Abstract
Purpose
The aim of this study is to evaluate the safety of fertility-sparing surgery as the treatment for patients with primary mucinous epithelial ovarian cancer.
Materials and Methods
A retrospective study of patients with mucinous ovarian cancer between 1991 and 2010 was performed. The demographics and survival outcomes were compared between patients who underwent fertility-sparing surgery and those who underwent radical surgery.
Results
A total of 110 patients underwent primary surgery. At the time of surgery, tumors appeared to be grossly confined to the ovaries in 90 patients, and evidence of metastasis was definite in 20 patients. Of the 90 patients with tumors that appeared to be grossly confined to the ovaries at surgical exploration, 35 (38.9%) underwent fertility-sparing surgery. The Kaplan- Meier curve and the log rank test showed no difference in either recurrence-free survival (p=0.792) or disease-specific survival (p=0.706) between the two groups. Furthermore, there was no significant difference in recurrence-free survival (p=0.126) or disease-specific survival (p=0.377) between the two groups, even when the analysis was limited to women below the age of 40. In a multivariate Cox model, fertility-sparing surgery had no effect on either recurrence-free survival (recurrence hazard ratio [HR], 1.20; 95% confidence interval [CI], 0.25 to 5.71) or disease-specific survival (death HR, 0.88; 95% CI, 0.17 to 4.60).
Conclusion
Fertility-sparing surgery in primary mucinous cancer grossly confined to the ovaries may be a safe option and one not associated with an increase in recurrence or mortality.
Keywords: Mucinous adenocarcinoma, Ovarian epithelial cancer, Fertility-sparing surgery, Survival
Introduction
Epithelial ovarian cancer is the most lethal of the gynecologic cancers. Approximately 20% of all epithelial ovarian cancer are diagnosed at an early stage, and thus high survival rates have been reported for patients whose disease was identified at this stage [1]. Moreover, approximately 10% of all epithelial ovarian cancer occurs in women under the age of 40 years [2]. The safety of fertility-sparing surgery for women of childbearing age with early-stage disease has been evaluated over the past two decades [3-11]. The current guidelines recommend fertility-sparing surgery only in selected cases of early-stage ovarian cancer [12].
Primary mucinous carcinoma of the ovary accounts for 7% to 14% of all invasive epithelial ovarian cancers [13]. When compared to high-grade serous carcinoma, mucinous carcinoma has distinct presentation, clinical course, and response to therapy [14]. Moreover mucinous carcinoma is usually confined to the ovary at surgery, and the prognosis is favorable when found at an early stage [15]. For mucinous tumors apparently confined to the ovary, some studies have reported that complete surgical staging is unnecessary due to a low risk of upstaging [16,17]. Despite the infrequency of this subtype, previous studies have shown that about 27% of patients with stage I epithelial ovarian cancer have mucinous histology [18,19]. In Korea, mucinous carcinoma is the most common histologic subtype of stage I epithelial ovarian cancer [16]. Because early-stage mucinous carcinoma is usually found in young women [7-11], fertility preservation is a matter of great interest in considering mucinous tumors than for other histologic subtypes. However, the safety of fertility-sparing surgery in mucinous histology has not yet been determined.
In this study, we compare the long-term oncologic outcomes of fertility-sparing surgery with those of radical surgery in patients with apparent early-stage mucinous carcinoma.
Materials and Methods
1. Patients
After receiving approval from the Institutional Review Board, we reviewed all patients with primary mucinous carcinoma of the ovary diagnosed at Seoul National University Hospital between January 1991 and December 2010. A gynecologic oncology pathologist evaluated all cases. Patients with borderline malignancies or with metastatic mucinous ovarian carcinomas from other primary sites were excluded. Histological diagnosis was established according to the World Health Organization (WHO) classification.
Clinical and pathologic variables, such as age, International Federation of Gynecology and Obstetrics (FIGO) stage, year of diagnosis, grade, adjuvant chemotherapy, surgical procedure, and date of last contact or date of recurrence/ death, were collected. In addition, we gathered information on the cause and date of death from death certificates obtained from the Korean National Statistical Office.
2. Treatment methods
Fertility-sparing surgery was performed if patients of childbearing age had a strong desire to retain fertility. These women were informed of the possible risks and benefits of fertility-sparing surgery and signed a consent form during a preoperative counseling session. Patients in the fertility-sparing surgery group underwent unilateral salpingo-oophorectomy on the side of the ovarian tumor with surgical exploration (cytology of peritoneal washing or ascites, careful palpation and inspection of the peritoneal cavity). Surgical staging procedures, such as peritoneal biopsy of suspicious lesions or random biopsy, appendectomy, omentectomy, and lymphadenectomy, were optional and conducted at the surgeon’s discretion. Otherwise, patients were categorized as being in the radical surgery group.
Adjuvant chemotherapy is based on the pathologic findings of the surgical specimen. Patients with stage IAG3 or IBG3, or any grades of IC, were treated with three to six courses of platinum-based chemotherapy. Two patients did not receive adjuvant chemotherapy despite indication.
3. Statistical analysis
Disease-specific survival was defined as the time from surgery to the date of death due to ovarian cancer. Recurrence- free survival was defined as the time from surgery to the date of recurrence.
The differences between clinical and demographic characteristics of the fertility-sparing surgery and radical surgery groups were compared with Student’s t-test for continuous variables and chi-square test or Fisher’s exact test for categorical variables. The recurrence-free and disease-specific survival curves were estimated using the Kaplan-Meier method, and the differences in survival between the groups were compared using the log rank test. Cox proportional hazards models were developed to examine the survival. In Cox proportional hazards analyses, the recurrence and death hazard ratios (HRs) were modeled and patients who underwent fertility preservation were compared with those who did not. All analyses were performed using STATA ver. 11.0 (StataCorp, College Station, TX). All p-values are two-sided.
Results
1. Patient characteristics
In total, 110 patients with mucinous epithelial ovarian cancer were identified in our cancer registry, all of which had undergone primary surgery. At the time of surgery, 90 patients (81.8%) had tumors grossly confined to the ovaries, and 20 patients (18.2%) had definite evidence of metastasis. Of the 90 patients with tumors grossly confined to the ovaries, 35 patients (38.9%) underwent fertility-sparing surgery based on the surgeon’s decision.
The baseline characteristics are shown in Table 1. The mean age at diagnosis was 28.6 years in the fertility-sparing surgery group and 50.3 years in the radical surgery group (p < 0.001). With the exception of age, the clinical characteristics were similar for both groups, including stage, year of diagnosis, adjuvant chemotherapy, tumor grade, and recurrence rate.
Table 1.
Clinical and demographic characteristics
| Fertility-sparing surgery |
p-value | ||
|---|---|---|---|
| Yes (n=35) | No (n=55) | ||
| Age (yr) | 28.6 ±10.1 | 50.3±14.0 | < 0.001 |
| Stage | 0.496 | ||
| IA | 21 (60) | 34 (61.8) | |
| IB | 0 (0) | 1 (1.8) | |
| IC | 13 (37.1) | 17 (30.9) | |
| IIA | 0 (0) | 1 (1.8) | |
| IIB | 0 (0) | 2 (3.6) | |
| IIC | 1 (2.9) | 0 (0) | |
| Year of diagnosis | 0.067 | ||
| 1991-2000 | 15 (42.9) | 18 (32.7) | |
| 2001-2005 | 13 (37.1) | 13 (23.6) | |
| 2006-2010 | 7 (20.0) | 24 (43.6) | |
| Adjuvant chemotherapy | 0.498 | ||
| Yes | 14 (40.0) | 26 (47.3) | |
| Conventional platinum-based | 5 (14.3) | 9 (16.4) | |
| Taxane plus platinum | 9 (25.7) | 17 (30.9) | |
| No | 21 (60.0) | 29 (52.7) | |
| Grade | 0.479 | ||
| 1 | 27 (77.1) | 34 (61.8) | |
| 2 | 5 (14.3) | 15 (27.3) | |
| 3 | 1 (2.9) | 2 (3.6) | |
| NA | 2 (5.7) | 4 (7.3) | |
| Recurrence (%) | 0.923 | ||
| Yes | 6 (17.1) | 9 (16.4) | |
| No | 29 (82.9) | 46 (83.6) | |
Values are presented as mean±standard deviation or number (%). NA, not available.
2. Upstaging after surgical staging
Table 2 shows the procedures performed at the time of surgical exploration and cases of microscopic metastasis after additional biopsies. Surgical staging led to upstaging for four patients who were identified as having invasive cancer following a peritoneal biopsy of a suspicious lesion or adhesion site. However, there were not any cases with upstaging occurrence based on random peritoneal biopsies without suspicious lesions. In addition, a positive peritoneal washing cytology was identified in eight patients. Among these patients, ovarian surface involvement or spontaneous rupture had already been identified in three cases, intraoperative rupture occurred in two patients, and invasive cancer was found following peritoneal biopsy in one patient. Therefore, only two patients were upstaged based solely on washing cytology, both of which were in the fertility-sparing surgery group. There was no case of upstaging following lymphadenectomy, appendectomy, omentectomy, or hysterectomy. Moreover, of the 49 patients who had tumors that were unilateral and confined to the ovary at the time of surgery in the radical surgery group, no tumor was identified on the opposite ovary following bilateral salpingooophorectomy.
Table 2.
Procedures performed during surgical staging and cases of microscopic metastasis after surgery (cases of metastasis/cases of surgery performed)
| Fertility-sparing surgery |
||
|---|---|---|
| Yes (n=35) | No (n=55) | |
| Hysterectomy | 0/0 | 0/55 |
| Contralateral oophorectomy | 0/0 | 0/49a) |
| Peritoneal biopsy | 1/7 | 3/20 |
| Appendectomy | 0/9 | 0/41 |
| Omentectomy | 0/12 | 0/45 |
| Lymphadenectomy | 0/2 | 0/26 |
| Cytology | 6/35 | 2/55 |
Among patients who had tumors grossly confined to unilateral ovary at the time of surgery.
3. Survival analysis
The median follow-up duration was 104 months (range, 8.0 to 231.6 months). The Kaplan-Meier curves and the log rank test showed no difference in either recurrence-free survival (p=0.792) or disease-specific survival (p=0.706) between patients who underwent fertility-sparing surgery and radical surgery (Fig. 1). Five-year disease-specific survival was 91.3% for those who had fertility-sparing surgery, compared with 86.4% for patients who underwent radical surgery. In addition, 10-year disease-specific survival was 81.4% for those who had fertility-sparing surgery, compared with 81.8% for patients who underwent radical surgery.
Fig. 1.
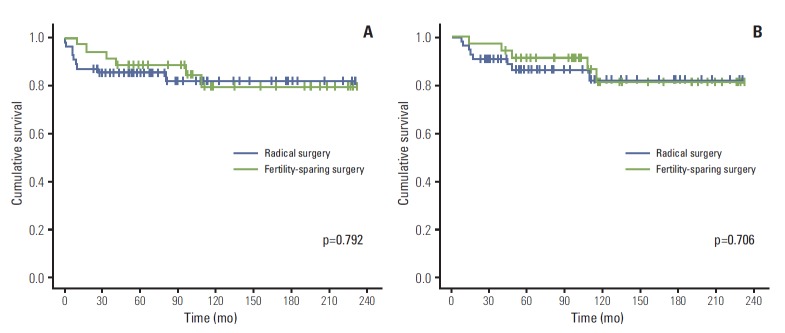
Recurrence-free survival (A) and disease-specific survival (B) in all patients with tumors grossly confined to the ovaries.
Of the 90 patients with tumors that appeared to be grossly confined to the ovaries upon surgical exploration, 15 (16.7%) eventually experienced disease recurrence, including six of the 35 patients (17.1%) who underwent fertility-sparing surgery and nine of the 55 patients (16.4%) who underwent radical surgery (p=0.923). Contralateral adnexal recurrence was suspected in just one of the patients with recurrence in the fertility-sparing surgery group, based on imaging study findings. Only two of the 15 patients who experienced recurrence went on to live without the disease.
A subgroup analysis was performed with women below the age of 40; there were 32 patients in the fertility-sparing surgery group and 11 in the radical surgery group. Even when the analysis was confined to women less than 40 years old, Kaplan-Meier curves and the log rank test showed no difference in either recurrence-free survival (p=0.126) or disease-specific survival (p=0.377) between the two groups (Fig. 2).
Fig. 2.
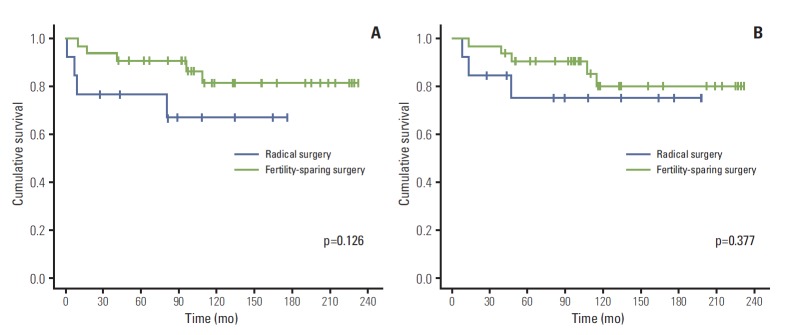
Recurrence-free survival (A) and disease-specific survival (B) in all patients under the age of 40 with tumors grossly confined to the ovaries.
Cox proportional hazards analysis was performed to adjust for the baseline characteristics (Table 3). Fertility-sparing surgery had no effect on either recurrence-free survival (HR, 1.20; 95% confidence interval [CI], 0.25 to 5.71) or disease-specific survival (HR, 0.88; 95% CI, 0.17 to 4.60). Stage was the most important prognostic factor for recurrence and survival in our cohort. This finding did not change when the analysis was confined to women younger than 40. The surgical procedure was not an independent prognostic factor for either recurrence-free survival (HR, 0.22; 95% CI, 0.04 to 1.24) or disease-specific survival (HR, 0.52; 95% CI, 0.09 to 3.11) in patients below the age of 40.
Table 3.
Cox proportional hazards model of factors associated with recurrence and disease-specific survival for primary mucinous cancer grossly confined to the ovaries
| Recurrence HR (95% CI) | Death HR (95% CI) | |
|---|---|---|
| FSS | ||
| No | Reference | Reference |
| Yes | 1.20 (0.25-5.71) | 0.88 (0.17-4.60) |
| Age (yr) | ||
| < 40 | Reference | Reference |
| ≥ 40 | 2.08 (0.38-11.55) | 1.04 (0.18-6.08) |
| Stage | ||
| IA or IB | Reference | Reference |
| IC | 1.98 (0.46-8.61) | 1.56 (0.35-7.03) |
| II | 28.99 (2.72-308.91) | 11.47 (1.16-113.17) |
| Year of diagnosis | ||
| 1991-2000 | Reference | Reference |
| 2001-2005 | 0.53 (0.12-2.31) | 0.24 (0.04-1.56) |
| 2006-2010 | 0.14 (0.01-1.33) | 0.29 (0.04-2.17) |
| Adjuvant chemotherapy | ||
| No | Reference | Reference |
| Yes | 2.24 (0.48-10.46) | 1.86 (0.39-8.83) |
| Grade | ||
| 1 | Reference | Reference |
| 2 | 1.35 (0.27-6.78) | 1.40 (0.25-7.98) |
| 3 | 3.09 (0.25-37.76) | 9.26 (0.60-141.82) |
HR, hazard ratio; CI, confidence interval; FSS, fertility-sparing surgery.
Discussion
This study highlights the safety of fertility-sparing surgery in cases of mucinous epithelial ovarian cancer when tumors appear to be grossly confined to the ovaries. We compared the oncologic outcomes of patients who underwent fertilitysparing and radical surgery using long-term follow-up data. In addition, the risk of upstaging is minimal in patients with early-stage mucinous ovarian cancer when no suspicious lesions on the peritoneum are found during careful intraoperative exploration.
Previous studies have reported the outcomes of fertilitysparing surgery in women with early-stage epithelial ovarian cancer [3-11]. The preservation of the female reproductive organ is one of the key issues for young women with epithelial ovarian cancer, and in particular for nulliparous women. However, the criteria for guaranteeing the safety of fertilitysparing surgery in early-stage ovarian cancer have not been identified thus far. Although the current guidelines recommend that fertility-sparing surgery may be considered in selected cases, it is not clear what these ‘selected cases’ are [12]. Most studies propose that fertility-sparing surgery can be performed on all young women with stage IA grade 1 ovarian cancer [20]. In many of the above-mentioned series, fertility-sparing surgery was also performed on invasive ovarian cancer patients with unfavorable grades, as well as on patients with disease not confined to one ovary [7,8,10,11,21]. Although the recurrence rate remains low even for these patients, the data has limitations due to the retrospective nature of this study and the small sample size.
Recent studies have demonstrated that epithelial ovarian cancer is not a single disease but is composed of a diverse group of tumors. Based on distinctive morphologic and molecular genetic features, a dualistic model that classifies various types of ovarian cancer into two groups (type I and type II) was proposed by Kurman and Shih [22]. Type I tumors are clinically indolent and usually present with low-grade carcinoma, including low-grade serous, low-grade endometrioid, and mucinous carcinoma. Mucinous carcinoma is indolent and has an excellent prognosis when identified at an early stage [23]. By contrast, advanced-stage mucinous ovarian cancer has a poor prognosis due to its resistance to platinum-based chemotherapy [24]. In particular, mucinous invasive ovarian cancer is a different disease entity and has distinct characteristics compared to highgrade serous carcinoma. Therefore, these favorable histologic subtypes should be distinguished from others.
In patients with mucinous ovarian cancer grossly confined to the ovaries, the risk of upstaging after surgical staging procedure has been reported to be minimal [16,17]. In addition, these histological subtypes are commonly found in younger women. As a result, fertility preservation is an important consideration in this disease subset. Of the 90 patients in our cohort with tumors grossly confined to the ovaries, only six cases were upstaged following surgical staging, and there were no cases of upstaging due to microscopic metastasis following omentectomy, appendectomy, or lymphadenectomy. This finding was in accordance to those of previous studies. Recently, a number of studies have reported that occult lymph node metastasis was not found in any patients with apparent early-stage mucinous epithelial ovarian cancer [16,17]. Cho et al. [16] reported that out of 85 patients with stage I mucinous epithelial ovarian cancers, only five were upstaged due to positive peritoneal cytology. Schmeler et al. [17] demonstrated that 13 out of 93 patients with mucinous tumors grossly confined to the ovary were upstaged based on additional biopsies at the time of surgery. Moreover, they reported no significant difference in survival between the group that underwent lymphadenectomy and the group that did not.
When considering previously described indolent aspects of early-stage mucinous adenocarcinoma, fertility-sparing surgery may be an acceptable option for younger women. However, concerns surrounding suboptimal surgery remain. One major concern with fertility-sparing surgery is the very small or microscopic foci involvement of the normal-looking uterus and contralateral ovary. Previous studies have shown that the risk of contralateral ovary involvement during surgery and recurrence in the contralateral residual ovary is low. Considering the 118 normal-appearing ovaries of epithelial ovarian cancer patients, Benjamin et al. [25] reported that only three patients (2.5%) had microscopic involvement in the contralateral ovary. Moreover, of the 209 patients treated conservatively, 14 patients (6.7%) experienced recurrence in the contralateral ovary [20]. Our results suggest more promising findings in mucinous histological subtypes. No cases of upstaging due to microscopic metastasis were identified in our cohort after hysterectomy. Of the patients with tumors confined to one ovary at surgical exploration in the radical surgery group, 49 underwent bilateral salpingo-oophorectomy, where no tumor was identified on the contralateral ovary. Moreover, of the 35 patients who underwent fertility-sparing surgery, only one experienced recurrence in the contralateral ovary. As a result, preservation of the uterus and contralateral ovary should be considered in patients with mucinous tumors confined grossly to one ovary.
Our study is limited by retrospective data collection, a long study period, small sample size, and possible referral bias. Moreover, varying surgical treatment types were used at the clinician’s discretion. Finally, it is unclear whether the lack of significant differences in recurrence and survival between patients who underwent fertility-sparing surgery and those who had radical surgery is attributable to our small sample size. However, only a small proportion of patients with invasive epithelial ovarian cancer underwent fertility-sparing surgery. In addition, no prospective study on the fertility- sparing issue in invasive epithelial ovarian cancer has been designed, and such a study is unlikely to perform a randomized controlled trial in the near future, due particularly to ethical problems and the anticipated difficulty in patient recruitment. Given these circumstances, our retrospective analysis opens up a discussion of the possibility that fertility-sparing surgery may be safe in mucinous histology.
Conclusion
In conclusion, our findings demonstrate that fertility-sparing surgery does not appear to have an adverse impact on the recurrence and survival rates in patients with early-stage mucinous ovarian carcinoma. This study has useful implications for physicians counseling patients who want to preserve their fertility. To validate our results, further largescale studies considering a sufficient number of patients with mucinous histology are required.
Acknowledgments
This work was supported by WCU (World Class University) program (R31-10056) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Young RC, Decker DG, Wharton JT, Piver MS, Sindelar WF, Edwards BK, et al. Staging laparotomy in early ovarian cancer. JAMA. 1983;250:3072–6. [PubMed] [Google Scholar]
- 2.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 Suppl 1:S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 3.Borgfeldt C, Iosif C, Masback A. Fertility-sparing surgery and outcome in fertile women with ovarian borderline tumors and epithelial invasive ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2007;134:110–4. doi: 10.1016/j.ejogrb.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Fruscio R, Corso S, Ceppi L, Garavaglia D, Garbi A, Floriani I, et al. Conservative management of early-stage epithelial ovarian cancer: results of a large retrospective series. Ann Oncol. 2013;24:138–44. doi: 10.1093/annonc/mds241. [DOI] [PubMed] [Google Scholar]
- 5.Kajiyama H, Shibata K, Mizuno M, Nawa A, Mizuno K, Matsuzawa K, et al. Fertility-sparing surgery in young women with mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2011;122:334–8. doi: 10.1016/j.ygyno.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Kwon YS, Hahn HS, Kim TJ, Lee IH, Lim KT, Lee KH, et al. Fertility preservation in patients with early epithelial ovarian cancer. J Gynecol Oncol. 2009;20:44–7. doi: 10.3802/jgo.2009.20.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morice P, Leblanc E, Rey A, Baron M, Querleu D, Blanchot J, et al. Conservative treatment in epithelial ovarian cancer: results of a multicentre study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer) and SFOG (Societe Francaise d'Oncologie Gynecologique) Hum Reprod. 2005;20:1379–85. doi: 10.1093/humrep/deh777. [DOI] [PubMed] [Google Scholar]
- 8.Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, et al. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: oncologic safety and reproductive outcomes. Gynecol Oncol. 2008;110:345–53. doi: 10.1016/j.ygyno.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Satoh T, Hatae M, Watanabe Y, Yaegashi N, Ishiko O, Kodama S, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol. 2010;28:1727–32. doi: 10.1200/JCO.2009.24.8617. [DOI] [PubMed] [Google Scholar]
- 10.Schilder JM, Thompson AM, DePriest PD, Ueland FR, Cibull ML, Kryscio RJ, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1–7. doi: 10.1006/gyno.2002.6805. [DOI] [PubMed] [Google Scholar]
- 11.Zanetta G, Chiari S, Rota S, Bratina G, Maneo A, Torri V, et al. Conservative surgery for stage I ovarian carcinoma in women of childbearing age. Br J Obstet Gynaecol. 1997;104:1030–5. doi: 10.1111/j.1471-0528.1997.tb12062.x. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Center . Ovarian cancer clinical practice guidelines in oncology (v.I.2010) [Internet] Fort Washington: National Comprehensive Cancer Network; 2010. [cited 2013 Feb 4]. Available from: http://www.nccn.org. [Google Scholar]
- 13.McGuire V, Jesser CA, Whittemore AS. Survival among U.S. women with invasive epithelial ovarian cancer. Gynecol Oncol. 2002;84:399–403. doi: 10.1006/gyno.2001.6536. [DOI] [PubMed] [Google Scholar]
- 14.Hess V, A'Hern R, Nasiri N, King DM, Blake PR, Barton DP, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004;22:1040–4. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 15.Mizuno M, Kikkawa F, Shibata K, Kajiyama H, Suzuki T, Ino K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29–36. doi: 10.1159/000071202. [DOI] [PubMed] [Google Scholar]
- 16.Cho YH, Kim DY, Kim JH, Kim YM, Kim KR, Kim YT, et al. Is complete surgical staging necessary in patients with stage I mucinous epithelial ovarian tumors? Gynecol Oncol. 2006;103:878–82. doi: 10.1016/j.ygyno.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Schmeler KM, Tao X, Frumovitz M, Deavers MT, Sun CC, Sood AK, et al. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet Gynecol. 2010;116(2 Pt 1):269–73. doi: 10.1097/AOG.0b013e3181e7961d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kajiyama H, Shibata K, Mizuno M, Umezu T, Suzuki S, Nawa A, et al. Long-term survival of young women receiving fertility- sparing surgery for ovarian cancer in comparison with those undergoing radical surgery. Br J Cancer. 2011;105:1288–94. doi: 10.1038/bjc.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergote I, De Brabanter J, Fyles A, Bertelsen K, Einhorn N, Sevelda P, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176–82. doi: 10.1016/S0140-6736(00)03590-X. [DOI] [PubMed] [Google Scholar]
- 20.Menczer J. Conservative fertility-sparing surgical treatment of invasive epithelial ovarian cancer: when is it acceptable? Isr Med Assoc J. 2013;15:116–20. [PubMed] [Google Scholar]
- 21.Raspagliesi F, Fontanelli R, Paladini D, di Re EM. Conservative surgery in high-risk epithelial ovarian carcinoma. J Am Coll Surg. 1997;185:457–60. doi: 10.1016/s1072-7515(97)00066-5. [DOI] [PubMed] [Google Scholar]
- 22.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison ML, Jameson C, Gore ME. Mucinous ovarian cancer. Int J Gynecol Cancer. 2008;18:209–14. doi: 10.1111/j.1525-1438.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 24.Karabuk E, Kose MF, Hizli D, Taskin S, Karadag B, Turan T, et al. Comparison of advanced stage mucinous epithelial ovarian cancer and serous epithelial ovarian cancer with regard to chemosensitivity and survival outcome: a matched case-control study. J Gynecol Oncol. 2013;24:160–6. doi: 10.3802/jgo.2013.24.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin I, Morgan MA, Rubin SC. Occult bilateral involvement in stage I epithelial ovarian cancer. Gynecol Oncol. 1999;72:288–91. doi: 10.1006/gyno.1998.5260. [DOI] [PubMed] [Google Scholar]


