Abstract
Borrelia spp. are agents of Lyme disease and relapsing fever, diseases which use Ixodes hard ticks and Ornithodoros soft ticks, respectively, as primary vectors. Some relapsing fever spirochetes, such as B. miyamotoi, are also found in hard ticks. To date, no Borrelia sp. is known to use the hard tick, Amblyomma maculatum, as a vector. However, both B. burgdorferi and B. lonestari were recently detected in A. maculatum removed from hosts. In our study, DNA extracts from 306 questing adult A. maculatum collected in Mississippi in 2009 and 2010 were tested for Borrelia spp. DNA by PCR amplification of flaB and 16S rRNA gene targets. An additional 97 A. maculatum collected in 2013 were tested by amplification of 16S rRNA gene target. Two ticks, one collected in 2009 and the other in 2010, were positive by PCR of the flaB and 16S rRNA gene targets; both were collected from the same location in central Mississippi. Interestingly, 16S rRNA gene amplicons from these two tick extracts were 98% identical to twelve Borrelia spp. including the reptile-associated spirochete B. turcica and Borrelia sp. “tAG158M”; flaB amplicons from these two ticks shared closest identity (89%) to the reptile-associated spirochete, B. turcica. These results demonstrate a Borrelia sp. in unfed A. maculatum ticks that is unique from other species in the NCBI database and in a clade with reptile-associated Borrelia species. Detection of a previously unrecognized Borrelia in a hard tick species generates additional questions regarding the bacterial fauna in these arthropods and warrants further studies to better understand this fauna.
Keywords: Borrelia, Gulf Coast ticks, Amblyomma maculatum, Spirochetes
Introduction
Lyme disease is the most commonly reported vector-borne disease and perhaps the most socio-politically charged infectious disease in the United States, where the etiologic agent, Borrelia burgdorferi sensu stricto, is transmitted by the hard tick, Ixodes scapularis Say (1821) (Burgdorfer et al., 1982). Relapsing fever, the other endemic borrelial disease in the United States, is most commonly caused by B. hermsii (Schwan and Piesman, 2002) and transmitted by soft ticks in the genus Ornithodoros. Borrelia species within the relapsing fever group have also been identified in hard ticks. For example, B. miyamotoi, first discovered in I. persulcatus ticks from Japan (Fukunaga et al., 1995), has been found in I. ricinus in Europe (Fraenkel et al., 2002; Richter et al., 2003), I. scapularis in the eastern United States (Scoles et al., 2001; Ullmann et al., 2005), and I. pacificus in the western U.S. (Mun et al., 2006). Interestingly, B. miyamotoi was recently associated with human disease in both the U.S. and Russia (Gugliotta et al., 2013; Krause et al., 2013; Platonov et al., 2011). Borrelia lonestari, another hard-tick relapsing fever group spirochete, was first detected in Amblyomma americanum, ticks common in the southeastern and south-central U.S. (Barbour et al., 1996). While this spirochete was associated with one case of human disease, “southern tick-associated rash illness,” over a decade ago (James et al., 2001), additional evidence implicating B. lonestari as a human disease agent is lacking (Feder et al., 2011). The Gulf Coast tick, Amblyomma maculatum Koch (1844) is a hard tick found primarily along the Gulf and Atlantic Coasts of the U.S., with a host range that includes rodents and ground-dwelling birds for immature stages, and large mammals, such as cattle, for adult stages (Teel et al., 2010). While A. maculatum is a known vector for the human spotted fever rickettsial pathogen, Rickettsia parkeri (Paddock et al., 2004), and the canine protozoal pathogen Hepatozoon americanum (Ewing and Panciera, 2003), A. maculatum is not known to harbor or transmit a Borrelia species. However, a recent study of A. maculatum in Arkansas reported the presence of a Borrelia flaB gene amplicon (B. lonestari and B. burgdorferi) in approximately 28% of A. maculatum collected from canines and white-tailed deer (Fryxell et al., 2012). To our knowledge, the presence of a Borrelia sp. in unfed A. maculatum has not been shown.
The objective of this study was to evaluate questing (unfed) adult A. maculatum for the presence of Borrelia species. Based on previous evidence of B. burgdorferi and B. lonestari in A. maculatum removed from hosts, the possibility of acquiring these spirochetes during the blood meal and the lack of evidence for A. maculatum as a vector for either Borrelia sp., we hypothesized that B. burgdorferi and B. lonestari would not be present in questing A. maculatum. We tested A. maculatum collected in Mississippi (MS) in 2009 and 2010 as part of another study (Ferrari et al., 2012), and an additional 97 samples collected in 2013, using PCR assays and amplicon sequencing.
Materials and methods
Amblyomma maculatum collection
A subset of 306 (177 in 2009 and 129 in 2010) questing adult A. maculatum that were collected as part of a previous study was used in the present study (Ferrari et al., 2012). An additional 97 questing A. maculatum that was collected in May and June of 2013 from 2 sites in northern MS and one site in central MS were also tested. The three 2013 sites were among those included in the previous study (Ferrari et al., 2012).
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted using an Illustra Tissue and Cells GenomicPrep Mini Spin Kit (GE Healthcare Life Sciences, Piscataway, NJ, USA) from the 306 whole-individual A. maculatum collected previously and from the 97 half tick bodies collected in 2013. Amblyomma maculatum collected in 2013 were first cleaned with 20% sodium hypochlorite (NaClO), 0.5% benzalkonium chloride, and 70% ethyl alcohol, rinsed in sterile PBS and then bisected sagittally with the half tick used for DNA extraction and the other half used as part of another study.
DNA extracts from 2009 and 2010 were used in 2 nested PCR assays to amplify approximately 330-base-pair (bp) and 650-bp regions of the flaB and 16S rRNA genes, respectively, for Borrelia spp. based on previously published assays (Barbour et al., 1996; Richter et al., 2003). Samples from 2013 were tested by Borrelia spp. 16S rRNA gene PCR only (Richter et al., 2003). All ticks were also tested in a PCR amplifying a portion of the tick mitochondrial 16S rRNA gene to ensure presence of amplifiable DNA (Black and Piesman, 1994; Ferrari et al., 2012). DNA extracted from cultured B. burgdorferi strain B31 and B. hermsii strain DAH were used as positive controls; molecular grade water was used as a non-template negative control. PCR products were detected by electrophoresis in a 1.5% agarose gel and visualized with ethidium bromide. PCR amplicons were purified using a DNA Clean and Concentrator Kit (Zymo Research, Irvine, CA, USA), bidirectionally sequenced (Eurofins MWG Operon, Huntsville, AL, USA), aligned using ClustalX2 (Larkin et al., 2007), and compared to available sequences in the National Center for Biotechnology Information (NCBI) database. Alignments were also used in MEGA 5 (Molecular Evolutionary Genetics Analysis) (Tamura et al., 2011) to generate phylogenetic trees.
Results
Amblyomma maculatum collection and amplicon sequence
A total of 177 adult A. maculatum collected in 2009, comprised of 84 females (48%) and 93 males (53%), were tested by PCR assay. Of these ticks, 78 (44%), 26 (15%), and 73 (41%) were collected from northern, central, and southern Mississippi, respectively. In 2010, 129 ticks were tested of which 81 were females (63%) and 48 were males (37%). The majority (n = 123; 95%) of these ticks was collected from sites in northern Mississippi, while 6 (5%) were collected from central Mississippi. In 2013, 97 ticks were tested, of which 55 were females (57%) and 42 were males (43%). The majority (n = 95; 98%) of these ticks were collected from northern Mississippi, while 2 (2%) ticks were collected from the central Mississippi site.
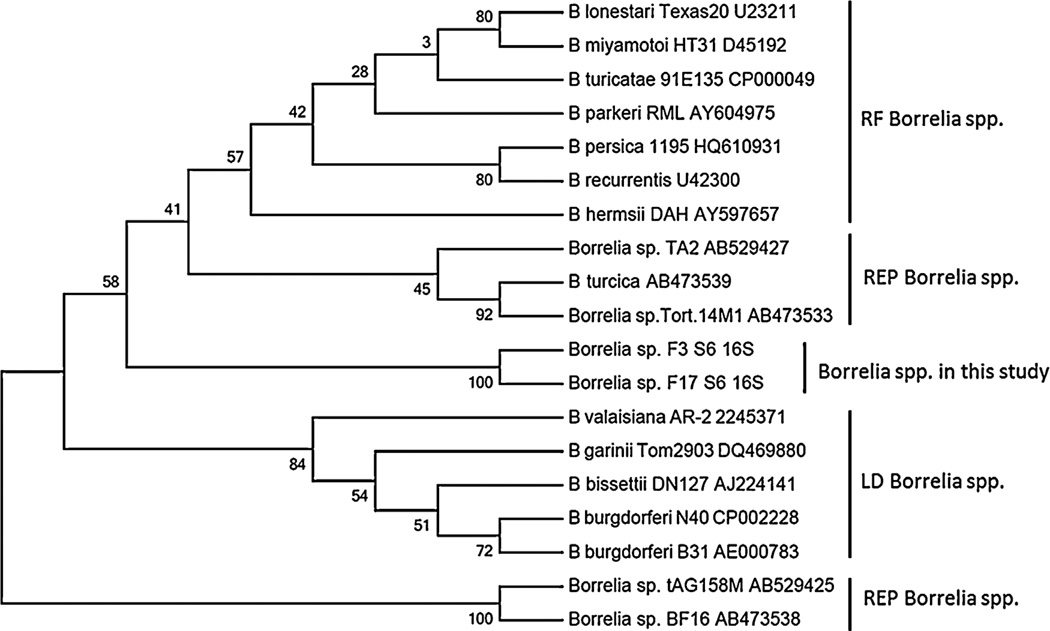
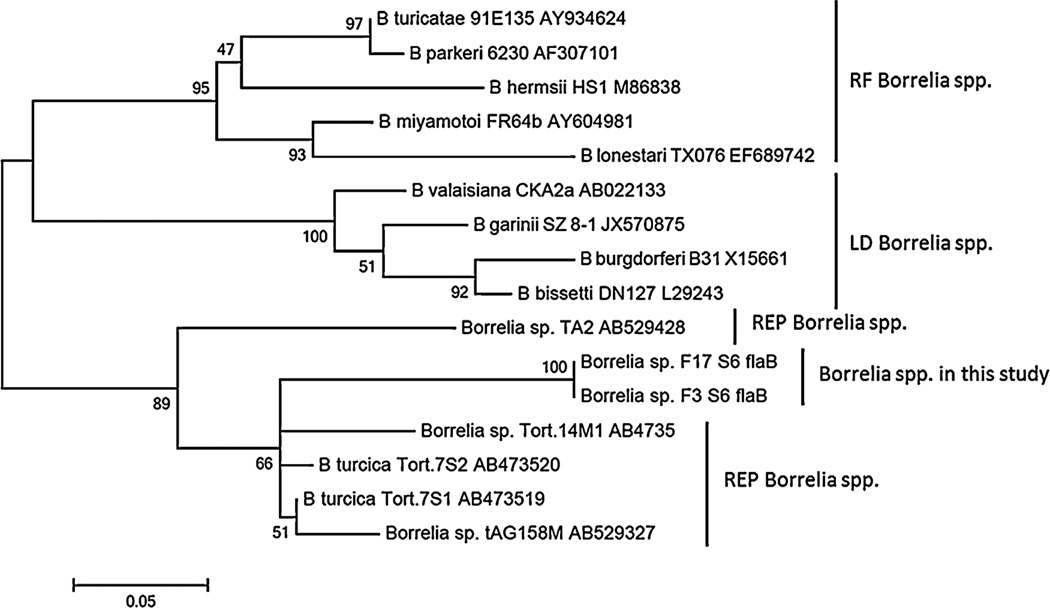
Two A. maculatum tick extracts out of 306 (0.65%) that were collected in 2009 and 2010 generated bands of appropriate size on PCR amplification of both the flaB and 16SrRNA gene targets. The positive tick samples were collected from the same location in central Mississippi (Byram, MS, USA). No tick extracts collected in 2013 were positive by PCR assay of the Borrelia 16S rRNA gene target. 16S rRNA gene sequences amplified from the 2 positive A. maculatum samples were 98% identical to 12 Borrelia spp. including B. turcica (AB473539.1), Borrelia sp. tAG158 M (AB529425.1) and Borrelia sp. Tortoise 14M1 (AB473533.1). FlaB amplicon sequences from these same 2 tick samples were both 89% identical to B. turcica flaB, isolate Tortoise 7S2 and 7S1 (Accession: AB473520.1 and AB473519.1). Phylogenetic analyses using partial 16S rRNA gene sequences (Fig. 1) and flaB (Fig. 2) sequences from this study with selected sequences available in GenBank showed that sequences detected from the 2 ticks in this study were not closely related to Lyme disease group or relapsing fever Borrelia group. Rather, they were more closely related to reptile-associated borreliae. Two additional tick extracts demonstrated flaB amplicons on PCR that shared 100% identity to multiple strains of B. hermsii including DAH. A flaB amplicon from a third sample shared 100% identity to B. burgdorferi sensu stricto including strain N40 and 99% identity to B. burgdorferi strain B31. However, these 3 tick extracts were negative using the 16S rRNA gene target and on subsequent PCR assays to reamplify the flaB gene. Given that we could not amplify a 16S rRNA gene product from these samples and both B. hermsii DAH and B. burgdorferi B31 were included as PCR controls, we suspect that the flaB PCR results were false-positive and likely a result of PCR contamination.
Fig. 1.
Unrooted maximum likelihood phylogenetic tree based on 568-bp partial 16S rRNA gene sequences from Borrelia spp. F3S6 and F17S6. Numbers at the branch notes denote bootstrap values as a percentage of 1000 replications. GenBank accession numbers for sequences used in tree construction are included for each species.
Fig. 2.
Unrooted maximum likelihood phylogenetic tree based on 267 and 226 bp partial flaB gene sequences from Borrelia spp. F3S6 and F17S6, respectively. Numbers at the branch notes denote bootstrap values as a percentage of 1000 replications. GenBank accession numbers for sequences used in tree construction are included for each species. The scale bar demonstrates degree of divergence and corresponds to a distance of 0.05.
GenBank accession numbers for Borrelia sp. partial 16S rRNA gene sequences are KF395228 and KF395229. Accession numbers for Borrelia sp. partial flaB gene sequences are KF395230 and KF395231.
Discussion
Of A. maculatum extracts tested in this study, 2 out of 403 (0.5%) of the extracts were positive by PCR for Borrelia species. Both ticks were collected from the same central Mississippi site (Byram, MS, USA) during 2009 and 2010. Sequences of the 16S rRNA gene amplicons from the 2 samples were 100% identical to each other; flaB amplicon sequences for the 2 samples were also identical to each other. The identification of a Borrelia sp. in unfed A. maculatum is intriguing considering Borrelia spp. have been detected by PCR from only fed A. maculatum, and were limited to B. lonestari and B. burgdorferi (Fryxell et al., 2012). The absence of B. burgdorferi and B. lonestari amplicons in over 400 questing adult A. maculatum tested here suggests that the spirochetes detected previously in A. maculatum attached to canids and deer may have been acquired from the blood meal (Fryxell et al., 2012). Alternatively, the geographic areas sampled here differed from the previous report and there may be differences in infection rates with Borrelia spp. between A. maculatum in these areas.
Phylogenetic analyses have revealed that spirochetes in the genus Borrelia fall into 3 groups (Parola and Raoult, 2001; Takano et al., 2010, 2011). The Lyme disease group of Borrelia spp. contains the most common tick-borne disease agents in the northern Hemisphere, including B. burgdorferi sensu stricto, B. afzelii, and B. garinii. The relapsing fever group of Borrelia spp. includes B. hermsii, B. duttonii, and B. turcicatae, all transmitted by soft ticks (Ornithodoros spp.). There is also a sister group of hard tick-associated relapsing fever spirochetes, including the species B. miyamotoi and B. lonestari. The third recently proposed group of borreliae consists of reptile-associated Borrelia spp. found in hard ticks of the genera Amblyomma and Hyalomma (Güner et al., 2004; Takano et al., 2011). Phylogenetic analyses of the flaB and 16S rRNA partial gene sequences identified in this study placed these A. maculatum associated Borrelia sequences in a clade with or as a sister group to the reptile-associated borreliae, and distinct from the relapsing fever group and Lyme disease groups. These results are consistent with the GenBank query results which showed closest flaB sequence identity (89%) to the B. turcica isolates Tortoise 7S1 and 7S2 from the hard tick, Hyalomma aegyptium, removed from a tortoise in Turkey (Güner et al., 2004), and closest 16S rRNA gene sequence identity (98%) to species including B. turcica. That the sequences identified here are closest to reptile-associated Borrelia species is curious considering A. maculatum are not known to feed on reptiles, and opens additional questions.
In this study, both 16S rRNA and flaB gene sequences amplified from A. maculatum-associated Borrelia sp. were unique compared to available Borrelia spp. sequences in the NCBI database. The presence of this previously uncharacterized Borrelia sp. and absence of other borreliae suggests that a distinct Borrelia sp. is present and supports absence of B. burgdorferi and B. lonestari in unfed A. maculatum tested in Mississippi. While infected A. maculatum may be rare, the existence of an additional Borrelia sp. in a southern tick species known to bite humans (Goddard, 2002) is significant. Additional studies that include continued testing to determine prevalence in A. maculatum throughout their range and more in depth characterization of the Borrelia species are warranted.
Acknowledgments
The authors would like to thank Jerome Goddard and Christopher Paddock for intellectual contributions to this study and the Mississippi State University College of Veterinary Medicine for funding support. It is specifically administered through the Office of Research and Graduate Studies MSU-CVM.
References
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- Black WC, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease - a tick-borne spirochetosis? Science (New York, NY) 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Ewing SA, Panciera RJ. American canine hepatozoonosis. Clin. Microbiol. Rev. 2003;16:688–697. doi: 10.1128/CMR.16.4.688-697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HM, Jr, Hoss DM, Zemel L, Telford SR, 3rd, Dias F, Wormser GP. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin. Infect. Dis. 2011;53(10):e142–e146. doi: 10.1093/cid/cir553. [DOI] [PubMed] [Google Scholar]
- Ferrari FA, Goddard J, Paddock CD, Varela-Stokes AS. Rickettsia parkeri and Candidatus Rickettsia andeanae in Gulf Coast ticks, Mississippi, USA. Emerg. Infect. Dis. 2012;18:1705–1707. doi: 10.3201/eid1810.120250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel CJ, Garpmo U, Berglund J. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J. Clin. Microbiol. 2002;40:3308–3312. doi: 10.1128/JCM.40.9.3308-3312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryxell RT, Steelman CD, Szalanski AL, Kvamme KL, Billingsley PM, Williamson PC. Survey of Borreliae in ticks, canines, and white-tailed deer from Arkansas, USA. Parasit. Vectors. 2012;5:139. doi: 10.1186/1756-3305-5-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov. isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J. Agromed. 2002;8:25–32. doi: 10.1300/J096v08n02_06. [DOI] [PubMed] [Google Scholar]
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güner ES, Watanabe M, Hashimoto N, Kadosaka T, Kawamura Y, Ezaki T, Kawabata H, Imai Y, Kaneda K, Masuzawa T. Borrelia turcica sp. nov. isolated from the hard tick Hyalomma aegyptium in Turkey. Int. J. Syst. Evol. Microbiol. 2004;54:1649–1652. doi: 10.1099/ijs.0.03050-0. [DOI] [PubMed] [Google Scholar]
- James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson BJ. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 2001;183:1810–1814. doi: 10.1086/320721. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J. Med. Entomol. 2006;43:120–123. doi: 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SLF, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Schlee DB, Matuschka FR. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis. 2003;9:697–701. doi: 10.3201/eid0906.020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 2002;8:115–121. doi: 10.3201/eid0802.010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Takano A, Fujita H, Kadosaka T, Konnai S, Tajima T, Watanabe H, Ohnishi M, Kawabata H. Characterization of reptile-associated Borrelia sp. in the vector tick Amblyomma geoemydae, and its association with Lyme disease and relapsing fever Borrelia spp. Environ. Microbiol. Rep. 2011;3:632–637. doi: 10.1111/j.1758-2229.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- Takano A, Goka K, Une Y, Shimada Y, Fujita H, Shiino T, Watanabe H, Kawabata H. Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ. Microbiol. 2010;12:134–146. doi: 10.1111/j.1462-2920.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teel PD, Ketchum HR, Mock DE, Wright RE, Strey OF. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 2010;47:707–722. doi: 10.1603/me10029. [DOI] [PubMed] [Google Scholar]
- Ullmann AJ, Gabitzsch ES, Schulze TL, Zeidner NS, Piesman J. Three multiplex assays for detection of Borrelia burgdorferi sensu lato and Borrelia miyamotoi sensu lato in field-collected Ixodes nymphs in North America. J. Med. Entomol. 2005;42:1057–1062. doi: 10.1093/jmedent/42.6.1057. [DOI] [PubMed] [Google Scholar]