Abstract
Purpose
Colorectal cancer patients with liver-confined metastases are classified as stage IV, but their prognoses can differ from metastases at other sites. In this study, we suggest a novel method for risk stratification using clinically effective factors.
Materials and Methods
Data on 566 consecutive patients with colorectal liver metastasis (CLM) between 1989 and 2010 were analyzed. This analysis was based on principal component analysis (PCA).
Results
The survival rate was affected by carcinoembryonic antigen (CEA) level (p < 0.001; risk ratio, 1.90), distribution of liver metastasis (p=0.014; risk ratio, 1.46), and disease-free interval (DFI; p < 0.001; risk ratio, 1.98). When patients were divided into three groups according to PCA score using significantly affected factors, they showed significantly different survival patterns (p < 0.001).
Conclusion
The PCA scoring system based on CEA level, distribution of liver metastasis, and DFI may be useful for preoperatively determining prognoses in order to assist in clinical decisionmaking and designing future clinical trials for CLM treatment.
Keywords: Risk calculation, Patient stratification, Colorectal neoplasms, Liver metastasis
Introduction
Colorectal cancer (CRC) is the fourth most commonly diagnosed type of cancer as well as second leading cause of cancer-related death [1]. About one-fourth of all CRC patients have liver metastases at the time of their diagnosis [2,3], and 20% of patients develop liver metastases after surgical resection of the primary tumor [2]. For patients with colorectal liver metastasis (CLM), hepatic resection is a potentially curative option [4-6], but only 20% of such patients are suitable for resection at presentation. As another option, chemotherapeutic agents such as oxaliplatin and irinotecan allow an additional 10%-20% of patients to undergo hepatic resection for initially unresectable CLM [7-9]. In addition, recent developments in surgical techniques, radiologic imaging, and chemotherapy are changing the definition of surgical resectability.
Previous studies have identified several risk factors that can be used to select patients suitable for hepatic resection [10-15]. Since 1996, 12 prognostic scoring systems have been developed [16]. All studies that developed scoring systems were based on retrospective data and proposed groups ranging in number from three to six. The studies were also based exclusively on patients who underwent surgical resections, and some of the methods were based on surgical-pathological factors that cannot be easily obtained. Given this background, reliable, easily accessible, and accurate clinical factors predicting outcomes need to be identified and consolidated in referred patients with CLM.
In this study, principal component analysis (PCA) was developed as a new stratification method for CLM using prognostic factors selected by multivariate analysis. We also compared PCA with the Fong's scoring method.
Materials and Methods
1. Patients
Analysis was conducted using prospective databases containing patients diagnosed with confined liver metastasis due to CRC at Severance Hospital of the Yonsei University Health System between 1989 and 2010. All patients were given a physical examination, and medical histories were collected. Serum laboratory tests and appropriate imaging studies, including computed tomographic (CT) or magnetic resonance (MR) imaging of the chest, abdomen, and pelvis, were done to evaluate the liver and check for extrahepatic disease. Patients also underwent colonoscopies to assess local recurrence of the primary tumor. After surgery, serial serum carcinoembryonic antigen (CEA) levels, CT imaging of the abdomen, and chest radiography were obtained for each patient every 3 to 4 months for 2 years, and every 6 months thereafter. For induction chemotherapy, response to chemotherapy was evaluated every 2 months by a multidisciplinary team that included surgeons, oncologists, and radiologists. The team used CT or MR imaging according to World Health Organization guidelines during the initial study period as well as the Response Evaluation Criteria in Solid Tumors during the final period. Patients responding to chemotherapy were reconsidered for surgery when the overall strategy achieved complete clearance of hepatic metastases.
2. Statistical method
To stratify patients according to survival risk, we applied PCA. PCA is a simple, nonparametric statistical method that reduces data dimensionality by using an orthogonal transformation to convert correlated variables into uncorrelated variables, which are termed principal components. PCA has a lower or equal number of principal components compared to the number of original variables. Each principal component is represented in the form of linear combinations of original variables. Therefore, some value for each patient can be calculated according to the following formula, which we call the PCA score:
, where V1, V2, V3 … Vn are the values of original variables, whereas C1, C2, C3 … Cn are coefficients of each of the variables.
We applied PCA to one patient group in order to determine the coefficient of each variable, and a PCA score was generated for each patient. The variables included in the PCA analysis were defined by multivariate Cox regression analysis. The patient groups were stratified into three groups by the lower (Q1) and upper (Q3) quartiles of the PCA score. The presence of significantly different survival patterns was then tested among these three patients groups.
To investigate the performance of the new PCA-based method, we explored the distribution of accuracy, specificity, and sensitivity. For this, we simulated the patient stratification process 100 times by resampling. The patients wer randomly divided into training (396 patients, 70%) and test set (170 patients, 30%) groups. PCA was applied to the training data set to determine the coefficient of each variable, and a PCA score was generated for each patient. The training set was then stratified into two groups based on the mean value of the PCA scores. Based on the final outcome (survival or death), accuracy, specificity, and sensitivity were calculated as follows:
, where TP, TN, FP, and FN are true positive, true negative, false positive, and false negative, respectively.
Multivariate Cox regression was used to select candidate significant factors before PCA, and chi-square tests were used to test dependency between survival patterns and different risk groups. Statistical analyses were conducted using R ver. 2.12.0, and a p-value of < 0.05 was considered statistically significant. The PCA algorithm was also performed using R.
3. Fong’s risk score
To analyze our patients by the Fong's method, the following five prognostic factors were assessed for each patient, and each factor scored one point: 1) lymph node-positive primary tumor; 2) disease-free interval (DFI) < 12 months; 3) number of liver metastases > 1; 4) preoperative CEA level of > 200 ng/mL; 5) size of largest liver metastasis > 5 cm. A total score between 0 and 5 points was obtained for each subject in the population, which was then divided into two risk groups: 0-2 points, low-risk group; 3-5 points, high-risk group.
Results
1. Patient demographics
A total of 566 patients with liver-confined metastasis were included in this study. The median age at time of liver metastasis was 57.8 years. Colon was the primary site of metastasis in patients at 60.6%. Among 334 patients with initially unresectable CLM, 264 patients remained unresectable after chemotherapy, whereas 70 patients underwent liver resection after tumor response to induction treatment. Among them, lobectomy was performed in 31.4% of patients (n=22) and segmentectomy or wedge resection in 65.7% (n=46). Local treatment (radio frequency ablation [RFA]) was necessary in 40% of patients (n=28) to achieve complete resection. RFA was conducted when patients had anatomically ill-located lesions, insufficient hepatic reserves after surgery, or combined comorbidities that inhibited major surgery. The regimen of induction treatment consisted most often of fluorouracil and leucovorin alone (12.9%) or combination with oxaliplatin (58.6%), irinotecan (28.6%), or targeted agents (34.3%). Two hundred and thirty-two patients with initially resectable CLM underwent liver resection followed by adjuvant chemotherapy. Lobectomy was performed in 37 patients (15.9%), segmentectomy or wedge resection in 161 patients (69.4%), and RFA in 69 patients (29.7%). Thus, a total of 302 patients underwent hepatic resection. After a median follow-up period of 19.5 months (range, 0.3 to 252.6 months), the median overall survival time was 27.2 months for all patients (95% confidence interval, 23.9 to 30.5 months). Table 1 summarizes the clinical and pathologic characteristics of patients.
Table 1.
Characteristics of patients
| Variable | No. (%) (n=566) |
|---|---|
| Age (yr) | |
| < 60 | 302 (53.4) |
| ≥ 60 | 264 (46.6) |
| Median (range) | 58 (22-85) |
| Gender | |
| Male | 382 (67.5) |
| Female | 184 (32.5) |
| Primary tumor T stage | |
| T1 | 5 (0.9) |
| T2 | 9 (1.6) |
| T3 | 467 (82.5) |
| T4 | 63 (11.1) |
| NA | 22 (3.9) |
| Primary tumor N stage | |
| N0 | 151 (22.7) |
| N1 | 197 (34.8) |
| N2 | 193 (34.1) |
| NA | 25 (4.4) |
| Tumor grade | |
| Well differentiated | 54 (9.5) |
| Moderately differentiated | 422 (74.6) |
| Poorly differentiated | 54 (9.5) |
| Mucinous | 2 (0.4) |
| Signet ring cell | 14 (2.5) |
| NA's | 20 (3.5) |
| No. of metastases | |
| 1 | 151 (26.7) |
| > 1 | 415 (73.4) |
| Size of largest metastasis (cm) | |
| ≤ 5 | 457 (80.7) |
| > 5 | 109 (19.3) |
| Distribution of liver metastasis | |
| Single | 244 (43.1) |
| Both | 322 (56.9) |
| CEA (ng/mL) | |
| ≤ 200 | 469 (82.9) |
| > 200 | 97 (17.1) |
| Disease-free interval (mo) | |
| < 12 | 152 (26.9) |
| ≥ 12 | 414 (73.1) |
| Fong's score | |
| 0-2 | 359 (63.4) |
| 0 (low) | 28 (4.9) |
| 1 | 128 (22.6) |
| 2 | 203 (35.9) |
| 3-5 | 207 (36.6) |
| 3 (high) | 147 (26.0) |
| 4 | 49 (8.7) |
| 5 | 11 (1.9) |
NA, not available; CEA, carcinoembryonic antigen.
2. Analysis of data set
Multivariate Cox regression was applied to the patient data set to explore the significance of clinopathologic variables. The most influential variable for prognosis was DFI < 12 months (from primary to discovery of liver metastasis). In terms of influence, DFI was followed by CEA level. Distribution of liver metastasis seemed significant, whereas the number of metastases, size of largest metastasis, and node-positive primary tumors were unrelated with prognosis (Table 2).
Table 2.
Multivariate analysis for clinicopathological variables by Cox regression analysis
| Variable | Parameter | p-value | Hazard ratio (90% CI ) |
|---|---|---|---|
| Distribution of liver metastasis in both lobes | 0.377 | 0.014* | 1.459 (1.080-1.969) |
| No. of metastasis > 1 | 0.249 | 0.153 | 1.282 (0.912-1.804) |
| Size of largest metastasis > 5 cm | 0.147 | 0.296 | 1.159 (0.879-1.527) |
| CEA > 200 ng/mL | 0.643 | < 0.001** | 1.902 (1.416-2.554) |
| Disease-free interval < 12 mo | 0.684 | < 0.001** | 1.982 (1.561-2.518) |
| Node-positive primary tumor | 0.237 | 0.064 | 1.267 (0.987-1.627) |
CEA, carcinoembryonic antigen.
p < 0.05,
p < 0.001.
There was a highly significant relationship between the number and distribution of metastases. Although the number of metastases was not a significant factor for survival, it was reported as a significant prognostic factor in previous studies. Of the two, distribution of liver metastasis is likely to be a more robust factor since distribution of tumors is more easily determined compared to the absolute number of tumors.
PCA was applied to CEA, distribution of liver metastasis, and DFI as variables in the training data set. From 500 rounds of simulation, mean accuracy, specificity, and sensitivity values were calculated to be 0.54, 0.12, and 0.81, respectively. The results of PCA of the test data set were similar to those of the training set with respect to accuracy and sensitivity, but the property of specificity was not similar. In the case of Fong’s criteria, the same three properties were 0.52, 0.47, and 0.65, respectively. Thus, the new PCA-based risk stratification method showed better performance in terms of accuracy and sensitivity, whereas specificity was lower than that of Fong’s criteria (Fig. 1).
Fig. 1.
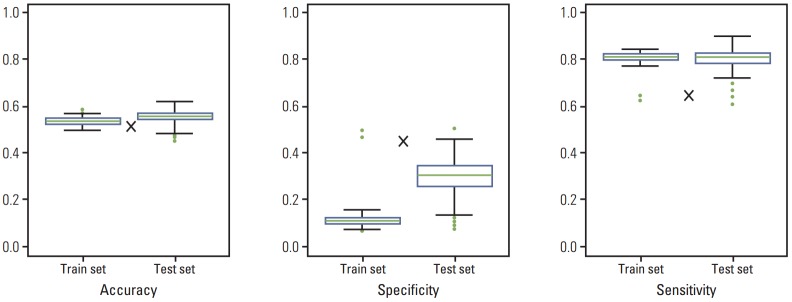
Comparison of accuracy, specificity, and sensitivity in training and test data sets. Results are from 500 rounds of simulation. The sizes of training and test data sets were 396 (70%) and 170 (30%), respectively. The symbol×indicates accuracy, specificity, and sensitivity of Fong’s criteria.
The coefficients for distribution of liver metastasis were 0.9000793 in a single lobe and –0.7349271 in both lobes; for CEA, 0.3480379 for ≤ 200 ng/mL and –1.8054465 for > 200 ng/mL; and for DFI, 0.1686233 for ≥ 12 months and –0.30495 70 for < 12 months. This depicts the coefficients assigned by the PCA algorithm for each variable. All of the predictors were categorical, which means each coefficient in a variable corresponds to each category. The probability of survival increased in direct proportion to the PCA score. The PCA score ranged from –2.98 to 1.56. Based on the Q1 and Q3 quartiles, patients were stratified into three groups. The survival curves of each subgroup based on PCA score are shown in Fig. 2. This analysis indicates that there were significantly different survival patterns among the three risk groups. In a pairwise comparison, groups 1 and 2 as well as groups 2 and 3 were significantly different in terms of survival patterns. Therefore, these stratification criteria can be a reliable tool for selecting patients for surgical resection of CLM.
Fig. 2.
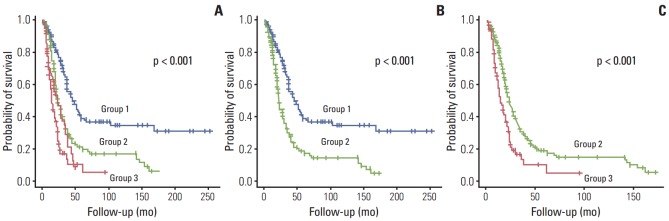
Kaplan-Meier survival curves of risk groups defined by principal component analysis scores. Comparison of three risk groups (A); group 1 vs. group 2 (B); and group 2 vs. group 3 (C).
Survival patterns between two vs. six groups based on Fong’s criteria are shown in Fig. 3. The survival patterns were significantly different (Fig. 3A), although analysis showed that a four-group stratification (0-1, 2, 3, and 4-5) was more reliable than a six-group one.
Fig. 3.
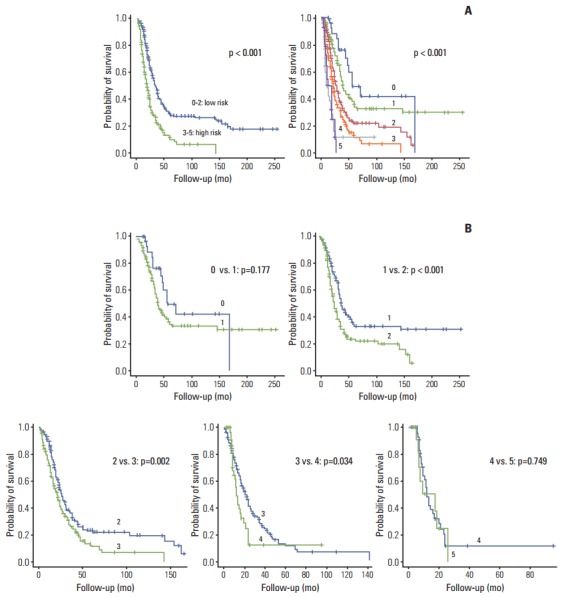
Kaplan-Meier survival curves of risk groups defined by Fong’s score. (A) Comparison of survival patterns between two vs. six groups. (B) Pairwise comparisons.
Common factors in Fong’s criteria and the PCA-based method were CEA and DFI. In the PCA method, distribution of metastases was used, as it was found to be a more significant factor than size and number of liver metastases. Three factors in the PCA method were very significantly different among the three risk groups, according to PCA score (Table 3). Table 3 shows that age, sex, and histology were randomly distributed among the three risk groups. It is noteworthy that T and N stages are not related to the three risk groups. Fong’s score was significantly associated with the PCA risk groups. The significance of metastasis size was weaker than the other five factors, although it was also significant. Although size of metastasis was significant among the three risk groups, it was not significant in the whole patient data set.
Table 3.
Comparison of clinopathologic variables among different risk groups
| Variable | Group 3 | Group2 | Group1 | p-value |
|---|---|---|---|---|
| Age (yr) | 0.341 | |||
| < 60 | 88 | 119 | 95 | |
| ≥ 60 | 91 | 101 | 72 | |
| Gender | 0.228 | |||
| Male | 124 | 154 | 204 | |
| Female | 55 | 66 | 63 | |
| Primary T stage | 0.661 | |||
| T1 | 1 | 3 | 1 | |
| T2 | 2 | 5 | 2 | |
| T3 | 149 | 177 | 141 | |
| T4 | 18 | 30 | 15 | |
| NA | 9 | 5 | 8 | |
| Primary N stage | 0.368 | |||
| N0 | 50 | 55 | 46 | |
| N1 | 60 | 84 | 53 | |
| N2 | 52 | 73 | 68 | |
| NA | 17 | 8 | 0 | |
| Tumor grade | 0.215 | |||
| Well differentiated | 17 | 20 | 17 | |
| Moderately differentiated | 134 | 169 | 119 | |
| Poorly differentiated | 19 | 16 | 19 | |
| Mucinous | 2 | 0 | 0 | |
| Signet ring cell | 1 | 7 | 6 | |
| NA | 6 | 8 | 6 | |
| No. of metastases | < 0.001 | |||
| 1 | 3 | 40 | 109 | |
| > 1 | 176 | 180 | 58 | |
| Size of largest metastasis (cm) | 0.012 | |||
| ≤ 5 | 134 | 183 | 145 | |
| > 5 | 45 | 37 | 22 | |
| Distribution of liver metastasis | < 0.001 | |||
| Single | 2 | 75 | 167 | |
| Both | 177 | 145 | 0 | |
| CEA (ng/mL) | < 0.001 | |||
| ≤ 200 | 88 | 214 | 167 | |
| > 200 | 91 | 6 | 0 | |
| Disease-free interval (mo) | < 0.001 | |||
| ≥ 12 | 52 | 151 | 167 | |
| < 12 | 127 | 69 | 0 | |
| Fong's score | < 0.001 | |||
| 0-2 | 34 | 166 | 159 | |
| 0 (low) | 0 | 0 | 28 | |
| 1 | 2 | 41 | 85 | |
| 2 | 32 | 125 | 46 | |
| 3-5 | 145 | 54 | 8 | |
| 3 (high) | 89 | 50 | 8 | |
| 4 | 45 | 4 | 0 | |
| 5 | 11 | 0 | 0 |
Chi-square test was used. NA, not available; CEA, carcinoembryonic antigen.
Discussion
For patients with CLM, predictive models can help patients who are at increased risk of recurrent disease by offering appropriate treatment modalities, such as neoadjuvant, perioperative, or adjuvant chemotherapy, and support decision-making in terms of the timing of surgery [17].
The first predictive model was reported by Nordlinger et al. [11], and 11 additional prognostic scoring systems were developed since then. In terms of comparing the different methods, Merkel et al. [18] analyzed the predictive value of Fong’s score, that of Nordlinger et al. [11], and TNM classification. They found that Fong’s score was the most useful for prognosis and selecting adequate patients for hepatic resection. Reissfelder et al. [19] also found that both Fong’s and Iwatsuki’s scoring systems were effective in stratifying disease-specific survival among five prognostic scoring systems (Nordlinger score, Basingstoke index, Fong’s risk score, Iwatsuki score, and Mayo Clinic scoring system) [13,20].
Fong’s method originally used five factors (nodal status of primary, DFI of < 12 months, more than one metastasis, pre-operative CEA of > 200 ng/mL, and tumor size of > 5 cm) as criteria for clinical risk score (CRS) [20]. Patients with 0 points had an improved long-term outcome (p < 0.001) with a 5-year actuarial survival rate of 60%, in comparison with that of 14% in patients with 5 points. This model is simple and easy to apply in a clinical setting.
Similar to previous reports of Mann et al. [21] and Arru et al. [22], Fong’s CRS in our study was also highly predictive of survival when patients were grouped by scores of 0-2 and 3-5 (p < 0.001); 5-year survival rates were reported as 29.1% and 10.6%, whereas 1-year survival rates were 90.4% and 67.1%, respectively. Fong’s CRS was calculated for each patient, and the long-term outcomes of the six classes (0-5) were compared and worsened as Fong’s score increased. Although Fong’s CRS proved to be a useful instrument for identifying poor overall survival, a four-group stratification (0-1, 2, 3, and 4-5) was more reliable than a six-group one.
The PCA scoring system has good discriminatory ability. The PCA score can identify patients with a relatively favorable prognosis, demonstrating efficacy of therapeutic strategies. In our study, PCA score was based on retrospective analysis of patients at a single institution. Nonetheless, it retained its high prognostic significance when applied to an internal validation set of patients, and it compared favorably to Fong’s CRS.
The PCA score is calculated using three clinical variables: CEA, DFI, and distribution of liver metastasis. In our study, the number of liver metastases was highly correlated with distribution of liver metastasis (data not shown). By using distribution rather than number or size, one is able to save time and avoid counting errors. Distribution of liver metastasis is considered as a prognostic factor in the H-number system of the Japanese Classification Colorectal Carcinoma [23]. In addition, recent effective chemotherapeutic agents and techniques can reduce the importance of tumor size, especially when metastases are confined to one lobe, thus making it easy to operate radically [17].
Prognostic scores should ideally be available for all patients during preoperative assessment and not restricted to liver resection [24]. Our scoring system showed more satisfactory and predictive efficacy in all patients with CLM. Importantly, the PCA method is simple and easy to apply, and all necessary clinical parameters can be easily obtained preoperatively through imaging and serum biochemical tests.
Conclusion
Our results suggest that the PCA scoring system based on three variables may be useful for determining prognosis preoperatively, assisting in clinical decision-making, and designing future clinical trials for treatment of CLM patients. Prospective validation of this scoring system through a multicenter collaboration would confirm its utility.
Acknowledgments
The authors gratefully acknowledge Eun Sil Baek and Han Na Park for data collection and management of the database.
This work was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0031396).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Adam R, Hoti E, Folprecht G, Benson AB. Accomplishments in 2008 in the management of curable metastatic colorectal cancer. Gastrointest Cancer Res. 2009;3(5 Suppl 2):S15–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–10. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 3.Mella J, Biffin A, Radcliffe AG, Stamatakis JD, Steele RJ. Population-based audit of colorectal cancer management in two UK health regions. Colorectal Cancer Working Group, Royal College of Surgeons of England Clinical Epidemiology and Audit Unit. Br J Surg. 1997;84:1731–6. [PubMed] [Google Scholar]
- 4.Poston GJ, Adam R, Alberts S, Curley S, Figueras J, Haller D, et al. OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–34. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 5.Rees M, John TG. Current status of surgery in colorectal metastases to the liver. Hepatogastroenterology. 2001;48:341–4. [PubMed] [Google Scholar]
- 6.Papadimitriou JD, Fotopoulos AC, Prahalias AA, Vassiliou JG, Papadimitriou LJ. The impact of new technology on hepatic resection for malignancy. Arch Surg. 2001;136:1307–13. doi: 10.1001/archsurg.136.11.1307. [DOI] [PubMed] [Google Scholar]
- 7.Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–53. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 8.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 9.Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–9. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 10.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 11.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 12.Taylor M, Forster J, Langer B, Taylor BR, Greig PD, Mahut C. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg. 1997;173:467–71. doi: 10.1016/s0002-9610(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 13.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–9. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–66. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambiru S, Miyazaki M, Isono T, Ito H, Nakagawa K, Shimizu H, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–9. doi: 10.1007/BF02234142. [DOI] [PubMed] [Google Scholar]
- 16.Gomez D, Cameron IC. Prognostic scores for colorectal liver metastasis: clinically important or an academic exercise? HPB (Oxford) 2010;12:227–38. doi: 10.1111/j.1477-2574.2010.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh FK, Tekkis PP, John TG, Rees M. Predictive models in colorectal liver metastases--can we personalize treatment and outcome? Dig Surg. 2008;25:406–12. doi: 10.1159/000184731. [DOI] [PubMed] [Google Scholar]
- 18.Merkel S, Bialecki D, Meyer T, Muller V, Papadopoulos T, Hohenberger W. Comparison of clinical risk scores predicting prognosis after resection of colorectal liver metastases. J Surg Oncol. 2009;100:349–57. doi: 10.1002/jso.21346. [DOI] [PubMed] [Google Scholar]
- 19.Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, et al. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:3279–88. doi: 10.1245/s10434-009-0654-7. [DOI] [PubMed] [Google Scholar]
- 20.Fong Y, Salo J. Surgical therapy of hepatic colorectal metastasis. Semin Oncol. 1999;26:514–23. [PubMed] [Google Scholar]
- 21.Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–72. doi: 10.1001/archsurg.139.11.1168. [DOI] [PubMed] [Google Scholar]
- 22.Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, et al. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 23.Nanashima A, Sumida Y, Abo T, Tobinaga S, Takeshita H, Hidaka S, et al. A modified grading system for post-hepatectomy metastatic liver cancer originating from colorectal carcinoma. J Surg Oncol. 2008;98:363–70. doi: 10.1002/jso.21114. [DOI] [PubMed] [Google Scholar]
- 24.Schindl M, Wigmore SJ, Currie EJ, Laengle F, Garden OJ. Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg. 2005;140:183–9. doi: 10.1001/archsurg.140.2.183. [DOI] [PubMed] [Google Scholar]


