI] [PubMed] [Google Scholar]
- Maki H, Ogawa H, Matsuoka S, Yonezawa Y, Caldwell WM. A daily living activity remote monitoring system for solitary elderly people. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE; IEEE; Aug, 2011. pp. 5608–5611. [DOI] [PubMed] [Google Scholar]
- Mellone S, Tacconi C, Chairi L. Validity of a smartphone-based instrument Timed Up and Go. Gait & Posture. 2012;36(1):163–165. doi: 10.1016/j.gaitpost.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Mellone S, Tacconi C, Schwickert L, Klenk J, Becker C, Chiari L. Smartphone-based solutions for fall detection and prevention: the FARSEEING approach. Zeitschrift Für Gerontologie Und Geriatrie. 2012;45(8):722–727. doi: 10.1007/s00391-012-0404-5. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Sharma P, Modi J, Simpson M, Thomas M, Hill MD, Goyal M. A smartphone client-server teleradiology system for primary diagnosis of acute stroke. Journal of medical Internet research. 2011;13(2) doi: 10.2196/jmir.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus DD, Lee J, Maitas O, Esa N, Pidikiti R, Carlucci A, Chon KH. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm. 2013;10(3):315–319. doi: 10.1016/j.hrthm.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi S, Yamada M, Nagai K, Mori S, Kajiwara Y, Sonoda T, Aoyama T. Reliability and validity of gait analysis by android-based smartphone. Telemedicine and e-Health. 2012;18(4):292–296. doi: 10.1089/tmj.2011.0132. [DOI] [PubMed] [Google Scholar]
- Ockendon M, Gilbert RE. Validation of a novel smartphone accelerometer-based knee goniometer. Journal of Knee Surgery. 2012;25(4):341–346. doi: 10.1055/s-0031-1299669. [DOI] [PubMed] [Google Scholar]
- Shaw M, Adam CJ, Izatt MT, Licina P, Askin GN. Use of the iPhone for Cobb angle measurement in scoliosis. European Spine Journal. 2012;21(6):1062–1068. doi: 10.1007/s00586-011-2059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sposaro F, Tyson G. iFall: an Android application for fall monitoring and response. Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE; IEEE; Sep, 2009. pp. 6119–6122. [DOI] [PubMed] [Google Scholar]
- Stopczynski A, Stahlut C, Larsen JE, Petersen MK, Hansen LK. The smartphone brain scanner: A portable real-time neuroimaging system. PlosOne. 2014;9(2):e86733. doi: 10.1371/journal.pone.0086733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wieringen M, Eklund JM. Real-time signal processing of accelerometer data for wearable medical patient monitoring devices. Engineering in Medicine and Biology Society, 2008. EMBS 2008. 30th Annual International Conference of the IEEE; IEEE; Aug, 2008. pp. 2397–2400. [DOI] [PubMed] [Google Scholar]
- Visual Networking Index (VNI), Cisco Systems Global mobile data traffic forecast update, 2013-2018 [Press release] 2014 Retrived from http://www.cisco.com/c/en/us/solutions/collateral/service-provider/visual-networking-index-vni/white_paper_c11-520862.html.
- Virtanen J, Noponen T, Kotilahti K, Virtanen J, Llmoniemi RJ. Accelerometer-based method for correcting signal baseline changes caused by motion artifacts in medical near-infrared spectroscopy. Journal of Biomedical Optics. 2011;16(8):087005.1–087005.9. doi: 10.1117/1.3606576. [DOI] [PubMed] [Google Scholar]
- Zeng LL, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, Buckner RL, Liu H. Neurobiological basis of head motion in brain imaging. Proceedings of the National Academy of Sciences. 2014;111(16):6058–6062. doi: 10.1073/pnas.1317424111. [DOI] [PMC free article] [PubMed] [Google Scholar]