Abstract
BACKGROUND
Studies comparing survival of adolescent and young adult (AYA) patients to that of younger patients with newly diagnosed acute myeloid leukemia (AML) have yielded conflicting results. In order to more accurately characterize relative survival and other outcomes of AYA patients, a cross-study analysis was conducted using data from recent trials conducted by the Children's Cancer Group (CCG) and Children's Oncology Group (COG).
METHODS
Data were combined from the CCG-2891, CCG-2941, CCG-2961, and AAML03P1 trials. The data set included 1840 patients, comprising 238 AYA and 1602 younger patients.
RESULTS
Overall survival was not significantly different in the 2 groups (AYA, 49% ± 7% versus younger, 54% ± 3% (6 2 standard errors), P =.058). Relapse was lower in AYA patients (30% ± 7% versus 41% ± 3%, P = .002), but treatment-related mortality (TRM) was higher (25% ± 6% versus 12% ± 2%, P < .001). After adjustment for other factors, older age remained strongly associated with TRM (hazard ratio = 2.30, 95% CI = 1.59-3.33, P < .001). Infection accounted for the excess TRM in AYA patients.
CONCLUSIONS
Survival in AYA and younger patients with newly diagnosed AML is similar; however, older patients are at higher risk for TRM. More effective strategies for preventing mortality from infection in AYA patients are needed.
Keywords: acute myeloid leukemia, adolescent and young adult, children, infection, cancer
INTRODUCTION
Although enormous gains have been made in the treatment of newly diagnosed pediatric acute myeloid leukemia (AML),1-6 with long-term overall survival now exceeding 50%, several studies suggest that adolescent and young adult (AYA) patients may be less likely to benefit from this treatment than younger patients.4,7,8 Uncertainty remains regarding the impact of older age on survival, however, because other studies have shown no differences between AYA and younger patients.9,10 In some of these studies, AYA patients, unlike younger patients, were either treated on protocols for adult patients or treated at adult medical centers.7-9 It is possible, then, that associated differences in treatment may have influenced the findings.
The Children's Oncology Group (COG), unlike most other pediatric cooperative groups, enrolls patients with AML through the age of 20 years on its trials, which provides the unique opportunity to compare outcomes of a segment of the AYA age group (usually considered as extending from mid-adolescence to 39 years11) to outcomes of younger patients receiving the same treatment delivered in the same setting. We examined the outcomes of AYA patients (16 through 20 years) relative to younger patients, by combining and analyzing data from 4 consecutive COG trials: CCG-2891, CCG-2941, CCG-2961, and AAML03P1.
MATERIALS AND METHODS
Treatment Protocols
We aggregated data from four consecutive cooperative group trials for newly diagnosed pediatric AML: CCG-2891, CCG-2941, CCG-2961 and AAML03P1.2,4,6,12 Collectively, these studies accrued patients between 1989 and 2006. In the CCG-2891 study (a phase 2 trial), 4 cycles of induction therapy, comprising dexamethasone, cytarabine, 6-thioguanine, etoposide, and daunorubicin (DCTER), were administered. Patients were randomized to receive intensive timed or standard timed DCTER. For the intensive timed group, the second and fourth cycles of DCTER were initiated 6 days after completion of the previous cycle. The third cycle was initiated after count recovery. For the standard timed group, the second, third and fourth cycles were started after blood count recovery.6 Because intensive induction therapy, whether achieved by compressing the interval between induction cycles as in the 2891 trial (and in the 2941 and 2961 trials) or by increasing the number of days of chemotherapy for the initial induction cycle (UK Medical Research Council5) or adding chemotherapy agents to an existing backbone (German BFM3), has become standard practice, only data from the 2891 patients assigned to intensively timed induction therapy were used in this analysis. In this trial patients with a human leukocyte antigen (HLA)-matched related donor were assigned to allogeneic bone marrow transplant (BMT) in first remission, and patients without a donor were randomly assigned to autologous BMT or intensive consolidation chemotherapy. There was no difference in survival between patients assigned to autologous transplant and those assigned consolidation chemotherapy alone,6 and the data for these 2 groups were combined.
In the 2961 study, another phase 3 trial, all patients received intensively timed induction therapy. All received dexamethasone, cytarabine, 6-thioguanine, etoposide, and idarubicin (DCTEI) for the first cycle and DCTER for the second. Patients in remission were then randomly assigned to repeat this sequence or to receive fludarabine, high-dose cytarabine and idarubicin. Patients in remission at the end of induction therapy were assigned to allogeneic BMT if they had an HLA-matched related donor. Others were assigned to get consolidation chemotherapy. At the end of consolidation chemotherapy, patients were randomly assigned to IL-2 infusions or no further therapy. Enrollment was temporarily suspended midway through this study to address concerns regarding treatment-related mortality (TRM). There was a marked reduction in TRM after the study was amended to incorporate more aggressive supportive care measures.4 To better gauge the impact of the pre- and postamendment guidelines on our analyses, we analyzed the pre- and postamendment data separately (pre-2961 and post-2961).
CCG-2941 was a single-arm pilot study conducted in preparation for the 2961 study. Patients received intensively timed DCTEI and DCTER for the first and second induction cycles and again for the third and fourth cycles. Patients with an HLA-matched related donor were assigned to transplant in remission, whereas others were assigned to consolidation chemotherapy.12 COG 03P1 was also a single-arm pilot study. Patients received 2 induction cycles consisting of cytarabine, daunorubicin, and etoposide (ADE). The first was 10 days and included gemtuzumab ozogamycin (GO). The second was 8 days and did not include GO. Patients then received 3 cycles of consolidation therapy. The second induction cycle included GO. Patients with a matched related donor proceeded to BMT after the first round of consolidation therapy.2
Adjustments for obesity when calculating doses of chemotherapy or of agents prescribed for supportive care were not made in any of the trials with one exception: in the 2891 trial, the doses of busulfan and cyclophosphamide used for pretransplant conditioning were calculated using ideal body weight for all patients 13 years or older and in younger patients who were obese.6
Analysis
Overall, there were minor differences in the eligibility criteria employed in the four trials. Our data set included all patients between the ages of 1 month and 21 years with newly diagnosed AML except those who had Down syndrome, acute promyelocytic leukemia, a constitutional bone marrow failure syndrome or had been treated previously for another malignancy. Patients were grouped by age; those aged 16 through 20 years were defined as the AYA group, and then compared to patients younger than 16 years. Patients who were 1 year of age and older were also classified as being underweight, normal weight or overweight by weight-for-length (WT/LT) percentile for patients 1 to 2 years of age, and by body mass index (BMI) percentile for patients 2 years of age and older.13 The patients were stratified into favorable-, standard-, and poor-risk disease groups in 2 ways: 1) by the results of cytogenetic testing alone, and 2) by the results of molecular and cytogenetic testing. Inv(16), t(8;21), CEBPA (single or double)14 and nucleophosmin15 mutations were considered to be favorable-risk abnormalities; monosomy 7, monosomy 5, deletions of 5q, or high FLT3-ITD allelic ratio16 were considered to be poor-risk abnormalities. Patients lacking these abnormalities were deemed to have standard-risk disease.
All analyses were performed on an intent-to-treat basis. To account for the influence of allogeneic transplant, we performed the analyses with and without censoring these patients at the time of allogeneic transplant. The significance of observed differences in proportions was tested using the chi-square test and Fisher's exact test when data were sparse. The Mann-Whitney test was used to determine the significance between differences in medians. The Kaplan-Meier method was used to estimate overall survival (OS) and event-free survival (EFS). Estimates of relapse risk (RR) and TRM were obtained using methods that account for competing events. In patients dying after relapse, the death was considered relapse-related even if they died in a second or subsequent remission. OS, EFS, and TRM were defined from study entry. OS was defined as time to death from any cause. EFS was defined as time to failure to achieve remission by the end of induction, relapse, or death from any cause. TRM was defined as time to death due to nonprogressive disease where relapses and deaths due to progressive disease were competing events. RR was defined from the end of course one for patients in complete remission (CR). RR was defined as time to relapse or death due to progressive disease where deaths due to nonprogressive disease were competing events. The significance of predictor variables was tested with the log-rank statistic for OS and EFS and with Gray's statistic for RR and TRM. Patients lost to follow-up were censored at their date of last known contact or at a cutoff 6 months before the data set creation date for each study. All reported estimates of OS, EFS, RR, and TRM were at 5 years with corresponding Greenwood standard errors multiplied by 2. Cox proportional hazard models were used to estimate hazard ratios (HR) for univariate and multivariate analyses of OS and EFS. Competing risk regression models were used to estimate HRs for univariate and multivariate analyses of TRM and RR. Multivariate models included age (16-20 years versus younger), study, cytogenetic risk group, white blood cell count (WBC) at study entry, weight group and race/ethnic group (African American; Hispanic versus caucasian; Asian; non-Hispanic caucasian).
Cause of death in patients in remission was considered in 2 ways. First, we used the primary cause of death as reported by the treating center. Second, we categorized the causes into 1 of 4 groups. In nontransplant patients, if the primary cause of death was listed as infection or infection was listed a contributing factor and a common sequela to serious infection, such as multiorgan failure or acute respiratory distress syndrome, was listed as the primary cause listed, the death was classified as infection-related. Other cases were classified as non-infection-related. In BMT patients, if the patient had graft-versus-host disease (GVHD) or veno-occlusive disease (VOD) at the time of death, even if infection was present or was listed as the primary cause of death, the death was classified as related to GVHD or VOD. In the absence of GVHD and VOD, deaths were classified as infection-related or non-infection-related as in nontransplant patients.
RESULTS
Patient and Disease Characteristics
The data set included 1840 patients, 238 AYA (12.9%, median 17.2 years) and 1602 younger patients (87.1%, median 6.9 years). Comparing baseline patient and disease-related characteristics (Table 1) for the 2 groups, there were no significant differences in race/ethnicity or sex. Obesity was more prevalent in the AYA group (19.9% versus 13.2%, P = .009). AYA patients were less likely to have M5 (10.8% versus 18.7%, P = .005) or M7 (1.3% versus 7.9%, P < .001) disease. Cytogenetic testing results were known for 1155 patients. Among these, AYA patients were more likely to have normal cytogenetics (35.4% versus 21.1%, P < .001), but less likely to have t(9;11)(p22;q23) or other abnormality involving 11q23 (8.9% versus 23.5%, P < .001). When cytogenetic testing results alone were considered there were no differences between the 2 groups in the proportion of patients with poor-, standard-, and high-risk disease. When risk was determined by both molecular and cytogenetic testing results, AYA patients were more likely to have poor risk disease (16.6% versus 10.4%, P = .026) and less likely to have standard-risk disease (50.8% versus 61.8%, P = .006). The AYA patients were also more likely to have a matched related donor (29% versus 21.7%, P = .016) and to have received a matched related donor transplant (22.3% versus 14.79%, P = .005).
TABLE 1.
Patient Characteristics, Age <16 years and 16-20 years
| Age <16y (n = 1602) |
Age 16-20 y (n = 238) |
||||
|---|---|---|---|---|---|
| Characteristics | N | % | N | % | P |
| Age, y, median (range) | 6.9 | (0.01-15.99) | 17.2 | (16-20.9) | - |
| Study | |||||
| CCG-2941 | 80 | 5.0% | 6 | 2.5% | .128 |
| CCG-2961 pre-suspension | 427 | 26.7% | 68 | 28.6% | .586 |
| CCG-2961 post-suspension | 351 | 21.9% | 55 | 23.1% | .740 |
| AAML03P1 | 286 | 17.9% | 53 | 22.3% | .121 |
| CCG-2891 INT | 458 | 28.6% | 56 | 23.5% | .122 |
| Sex | |||||
| Male | 816 | 50.9% | 130 | 54.6% | .321 |
| Female | 786 | 49.1% | 108 | 45.4% | |
| Race | |||||
| White | 1026 | 65.3% | 165 | 70.5% | .132 |
| Black | 164 | 10.4% | 20 | 8.5% | .439 |
| Hispanic | 263 | 16.7% | 32 | 13.7% | .278 |
| Asian | 54 | 3.4% | 10 | 4.3% | .647 |
| Other | 65 | 4.1% | 7 | 3.0% | .512 |
| Unknown | 30 | 4 | |||
| Weight group (1- to 20-y-olds) | |||||
| Underweight | 181 | 13.1% | 21 | 9.1% | .114 |
| Middleweight | 1022 | 73.7% | 164 | 71.0% | .428 |
| Overweight | 183 | 13.2% | 46 | 19.9% | .009 |
| Unknown | 216 | 7 | |||
| WBC (×103/iL), median (range) | 20.1 | (0.3-684) | 18.9 | (0.5-860) | .090 |
| FAB Classification | |||||
| M0 | 71 | 4.6% | 12 | 5.2% | .833 |
| M1 | 234 | 15.3% | 46 | 19.9% | .088 |
| M2 | 411 | 26.8% | 75 | 32.5% | .086 |
| M4 | 374 | 24.4% | 65 | 28.1% | .252 |
| M5 | 287 | 18.7% | 25 | 10.8% | .005 |
| M6 | 35 | 2.3% | 5 | 2.2% | .901 |
| M7 | 121 | 7.9% | 3 | 1.3% | <.001 |
| Other NOS | 66 | 7 | |||
| Unknown | 3 | 0 | |||
| Cytogenetics | |||||
| Normal | 210 | 21.1% | 56 | 35.4% | <.001 |
| t(8;21) | 134 | 13.4% | 24 | 15.2% | .638 |
| inv(16) | 98 | 9.8% | 15 | 9.5% | .990 |
| t(9;11)/11q23 | 234 | 23.5% | 14 | 8.9% | <.001 |
| t(6;9)(p23;q34) | 14 | 1.4% | 4 | 2.5% | .293 |
| del(7q) | 17 | 1.7% | 1 | 0.6% | .495 |
| monosomy 7 | 22 | 2.2% | 8 | 5.1% | .068 |
| -5/5q- | 11 | 1.1% | 2 | 1.3% | .695 |
| +8 | 70 | 7.0% | 12 | 7.6% | .925 |
| Other | 187 | 18.8% | 22 | 13.9% | .143 |
| Unknown | 605 | 80 | |||
| FLT3/ITD status | |||||
| ITD-positive | 84 | 11.0% | 23 | 16.7% | .082 |
| ITD-negative | 678 | 89.0% | 115 | 83.3% | |
| Missing | 840 | 100 | |||
| Of ITD-positive patients: | |||||
| Low allelic ratio (AR) | 25 | 29.8% | 6 | 26.1% | .932 |
| High allelic ratio (AR) | 59 | 70.2% | 17 | 73.9% | |
| Risk group (by cytogenetics only) | |||||
| Standard | 732 | 73.4% | 109 | 69.0% | .286 |
| Low (t(8;21 or inv(16)) | 232 | 23.3% | 39 | 24.7% | .773 |
| High (Mono 7 or -5/5q-) | 33 | 3.3% | 10 | 6.3% | .102 |
| Risk group (by molecular and cytogenetic status) | |||||
| Standard | 643 | 61.8% | 85 | 50.3% | .006 |
| Low | 290 | 27.9% | 56 | 33.1% | .188 |
| High | 108 | 10.4% | 28 | 16.6% | .026 |
| Unknown | 561 | 69 | |||
| Course 1 response | |||||
| Complete response | 1255 | 81.2% | 190 | 81.5% | .965 |
| Partial response | 106 | 6.9% | 16 | 6.9% | .894 |
| Fail | 108 | 7.0% | 14 | 6.0% | .681 |
| Death | 77 | 5.0% | 13 | 5.6% | .819 |
| Unevaluable | 56 | 5 | |||
| Matched family donor (MFD)? | |||||
| Yes | 348 | 21.7% | 69 | 29.0% | 0.016 |
| No | 1254 | 78.3% | 169 | 71.0% | |
| Received protocol MFD transplant? | |||||
| Yes | 238 | 14.9% | 53 | 22.3% | .005 |
| No | 1364 | 85.1% | 185 | 77.7% | |
Abbreviations: NOS, not otherwise specified; WBC, white blood cell.
Outcomes
Unadjusted actuarial OS at 5 years was not statistically different for the AYA and younger patients (49% ± 7% versus 54% ± 3% (± 2SE), P = .058; Table 2, Fig. 1). EFS, was also not statistically different (AYA, 40% ± 7% versus younger, 44% ± 3%, P = .324). RR was significantly lower (30% ± 7% versus 41% ± 3%, P = .002) in AYA patients. When we excluded the preamendment 2961 patients, OS for the AYA and younger groups was equivalent (57% ± 8% versus 56% ± 3%, P = .967), respectively (P = .967) and RR was 33% (± 8%) and 40% (± 3%, P = .060). After adjustment for study, cytogenetic-defined risk group, weight, race/ethnicity, and presenting WBC, older age remained marginally associated with worse OS (Table 4; HR = 1.28, 95% CI = 1.00-1.65, P = .053). Older age was not associated with EFS (HR = 1.19, 95% CI = 0.95-1.50, P = .136) or RR (HR = 0.80, 95% CI = 0.57-1.13, P = .206). In the multivariate analyses, being overweight, being African American or Hispanic, having a high-risk cytogenetic abnormality, and having a WBC ≥ 100,000/μL were all negatively associated with OS and EFS (Table 3). Having a low-risk cytogenetic abnormality and enrollment on COG AAML03P1 were associated with improved OS and EFS. Being African American or Hispanic and having a WBC ≥ 100,000/μL were associated with an increased RR, whereas having a low-risk cytogenetic abnormality and enrollment on COG 03P1 were associated with lower RR.
TABLE 2.
Patient Outcomes Separated by Age
| Age |
|||
|---|---|---|---|
| Age <16 y (n = 1602) | Age 16-20 y (n = 238) | ||
| % ± 2 SE% | % ± 2 SE% | P | |
| 5-y OS from study entry | 54 ± 3 | 49 ± 7 | .058 |
| 5-y EFS from study entry | 44 ± 3 | 40 ± 7 | .324 |
| 5-y TRM from study entry | 12 ± 2 | 25 ± 6 | <.001 |
| 5-y RR from end of course 1 | 41 ± 3 | 30 ± 7 | .002 |
| Excluding presuspension 2961 patients | |||
| Age |
|||
|---|---|---|---|
| Age <16 y (n = 1175) | Age 16-20 y (n = 170) | ||
| % ± 2 SE% | % ± 2 SE% | P | |
| 5-y OS from study entry | 56 ± 3 | 57 ± 8 | .967 |
| 5-y EFS from study entry | 45 ± 3 | 46 ± 8 | .703 |
| 5-y TRM from study entry | 11 ± 2 | 18 ± 6 | .012 |
| 5-y RR from end of course 1 | 40 ± 3 | 33 ± 8 | .6 |
| BMT patients censored in analyses | |||
| 5-y TRM from study entry | 11 ± 2 | 22 ± 6 | <.001 |
Abbreviations: BMT, bone marrow transplant; EFS, event-free survival; OS, overall survival; SE, standard error; TRM, treatment-related mortality; RR, risk of relapse.
Figure 1.
Graph shows 5-year overall survival from study entry: patients aged < 16 years, 54% ± 3% versus 16-20 years, 49% ± 7%, P =.058.
TABLE 3.
Multivariate Analyses (Adjusted for Age, Study, Weight, Ethnicity, Cytogenetic-Defined Risk Group, and Presenting White Blood Cell Count)
| Characteristic | OS From Study Entry |
EFS From Study Entry |
TRM From Study Entry |
RR From End of Course 1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age, 16-20 y | 148 | 1.28 | 1.00-1.65 | .053 | 1.19 | 0.95-1.50 | .136 | 2.3 | 1.59-3.33 | <.001 | 0.80 | 0.57-1.13 | .206 |
| Underweight | 120 | 1.26 | 0.95-1.67 | .111 | 1.16 | 0.89-1.50 | .265 | 1.50 | 0.92-2.44 | .100 | 1.12 | 0.78-1.61 | .550 |
| Overweight | 131 | 1.56 | 1.21-2.03 | <.001 | 1.36 | 1.08-1.73 | .011 | 2.18 | 1.42-3.36 | <.001 | 1.00 | 0.70-1.42 | .995 |
| Black/Hispanic | 262 | 1.46 | 1.19-1.79 | <.001 | 1.33 | 1.11-1.61 | .003 | 1.23 | 0.85-1.78 | .279 | 1.38 | 1.06-1.79 | .016 |
| WBC count (>100,000) | 155 | 1.45 | 1.14-1.84 | .003 | 1.56 | 1.25-1.94 | <.001 | 1.17 | 0.75-1.81 | .496 | 1.45 | 1.05-2.01 | .024 |
| Low (t(8;21 or inv(16)) | 261 | 0.45 | 0.35-0.58 | <.001 | 0.52 | 0.42-0.64 | <.001 | 1.10 | 0.77-1.59 | .591 | 0.46 | 0.34-0.61 | <.001 |
| High (Mono 7 or -5/5q-) | 41 | 1.85 | 1.26-2.72 | .016 | 1.83 | 1.27-2.65 | .001 | 1.18 | 0.55-2.56 | .671 | 1.29 | 0.67-2.50 | .440 |
Abbreviations: CI, confidence interval; EFS, event-free survival; HR, hazard ratio; OS, overall survival; TRM, treatment-related mortality; RR, risk of relapse; WBC, white blood cell count.
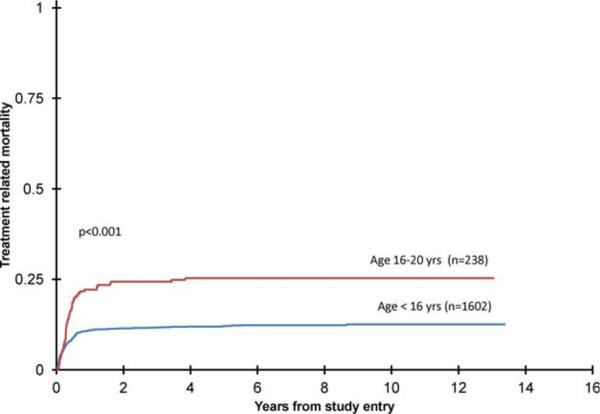
AYA patients had a higher incidence of TRM from study entry (25% ± 6% versus 12% ± 2%, P < .001). For the AYA group, it varied significantly (P = .006) by study, ranging from 16% (2891) to 43% (preamendment 2961) at 5 years (Fig. 2). There was less variation in the younger patients (P = .087), where it ranged from 8% (AAML03P1) to 15% (2941). When preamendment 2961 patients were excluded, TRM for the AYA and younger patients was 18% (± 6%) and 11% (± 2%), respectively (P = .012). AYA patients receiving allogeneic transplants had a higher incidence of transplant-related mortality (25% versus 12%, P = .010). When allogeneic transplant patients were censored at the time of transplant, TRM for the AYA and younger patients was 22% (± 6%) and 11% (± 2%), respectively (P = .012). When we excluded preamendment 2961 patients and censored for allogeneic BMT, TRM for the 2 groups was 15% (± 6%) and 10% (± 2%, P = .071). In 56% of the AYA and 67% of the younger patients with TRM, infection was identified on institutional report forms as the primary cause of death. When deaths were classified (see Materials and Methods) in the AYA patients as infection-related, non-infection-related, GVHD- (BMT only) or VOD-related (BMT only), 36 of 42 deaths (85.7%) in nontrans-plant patients were deemed to be infection-related. In the BMT patients, on the other hand, 13 of 17 deaths were classified as related to GVHD or VOD. Of the 40 infection-related deaths (BMT and nontransplant combined), in 14 bacterial infections alone (4 gram-positive, 6 gram-negative, 4 anaerobic or mixed) were identified. In 22, fungal infections (with or without other infection) were identified (10 Candida species, 10 Aspergillus species, 2 other molds).
Figure 2.
Graph shows 5-year treatment-related mortality from study entry: patients aged < 16 years, 12% ± 2% versus 16-20 years, 25% ± 6%, P < .001.
After adjustment for study, cytogenetic-defined risk group, weight, race/ethnicity, and presenting WBC, older age remained strongly associated with TRM (HR = 2.30, 95% CI = 1.59-3.33, P < .001). Being overweight was independently associated with TRM (HR = 2.18,95% CI = 1.42-3.36, P < .001). Using 2891 INT as the baseline, AAML03P1 was associated with a lower risk for TRM (HR = 0.53, 95% CI = 0.31-0.90, P = .018).
DISCUSSION
In this study, we sought to better define the impact of older age (16 through 20 years) on major outcomes in patients with newly diagnosed AML. By relying on experience from pediatric trials alone, we were able to avoid any potential for bias due to differences in chemotherapeutic approach, supportive care practices and institutional settings potentially introduced through combining experience from adult and pediatric trials. Two major findings emerge from our analyses: one, that the survival, both overall and event-free of AYA patients with newly diagnosed AML is similar to that of younger patients; second, that AYA patients are at increased risk for TRM. These findings raise 2 intriguing questions: first, are there fundamental differences in the susceptibility of AYA and younger patients to treatment-related toxicity; and second, could reducing TRM in the AYA population result in improved, even superior, survival compared with younger patients?
Our findings are similar to a Japanese study, in which 15- through 19-year-old patients had similar survival to younger patients.9 They differ from the experience of the MRC 10 trial (descriptive comparison only) and German experience. In these 2 studies, patients ages 13 through 20 years (German) and ages 15 through 24 years (MRC 10) had slightly inferior survival.7,8
On the other hand, we noted a strong association between age and TRM. To our knowledge, this is the first time this observation has been published. This may be because previous reports have focused almost exclusively on survival.4,7-10 Although the difference in TRM between AYA and younger patients was greatest in those enrolled on the 2961 trial prior to its amendment, the association between older age and TRM was not entirely explained by the early 2961 experience, as a significant difference persisted even after excluding the preamendment 2961 patients. In fact, the results of the multivariate analysis, adjusted for study, indicate that the association between older age and TRM transcends study. Even in the AAML03P1 trial, which had the lowest incidence of TRM of those analyzed, there was a sizeable difference in TRM between AYA and younger patients (17% versus 8%). The heightened risk for TRM among AYA patients also persisted after we censored for allogeneic BMT, indicating that the more frequent use of BMT in the AYA patients was not primarily responsible for the age-dependent differences.
From our analysis, it is clear that most treatment related deaths in nontransplant patients stem from infections, primarily bacterial and fungal, with Candida and Aspergillus species accounting for nearly all of the latter. Adopting measures, such as fluoroquinolone prophylaxis against bacterial infections and antifungal prophylaxis, using agents active against both Candida and Aspergillus, could help to reduce the risk of infection-related mortality in AYA patients.17 The results of an ongoing COG trial of levofloxacin prophylaxis (Clinicaltrials.gov; identifier NCT01371656) and another ongoing COG trial comparing caspofungin and fluconazole prophylaxis (Clinical-trials.gov; identifier NCT01307579) may help to define the benefits of these strategies for AYA patients.
Data from the recently completed COG AAML0531 phase 3 trial and its recently opened successor, AAML1031, should be helpful, because these 2 trials were designed to capture more detailed information on risk factors for infection. One possible explanation is that the pharmacology of chemotherapy agents differs in older and younger patients. Although the research in this area remains limited, a number of small studies have demonstrated that AYA patients have relatively decreased clearance of a variety of chemotherapy agents.18 If this is true in the setting of AML, then the attendant increase in drug exposure could increase the severity of their toxicities, such as myelosuppression and mucositis, and thus, predis-pose AYA patients to infection. Greater insight in this area could potentially lead to more effective strategies to prevent infections and reduce TRM in AYA patients. Another potentially important factor, one that to the best of our knowledge has not been systematically explored, is differences between AYA patients and younger patients in adherence to supportive care measures, such as oral, skin and central venous line care as well as prophylactic antibiotics.
Although AYA patients with other malignancies, such as acute lymphoblastic leukemia, have been shown to be at higher risk for nonfatal treatment-related toxicities,19 it is unclear whether AYA patients with other malignancies are also at heightened risk for TRM. Studying the impact of age on TRM would be more challenging in diseases like acute lymphoblastic leukemia, where treatment is less intense and TRM much less common.20,21
The impact of the AYA patients’ higher incidence of TRM on survival was largely offset by a lower incidence of relapse. The lower incidence of relapse was unexpected, especially because AYA patients were more likely to have poor-risk disease. Slower drug clearance, mentioned above as a possible explanation for increased risk for TRM in AYA patients, could likewise account for the decreased risk of relapse. As the association between age and relapse dissipated with adjustment for other variables in the multivariate analysis, an argument can be made against this supposition. The relationship between age and relapse needs to be examined further in future studies.
In conclusion, our study demonstrates that whereas survival for AYA and younger patients with newly diagnosed AML is similar, AYA patients have a greater risk for TRM. Future research should focus on furthering our understanding of TRM in these patients and identifying strategies to prevent it.
Acknowledgments
FUNDING SOURCES
Supported in part by the AYA Cancer Research Program of the Aflac Foundation/CureSearch for Children's Cancer, and by grants from the National Institutes of Health (U10 CA98543 and U10 CA98413).
Footnotes
Presented at the American Society for Clinical Oncology Annual Meeting, Chicago, IL, June 2011.
CONFLICT OF INTEREST DISCLOSURE
Dr. Franklin is a current employee of Amgen, owns stock and stock options in Amgen, and has received grants and travel/meeting support from USC/Children's Hospital Los Angeles. All other authors made no disclosure.
REFERENCES
- 1.Becton D, Dahl GV, Ravindranath Y, et al. Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood. 2006;107:1315–1324. doi: 10.1182/blood-2004-08-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemo-therapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children's Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 3.Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 4.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children's oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the Children's Oncology Group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb DK, Harrison G, Stevens RF, et al. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia. Blood. 2001;98:1714–1720. doi: 10.1182/blood.v98.6.1714. [DOI] [PubMed] [Google Scholar]
- 6.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 7.Creutzig U, Buchner T, Sauerland MC, et al. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer. 2008;112:562–571. doi: 10.1002/cncr.23220. [DOI] [PubMed] [Google Scholar]
- 8.Hann IM, Stevens RF, Goldstone AH, et al. Randomized comparison of DAT versus ADE as induction chemotherapy in children and younger adults with acute myeloid leukemia. Results of the Medical Research Council's 10th AML trial (MRC AML10).Adult and Childhood Leukaemia Working Parties of the Medical Research Council. Blood. 1997;89:2311–2318. [PubMed] [Google Scholar]
- 9.Horibe K, Tsukimoto I, Ohno R. Clinicopathologic characteristics of leukemia in Japanese children and young adults. Leukemia. 2001;15:1256–1261. doi: 10.1038/sj.leu.2402194. [DOI] [PubMed] [Google Scholar]
- 10.Woods WG, Alonzo TA, Lange BJ. Actue Myeloid Leukemia (AML) in adolescents and young adults (AYAs): a comparison of outcomes between patients treated on childhood or adult protocols. Blood. 2001:98. (abstract 1934) [Google Scholar]
- 11.Thomas DM, Albritton KH, Ferrari A. Adolescent and young adult oncology: an emerging field. J Clin Oncol. 2010;28:4781–4782. doi: 10.1200/JCO.2010.30.5128. [DOI] [PubMed] [Google Scholar]
- 12.Lange BJ, Dinndorf P, Smith FO, et al. Pilot study of idarubicin-based intensive-timing induction therapy for children with previously untreated acute myeloid leukemia: Children's Cancer Group Study 2941. J Clin Oncol. 2004;22:150–156. doi: 10.1200/JCO.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Rogers PC, Melnick SJ, Ladas EJ, et al. Children's Oncology Group (COG) Nutrition Committee. Pediatric blood Cancer. 2008;50(2 suppl):447–450. doi: 10.1002/pbc.21414. [DOI] [PubMed] [Google Scholar]
- 14.Ho PA, Alonzo TA, Gerbing RB, et al. Prevalence and prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia (AML): a report from the Children's Oncology Group. Blood. 2009;113:6558–6566. doi: 10.1182/blood-2008-10-184747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown P, McIntyre E, Rau R, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007;110:979–985. doi: 10.1182/blood-2007-02-076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 18.Veal GJ, Hartford CM, Stewart CF. Clinical pharmacology in the adolescent oncology patient. J Clin Oncol. 2010;28:4790–4799. doi: 10.1200/JCO.2010.28.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen EC, Salzer WL, Nachman JB, et al. Treatment toxicity in adolescents and young adult (AYA) patients compared with younger patients treated for high risk B-precursor acute lymphoblastic leukemia (HR-ALL): a report from the Children's Oncology Group Study AALL0232. Blood. 2011:118. Abstract 1510. [Google Scholar]
- 20.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children's oncology group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]