Abstract
Background
We report our initial experiences of robot-assisted cardiac surgery using the da Vinci Surgical System.
Methods
Between February 2010 and March 2014, 50 consecutive patients underwent minimally invasive robot-assisted cardiac surgery.
Results
Robot-assisted cardiac surgery was employed in two cases of minimally invasive direct coronary artery bypass, 17 cases of mitral valve repair, 10 cases of cardiac myxoma removal, 20 cases of atrial septal defect repair, and one isolated CryoMaze procedure. Average cardiopulmonary bypass time and average aorta cross-clamping time were 194.8±48.6 minutes and 126.1±22.6 minutes in mitral valve repair operations and 132.0±32.0 minutes and 76.1±23.1 minutes in myxoma removal operations, respectively. During atrial septal defect closure operations, the average cardiopulmonary bypass time was 128.3±43.1 minutes. The median length of stay was between five and seven days. The only complication was that one patient needed reoperation to address bleeding. There were no hospital mortalities.
Conclusion
Robot-assisted cardiac surgery is safe and effective for mitral valve repair, atrial septal defect closure, and cardiac myxoma removal surgery. Reducing operative time depends heavily on the experience of the entire robotic surgical team.
Keywords: Robotics, Thoracic surgery, Minimally invasive surgical procedures
INTRODUCTION
In 1998, Carpentier and Falk independently performed the first true robot-assisted mitral valve (MV) repair using a prototype of the da Vinci Surgical System. Since then, robot-assisted cardiac surgery has rapidly expanded in terms of case numbers and indications. We performed a retrospective review to examine our initial experiences with robot-assisted cardiac surgery using the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) and evaluate its safety and feasibility.
METHODS
1) Patient characteristics and evaluation
From February 2010 to March 2014, 50 consecutive patients underwent robot-assisted cardiac surgery using the da Vinci Robotic Surgical System. Indications for robot-assisted cardiac surgery were simple diagnoses, such as a secundum-type atrial septal defect (ASD), left atrial myxoma, mitral regurgitation, and single-vessel coronary artery disease, as well as the absence of any significant complicating factors in the patient’s condition.
All patients underwent transthoracic echocardiography and computerized tomography scans. Coronary angiography, heart catheterization, or transesophageal echocardiography (TEE) were performed if necessary, depending on the disease. All patients were monitored with TEE during the operation. Postoperative transthoracic echocardiography was performed on all patients before discharge.
2) Surgical technique
After appropriate anesthesia, the patient was intubated with a double-lumen endotracheal tube and transthoracic (Zoll-type) defibrillator pads were applied. The patient was heparinized in the supine position with the right side of the chest elevated, and a femoral arterial cannula (Medtronic Inc., Minneapolis, MN, USA) was placed through the right internal jugular vein into the superior vena cava under TEE guidance. This cannula was extended into the venous circuit using a Y-connector. Through a 2-cm incision, the right femoral artery and vein were exposed and cannulated using the Seldinger technique under TEE guidance. We did not perform special monitoring for leg ischemia.
The right lung was deflated and an anterior mini-thoracotomy (4–5 cm) was made through the fourth intercostal space (ICS) as a working port and camera access port. Two additional ports were made for robotic instruments. The left instrument arm port (8 mm) was always placed in the third ICS, near the anterior axillary line. The right instrument port was placed in either the fifth or sixth ICS near the anterior axillary line, depending on the position of the heart and the size of the chest cage. The working port and the instrument ports were placed in a triangular manner with a minimal distance of 10 cm from each other. An extra port for the left atrial retractor was made through the fifth ICS at the midclavicular line. In order to maintain a clear operative field, a separate stab-wound incision was used to place a 20F DLP intracardiac sump drain (Medtronic Inc.) in the fifth ICS at the midaxillary line.
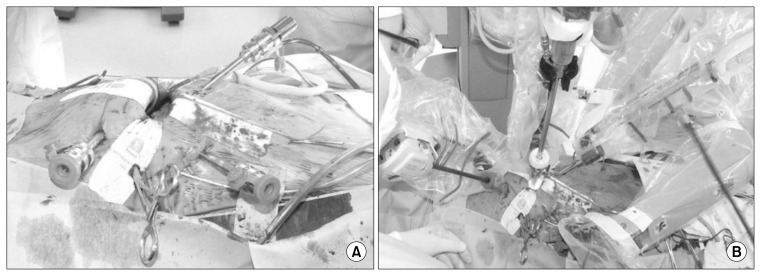
A Chitwood transthoracic aortic cross-clamp (Scanlan International, Minneapolis, MN, USA) positioned through the second ICS at the midaxillary line and intermittent cold (4°C) antegrade blood cardioplegia were used to achieve cardiac arrest and for myocardial protection. However, ASD closure operations were sometimes performed with a beating or fibrillating heart. The left atrium was opened along the Waterston groove, and MV or left atrium exposure was achieved with the dynamic left atrial retractor for MV repair and cardiac myxoma removal. For ASD closure, the right atrium was opened and retracted with the fourth arm (Fig. 1).
Fig. 1.
Port placement for mitral valve repair surgery. (A) Four ports and a Chitwood transthoracic aortic cross clamp are in place before aorta cross clamp. (B) Docking of the camera and robotic instruments is complete.
Robotic instruments were used for all leaflet resections and repairs, chordal transpositions, ring implantations, sutures, and knot tying. For patients with concomitant atrial fibrillation, a robot-assisted CryoMaze procedure was performed with a Cardioblate CryoFlex surgical ablation probe (Medtronic Inc.) to produce the Cox Maze III lesion.
The CryoMaze procedure was performed sequentially from left to right. After cardiac arrest was achieved, the left-side CryoMaze procedure was performed via a left atrial incision. After repairing the left atrial incision, the aortic cross-clamp was released and the right-side CryoMaze procedure was performed via a right atrial incision with a beating heart. After the operation, all patients were transferred to the intensive care unit without being extubated.
RESULTS
A total of 50 patients, including 30 men and 20 women, with a mean age of 55 years underwent robot-assisted cardiac surgery. There were two cases of minimally invasive direct coronary artery bypass, 17 cases of MV repair, 10 cases of cardiac myxoma removal, 20 cases of ASD repair, and one isolated CryoMaze procedure. Both minimally invasive coronary artery bypasses were single anastomoses of the left internal mammary artery to the left anterior descending coronary artery. Two CryoMaze procedures and one tricuspid annuloplasty were performed during MV repair operations. Likewise, three CryoMaze procedures and five tricuspid annuloplasties were performed during ASD closure operations (Table 1).
Table 1.
Summary of cases of robot-assisted cardiac surgery by type
| Type of robot-assisted cardiac surgery | No. of patients |
|---|---|
| Minimal invasive coronary artery bypass | 2 |
| Mitral valve repair | 17 |
| MVP+CryoMaze procedure | 2 |
| MVP+TAP | 1 |
| Myxoma removal | 10 |
| ASD closure | 20 |
| ASD+CryoMaze procedure | 3 |
| ASD+TAP | 5 |
| Isolated CryoMaze procedure | 1 |
MVP, mitral valvuloplasty; TAP, tricuspid annuloplasty; ASD, atrial septal defect.
All patients who underwent MV repair were diagnosed with moderate or severe mitral regurgitation by transthoracic echocardiography. The etiology of mitral regurgitation was degenerative in 13 cases, rheumatic in one case, and infectious in three cases. Mitral ring annuloplasty was performed in all MV operations, along with other techniques as necessary (Table 2). The average cardiopulmonary bypass (CPB) time in MV repair operations was 194.8±48.6 minutes (range, 114 to 307 minutes), and the average aorta cross-clamp (ACC) time was 126.1±22.6 minutes (range, 95 to 184 minutes). In MV repair operations involving no additional procedures, the average CPB and ACC times were 185.5±48.3 minutes and 123.2±24.0 minutes, respectively, whereas in MV repair operations that involved additional procedures, the average CPB and ACC times were 238.3±18.0 minutes and 139.3±2.6 minutes, respectively (Table 3). The average length of stay of patients who underwent MV repair was 7.4 days, with a median of seven days. Postoperative transthoracic echocardiography showed either no or trivial mitral regurgitation in all patients (Table 4).
Table 2.
Mitral valve repair techniques
| Techniques | No. (%) |
|---|---|
| Annuloplasty | 17 (100) |
| Quadrangular or triangular resection | 13 (76.5) |
| Commissuroplasty | 2 (11.8) |
| Chordal transposition | 2 (11.8) |
Table 3.
Perioperative data
| Variable | Cardiopulmonary bypass time (min) | Aortic cross clamp time (min) | Length of stay (day) |
|---|---|---|---|
| Minimal invasive coronary artery bypass | - | - | 6.5 (5–8) |
| Mitral valve repair | |||
| Total | 194.8±48.6 | 126.1±22.6 | 7.4 (5–13) |
| Isolated | 185.5±48.3 | 123.2±24.0 | 7.1 (5–10) |
| Combined | 238.3±18.0 | 139.3±2.51 | 8.6 (6–13) |
| Atrial septal defect closure | |||
| Total | 128.3±43.1 | 7.9 (4–26) | |
| Isolated | 118.0±32.3 | 5.4 (3–9) | |
| Combined | 143.6±56.2 | 11.5 (5–26) | |
| Cardiac myxoma removal | 132.0±32.0 | 76.1±23.1 | 8 (4–20) |
Values are presented as mean±standard deviation or mean (range).
Table 4.
Perioperative transesophageal echocardiography results after MV repair
| MV regurgitation | Pre-operation | Post-operation |
|---|---|---|
| None | - | 9 (52.9) |
| Trivial | - | 8 (47.1) |
| Moderate | 3 (17.6) | - |
| Severe | 14 (82.4) | - |
Values are presented as number (%).
MV, mitral valve.
In robot-assisted myxoma removal operations, the average maximum diameter of the mass that was removed was 2.82±1.3 cm (range, 1 to 5 cm). The average CPB time was 132.0±32.0 minutes (range, 100 to 204 minutes), and the average ACC time was 76.1±23.1 minutes (range, 54 to 132 minutes). The average length of stay was eight days, with a median of six days. One patient was discharged on postoperative day 20, due to atrial fibrillation that occurred after the surgery.
During ASD closure operations, the average CPB time was 128.3±43.1 minutes. Of the 20 total cases, eight were performed with the ascending aorta clamped, and in these operations the average ACC time was 92.6±38.8 minutes. Six ASD closures were performed with a fibrillating heart, with an average fibrillation time of 36.1±10.0 minutes. The other six cases were performed with a beating heart. The overall average length of stay was 7.9 days, with a median of five days (Table 3).
There were no intraoperative conversions to sternotomy, and all patients were extubated within 24 hours. The average length of stay in the intensive care unit was 1±0.3 days. In one case of MV repair, CPB was implemented twice due to significant MV regurgitation in the intraoperative TEE. There was one case of iatrogenic liver laceration during cardiac myxoma removal, which was repaired intraoperatively. A re-operation had to be performed in one case on the first day after ASD closure surgery, due to a significant amount of bleeding originating from the port insertion site. One case of newly appearing atrial fibrillation occurred as well as two cases of significant pericardial effusion requiring medication. All three cases were resolved during follow up. There were no deaths from any cause during the follow-up period.
DISCUSSION
Most cardiac operations have traditionally been performed through a median sternotomy. However, in the mid-1990s, videoscopes and instruments specifically designed for cardiac operations were developed, along with specific peripheral CPB cannulas and aortic endoclamps. These innovations allowed cardiac surgeons to perform minimally invasive cardiac operations, and excellent outcomes have been reported in large series of patients [1,2].
The introduction of robotic surgical systems has opened a new frontier in cardiac surgery. In the early period of robot-assisted cardiac surgery, the Automatic Endoscopic System for Optimal Positioning (AESOP; Computer Motion Inc., Goleta, CA, USA) and the ZEUS robotic system (Computer Motion Inc.) were used. Cho et al. [3] reported their initial experience of robot-assisted cardiac surgery using the AESOP 3000 system, suggesting good operative outcomes and high patient satisfaction.
Currently, the da Vinci Surgical System (Intuitive Surgical Inc.) is the most widely used robotic surgical system. It has several key features that make it ideal for minimally invasive surgery. It uses a dual-camera endoscope, thus enabling three-dimensional vision and allowing depth perception of the operative field. Surgical instruments have been minimized and given seven degrees of freedom by the multi-jointed EndoWrist. This allows the surgeon to operate within a small area such as the left atrium. Furthermore, the system reduces the extent of physiological tremor, which enables precise surgical maneuvers. The most recent da Vinci Si System has added a fourth arm that can be utilized for left atrial retraction or as an assisting arm in coronary anastomosis.
Critics have questioned the reproducibility of robot-assisted procedures, the increased cost, and the real benefit for patients. However, recent reports have shown that robot-assisted cardiac surgery is overcoming these issues.
Nifong et al. [4] reported the largest series to date regarding their experiences with robot-assisted mitral valvuloplasty. Of 540 patients, 454 underwent simple robot-assisted mitral valvuloplasty and 86 underwent robot-assisted mitral valvuloplasty with a concomitant CryoMaze procedure to treat atrial fibrillation. They reported a high successful repair rate, low operative mortality (0.2%), and a low technical failure rate (0.8%), after a mean follow-up of 303 days. In patients who underwent a concurrent CryoMaze procedure, 96.5% were free of atrial fibrillation at a mean of 351±281 days after the surgery [4]. Bonaros et al. [5] reported that the success and safety rates were 80% and 95%, respectively, in 500 cases of totally endoscopic coronary artery bypass.
Compared to the traditional method, robot-assisted cardiac surgery has the significant advantage of involving a smaller incision size and less intraoperative trauma. This means that the patient benefits not only from improved cosmetic outcomes, but also from less pain and a shorter hospital stay. Ultimately, it may lead to faster recovery and improved quality of life. The robotic approach has been associated with improved quality of life after ASD closure and MV repair [6,7].
Differences between conventional and robot-assisted cardiac surgery in terms of CPB time and hospital stay have been reported in numerous studies. In our experience, the cost per patient of robot-assisted cardiac surgery is almost double that of conventional methods. However, this study does not attempt to analyze these issues.
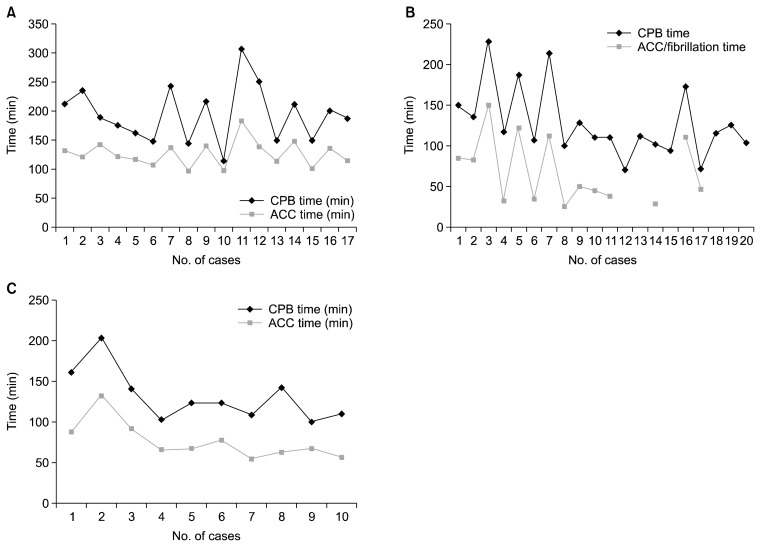
Some key criteria must be met for high-quality robot-assisted cardiac surgery to be performed with shorter operating times. First, a stable team dedicated to robot-assisted cardiac surgery must be organized. Cardiac surgery requires well-organized teamwork between the surgeon and the supporting team, which includes assistants, perfusionists, anesthesiologists, and nurses. Although the operating time can be reduced as the surgeon builds experience, other factors, such as proper patient positioning, one-lung ventilation, and device exchange time, depend on the supporting team members. In our experience, the operating time decreased in the early stages of implementation in Seoul National University Bundang Hospital, but showed fluctuation even after the surgeon gained experience (Fig. 2). During the later periods encompassed by this study, there were frequent changes of the first assistant or nurses, and we think that their learning curve affected the overall operating time. Therefore, the presence of a stable and experienced team is important in order to reduce the operating time. Second, simplified MV repair techniques such as a running suture for annuloplasty [8], triangular leaflet resections, and the haircut posterior leaflet-plasty [9] are necessary.
Fig. 2.
CPB time and ACC time of (A) Mitral valve repair. (B) Atrial septal defect closure. (C) Cardiac myxoma removal surgery. CPB, cardiopulmonary bypass; ACC, aortic cross clamp.
Gammie et al. [10] reported that in 2008, 20.1% of all MV operations in the United States were performed using minimally invasive techniques and that half of the surgeons used robot-assisted techniques. As patients continue to request less invasive operations, there is little doubt that this number will increase. The growing applications of robots in cardiac surgery now include MV operations, coronary artery revascularization, atrial fibrillation ablation, intracardiac tumor resection, and congenital heart surgery.
Based on our experience, robot-assisted surgery for MV repair, ASD closure, or cardiac myxoma removal operation using the da Vinci Surgical System is a feasible option for uncomplicated patients with simple diagnoses. The experience of the operating surgeon and of the entire robotic surgical team is vital in reducing the operative time. Although the system also can be utilized for minimally invasive direct coronary artery bypass and isolated CryoMaze procedures, more training and experience are needed to ensure its safety and efficacy for these procedures.
ACKNOWLEDGMENTS
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund No. KTCS04-020).
Footnotes
CONFLICT OF INTEREST
The authors confirm that there are no known conflicts of interest associated with this publication and that there was no significant financial support for this work that could have influenced its outcome.
REFERENCES
- 1.Casselman FP, Van Slycke S, Dom H, Lambrechts DL, Vermeulen Y, Vanermen H. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg. 2003;125:273–82. doi: 10.1067/mtc.2003.19. [DOI] [PubMed] [Google Scholar]
- 2.Grossi EA, Galloway AC, LaPietra A, et al. Minimally invasive mitral valve surgery: a 6-year experience with 714 patients. Ann Thorac Surg. 2002;74:660–3. doi: 10.1016/S0003-4975(02)03754-2. [DOI] [PubMed] [Google Scholar]
- 3.Cho SW, Chung CH, Kim KS, et al. Initial experience of robotic cardiac surgery. Korean J Thorac Cardiovasc Surg. 2005;38:366–70. [Google Scholar]
- 4.Nifong LW, Rodriguez E, Chitwood WR., Jr 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg. 2012;94:38–42. doi: 10.1016/j.athoracsur.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Bonaros N, Schachner T, Lehr E, et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: predictors of success and safety. Ann Thorac Surg. 2013;95:803–12. doi: 10.1016/j.athoracsur.2012.09.071. [DOI] [PubMed] [Google Scholar]
- 6.Morgan JA, Peacock JC, Kohmoto T, et al. Robotic techniques improve quality of life in patients undergoing atrial septal defect repair. Ann Thorac Surg. 2004;77:1328–33. doi: 10.1016/j.athoracsur.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 7.Suri RM, Antiel RM, Burkhart HM, et al. Quality of life after early mitral valve repair using conventional and robotic approaches. Ann Thorac Surg. 2012;93:761–9. doi: 10.1016/j.athoracsur.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 8.Mihaljevic T, Jarrett CM, Gillinov AM, Blackstone EH. A novel running annuloplasty suture technique for robotically assisted mitral valve repair. J Thorac Cardiovasc Surg. 2010;139:1343–4. doi: 10.1016/j.jtcvs.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Chu MW, Gersch KA, Rodriguez E, Nifong LW, Chitwood WR., Jr Robotic “haircut” mitral valve repair: posterior leaflet-plasty. Ann Thorac Surg. 2008;85:1460–2. doi: 10.1016/j.athoracsur.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Gammie JS, Zhao Y, Peterson ED, O’Brien SM, Rankin JS, Griffith BP. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery: less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2010;90:1401–8. 1410.e1. doi: 10.1016/j.athoracsur.2010.05.055. [DOI] [PubMed] [Google Scholar]