Abstract
Purpose
Gemcitabine-cisplatin combination chemotherapy has been regarded as standard regimen for advanced or metastatic biliary tract cancer (BTC), based on the ABC-02 trial. To date, however, no studies have compared the efficacies of gemcitabine-platinum and fluoropyrimidine- platinum combination chemotherapy, even though fluoropyrimidine has been widely used as a backbone agent for gastrointestinal cancer. This study compared the efficacy and toxicities of gemcitabine-cisplatin (GP) and capecitabine-cisplatin (XP) combination chemotherapy for treatment of advanced BTC.
Materials and Methods
We examined 49 patients treated with GP and 44 patients treated with XP from October 2009 to July 2012. All patients had unresectable BTC. The GP regimen comprised gemcitabine (1,000 mg/m2, intravenously [IV], days 1 and 8) and cisplatin (75 mg/m2, IV, day 1). The XP regimen comprised capecitabine (1,250 mg/m2 twice a day, peroral, days 1-14) and cisplatin (60 mg/m2, IV, day 1, every three weeks). We analyzed the response rate (RR), time to progression (TTP), overall survival (OS), and toxicity.
Results
The RRs were 27.3% and 6.1% in the XP and GP arms, respectively. XP resulted in longer TTP (5.2 months vs. 3.6 months, p=0.016), but OS was not statistically different (10.7 months vs. 8.6 months, p=0.365). Both regimens resulted in grade 3-4 hematologic toxicities, but febrile neutropenia was not noted. Grade 3-4 asthenia, stomatitis, and hand-foot syndrome occurred more frequently in the XP arm.
Conclusion
XP resulted in a superior TTP and RR compared to GP for treatment of advanced BTC, with comparable toxicity. Conduct of prospective large, randomized trials to evaluate the possibility of XP as another standard therapy is warranted.
Keywords: Biliary tract neoplasms, Gemcitabine, Capecitabine, Cisplatin
Introduction
Biliary tract cancers (BTCs) originate from epithelial cells of the biliary system, including the intrahepatic and extrahepatic bile duct, gallbladder, and ampulla of Vater [1]. They account for 3% of all gastrointestinal malignancies and show variable distribution [2]. BTCs are uncommon in Western countries, but are relatively common in Central America, Northern India, and Asian countries, including Korea [3,4]. Approximately 5,500 new patients develop BTC annually [5], and it accounts for 6% of all cancer-related deaths in Korea [6]. The only chance of cure is provided by surgical resection with a negative resection margin [7]. However, most patients present with locally advanced or metastatic disease, which is unresectable at the time of initial diagnosis [8]. Because of its late clinical manifestation and frequent recurrence after curative surgery, palliative systemic chemotherapy is the mainstay of treatment for BTCs [7,9]. In metastatic or recurrent BTC, systemic chemotherapy has been shown to improve overall survival (OS) and the quality of life [10].
Fluorouracil (5-FU) combined with platinum or an anthracycline agent has traditionally been the backbone of systemic chemotherapy [11,12]. In the 2000s, gemcitabine combined with platinum or gemcitabine alone was introduced for treatment of advanced BTCs, with comparable response rates and toxicity [7,13]. The ABC-02 phase III trial demonstrated the survival advantage of gemcitabine-cisplatin (GP) over gemcitabine alone [14], and, as a result, GP combination chemotherapy is currently the recommended first-line chemotherapy regimen for BTC [6]. However, a good response rate of 24% has been reported for 5-FU combined with cisplatin for advanced BTC [11], comparable to that of GP combination chemotherapy [7]. Since the development of oral fluoropyrimidines such as capecitabine, oral agents are rapidly replacing intravenous 5-FU because of the convenience of administration and comparable efficacy. In several phase II trials, a capecitabinecisplatin (XP) combination regimen showed modest activity with a response rate of 21.4%-40.6%, 9.1-12.4 months of survival outcome [9,15]. These results were comparable with those of GP combination chemotherapy [16]. However, to date, no trials comparing the GP regimen with combined 5-FU and platinum for BTC have been conducted.
In this study, we performed a retrospective analysis and comparison of the efficacy and toxicity of XP and GP as first-line chemotherapy for recurrent or metastatic BTC.
Materials and Methods
1. Patients
Patients treated with systemic GP or XP as a first-line treatment for advanced BTC between October 2009 and July 2012 were enrolled in this study. All of these patients had recurrent BTC after surgery or unresectable BTC at initial presentation. Other eligibility criteria were as follows: 1) age over 20 years; 2) pathologically confirmed biliary tract adenocarcinoma, excluding ampulla of Vater cancer; 3) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; 4) a measurable lesion based on Response Evaluation Criteria In Solid Tumors (RECIST) ver. 1.0; 4) adequate bone marrow function with a platelet count of ≥ 100,000/mm3, an absolute neutrophil count of ≥ 1,500/L, and a hemoglobin level of ≥ 8.0 g/dL; 5) adequate hepatic function with aspartate aminotransferase and alanine aminotransferase levels of ≤ 5×the upper normal limit and a bilirubin level of ≤ 1.5×the upper normal limit; and 6) adequate renal function, with a serum creatinine level of ≤ 2×the upper normal limit. We performed a retrospective analysis of clinical data through a review of the medical records. This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary’s Hospital, The Catholic University of Korea.
2. Treatment schedule and response evaluation
In the GP arm, gemcitabine 1,000 mg/m2 was administered intravenously (IV) for 30 minutes on days 1 and 8 and cisplatin was administered at 75 mg/m2 IV, on day 1; the schedule was repeated every three weeks. In the XP arm, capecitabine was administered orally at a dose of 1,250 mg/m2 twice per day over days 1-14 and cisplatin was administered at 60 mg/m2 IV, on day 1, every three weeks. One liter of half saline was administered before and after administration of cisplatin. Tumor response was evaluated after two cycles of chemotherapy in accordance with RECIST ver. 1.0 according to computed tomography (CT) findings. Toxicity was monitored at every visit in accordance with the National Cancer Institute-Common Terminology Criteria for Adverse Events ver. 3.0.
3. Statistical analysis
Overall survival (OS) was calculated from the start date of systemic chemotherapy to the date on which the patient died or the date of the last follow-up. Time to progression (TTP) was measured from the first day of the first chemotherapy cycle to the date of disease progression confirmed by CT, the date of the last follow-up, or death. The distributions of OS and TTP were analyzed using the Kaplan-Meier method. All statistical analysis was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
Ninety-three cases of advanced BTC treated at Seoul St. Mary’s Hospital from October 1, 2009, to July 31, 2012 were analyzed retrospectively. Forty-nine patients were treated with the GP regimen, and 44 with the XP regimen. The baseline characteristics of the patients are listed in Table 1. Three patients (6%) in the GP arm and 12 (27.3%) in the XP arm stopped chemotherapy because of treatment intolerance. There was no difference with respect to age, sex, type of cancer, and disease status between the two groups.
Table 1.
Patient characteristics
| GP (%) | XP (%) | p-value | |
|---|---|---|---|
| No. of patients | 49 | 44 | |
| Age (yr) | 0.153 | ||
| Median (range) | 62 (45-81) | 65 (39-80) | |
| > 65 | 19 (38.8) | 23 (52.3) | 0.194 |
| > 70 | 7 (14.3) | 9 (20.4) | 0.434 |
| Gender | 0.62 | ||
| Male | 31 (63.3) | 30 (68.2) | |
| Female | 18 (36.7) | 14 (31.8) | |
| Type | 0.196 | ||
| Intrahepatic cholangiocarcinoma | 12 (24.5) | 16 (36.4) | |
| Extrahepatic cholangiocarcinoma | 19 (38.8) | 16 (36.4) | |
| Gallbladder cancer | 18 (36.7) | 12 (27.2) | |
| Disease status | 0.831 | ||
| Recurrent | 20 (40.8) | 14 (70) | |
| Adjuvant treatmenta) | 17 (38.6) | 12 (70.5) | |
| Metastatic | 29 (59.2) | 27 (61.4) | |
| Median no. of cycles (range) | 4(1-8) | 3(1-13) | |
| Treatment termination due to drug intolerance | 3 (6.1) | 12 (27.3) |
GP, gemcitabine-cisplatin; XP, capecitabine-cisplatin.
Adjuvant treatment: fluorouracil (5-FU) chemoradiation and four cycles of 5-FU.
2. Treatment response
A total of 180 cycles of chemotherapy (median, four cycles per patient) were administered in the GP arm and 188 cycles of chemotherapy (median, three cycles per patient) were administered in the XP arm. In the GP arm, no patient showed complete response (CR) and three patients (6.1%) achieved partial response (PR); the disease control rate (DCR) was 61.2%. In the XP arm, one patient (2.3%) showed CR and 11 patients (25%) achieved PR; the DCR was 65.9% (Table 2). We also performed a sub-analysis of patients aged over 70 years. In the GP arm, seven patients (14.3%) in this age group received a total of 27 cycles of chemotherapy, with no objective response but with a DCR of 71.4%. In the XP arm, nine patients (20.4%) received 36 cycles of chemotherapy resulting in a response rate (RR) of 33.3% and a DCR of 44.4%.
Table 2.
Response rate
| Response | GP (%) | XP (%) |
|---|---|---|
| CR | 0 | 1 (2.3) |
| PR | 3 (6.1) | 11 (25) |
| SD | 27 (55.1) | 17 (38.6) |
| PD | 16 (32.7) | 9 (20.5) |
| NA | 3 (6.1) | 6 (13.6) |
| Overall response rate | 6.1 | 27.3 |
| Disease control ratea) | 61.2 | 65.9 |
GP, gemcitabine-cisplatin; XP, capecitabine-cisplatin; PD, progressive disease; NA, not assessed.
Including complete response (CR), partial response (PR), and stable disease (SD).
3. Survival outcomes
The overall median TTP was 5.1 months (range, 0.4 to 19.4 months), and the overall median OS was 9.6 months (range, 1.0 to 23.7 months). The XP arm showed a longer median TTP (5.2 months; 95% confidence interval [CI], 2.56 to 7.83 months) than the GP arm (3.6 months; 95% CI, 2.73 to 4.47; p=0.015), but OS did not differ significantly (10.7 months for XP and 8.6 months for GP, p=0.365) (Fig. 1). In subgroup analysis of patients aged over 70 years, XP resulted in a longer median TTP than GP; however, this difference was not statistically significant (7.4 months vs. 4.0 months, respectively; p=0.258) (Fig. 2). TTP did not differ according to the type of cancer or disease status (data not shown).
Fig. 1.
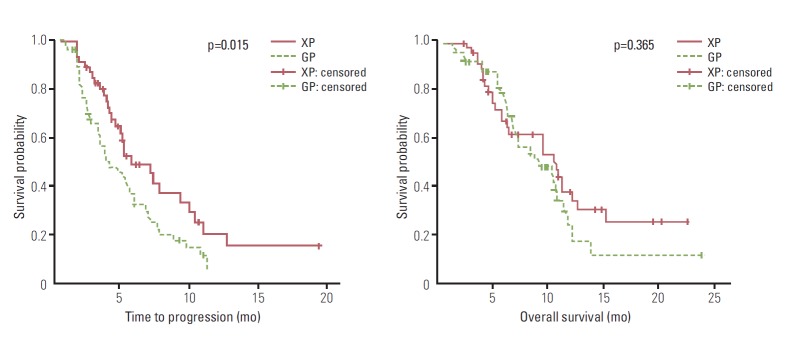
Comparison of survival outcomes between the capecitabine-cisplatin (XP) and gemcitabine-cisplatin (GP) regimen.
Fig. 2.
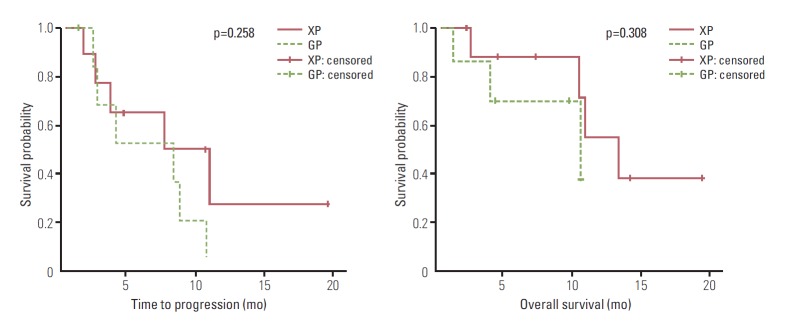
Survival outcomes in patients older than 70 years.
4. Safety
The hematologic and non-hematologic toxicities encountered are summarized in Table 3. In this study, there was no occurrence of chemotherapy-related death, and there were no cases of febrile neutropenia. The most common hematologic grade 3-4 toxicity was neutropenia in both the GP and XP arms (15% and 14.4%, respectively). Among the non-hematologic toxicities, grade 3-4 vomiting was most common in the GP arm (3.3%). Grade 3-4 asthenia, stomatitis, and hand-foot syndrome all occurred in the XP arm (6.9%, 3.7%, and 3.2%, respectively). In patients aged 70 years or older, grade 3-4 anemia (22.2%) was the dominant toxicity in the GP arm and grade 3-4 hand-foot syndrome (8.3%) was the most common toxicity in the XP arm (Table 4).
Table 3.
Toxicity profiles
| No. of episodes (%) |
||||||
|---|---|---|---|---|---|---|
| GP |
XP |
|||||
| Grade 1-2 | Grade 3-4 | Total | Grade 1-2 | Grade 3-4 | Total | |
| Hematologic | ||||||
| Anemia | 61 (33.9) | 17 (9.4) | 78 (43.3) | 35 (18.6) | 5 (2.7) | 40 (21.3) |
| Neutropenia | 25 (13.9) | 27 (15) | 52 (28.9) | 21 (11.2) | 27 (14.4) | 48 (25.5) |
| Thrombocytopenia | 48 (26.7) | 19 (10.6) | 67 (37.2) | 40 (21.3) | 17 (9) | 57 (30.3) |
| Non-hematologic | ||||||
| Nausea | 11 (6.1) | 5 (2.8) | 16 (8.9) | 7 (3.7) | 7 (3.7) | 14 (7.4) |
| Vomiting | 7 (3.9) | 6 (3.3) | 13 (7.2) | 4 (2.1) | 3 (1.6) | 7 (3.7) |
| Anorexia | 4 (2.2) | 3 (1.7) | 7 (3.9) | 8 (4.3) | 5 (2.7) | 13 (6.9) |
| Asthenia | 12 (6.7) | 4 (2.2) | 16 (8.9) | 4 (2.1) | 13 (6.9) | 17 (9) |
| Stomatitis | 1 (0.6) | 0 | 1 (0.6) | 10 (5.3) | 7 (3.7) | 17 (9) |
| Diarrhea | 0 | 0 | 0 | 5 (2.7) | 1 (0.5) | 6 (3.2) |
| Neuropathy | 2 (1.1) | 2 (1.1) | 4 (2.2) | 4 (2.1) | 4 (2.1) | 8 (4.3) |
| Nephropathy | 2 (1.1) | 1 (0.6) | 3 (1.7) | 4 (2.1) | 4 (2.1) | 8 (4.3) |
| Hand-foot syndrome | 0 | 0 | 0 | 15 (8) | 6 (3.2) | 21 (11.2) |
| Infection | 0 | 2 (1.1) | 2 (1.1) | 0 | 0 | 0 |
GP, gemcitabine-cisplatin; XP, capecitabine-cisplatin.
Table 4.
Toxicity profiles in patients aged over 70 years
| No. of episodes (%) |
||||||
|---|---|---|---|---|---|---|
| GP |
XP |
|||||
| Grade 1-2 | Grade 3-4 | Total | Grade 1-2 | Grade 3-4 | Total | |
| Hematologic | ||||||
| Anemia | 8 (29.6) | 6 (22.2) | 14 (51.8) | 1 (2.8) | 0 | 1 (2.8) |
| Neutropenia | 2 (7.4) | 2 (7.4) | 4 (14.8) | 6 (16.7) | 2 (5.6) | 8 (22.2) |
| Thrombocytopenia | 7 (25.9) | 4 (14.8) | 11 (40.7) | 4 (11.1) | 4 (11.1) | 8 (22.2) |
| Non-hematologic | ||||||
| Nausea | 2 (7.4) | 1 (3.7) | 3(11.1) | 0 | 2 (5.6) | 2 (5.6) |
| Vomiting | 2 (7.4) | 1 (3.7) | 3(11.1) | 1 (2.8) | 1 (2.8) | 2 (5.6) |
| Anorexia | 2 (7.4) | 1 (3.7) | 3 (11.1) | 1 (2.8) | 1 (2.8) | 2 (5.6) |
| Asthenia | 3 (11.1) | 1 (3.7) | 4 (14.8) | 0 | 3 (8.3) | 3 (8.3) |
| Stomatitis | 0 | 0 | 0 | 1 (2.8) | 1 (2.8) | 2 (5.6) |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 |
| Neuropathy | 0 | 0 | 0 | 0 | 0 | 0 |
| Nephropathy | 0 | 0 | 0 | 0 | 0 | 0 |
| Hand-foot syndrome | 0 | 0 | 0 | 1 (2.8) | 3 (8.3) | 4 (11.1) |
| Infection | 0 | 0 | 0 | 0 | 0 | 0 |
GP, gemcitabine-cisplatin; XP, capecitabine-cisplatin.
Discussion
BTCs follow an aggressive clinical course with a poor prognosis, and until 2010, there was no effective treatment for unresectable or metastatic disease. Since publication of the results of the ABC-02 phase III trial in that year [14], GP combination chemotherapy has been adopted as the standard first-line chemotherapy regimen for this cancer, replacing traditional 5-FU and platinum combination therapy [7]. However, few clinical trials directly compared the efficacy and toxicity of gemcitabine and 5-FU, and in these trials, no significant difference was found between two regimens [15,17]. Recently, capecitabine, the oral prodrug of fluoropyrimidine, has replaced continuous intravenous 5-FU because of its similar efficacy, as in colorectal cancer and advanced gastric cancer [18,19]. Based on these data, capecitabine might also be evaluated as an alternative regimen for BTC, and we therefore compared the efficacy and safety of XP and GP combination chemotherapy in the current study.
In our analysis, we found no significant difference in OS between the GP and XP treatment arms. However, XP resulted in a significantly longer TTP and a better RR and DCR compared with the GP regimen. Both GP and XP regimens showed similar toxicity profiles to those described in previous studies [9,14,15,20]. However, drug intolerance was more common in the XP arm (27.3% and 6.1%, respectively), resulting in frequent treatment termination, although both regimens had similar grade 3-4 hematologic toxicities. Of the other toxicities, both stomatitis and hand-foot syndrome were significantly more common in the XP arm than in the GP arm. Even moderate to severe stomatitis can lead to poor oral intake and malnutrition, and hand-foot syndrome can affect daily life. These toxicities therefore affect patients’ quality of life and can ultimately lead to treatment termination. In addition, patients with advanced cancer take many other oral medications as well; for example, control of pain and underlying chronic disease. This could also cause patients to be less willing to take oral chemotherapy agents, even if it is more convenient than continuous IV infusion.
In the GP arm of our study, cisplatin was administered at the dose of 75 mg/m2 on day 1, repeated every three weeks. This treatment schedule differs from that of the ABC-02 trial. We started this study in 2009, so that the treatment schedule was based on two previous phase II trials [21,22]. In our study, the response rate and DCR of the GP regimen showed somewhat inferior results to those of the ABC-02 trial (response rate: 6.1% vs. 26.1%, respectively; DCR: 61.2% vs. 81.4%, respectively) [14]. This difference may be due to different characteristics of patients enrolled between two studies. We analyzed only patients who had measurable lesions, but patients with evaluable lesions based on RECIST 1.0 criteria were enrolled in the ABC-02 trial. Second, patients with ampulla of Vater cancer were enrolled in the ABC-02 trial, even though only a small number were included. Relatively few cases of hematologic and non-hematologic toxicity occurred in the subgroup of patients aged over 70 years. Among these, grade 3-4 anemia was the most common (22.2%) in the GP arm, and grade 3-4 hand-foot syndrome (8.3%) was the most common in the XP arm. These complications were easily managed and did not result in treatment-related mortality.
The current study has some limitations. One of these is the fact that it was conducted retrospectively, thus, conclusions must be interpreted with caution. In addition, the chemotherapy regimen used for each patient was selected by investigators. This may have influenced the results of the study, for example, through selection bias. Nonetheless, our results suggest that the XP combination regimen could be an alternative to the standard GP combination regimen.
Recently, oxaliplatin has been evaluated in combination with gemcitabine or 5-FU instead of cisplatin and showed promising results with fewer side effects. Gemcitabine combined with oxaliplatin (GEMOX) resulted in a reported RR of 19% to 36% and a progression-free survival of 4.8 to 5.7 months in two different studies [2,23], and capecitabine-oxaliplatin combination (XELOX) resulted in a RR of 27% and a TTP of 6.5 months [24]. These oxaliplatin-based combination regimens are currently the subject of phase II clinical trials to determine their efficacy and safety.
Conclusion
In summary, combination of capecitabine and cisplatin might be a candidate for another treatment option for treatment of advanced or metastatic BTCs. While GP combination regimen is regarded as the standard treatment of care in light of the ABC-02 trial, conduct of a large, prospective study based on new drug combinations such as capecitabine or oxaliplatin should be warranted.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang JS, Lim HY, Hwang IG, Song HS, Yoo N, Yoon S, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol. 2010;65:641–7. doi: 10.1007/s00280-009-1069-7. [DOI] [PubMed] [Google Scholar]
- 3.Valle JW. Advances in the treatment of metastatic or unresectable biliary tract cancer. Ann Oncol. 2010;21 Suppl 7:vii345–8. doi: 10.1093/annonc/mdq420. [DOI] [PubMed] [Google Scholar]
- 4.Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009;20:146–59. doi: 10.1093/annonc/mdn533. [DOI] [PubMed] [Google Scholar]
- 5.Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012;44:25–31. doi: 10.4143/crt.2012.44.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–8. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 7.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–23. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 8.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 9.Hong YS, Lee J, Lee SC, Hwang IG, Choi SH, Heo JS, et al. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol. 2007;60:321–8. doi: 10.1007/s00280-006-0380-9. [DOI] [PubMed] [Google Scholar]
- 10.Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 11.Ducreux M, Rougier P, Fandi A, Clavero-Fabri MC, Villing AL, Fassone F, et al. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol. 1998;9:653–6. doi: 10.1023/a:1008241008379. [DOI] [PubMed] [Google Scholar]
- 12.Ellis PA, Norman A, Hill A, O'Brien ME, Nicolson M, Hickish T, et al. Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer. 1995;31:1594–8. doi: 10.1016/0959-8049(95)00323-b. [DOI] [PubMed] [Google Scholar]
- 13.Dingle BH, Rumble RB, Brouwers MC, Cancer Care Ontario's Program in Evidence-Based Care's Gastrointestinal Cancer Disease Site Group The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol. 2005;19:711–6. doi: 10.1155/2005/565479. [DOI] [PubMed] [Google Scholar]
- 14.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 15.Kim TW, Chang HM, Kang HJ, Lee JR, Ryu MH, Ahn JH, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol. 2003;14:1115–20. doi: 10.1093/annonc/mdg281. [DOI] [PubMed] [Google Scholar]
- 16.Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, et al. A phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106:1339–46. doi: 10.1002/cncr.21741. [DOI] [PubMed] [Google Scholar]
- 17.Kim MJ, Oh DY, Lee SH, Kim DW, Im SA, Kim TY, et al. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer. 2008;8:374. doi: 10.1186/1471-2407-8-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 19.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 20.Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–74. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Kim TY, Lee MA, Ahn MJ, Kim HK, Lim HY, et al. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol. 2008;61:47–52. doi: 10.1007/s00280-007-0444-5. [DOI] [PubMed] [Google Scholar]
- 22.Giuliani F, Gebbia V, Maiello E, Borsellino N, Bajardi E, Colucci G, et al. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM) Ann Oncol. 2006;17 Suppl 7:vii73–7. doi: 10.1093/annonc/mdl956. [DOI] [PubMed] [Google Scholar]
- 23.Andre T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–43. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 24.Nehls O, Oettle H, Hartmann JT, Hofheinz RD, Hass HG, Horger MS, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309–15. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]


