Summary
Systemic lupus erythematosus is an autoimmune disease characterized by antibodies that bind target autoantigens in multiple organs in the body. In peripheral organs, immune complexes engage the complement cascade, recruiting blood-borne inflammatory cells and initiating tissue inflammation. Immune complex-mediated activation of Fc receptors on infiltrating blood-borne cells and tissue resident cells amplifies an inflammatory cascade with resulting damage to tissue function, ultimately leading to tissue destruction. This pathophysiology appears to explain tissue injury throughout the body, except in the central nervous system. This review addresses a paradigm we have developed for autoantibody-mediated brain damage. This paradigm suggests that antibody-mediated brain disease does not depend on immune complex formation but rather on antibody-mediated alterations in neuronal activation and survival. Moreover, antibodies only access brain tissue when blood-brain barrier integrity is impaired, leading to a lack of concurrence of brain disease and tissue injury in other organs. We discuss the implications of this model for lupus and for identifying other antibodies that may contribute to brain disease.
Keywords: autoimmunity, systemic lupus erythematosus, antibodies
Systemic lupus erythematosus and anti-DNA antibodies
Systemic lupus erythematosus (SLE) is an autoimmune disease occurring primarily in women of child-bearing age. It is characterized by high serum titers of antibodies to nuclear antigens, the most common antigen being double-stranded (ds) DNA. Antibodies to nuclear antigen are present in essentially all patients with SLE but are present also in 5–10% percent of the healthy population. Anti-dsDNA antibodies are present in approximately 70% of SLE patients and, when present, are diagnostic of the disease. As anti-DNA antibodies are the most common autoantibody in lupus, much effort has been expended to understand their origins and potential pathogenicity.
The molecular characterization of anti-DNA antibodies derived from mouse strains that spontaneously develop SLE, NZB/W, and the MRLlpr has shown that there is extensive somatic mutation of the immunoglobulin variable region genes (1–5). This is a characteristic of B cells activated in a T-cell-dependent fashion to form germinal centers, where heavy chain class switching and somatic mutation occur at high frequency at immunoglobulin gene loci (6). More detailed analysis of the antibodies has shown that back mutation to the germline-encoded immunoglobulin most commonly generates an antibody that does not bind DNA (7–10). The implication is that an antigen that is not chromatin or DNA itself triggers the activation of the B-cell. Similar studies, performed over decades, analyzing anti-DNA antibodies from patients with SLE have yielded similar observations. Efforts to identify one or more triggering antigen have shown that anti-DNA antibodies often cross-react with bacterial antigen (11–14). Thus, anti-DNA antibodies may arise by a failure to regulate B cells that acquire autoreactivity by somatic mutation during the response to microbial antigen.
Renal disease is present in 50% of patients with SLE (15). The pathogenesis of the renal injury appears to begin with immune complex-mediated glomerulonephritis, although a substantial subset of patients with kidney disease also has interstitial inflammation (16). Numerous studies of immunoglobulin sequestered in kidneys of patients and lupus-prone mice have shown DNA reactivity to be present. Chromatin can bind to glomerular basement membrane providing antigen for the deposition of anti-DNA antibodies (17). However, anti-DNA antibodies often bind ex vivo to glomeruli that have been treated with DNase; thus it has become clear that at least some anti-DNA antibodies bind to non-DNA, non-chromatin antigen in the kidney (18–20). Many studies have identified renal antigens that can be bound by anti-DNA antibodies, including laminin, heparan, or α actinin (21, 22). These studies showed that anti-DNA antibodies not only cross-react with microbial antigen (23–26) but also with non-nucleic acid self-antigen (27–29). As it will become important below, these studies more generally demonstrate that antibodies often display physiologically significant cross-reactivities. Antibodies can be elicited by a particular antigen and bind one or more structurally related self-antigens.
Probing the specificity of R4A
Our interest in autoantigenic cross-reactivity of anti-DNA antibodies arose from a structure: function analysis of a mouse monoclonal, glomerulotropic anti-DNA antibody (30). Mutation of three amino acids in the heavy chain variable region of the R4A antibody generated an antibody with a 10-fold higher apparent affinity for DNA. Surprisingly, unlike R4A itself, this antibody no longer deposited in glomeruli when injected into severe combined immunodeficient mice (20). The implication of this observation was that the parental R4A antibody was not binding DNA in the kidney, but rather a cross-reactive antigen. We therefore probed a decapeptide library for R4A binding and identified a consensus sequence D/E W D/E Y S/G within several decapeptides bound by the antibody. An inhibition enzyme-linked immunosorbent assay (ELISA) confirmed that the peptide, composed of either L or D amino acids, was bound by the R4A antibody (31).
Analysis of serum from NZB/W mice showed that approximately 60% of the DNA reactivity was peptide inhibitable, demonstrating this cross-reactivity to be frequent among murine anti-DNA antibodies (32, 33). A study of SLE patients with anti-DNA antibodies and renal disease showed that essentially all had some proportion, from 15% to 90%, of DNA reactivity that was peptide inhibitable, demonstrating this cross-reactivity to be reasonably common in SLE patients also. Subsequent studies have shown that about 40% of SLE patients have anti-DWEYS peptide antibodies. These antibodies are rarely present in the absence of anti-DNA antibodies and are present in about half of SLE patients with anti-DNA antibodies (34–36). Thus, the antibody specificity appeared to be sufficiently prominent to warrant further study.
A search of protein databases revealed the consensus peptide to be present in the NR2A and NR2B subunits of mouse, rat, and human N-methyl-D-aspartate receptor (NMDAR). ELISAs performed on the extracellular domains of NR2A and NR2B showed that the R4A antibody did indeed bind these antigens in a dose-dependent fashion (37, 38) (Fig. 1).
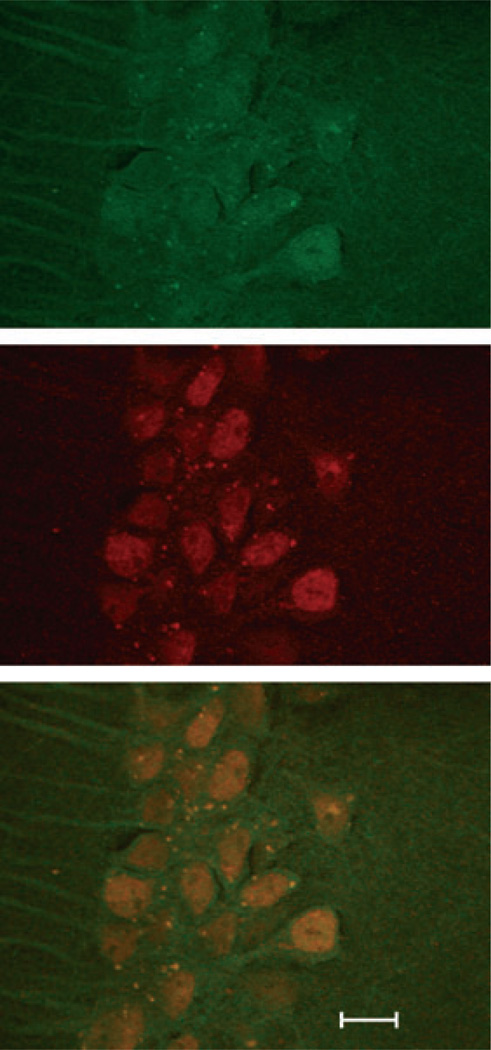
Fig. 1. R4A co-localizes in CA1 pyramidal neurons and their dendrites with anti-N-methyl-D-aspartate antibody.
Demonstrated in the merged figure on the bottom. R4A was visualized (in the top panel) with Alex 488 fluor (Invitrogen, Grand Island, NY, USA), and antibody to NR2A and 2B (Millipore, Billerica, MA, USA) was visualized with an Alexa 594 fluor (in the middle panel). Scale bar is 10 µm.
Mechanisms of tissue damage in SLE
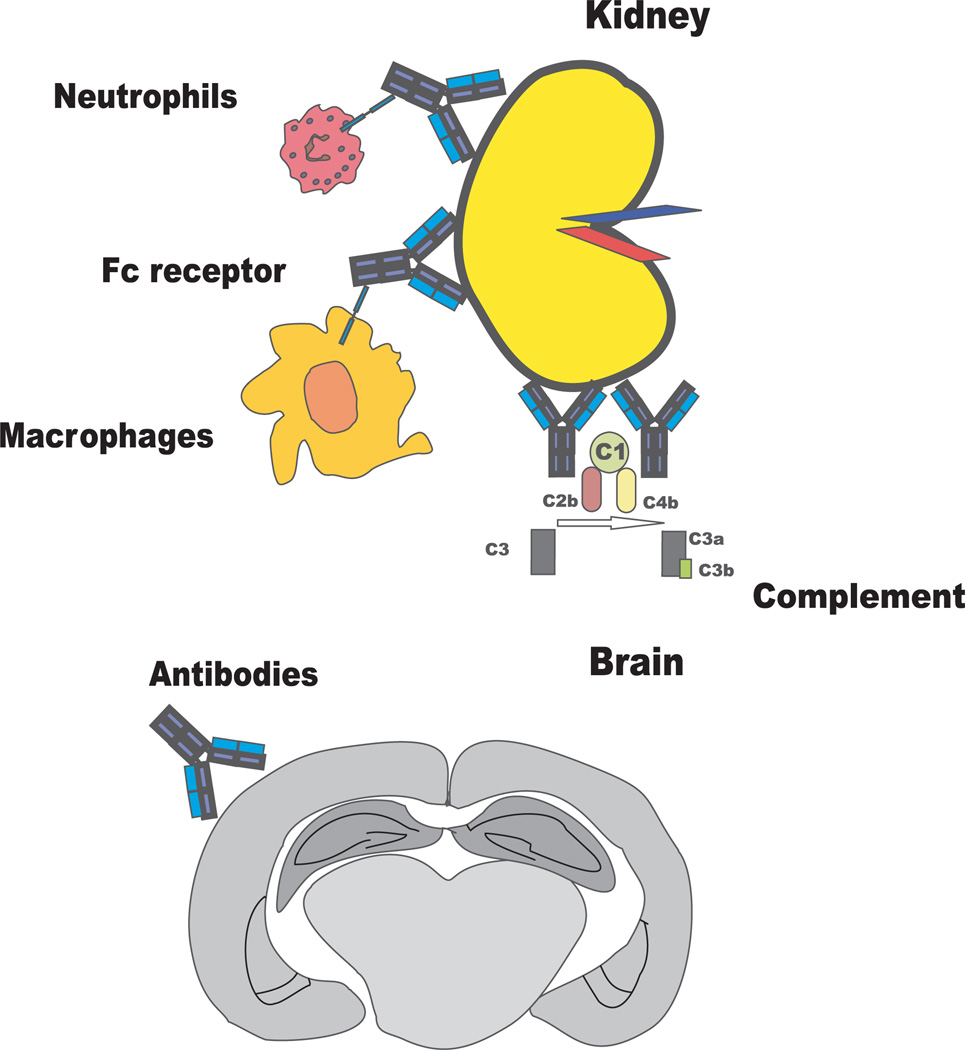
SLE can affect every organ in the body, but preferentially affects kidneys and skin. In both of these organs, it has been shown that immune complexes engage complement and activating Fc receptors and initiate an inflammatory cascade. In the skin, antibody and complement deposition can occur in both affected and apparently unaffected skin (39); thus complement binding alone does not trigger skin inflammation. In the kidney, antibody and complement deposition most commonly initiate an inflammatory response and subsequent tissue injury (40). Activation of the complement cascade and engagement of Fc receptors on Fc receptor-bearing cells amplifies tissue inflammation and ultimately leads to tissue damage. Immune complexes appear to be involved in all solid organ inflammation in SLE. Immune complexes containing nucleic acid, either DNA or RNA, are perhaps particularly pathogenic because they can be internalized by Fc receptor engagement and then activate Toll-like receptor 9 (TLR9) (DNA) or TLR7 (RNA) to activate inflammatory pathways in multiple cell types within tissues and to enhance antigen presentation by dendritic cells (41, 42), as well as contributing, through the same pathway, to systemic inflammation and accelerated atherosclerosis (43, 44).
Solid organ injury in SLE therefore is mediated by immune complexes. In contrast, some other manifestations of disease are mediated directly by antibody (Fig. 2). Hematologic cytopenias are mediated through cellular opsonization and destruction (45, 46). Thrombosis may be mediated by direct activation of the clotting cascade through antibody binding to β2-glycoprotein I (47, 48) or by direct endothelial cell activation mediated by anti-cardiolipin or anti-phospholipid antibodies (49, 50). The ensuing induction of inflammatory and vasoconstrictive mediators increases the possibility of thrombosis, which is particularly dangerous in the heart and the brain (51, 52).
Fig. 2. Kidney tissue injury involves antibody engagement of complement or Fc receptor-bearing cells; brain injury is mediated directly by antibody binding.
SLE causes a number of neuropsychiatric syndromes (53). Some occur due to pathology in the blood vessels. Vascular disease, at times with inflammation of the perivascular space, is often associated with accelerated atherosclerosis or with thrombosis (54, 55). Antibodies interacting with platelets or clotting factors contribute to local brain ischemia (56–58). Some \microinfarcts may be secondary to non-deformability of red blood cells coated with immune complexes (59). Other microinfarcts are secondary to thrombotic events that originate in the vasculature and are largely caused by anti-cardiolipin/anti-phospholipid antibodies, as described above. Peripheral neurologic syndromes may be caused, rarely, by vasculitis. More commonly they results from antibodies to glycolipids, myelin-associated proteins, or nicotinic ganglionic acetylcholine receptor leading to an inflammatory demyelinating polyneuropathy (60, 61).
Central nervous system manifestations of SLE have been recorded in extensive multi-center studies (62), but the mechanism for these are largely unexplained. There are multiple central nervous system manifestations of disease, but the three most common are cognitive impairment, mood disturbance, and headache (62). The cognitive impairment is most commonly memory impairment (63). Among the explanations for central nervous system disease that have been considered are medications. Patients with SLE are often treated with corticosteroids to suppress inflammation. Chronic steroid use carries a slight increased risk of stroke (64). There are compelling data supporting a role for steroids in neuropsychiatric symptoms. There are no convincing data to relate steroids to irreversible cognitive decline or mood disorder. The longitudinal studies to relate steroid use to persistent cognitive decline were based on mixed model analyses and a causal connection was not established (63, 65). The correlation between steroid use and emergent psychiatric symptoms, mania, depression, and delirium, and, less commonly, hallucinations, anxiety, and panic disorder is dose related, and symptoms abate with decreasing steroid dose (66). Thus, these symptoms are far more commonly ascribed to the disease itself.
The toxic side effects associated with steroid treatment, especially the increased risk of infection (67), have led to increased use of cytotoxic drugs that permit steroid taper (65). SLE patients often receive cytotoxic medications to eradicate autoreactive lymphocytes. These medications have been implicated in intellectual impairment when used to treat various malignancies. The doses of these medications used in SLE, however, are less than the doses used in patients with malignancy. Moreover, the clinical data suggest no relationship between brain disease in SLE, especially fixed cognitive impairment, and disease severity necessitating the use of cytotoxic medications.
SLE is characterized by high levels of a variety of cytokines in the circulation (68). There are data in both rodents and humans that high levels of cytokines can affect central nervous system function and lead to constitutional symptoms, such as fever, fatigue, and anorexia (69, 70). The clinical studies in SLE suggest that central nervous system disease may progress in the absence of these symptoms, and no studies have reproducibly associated high serum cytokine levels with central nervous system lupus (53).
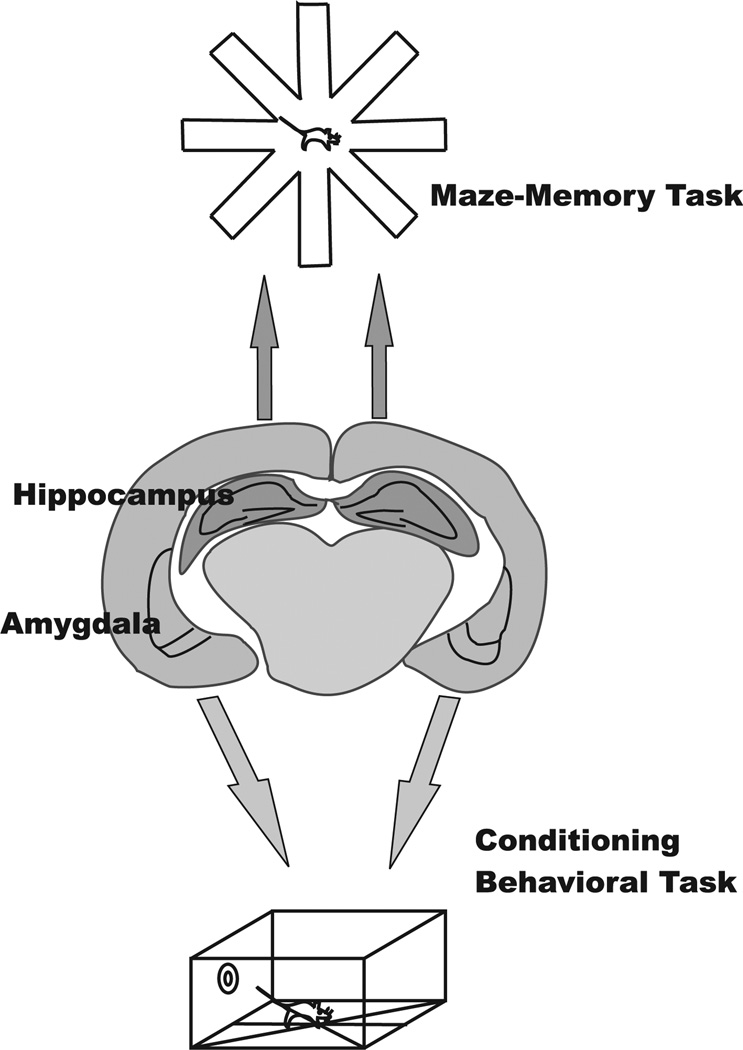
While both cytokines and medications may yet be found to contribute to neuropsychiatric lupus other explanations must be sought. In SLE, it would be difficult to consider pathogenesis of tissue injury without considering a potential role of antibody. Thus, the observation that some anti-DNA antibodies might cross-react with the N-methyl-D-aspartate receptor (NMDAR) on neurons was of great interest. NMDARs are composed of 2NR1 subunits and two of any of 4 NR2 subunits (A–D) (71). The NMDAR pore permits influx of calcium on ligand binding initiating a downstream signaling cascade. Indeed, our interest in potential antibody reactivity with NMDARs was enhanced because NMDARs expressed on neurons in the hippocampus are known to be critical in learning and memory (72). NMDARs expressed on neurons in the amygdala, in contrast, are known to modulate behavior in fear conditioning paradigms in rodents. Thus, the potential binding of antibody to NMDARs might provide insight into two common damages of neuropsychiatric lupus, memory impairment and mood disturbance.
Anti-DNA, anti-peptide antibodies bind NMDARs on neurons
To determine whether anti-DNA, anti-peptide cross-reactive antibodies actually bind the NMDARs on the neuronal surface, we first used the R4A monoclonal antibody to immunoprecipitate intact NMDAR from differentiated PC 12 cells and from brain lysates (37, 38). We next asked whether the R4A antibody would bind to and affect NMDARs in live brain tissue. We injected R4A into the hippocampus of a living mouse, as NR2A and NR2B containing NMDARs are highly expressed on hippocampal neurons (38). The antibody-mediated neuronal cell death in the hippocampus suggesting that it was functioning to amplify receptor function; overstimulation of the NMDAR with its natural ligand causes a form of cell death termed excitotoxic or glutamatergic (73). Human antibodies derived from serum of SLE patients and isolated on a peptide affinity column also caused neuronal death when injected into mouse hippocampus (37). Prior treatment of the mouse with the NMDAR antagonist, MK801, blocked neuronal death, confirming that the antibodies were activating the NMDAR pathway (Fig. 3). Thus, these antibodies are clearly cross-reactive with the NMDAR and are hereafter called anti-NMDAR antibodies. Moreover, Fab12 fragments of the antibody, lacking the Fc portion of the antibody molecule and therefore unable to activate Fc receptors or the complement cascade, also mediated neuronal death when injected into mouse hippocampus. Thus, the anti-DNA, anti-NMDAR antibodies did not cause tissue injury in the brain through immune complex formation as in other tissues, but rather through direct activation of a cell signaling pathway.
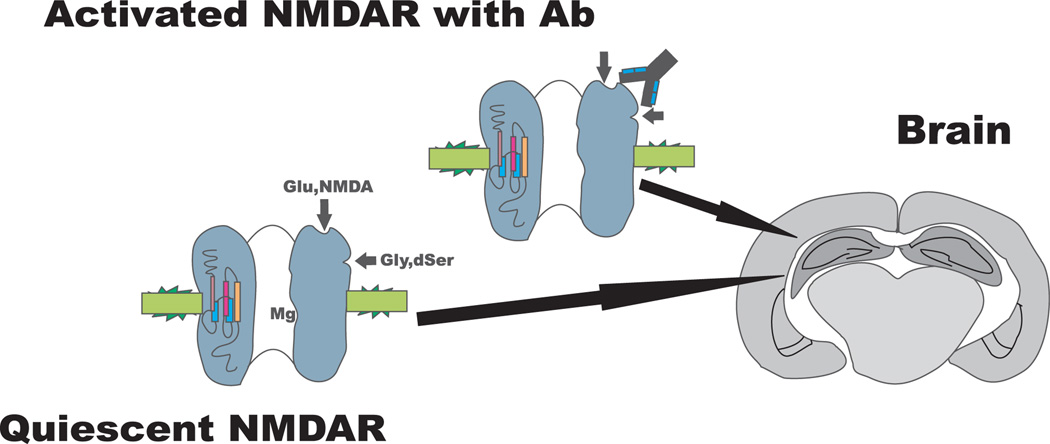
Fig. 3. Systemic lupus erythematosus anti-N-methyl-D-aspartate receptor (NMDAR) antibodies bind activated NMDA receptor.
Magnesium (Mg) occupies the pore of the quiescent NMDA receptor.
To confirm the effects of the antibody on hippocampal neurons and to understand their potential impact on memory function, we explored the effect of antibody in the ex vivo hippocampal slice. We asked whether the R4A antibody could enhance excitatory postsynaptic potentials (EPSPs). The antibody increased EPSPs in a dose-dependent fashion (74). Interestingly, and to our initial surprise, there was no effect of antibody in brain slices not treated with an NMDAR agonist. This demonstrated that the antibody did not function strictly as a pharmacologic agonist; rather it appeared to enhance the activity of a pharmacologic agonist. We reasoned that antibody might preferentially bind the active configuration of the receptor. If so, antibody would augment ligand activity, but would not lead to an activation of the resting NMDAR. We confirmed experimentally the preferential binding of R4A to the active NMDAR (74). Thus, the antibody modulates only the already activated NMDARs, and quiescent NMDARs are unaffected by the presence of the antibody.
Correlation of antibody titer with central nervous system manifestations of SLE
The next key question was whether we could relate antibody titer to brain dysfunction. Several studies have been performed by several groups of investigators examining serum titers of anti-NMDAR antibodies. Some studies suggested an association of high serum titers with memory impairment or depression, whereas other studies failed to confirm these associations (75–79). Studies were also performed looking for a correlation of antibody titers within the cerebrospinal fluid (CSF) with central nervous system symptoms (80, 81). These studies examined CSF of patients obtained during an acute episode marked by a change in alertness, cognitive function or mood in contrast to the studies of serum titers. Studies of serum titer, in contrast, assessed accumulated damage in clinically quiescent patients rather than focusing on patients experiencing an acute change in neurologic status. All studies of CSF titers of anti-NMDAR antibody have demonstrated a correlation with diffuse non-focal injury (81–86). Moreover, we were able to elute anti-NMDAR antibody from the brain of a patient with brain disease as a terminal event (37). These studies demonstrated a link between antibody in the brain and brain dysfunction and confirmed the importance of the blood-brain barrier in protecting the brain from routine exposure to circulating antibody.
A model for antibody penetration of brain
When BALB/c mice are immunized with an octameric form of a peptide (DWEYS) containing the pentapeptide NMDAR consensus sequence, they develop anti-peptide, anti-DNA cross-reactive antibodies. This response is T-cell dependent and requires the presence of the histocompatibility complex (MHC) class II Ed molecule (87). The titers of anti-DNA antibody in these mice are comparable with those in NZB/W lupus-prone mice. The brains of immunized BALB/c mice lack detectable pathology, and the mice performed comparably to controls in behavioral and cognitive tasks, suggesting in mice as in patients that antibody in the circulation is not noxious to the brain unless it penetrates the brain parenchyma through the blood-brain barrier.
There are many insults to the integrity of the blood-brain barrier (53, 88). Blood-brain barrier compromise has long been demonstrated in infection. More recent studies have shown that stress, epinephrine in particular, can abrogate barrier integrity (89–91). Hypertension, smoking, and various drugs have been reported also to compromise barrier integrity. We decided to explore the effect of infection and stress in the peptide-induced model of lupus serology. We chose to study infection as an insult to barrier integrity using lipopolysaccharide (LPS) administration to mimic the effects of infection. SLE patients experience an increased frequency of infection both because of the immunosuppression intrinsic to the disease and the immunosuppression caused by the medications used to treat the disease-specific autoreactivity and inflammation. When peptide-immunized mice were given LPS by intraperitoneal injection, there was evidence of antibody penetration into the brain with specific binding of antibody to hippocampal neurons. The mice exhibited a loss of hippocampal neurons (92). There was evidence of loss by 48 h, and no increased loss was observed thereafter. Most interestingly, there was no evidence of inflammation, no infiltration of blood cells into the brain, and no evidence of prolonged activation of glial cells. When these mice were subjected to a battery of behavioral and cognitive assessments, they displayed persistent memory impairment (92). To confirm that antibodies from SLE serum had the same ability to cause impaired memory function, we administered serum containing anti-DNA, anti-NMDAR from SLE patients to naive mice, and subsequently administered LPS. The human antibodies bound preferentially to hippocampal neurons, and the mice displayed memory impairment. If the serum was first depleted of anti-NMDAR antibodies on a peptide affinity column and then given to mice, followed by LPS, there was no specific binding to hippocampal neurons and no memory impairment (92). These studies provided a model for the persistent memory impairment experienced by patients with SLE. Indeed, these studies provided the first mechanistic model for any aspect of central nervous system SLE that is not a consequence of thrombosis.
Many SLE patients experience a transient impairment in cognitive function. The model we described involved a fixed impairment with neuronal death. We therefore explored the antibody concentration required to alter excitatory postsynaptic potentials in the hippocampal slice preparation and the antibody concentration required to mediate cell death (74). Although as little as 10 µg/ml could enhance synaptic potentials, 100 µg/ml was needed for neuronal death. Thus, lower concentrations of antibody might cause acute reversible symptomatology, whereas higher concentrations might lead to irreversible damage through neuronal death. When we determined antibody concentrations within the CSF of patients with acute neuropsychiatric lupus, we found that there was a broad spectrum from 10 to 300 µg/ml of specific anti-NMDAR antibody (74, 81). This spectrum would allow for both reversible and irreversible injury.
In an effort to confirm the antibody-mediated neuronal loss that followed abrogation of blood-brain barrier integrity by exposure to LPS, we treated peptide-immunized mice with epinephrine. These mice displayed a normal hippocampus and normal memory function. In contrast to LPS treated peptide-immunized mice, they displayed neuronal loss in the amygdala and impairment in a fear conditioning paradigm (93). This study demonstrated that neurons in both the amygdala and the hippocampus were vulnerable to antibody-mediated cell death and that both cognitive and behavioral impairments might be secondary to antibody-induced damage. Moreover, they demonstrated for the first time that insults to the blood-brain barrier have regional effects with LPS, compromising barrier integrity in the hippocampus and epinephrine in the amygdala (Fig. 4).
Fig. 4. Performance impairments depend on the brain regions exposed to antibody.
Hippocampal exposure leads to isolated memory performance impairments on maze tasks. Amygdala exposure leads to isolated behavioral impairments on conditioning tasks.
This model fulfilled several conditions of the human disease. The model requires two hits; the first is neurotoxic antibodies and the second an insult to blood-brain barrier integrity. The compromise to barrier integrity is not mediated by the disease itself, but by a disease-independent insult, such as infection or stress. Neuropsychiatric SLE also appears to require two hits, as the presence of memory or mood impairment is unrelated to concomitant disease activity or indeed to cumulative disease activity. Furthermore, the neuronal loss appears to be non-inflammatory. Most SLE patients with documented cognitive or behavioral impairment have never had a clinical episode of brain inflammation. Thus, the model reflects key aspects of the clinical disease.
Examining brains of SLE patients for damage to the amygdala and hippocampus
We examined brain function in SLE patients by functional magnetic resonance imaging (MRI), using paradigms to activate the hippocampus or the amygdala (94). We studied patients with less than 2 years disease duration and patients with more than 10 years disease duration. We used the Sternberg test, which assesses memory for shapes and depends on hippocampal function (95). In brief, there is an initial phase when the individual sees 1, 2, or 3 objects on a screen. The screen goes dark during a consolidation phase. One figure, either previously displayed or a novel figure, is then shown to the subject in the recall phase of the experiment. Patients with disease duration of less than 2 years showed little brain activity during the consolidation phase and increased blood flow not only in the hippocampus but also in the cingulate gyrus, prefrontal, and somatosensory cortex during the recall phase, a pattern associated with neurodegenerative disease (96). In contrast, patients with long disease duration exhibited diffuse activity during the consolidation phase and no specific hippocampal activity during the recall phase, a pattern likely associated with more extensive brain injury.
The test of amygdala function employs a fearful faces paradigm. When a fearful face is shown to an individual, there will be increased blood flow in the amygdala. If an individual is shown the fearful face very briefly, a subliminal exposure, followed by a neutral face, there will be increased activity in the amygdala in some individuals who are sensitive to the fearful face but not in all individuals (97). Patients with <2 years of disease behaved like a normal cohort with most responding to the subliminal exposure to the fearful face. Fifty percent of patients with more than 10 years of disease did not respond to the subliminal exposure. Thus, the functional MRI assessments showed that SLE patients accumulate damage to the hippocampus and the amygdala over time. One team of investigators showed that damage to the amygdala assessed by a volumetric analysis correlated with the presence of elevated serum titers of anti-NMDAR antibodies (98). Our study also showed that abnormal brain function did not correlate with lupus damage assessed by the SLICC damage index which measures accumulated, fixed damage in organs throughout the body. The discrepancy between the SLICC damage index and measures of damage in the brain can be explained by the blood-brain barrier, which protects the brain from antibody-mediated disease. Thus, the brain is protected while antibodies are destroying other tissues. Damage accrual in most organs therefore proceeds at a different rate from damage accrual in the brain; some non-SLE dependent insults to the blood-brain barrier must occur to initiate damage or at least antibody-mediated damage in the brain.
Recent studies of the CSF of SLE patients have shown increased interleukin-6 (IL-6) during active diffuse disease of the central nervous system (99–101). Another recent study has shown that the antibodies present in the CSF can bind apoptotic debris, and the resulting immune complexes can activate TLRs in dendritic cells (102). Thus, it is possible that anti-DNA antibodies, including those cross-reactive with NMDAR, activate myeloid or glial cells in the brain and induce local cytokine production, especially IL-6.
Antibody-mediated damage to the fetal brain
It has been reported in several studies, each relatively small, that the children of mothers with lupus display an increased incidence of learning disabilities (103–106). As this increased incidence is apparently not present in the children of men with lupus, we reasoned it reflected some aspect of the in utero environment.
Maternal antibody can cross the placenta after the first trimester of pregnancy. It is not known exactly when the blood-brain barrier forms, but it appears to be somewhat porous to immunoglobulin until almost to the end of pregnancy. Thus, throughout the second and much of the third trimester of pregnancy, maternal antibody can penetrate brain tissue. Our studies show that 50–70 times more immunoglobulin per gram of tissue is present in fetal than maternal brain. It is important to note, however, that even the porous blood-brain barrier restricts antibody access to brain, and more immunoglobulin is present in other fetal organs than in the brain.
On the basis of the information we had obtained in studies of antibody-mediated brain disease in adult mice, we asked whether maternal anti-NMDAR antibody might alter fetal brain development (107). BALB/c female mice were immunized with octameric peptide or irrelevant antigen. When antibody levels were high, the females were mated to male mice. Thus, we could examine mice that developed in utero in the presence or absence of anti-NMDAR antibodies. Those mice exposed in utero to lupus-like antibody displayed abnormal brain histology with a thin cortical plate, increased apoptosis, and increased numbers of mitotic cells during fetal development. Moreover, the mitotic cells were found not in the usual location, at the subventricular zone, but throughout the developing cortex. These mice displayed a delayed acquisition of neonatal reflexes. Once they became adults, they performed normally on a standard neurological battery (108–111) and a number of standardized cognitive and behavioral tasks (37, 74, 92, 93, 107). They did, however, exhibit significant impairment on three tasks that depend on cortical function. The first task was a novel object recognition that depends on the function of the rhinal and perirhinal cortex (112). The second task was a spatial recognition task that depends on the parietal cortex (113). The third task required intact fear conditioning, but focused on the extinction of the conditioned response that depends on the infra-limbic areas of the prefrontal cortex (114). The neuropathological characterization showed abnormal cortical thickness and organization present in the adult brain of mice exposed in utero to anti-NMDAR antibodies. These impairments cannot be termed learning disorders, as there is no murine correlate of a learning disability, but they are restricted performance deficits that reflect murine cortical dysfunction and in that way they are comparable to learning disorders. It will be important to study prospectively whether female SLE patients with anti-NMDAR antibodies are more likely to have a child with a learning disorder.
A peptide therapeutic
Current therapies for SLE involve immunosuppression. Indeed, approximately one-third of SLE patients die of infection, often secondary to immunosuppressive therapy. We reasoned that a decoy antigen would prevent antibody-mediated tissue damage and would not be immunosuppressive (80). As the anti-NMDAR antibody binds to the d-peptide as well as the l-peptide, we could use the D-peptide as a decoy antigen as it has a longer half-life than the l-peptide. D-DWEYS is not vulnerable to serum proteases and has a half-life of 6–8 h. We therefore tested whether it could protect kidneys from binding by the monoclonal R4A antibody in vivo. The d-peptide, but not the l-peptide which is immediately degraded in vivo, prevents tissue binding by R4A. The peptide was also able to protect neurons from antibody-mediated damage when given systemically to peptide-immunized mice, prior to epinephrine administration (115). We have demonstrated that the peptide can cross the blood-brain barrier, but it may also serve as a decoy antigen to protect neurons by binding antibody in the circulation, prior to penetration of brain tissue. While other investigators have considered the use of an NMDAR antagonist as a therapeutic strategy in neuropsychiatric lupus (116), the incapacitation of the NMDAR chronically over years will clearly have a significant impact on brain function. Thus, the strategy of a decoy antigen seems preferable. It is also of importance that the d-peptide is not immunogenic in mice, perhaps because a 5 amino acid peptide is too small to fit snugly in the peptide binding groove of a major MHC class II molecule. Even mice given prolonged treatment with peptide delivered by intraperitoneal injection, followed by a rest and then re-exposed to peptide, a regimen designed to induce an immune response, failed to generate detectable antibody titers to peptide.
A peptide mimetope as a therapeutic agent
While the therapeutic efficacy of D-DWEYS in protecting kidney and brain from antibody-mediated injury confirms the contribution of antibody to tissue injury and provides proof of principle for the therapeutic use of a decoy antigen, the D-DWEYS peptide, like most peptides, cannot be given orally. While individuals do self-inject daily or even more frequently in the case of some therapeutic agents, such as insulin, it may be difficult for patients to self-inject peptide 2 or 3 times daily. We therefore synthesized a small molecule mimetope of the DWEYS peptide, FISLE 412 (117). This molecule is orally absorbed and, like the d-peptide, will inhibit a significant percent of DNA reactivity in multiple lupus sera. It can prevent both R4A, the mouse monoclonal anti-DNA, anti-NMDAR antibody and G11, a human monoclonal antibody with the same cross-reactivity and cloned from a peripheral blood B-cell of a lupus patient, from binding glomeruli ex vivo or from causing neuronal death in vivo. FISLE 412 is an attractive therapeutic not only because it can be taken orally but also because it binds even more avidly to R4A and G11 than DWEYS, suggesting that it may have a competitive advantage over tissue antigen despite being monomeric.
It should now be possible to perform clinical trials to protect the brain from antibody-mediated damage and maintain cognitive function and mood stability. The remaining obstacle is to identify a metric for neuroprotection. Neuropsychiatric testing does not exhibit fixed and reproducible changes in lupus patients over a 6-month period of time, a reasonable time frame for a clinical trial. Moreover, fatigue, motivational status, steroid use may all influence test results. Similarly, functional MRI cannot be used to assess neuroprotection for all the same reasons. It is critical to identify an objective parameter of brain function that changes in many patients over a period of 6 months and is not affected by current steroid use or by aspects of performance that may change day to day.
Implications for brain disease: antigen targets
These studies have implications for brain disease. They demonstrate that autoantibodies that bind to a target in the brain and a cross-reactive target in another organ may mediate damage in each organ through different effector mechanisms. Anti-DNA, anti-NMDAR antibodies activate inflammatory cascades in the kidney; in the brain, they function as modulators of NMDAR activation. There is no evidence that they induce inflammation in the brain.
These studies also remind us that antibodies can bind a cellular receptor or other protein in one configuration and not another. The anti-NMDAR antibodies we study, preferentially bind the active configuration of the NMDAR. This means that in vivo, the antibodies react only with activated neurons. In vitro, antibody binding may be absent in some assays; for example, lupus-like anti-NMDAR antibodies will not bind to quiescent cells. Many brain-reactive autoantibodies bind neurotransmitter receptors or channel proteins. Whether these antibodies recognize epitopes that are unchanged by neuronal activation state is not currently known.
Implications for brain disease: the critical role of the blood-brain barrier
These studies emphasize the importance of the blood-brain barrier in limiting or permitting antibody access to brain tissue. An intact blood-brain barrier renders the antibodies harmless, as little antibody penetrates the central nervous system on a routine basis. It is important to remember, however, that if the antibody binds a target that is also present on peripheral nerves, the brain may be spared while the antibodies destroy or impair function of peripheral targets.
These studies have serendipitously shown that the compromise of barrier integrity is regional. An understanding of insults to barrier function is still in its infancy. Nevertheless, these studies provide precedent for broad, even ubiquitous expression of target neuronal antigen, and yet regional specificity of antibody-mediated disease. This is an important caveat in studies of central nervous system autoimmunity. While the antibody may bind throughout the brain, toxicity in vivo may be local and depend on the nature of the compromise of barrier integrity.
The importance of the blood-brain barrier in defending against autoantibody-mediated brain damage highlights the transient vulnerability of the fetal brain. The fetal brain is exposed during the second and third trimester of gestation to maternal antibody. Thus, the mother may show no untoward effect of harboring brain-reactive antibody in her circulation as she may have an intact blood-brain barrier, but her fetus may experience transient or permanent alterations in brain function. Importantly, the function of surface molecules on neurons many change over the lifespan. NMDARs in the developing brain have been shown to regulate neuronal migration; this is not a function that persists into adulthood. The implications of this observation are that the cellular alterations mediated by antibody in fetal brain may differ from those mediated by the same antibody in adult brain. How commonly a disparity in molecular function exists between fetal and adult brain is not known.
The blood-brain barrier not only limits access of molecules in the circulation to the brain, it also limits access of brain antigens to the systemic immune system. The B-cell repertoire is incredibly diverse, designed to protect the organism from a vast world of microbial antigens. The diversity of the repertoire is limited to a B-cell subset termed follicular B cells. B1 cells and marginal zone B cells have a relatively limited repertoire of antigen receptors. Moreover, they undergo limited mutation of their immunoglobulin genes, following antigen exposure. Follicular B cells, in contrast, have a much more diverse repertoire of antigen receptors and undergo a germinal center reaction on antigen activation that involves extensive mutation of their immunoglobulin genes, further diversifying the repertoire. As B cells develop, much autoreactivity is generated. Some studies show as many as 70% of immature B cells are autoreactive (118). Exposure to antigen at an immature stage tolerizes the B cells and effectively eliminates the autospecificity from the repertoire of mature, immunocompetent B cells. Obviously, tolerance is most effective to antigens that are present in the periphery at sufficiently high concentration to extinguish low as well as high affinity autoreactivity. The generation of follicular B cells begins after the blood-brain barrier is formed. Thus, there may be many brain antigens that are not present outside the brain at high enough concentration to tolerize B cells. Brain-reactive B cells may be present in the immunocompetent naive B-cell repertoire. We speculate that during the routine exposures to microbial challenge, B cells that recognize microbial antigen and cross-react with brain antigen are generated. In contrast, B cells that recognize microbial antigen and cross-react, for example, with liver or kidney antigen will have been tolerized. Thus, brain-reactive antibodies may routinely arise during the protective response to injection. Identifying these antibodies and determining whether they are potentially pathogenic requires further investigation.
There are a growing number of anti-brain antibodies in SLE and a growing number of diseases in which anti-neuronal antibodies contribute to symptoms. These include antibodies that engage the peripheral nervous system, the spinal cord, and the central nervous system (119). In most, it is not yet understood why the antibodies arise. In others, such as celiac disease, it appears the antibodies are triggered by a cross-reactive antigen (transglutaminase). In rheumatic fever, the cross-reactive antigen is clearly bacterial with antibodies to bacterial N acetyl glucosamine cross-reacting with the dopamine receptor and triggering a movement disorder (120).
We do not know how many microbial antigens elicit anti-brain cross-reactivity, or how often such antibodies can traverse the blood-brain barrier. We would suggest, however, that many acquired changes in cognition behavior or motor function may reflect antibody-mediated damage. In parallel, many congenital, non-genetic alterations in fetal brain development may be antibody-mediated (121). The role of antibodies in human pathobiology may be far greater than currently appreciated. The model of preventing antibody-mediated brain injury through the use of a decoy antigen suggests that as brain-reactive antibodies are identified, a therapeutic strategy is readily available.
Summary
Antibodies can mediate both cognitive and behavioral alterations in adults by binding to neuronal antigens. In SLE, some anti-DNA antibodies cross-react with the NMDAR to mediate insults to brain function that can be either transient or permanent. However, in the adult brain, anti-brain antibodies alone do not cause disease. There must also be an insult to blood-brain barrier integrity to provide the antibody access to brain tissue. Insults to barrier integrity display regional specificity, thereby accounting for the specificity of histologic damage and the nature of the cognitive or behavioral impairment. Antibodies can likewise alter fetal brain development. Maternal antibody has access to fetal brain tissue during the second and third trimesters of gestation.
The spectrum of antibody-mediated brain disease is probably greater than we currently appreciate. While identifying brain-reactive antibodies and relating their presence to clinical symptomatology is complex, the road from identification to therapy is relatively straightforward, making the effort immensely worthwhile.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Andrews BS, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon FJ, Andrews BS, Eisenberg RA, McConahey PJ, Theofilopoulos AN, Wilson CB. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum. 1978;21:S64–S67. doi: 10.1002/art.1780210909. [DOI] [PubMed] [Google Scholar]
- 3.Howie JB, Helyer BJ. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- 4.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotech. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talal N, Steinberg AD. The pathogenesis of autoimmunity in New Zealand black mice. Curr Top Microbiol Immunol. 1974;64:79–103. doi: 10.1007/978-3-642-65848-8_3. [DOI] [PubMed] [Google Scholar]
- 6.Diamond B, Scharff MD. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci USA. 1984;81:5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manheimer-Lory AJ, Davidson A, Watkins D, Hannigan NR, Diamond BA. Generation and analysis of clonal IgM- and IgG-producing human B cell lines expressing an anti-DNA-associated idiotype. J Clin Invest. 1991;87:1519–1525. doi: 10.1172/JCI115162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mietzner B, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler TH, Fehr H, Kalden JR. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol. 1992;22:1719–1728. doi: 10.1002/eji.1830220709. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Jacobi AM, Wang T, Diamond B. Pathogenic autoantibodies in systemic lupus erythematosus are derived from both self-reactive and non-self-reactive B cells. Mol Med. 2008;14:675–681. doi: 10.2119/2008-00066.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behar SM, et al. Impact of somatic mutation on the S107(T15) heavy-chain V region of antibodies reactive with self and nonself. Cold Spring Harb Symp Quant Biol. 1989;54:921–931. doi: 10.1101/sqb.1989.054.01.107. [DOI] [PubMed] [Google Scholar]
- 12.Behar SM, Corbet S, Diamond B, Scharff MD. The molecular origin of anti-DNA antibodies. Int Rev Immunol. 1989;5:23–42. doi: 10.3109/08830188909086988. [DOI] [PubMed] [Google Scholar]
- 13.Chien N, et al. Effect of somatic mutation on antibody affinity and specificity. Mt Sinai J Med. 1986;53:181–186. [PubMed] [Google Scholar]
- 14.Diamond B, Chien N, Scharff M. Somatic mutation alters affinity and specificity. Ann Inst Pasteur Immunol. 1985;136C:267–271. doi: 10.1016/s0769-2625(85)80057-x. [DOI] [PubMed] [Google Scholar]
- 15.Croca SC, Rodrigues T, Isenberg DA. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology. 2011;50:1424–1430. doi: 10.1093/rheumatology/ker101. [DOI] [PubMed] [Google Scholar]
- 16.Wakasugi D, et al. Frequency of class III and IV nephritis in systemic lupus erythematosus without clinical renal involvement: an analysis of predictive measures. J Rheumatol. 2012;39:79–85. doi: 10.3899/jrheum.110532. [DOI] [PubMed] [Google Scholar]
- 17.Dieker JW, van der Vlag J, Berden JH. Triggers for anti-chromatin autoantibody production in SLE. Lupus. 2002;11:856–864. doi: 10.1191/0961203302lu307rr. [DOI] [PubMed] [Google Scholar]
- 18.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genetics. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 19.van der Vlag J, Berden JH. Lupus nephritis: role of antinucleosome autoantibodies. Semin Nephrol. 2011;31:376–389. doi: 10.1016/j.semnephrol.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Katz JB, Limpanasithikul W, Diamond B. Mutational analysis of an autoantibody: differential binding and pathogenicity. J Exp Med. 1994;180:925–932. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanrotel-Saliou C, Segalen I, Le Meur Y, Youinou P, Renaudineau Y. Glomerular antibodies in lupus nephritis. Clin Rev Allergy Immunol. 2011;40:151–158. doi: 10.1007/s12016-010-8204-4. [DOI] [PubMed] [Google Scholar]
- 22.Raats CJ, Van Den Born J, Berden JH. Glomerular heparan sulfate alterations: mechanisms and relevance for proteinuria. Kidney Int. 2000;57:385–400. doi: 10.1046/j.1523-1755.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhry IA, Kowal C, Hardin J, Zhou Z, Diamond B. Autoantibodies that bind glomeruli: cross-reactivity with bacterial antigen. Arthritis Rheum. 2005;52:2403–2410. doi: 10.1002/art.21143. [DOI] [PubMed] [Google Scholar]
- 24.Kowal C, Weinstein A, Diamond B. Molecular mimicry between bacterial and self antigen in a patient with systemic lupus erythematosus. Eur J Immunol. 1999;29:1901–1911. doi: 10.1002/(SICI)1521-4141(199906)29:06<1901::AID-IMMU1901>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci USA. 1996;93:2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A, Isenberg DA, Diamond B. Cross-reactivity of human anti-dsDNA antibodies to phosphorylcholine: clues to their origin. J Autoimmun. 2001;16:479–484. doi: 10.1006/jaut.2001.0514. [DOI] [PubMed] [Google Scholar]
- 27.Briassouli P, Rifkin D, Clancy RM, Buyon JP. Binding of anti-SSA antibodies to apoptotic fetal cardiocytes stimulates urokinase plasminogen activator (uPA)/uPA receptor-dependent activation of TGF-beta and potentiates fibrosis. J Immunol. 2011;187:5392–5401. doi: 10.4049/jimmunol.1101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Defendenti C, et al. Clinical and laboratory aspects of Ro/SSA-52 autoantibodies. Autoimmun Rev. 2011;10:150–154. doi: 10.1016/j.autrev.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Provost TT, Razzaque A, Maddison PJ, Reichlin M. Antibodies to cytoplasmic antigens in lupus erythematosus. Serologic marker for systemic disease. Arthritis Rheum. 1977;20:1457–1463. doi: 10.1002/art.1780200803. [DOI] [PubMed] [Google Scholar]
- 30.Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991;173:287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc Natl Acad Sci USA. 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn BH, Singh RR, Wong WK, Tsao BP, Bulpitt K, Ebling FM. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis Rheum. 2001;44:432–441. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Monneaux F, Muller S. Peptide-based immunotherapy of systemic lupus erythematosus. Autoimmun Rev. 2004;3:16–24. doi: 10.1016/S1568-9972(03)00061-2. [DOI] [PubMed] [Google Scholar]
- 34.Bertsias GK, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010;69:2074–2082. doi: 10.1136/ard.2010.130476. [DOI] [PubMed] [Google Scholar]
- 35.Hanly JG, et al. Autoantibodies as biomarkers for the prediction of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:1726–1732. doi: 10.1136/ard.2010.148502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muscal E, Brey RL. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol Clin. 2010;28:61–73. doi: 10.1016/j.ncl.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kowal C, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci USA. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 39.Alahlafi A, Wordsworth P, Wojnarowska F. Activation/inactivation of the classical pathway of complement in non-lesional skin of patients with systemic lupus erythematosus. J Cutan Pathol. 2005;32:537–540. doi: 10.1111/j.0303-6987.2005.00373.x. [DOI] [PubMed] [Google Scholar]
- 40.Migliorini A, Anders HJ. A novel pathogenetic concept-antiviral immunity in lupus nephritis. Nat Rev Nephrol. 2012;8:183–189. doi: 10.1038/nrneph.2011.197. [DOI] [PubMed] [Google Scholar]
- 41.Munoz LE, et al. Autoimmunity and chronic inflammation - two clearance-related steps in the etiopathogenesis of SLE. Autoimmun Rev. 2010;10:38–42. doi: 10.1016/j.autrev.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Rahman AH, Eisenberg RA. The role of toll-like receptors in systemic lupus erythematosus. Springer Semin Immunopathol. 2006;28:131–143. doi: 10.1007/s00281-006-0034-3. [DOI] [PubMed] [Google Scholar]
- 43.Roman MJ, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3412–3419. doi: 10.1002/art.22924. [DOI] [PubMed] [Google Scholar]
- 44.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE-mechanisms and management. Nat Rev Rheumatol. 2012;8:214–223. doi: 10.1038/nrrheum.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreiber AD, Frank MM. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972;51:575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starkebaum G. Chronic neutropenia associated with autoimmune disease. Semin Hematol. 2002;39:121–127. doi: 10.1053/shem.2002.31918. [DOI] [PubMed] [Google Scholar]
- 47.Harris EN, et al. Anticardiolipin antibodies: detection by radioimmunoassay and association with thrombosis in systemic lupus erythematosus. Lancet. 1983;2:1211–1214. doi: 10.1016/s0140-6736(83)91267-9. [DOI] [PubMed] [Google Scholar]
- 48.Koike T, Sueishi M, Funaki H, Tomioka H, Yoshida S. Anti-phospholipid antibodies and biological false positive serological test for syphilis in patients with systemic lupus erythematosus. Clin Exp Immunol. 1984;56:193–199. [PMC free article] [PubMed] [Google Scholar]
- 49.Merrill JT. Endothelial cell damage and atherosclerosis. In: Lahita RG, editor. Systemic Lupus Erythematosus. 5th edn. Amsterdam, The Netherlands: Elsevier; 2011. pp. 967–983. [Google Scholar]
- 50.Asherson RA, Cervera R, Merrill JT, Erkan D. Antiphospholipid antibodies and the antiphospholipid syndrome: clinical significance and treatment. Semin Thrombosis Hemostasis. 2008;34:256–266. doi: 10.1055/s-0028-1082269. [DOI] [PubMed] [Google Scholar]
- 51.Pierangeli SS, Chen PP, Gonzalez EB. Antiphospholipid antibodies and the antiphospholipid syndrome: an update on treatment and pathogenic mechanisms. Curr Opin Hematol. 2006;13:366–375. doi: 10.1097/01.moh.0000239710.47921.d2. [DOI] [PubMed] [Google Scholar]
- 52.Roldan JF, Brey RL. Neurologic manifestations of the antiphospholipid syndrome. Curr Rheumatol Rep. 2007;9:109–115. doi: 10.1007/s11926-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 53.Mackay M, Ulug AM, Volpe BT. Neuropsychiatric systemic lupus erythematosus: mechanism of injury. In: Lahita RG, editor. Systemic Lupus Erythematosus. 5th edn. Amsterdam, The Netherlands: Elsevier; 2011. pp. 491–511. [Google Scholar]
- 54.Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun. 2007;28:69–75. doi: 10.1016/j.jaut.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Johnson RT, Richardson EP. The neurological manifestations of systemic lupus erythematosus. Medicine. 1968;47:337–369. doi: 10.1097/00005792-196807000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Joseph FG, Scolding NJ. Neurolupus. Practical Neurol. 2010;10:4–15. doi: 10.1136/jnnp.2009.200071. [DOI] [PubMed] [Google Scholar]
- 57.Scolding NJ. Central nervous system vasculitis. Semin Immunopathol. 2009;31:527–536. doi: 10.1007/s00281-009-0183-2. [DOI] [PubMed] [Google Scholar]
- 58.Scolding NJ, Joseph FG. The neuropathology and pathogenesis of systemic lupus erythematosus. Neuropathol Appl Neurobiol. 2002;28:173–189. doi: 10.1046/j.1365-2990.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 59.Ghiran IC, Zeidel ML, Shevkoplyas SS, Burns JM, Tsokos GC, Kyttaris VC. Systemic lupus erythematosus serum deposits C4d on red blood cells, decreases red blood cell membrane deformability, and promotes nitric oxide production. Arthritis Rheum. 2011;63:503–512. doi: 10.1002/art.30143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKeon A, Lennon VA, Lachance DH, Fealey RD, Pittock SJ. Ganglionic acetylcholine receptor autoantibody: oncological, neurological, and serological accompaniments. Arch Neurol. 2009;66:735–741. doi: 10.1001/archneurol.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willison HJ. Biomarkers in experimental models of antibody-mediated neuropathies. J Peripher Nerv Syst. 2011;16(Suppl):60–62. doi: 10.1111/j.1529-8027.2011.00310.x. [DOI] [PubMed] [Google Scholar]
- 62.Hanly JG, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2010;69:529–535. doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLaurin EY, Holliday SL, Williams P, Brey RL. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology. 2005;64:297–303. doi: 10.1212/01.WNL.0000149640.78684.EA. [DOI] [PubMed] [Google Scholar]
- 64.Zonana-Nacach A, Barr SG, Magder LS, Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. 2000;43:1801–1808. doi: 10.1002/1529-0131(200008)43:8<1801::AID-ANR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 65.Ioannou Y, Isenberg DA. Current concepts for the management of systemic lupus erythematosus in adults: a therapeutic challenge. Postgrad Med J. 2002;78:599–606. doi: 10.1136/pmj.78.924.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kenna HA, Poon AW, de los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65:549–560. doi: 10.1111/j.1440-1819.2011.02260.x. [DOI] [PubMed] [Google Scholar]
- 67.Gladman DD, Hussain F, Ibanez D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus. Lupus. 2002;11:234–239. doi: 10.1191/0961203302lu170oa. [DOI] [PubMed] [Google Scholar]
- 68.Apostolidis SA, Lieberman LA, Kis-Toth K, Crispin JC, Tsokos GC. The dysregulation of cytokine networks in systemic lupus erythematosus. J Interferon Cytokine Res. 2011;31:769–779. doi: 10.1089/jir.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cerami A. The value of failure: the discovery of TNF and its natural inhibitor erythropoietin. J Int Med. 2011;269:8–15. doi: 10.1111/j.1365-2796.2010.02319.x. [DOI] [PubMed] [Google Scholar]
- 70.Dinarello CA. Blocking interleukin-1beta in acute and chronic autoinflammatory diseases. J Int Med. 2011;269:16–28. doi: 10.1111/j.1365-2796.2010.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 72.LeDoux JE. Emotion circuits in the brain. Annu Rev Neuorsci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 73.Abramov AY, Duchen MR. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta. 2008;1777:953–964. doi: 10.1016/j.bbabio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Faust TW, et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc Natl Acad Sci USA. 2010;107:18569–18574. doi: 10.1073/pnas.1006980107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanly JG, Robichaud J, Fisk JD. Anti-NR2 glutamate receptor antibodies and cognitive function in systemic lupus erythematosus. J Rheumatol. 2006;33:1553–1558. [PubMed] [Google Scholar]
- 76.Harrison MJ, Ravdin LD, Lockshin MD. Relationship between serum NR2a antibodies and cognitive dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2515–2522. doi: 10.1002/art.22030. [DOI] [PubMed] [Google Scholar]
- 77.Lapteva L, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505–2514. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 78.Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R, Mellgren SI. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol. 2005;12:392–398. doi: 10.1111/j.1468-1331.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 79.Steup-Beekman G, Steens S, van Buchem M, Huizinga T. Anti-NMDA receptor autoantibodies in patients with systemic lupus erythematosus and their first-degree relatives. Lupus. 2007;16:329–334. doi: 10.1177/0961203307078224. [DOI] [PubMed] [Google Scholar]
- 80.Diamond B, Volpe BT. Antibodies and brain disease: a convergence of immunology and physiology. PLoS Med. 2006;3:e498. doi: 10.1371/journal.pmed.0030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fragoso-Loyo H, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS ONE. 2008;3:e3347. doi: 10.1371/journal.pone.0003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2008;58:1130–1135. doi: 10.1002/art.23399. [DOI] [PubMed] [Google Scholar]
- 83.Gono T, et al. Anti-NR2A antibody as a predictor for neuropsychiatric systemic lupus erythematosus. Rheumatol. 2011;50:1578–1585. doi: 10.1093/rheumatology/keq408. [DOI] [PubMed] [Google Scholar]
- 84.Husebye ES, et al. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann Rheum Dis. 2005;64:1210–1213. doi: 10.1136/ard.2004.029280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshio T, Okamoto H, Minota S. Antibodies to bovine serum albumin do not affect the results of enzyme-linked immunosorbent assays for IgG anti-NR2 glutamate receptor antibodies: reply to the letter by Hirohata et al. Arthritis Rheum. 2007;56:2813–2814. doi: 10.1002/art.22827. [DOI] [PubMed] [Google Scholar]
- 86.Yoshio T, Onda K, Nara H, Minota S. Association of IgG anti-NR2 glutamate receptor antibodies in cerebrospinal fluid with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2006;54:675–678. doi: 10.1002/art.21547. [DOI] [PubMed] [Google Scholar]
- 87.Wang C, Khalil M, Ravetch J, Diamond B. The naive B cell repertoire predisposes to antigen-induced systemic lupus erythematosus. J Immunol. 2003;170:4826–4832. doi: 10.4049/jimmunol.170.9.4826. [DOI] [PubMed] [Google Scholar]
- 88.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 89.Johansson BB. Implications of the Blood-Brain Barrier and Its manipulation. In: Neuwelt EA, editor. Implications of the blood brain barrier and its manipulation. NY: Plenum; 1989. pp. 389–410. [Google Scholar]
- 90.Kuang F, et al. Extravasation of blood-borne immunoglobulin G through blood-brain barrier during adrenaline-induced transient hypertension in the rat. Int J Neurosci. 2004;114:575–591. doi: 10.1080/00207450490422731. [DOI] [PubMed] [Google Scholar]
- 91.Tuor UI, McKenzie E, Tomanek B. Functional magnetic resonance imaging of tonic pain and vasopressor effects in rats. Magn Reson Imaging. 2002;20:707–712. doi: 10.1016/s0730-725x(02)00599-4. [DOI] [PubMed] [Google Scholar]
- 92.Kowal C, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 93.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mackay M, et al. Differences in regional brain activation patterns assessed by functional magnetic resonance imaging in patients with Systemic Lupus Erythematosus stratified by disease duration. Mol Med. 2011;17:1349–1356. doi: 10.2119/molmed.2011.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 96.Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. Eur Radiol. 2006;16:193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]
- 97.Etkin A, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Emmer BJ, van der Grond J, Steup-Beekman GM, Huizinga TW, van Buchem MA. Selective involvement of the amygdala in systemic lupus erythematosus. PLoS Med. 2006;3:e499. doi: 10.1371/journal.pmed.0030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fragoso-Loyo H, et al. Interleukin-6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus. Arthritis Rheum. 2007;56:1242–1250. doi: 10.1002/art.22451. [DOI] [PubMed] [Google Scholar]
- 100.Hirohata S, Kanai Y, Mitsuo A, Tokano Y, Hashimoto H, Subcommittee NR. Accuracy of cerebrospinal fluid IL-6 testing for diagnosis of lupus psychosis. A multicenter retrospective study. Clin Rheumatol. 2009;28:1319–1323. doi: 10.1007/s10067-009-1226-8. [DOI] [PubMed] [Google Scholar]
- 101.Katsumata Y, et al. Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: interleukin 6 (IL-6), IL-8, interferon-alpha, IgG index, and Q-albumin. J Rheumatol. 2007;34:2010–2017. [PubMed] [Google Scholar]
- 102.Kattah NH, Kattah MG, Utz PJ. The U1-snRNP complex: structural properties relating to autoimmune pathogenesis in rheumatic diseases. Immunol Rev. 2010;233:126–145. doi: 10.1111/j.0105-2896.2009.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lahita RG. Systemic lupus erythematosus: learning disability in the male offspring of female patients and relationship to laterality. Psychoneuroendocrinology. 1988;13:385–396. doi: 10.1016/0306-4530(88)90045-5. [DOI] [PubMed] [Google Scholar]
- 104.Neri F, et al. Neuropsychological development of children born to patients with systemic lupus erythematosus. Lupus. 2004;13:805–811. doi: 10.1191/0961203304lu2018oa. [DOI] [PubMed] [Google Scholar]
- 105.Ross G, Sammaritano L, Nass R, Lockshin M. Effects of mothers’ autoimmune disease during pregnancy on learning disabilities and hand preference in their children. Arch Ped Adolesc Med. 2003;157:397–402. doi: 10.1001/archpedi.157.4.397. [DOI] [PubMed] [Google Scholar]
- 106.Tincani A, et al. Impact of in utero environment on the offspring of lupus patients. Lupus. 2006;15:801–807. doi: 10.1177/0961203306071005. [DOI] [PubMed] [Google Scholar]
- 107.Lee JY, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Contet C, Rawlins JN, Deacon RM. A comparison of 129S2/SvHsd and C57BL/6JOlaHsd mice on a test battery assessing sensorimotor, affective and cognitive behaviours: implications for the study of genetically modified mice. Behav Brain Res. 2001;124:33–46. doi: 10.1016/s0166-4328(01)00231-5. [DOI] [PubMed] [Google Scholar]
- 109.Crawley JN. What’s Wrong With my Mouse: Behavioral Phenotyping of Transgenic and Knockout Mice. 2nd edn. Hoboken, NJ: Wiley Interscience; 2007. [Google Scholar]
- 110.Deacon RM, Croucher A, Rawlins JN. Hippocampal cytotoxic lesion effects on species- typical behaviours in mice. Behav Brain Res. 2002;132:203–213. doi: 10.1016/s0166-4328(01)00401-6. [DOI] [PubMed] [Google Scholar]
- 111.Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 112.Barker GR, et al. The different effects on recognition memory of perirhinal kainate and NMDA glutamate receptor antagonism: implications for underlying plasticity mechanisms. J Neurosci. 2006;26:3561–3566. doi: 10.1523/JNEUROSCI.3154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behav Neurosci. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- 114.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 115.Bloom O, et al. Generation of a unique small molecule peptidomimetic that neutralizes lupus autoantibody activity. Proc Natl Acad Sci USA. 2011;108:10255–10259. doi: 10.1073/pnas.1103555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Petri M, Naqibuddin M, Sampedro M, Omdal R, Carson KA. Memantine in systemic lupus erythematosus: a randomized, double-blind placebo-controlled trial. Semin Arthritis Rheum. 2011;41:194–202. doi: 10.1016/j.semarthrit.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diamond B, Bloom O, Al Abed Y, Kowal C, Huerta PT, Volpe BT. Moving towards a cure: blocking pathogenic antibodies in systemic lupus erythematosus. J Intern Med. 2011;269:36–44. doi: 10.1111/j.1365-2796.2010.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yurasov S, Nussenzweig MC. Regulation of autoreactive antibodies. Curr Opin Rheumatol. 2007;19:421–426. doi: 10.1097/BOR.0b013e328277ef3b. [DOI] [PubMed] [Google Scholar]
- 119.Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol. 2009;9:449–456. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 121.Rossi CC, Van de Water J, Rogers SJ, Amaral DG. Detection of plasma autoantibodies to brain tissue in young children with and without autism spectrum disorders. Brain Behav Immun. 2011;25:1123–1135. doi: 10.1016/j.bbi.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]