Abstract
Visceral pain is the most common form of pain caused by varied diseases and a major reason for patients to seek medical consultation. It also leads to a significant economic burden due to workdays lost and reduced productivity. Further, long-term use of non-specific medications is also associated with side effects affecting the quality of life. Despite years of extensive research and the availability of several therapeutic options, management of patients with chronic visceral pain is often inadequate, resulting in frustration for both patients and physicians. This is, most likely, because the mechanisms associated with chronic visceral pain are different from those of acute pain. Accumulating evidence from years of research implicates several receptors and ion channels in the induction and maintenance of central and peripheral sensitization during chronic pain states. Understanding the specific role of these receptors will facilitate to capitalize on their unique properties to augment the therapeutic efficacy while at the same time minimizing unwanted side effects. The aim of this review is to provide a concise review of the recent literature that reports on the role of principal ionotropic receptors and metabotropic receptors in the modulation visceral pain. We also include an overview of the possibility of these receptors as potential new targets for the treatment of chronic visceral pain conditions.
Keywords: Ligand-gated ion channels; Receptors, metabotropic glutamate; Visceral pain
Introduction
Chronic visceral pain is one of the most frequent and debilitating disorders in the general population, critically impacting economy and quality of life.1 Epidemiologic studies fail to identify the number of patients with this condition, perhaps due the lack of clear pathologic features associated with visceral pain conditions.2 In spite of this problem, surveys have shown prevalence rates among adults of 25% for intermittent abdominal pain, 20% for chest pain and 16–24% for pelvic pain in women.3 Functional gastrointestinal disorders underlie the most prevalent forms of visceral pain. Irritable bowel syndrome (IBS) is one functional gastrointestinal disorders characterized by abdominal pain, discomfort and altered bowel habits and creates tremendous pressure on the healthcare system affecting an estimated 10–15% of Europe and U.S. populations with consequent costs estimated to exceed US$ 40 billion.4,5 Pain is also one of the presenting symptoms in about 50–70% of patients experiencing the initial onset or exacerbations of inflammatory bowel disease.6 Even though visceral pain may be the response to noxious stimuli as distension or inflammation, the severity of pain does not always reflect the severity of the condition causing the pain.2 Although the precise pathophysiology of chronic visceral pain is still far from being elucidated, recent reports indicate that this disorder might be associated with a dysregulation at multiple levels of the brain-gut axis and might involve both the central nervous system (CNS) and the peripheral nervous system (Figure).7 In addition, aberrant central processing, as well as abnormalities within the stress responsive systems has also been reported to cause visceral pain.5,6 Moreover, early-in-life exposure to noxious and/or inflammatory stimuli has been reported to enhance the susceptibility of the organism to subsequent pathological challenges in the adult life by producing long-lasting neuroanatomical and neurophysiological changes in the nociceptive system.9–14 Nevertheless, our understanding of the etiology, pathophysiology and natural history of chronic abdominal pain has significantly increased in the past decade, in part because of the new tools to investigate the nervous system.15
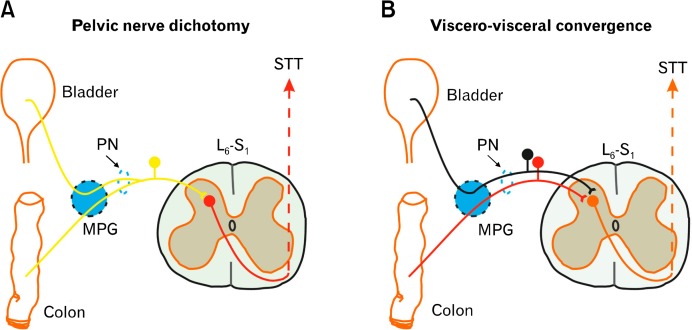
Figure.
Visceral pain pathway.
MPG, major pelvic ganglion; PN, pelvic nerve; STT, spino-thalamic tract.
Although development of visceral pain is considered to be an important defensive mechanism, development of hypersensitivity represents a significant clinical problem and is likely to be one of the major factors involved in the pathogenesis of abdominal and chest pain in functional bowel disorders.16 Research over the years has shown that the pathophysiology of the visceral pain is extremely complex. Hence, development of effective analgesics for the treatment of visceral pain has become an ongoing challenge for the pharmaceutical industry.17 For years, treatment recommendations for visceral pain were the same as that for somatic pain. However, of late it is widely accepted that visceral pain processing is distinctly different from somatic pain and as a result it should be treated differently from somatic pain.18 Currently, analgesics (opiates, nonsteroidal anti-inflammatory drugs, and benzodiazepine), antispasmodics and antidepressants are the most common medications for acute as well as chronic visceral pain conditions; yet they are always associated with the prominent adverse effects like addiction and constipation. Furthermore, the development of analgesic tolerance, inadequate pain relief and altered pain sensitivity with prolonged opioid use has also proved an unfortunate obstacle for their clinic applications.19–21 Complementary and alternative medicinal approaches like probiotics, herbal supplements, and electro-acupuncture are also being widely used to treat visceral pain although with varying degree of success.
For better understanding of the increase of visceral sensitivity in patients, a broader knowledge of the molecular components involved in the pathophysiology visceral pain is necessary. Accumulating evidence from extensive clinical and basic research in the last decade have shown the involvement of several receptors and ion channels in the development and maintenance of chronic visceral pain conditions.22 Receptors and channels have always been traditional targets for drug development to battle against a variety of diseases including chronic pain. Based on the epidemiological evidence and convincing basic science data, there is growing interest in the pharmaceutical industry to expand basic science and clinical research data and to explore specific potential targets for visceral pain therapy for this underserved patient population. Hence, a comprehensive review of the major receptors and ion channels involved in the modulation of visceral pain is necessary. While discussing all the receptors and ion channels will be beyond the scope and limitation of this article, we have limited our focus on the recent reports which have strongly indicated a role for these receptors and ion channels in modulating chronic visceral pain. These channels and receptors might in future be considered as potential targets for novel analgesics to treat visceral pain (Table). The contribution of ionotropic (membrane ion channels) and metabotropic (coupled to second messenger systems) receptors to visceral nociceptive processing is discussed below.
Table.
List of Ionotropic and Metabortopic Receptors and Their Ligands
| Receptor | Agonist | Antagonist | |
|---|---|---|---|
| GABA A receptor | GABA, Muscimol, HZ166 | Baclofen | |
| GABA B receptor | GABA, Baclofen, gamma-hydroxybutyrate, CGP7930, ADX71441, ADX71943 | Phaclofen, CGP-35348 | |
| NMDA receptor | NMDA, glutamate, aspartic acid | AP-5, AP-7, Ketamine | |
| Kainate receptor | Kainic acid | LY382884, CNQX, DNQX | |
| AMPA receptor | AMPA, glutamate | NBQX, Kynurenic acid | |
| Opioid receptor | |||
| MOR | Morphine, oxycodone | - | |
| KOR | Oxycodone, asimadoline | - | |
| DOR | Enkephalin, cannabidiol, tetrahydrocannabinol | Buprenorphine | |
| Cannabinoid (CB) receptor | CB1 | Dronabinol | - |
| CB2 | |||
| Serotonin (5-HT) receptor | 5-HT1 | DPAT | - |
| 5-HT3 | - | Alosetron | |
| 5-HT4 | Tegaserod | - | |
| TRP channels | TRPV1 | - | SB 366791 |
| TRPA1 | - | - | |
| TRPV4 | - | RN1734 | |
| TRPC4 | - | ML-204 | |
GABA, gamma aminobutyric acid; NMDA, N-methyl-D-aspartate receptor; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; MOR, μ-opioid receptor; KOR, κ-opioid receptor; DOR, δ-opioid receptor; DPAT, 8-hydroxy-DPAT hydrobromide; TRP, transient receptor potential.
Gamma Aminobutyric Acid Receptors
Gamma aminobutyric acid (GABA), the major inhibitory neurotransmitter in the brain, is essential for the overall balance between neuronal excitation and inhibition that is vital to normal brain function.21 GABA produces neuronal inhibition by acting on a diversity of membrane-bound receptors. These receptors can be divided into 2 major types: ionotropic receptors that are ligand-gated ion channels (GABAA receptors), and metabotropic receptors that are G-protein coupled receptors (GABAB receptors).23,24 GABAA receptor channels are ubiquitous in the mammalian CNS mediating fast inhibitory neurotransmission by becoming permeant to chloride ions.25 Similarly, GABAB receptors are expressed by almost all neurons as well as glial cells in the CNS and their activity influences many neural systems and behavioral states.26 Several recently published reports indicate that both GABAA and GABAB receptors are involved in the pathophysiology of chronic pain.27 While majority of the reports confirm the role of GABA receptors in somatic pain, few studies have also reported on their involvement in visceral pain. GABAA receptors have been widely reported to be involved in several models of somatic pain and are actively pursued as potential targets for novel analgesic drugs.28–30 In contrast, their role in visceral pain condition was confirmed only recently. Sengupta et al31 reported that down regulation of microRNA-mediated GABAAα1 receptor subunit in adult spinal cord following neonatal cystitis was responsible for the development of chronic visceral pain in rats. Microinjection of muscimol, (GABAA receptor agonist), into raphe magnus of rats reduced colorectal distension evoked suppression of withdrawals indicating that evidence that raphe magnus neurons contribute to this antinociception via the GABAA receptors.32
Studies have shown that GABAB receptor is involved in modulating mechanosensory traffic through the vagus nerve and brainstem pathways. While GABAB receptors blocked mechanosensory input, they were found not to modulate chemosensitivity. Agonists of GABAB receptors were found to directly influence the sensory input via binding enteric receptors or by way of activating inhibitory interneurons within the CNS.33,34 Baclofen, a GABAB receptor agonist, has been extensively used in animal models and clinical conditions to test somatic and visceral nociception.35 While the exact mechanism is not known, GABAB receptors were found to couple with calcium channels and potassium channels which may be the mechanism of antinociceptive effect. Activation of GABAB receptors were found to down regulate calcium channels and activate the inward rectify potassium channels which was found to improve repolarization of neurons.36–38 In rodent studies, baclofen was found to reduce afferent firing from a colorectal distention which is a well-established and widely used model for noxious visceral stimuli. This effect was found to occur in a dose-dependent manner and was unrelated to smooth muscle relaxation or improved colon compliance.39,40 Several other studies also reported that baclofen significantly reduces the visceromotor responses from colorectal distention.41,42 Baclofen was also found to inhibit the expression of c-fos in the dorsal horn to experimental colitis and capsaicin-induced bladder irritation in rodent models of visceral pain.43–45 Hara et al45 reported that intrathecal diltiazem (a calcium channel blocker) in combination with muscimol (GABAA agonist) or baclofen (GABAB agonist) was found to potentiate the GABA agonists-induced visceral antinociception without increasing motor paralysis. The authors report that when muscimol was administered with diltiazem, the increase in the threshold for colorectal distension was significantly larger than muscimol alone. Similarly, the colorectal distension threshold after the combination of baclofen and diltiazem also showed a significantly larger increase than that seen after baclofen alone. In addition to that, motor paralysis observed with muscimol did not increase when muscimol was co-administered with diltiazem. Brusberg et al40 reported that activation of GABAB receptors was found to produce antinociceptive effects in a rat model of mechanically induced visceral pain (noxious colorectal distension). Despite these effects, clinical use of baclofen has been limited by its short duration of action, narrow therapeutic margin and side effects, including sedation, dizziness, nausea, muscle weakness and mental confusion.46
Positive allosteric modulation (PAM) of GABA receptors may represent a valid approach in the treatment of visceral pain conditions, with the possibility of an improved safety profile compared to the effect of agonist. Kalinichev et al47 demonstrated that the GABA B receptor positive allosteric modulator, ADX71441, significantly improved micturition indices and cystometry variables in 2 models of overactive bladder, a disorder caused in part by urothelial dysfunction, increased excitability of the detrusor and abnormal functioning of neuronal circuits serving the micturition reflex. Similarly, ADX71943, a novel, potent and selective GABAB receptor PAM was recently reported to reduce pain-associated behaviors in acetic acid induced abdominal writhing test. This effect was blocked by GABAB receptor antagonist CGP63360. ADX71943 also reduced pain in the formalin induced paw withdrawal in mice and rats.48 Allosteric modulators for GABAA receptors have also been developed and tested in several models of pain. While they were found to offer effective analgesic effect in rodent models of somatic pain, their efficacy in visceral pain models is yet to be established. HZ166, a new PAM agonist with preferential activity at GABAAα2 and GABAAα3 receptors showed a dose-dependent anti-hyperalgesic effect in mouse models of neuropathic and inflammatory pain which was triggered by chronic constriction injury of the sciatic nerve and by subcutaneous injection of zymosan. At doses producing maximal antihyperalgesia, HZ166 was found to be devoid of sedation and motor impairment which is usually seen with muscimol and showed no loss of analgesic activity during chronic treatment period.49 Recently, Paul et al50 showed that the antihyperalgesic effect mediated by GABAAα2 receptors occurs exclusively via a genuine spinal action and does not involve supraspinal sites. These findings further support the use of allosteric modulators rather than using traditional ligands as they are devoid of side effects.
Glutamate Receptors
The excitatory amino acid, glutamate, is a major neurotransmitter in the mammalian central nervous system and plays an important role in nociceptive processing. via a diverse set of membrane receptors which include both ionotropic and metabotropic. Activation of these receptors is responsible for basal excitatory synaptic transmission and many forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression, mechanisms that are thought to underlie learning and memory process.51
Ionotropic Glutamate Receptors
Glutamate acts on 3 ionotropic receptor subtypes: N-methyl-D-aspartate receptor (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA), and kainite receptors that are widely reported to be involved in plasticity, brain development, learning, excitatory synaptic transmission, and LTP.52,53 Of these, NMDA receptors have received particular attention because of their crucial roles in excitatory synaptic transmission, plasticity, and neurodegeneration in the CNS.54,55
N-Methyl-D-aspartate receptors
NMDA receptors are calcium channels activated by glutamate and are slowly desensitized once activated. These high-calcium permeable channels generate synaptic neuroplasticity, wide dynamic range neuronal responses and gene expression within the CNS.54 NMDA receptors display a number of unique properties that distinguish them from other ligand-gated ion channels. First, the receptor controls a cation channel that is highly permeable to monovalent ions and calcium. Second, simultaneous binding of glutamate and glycine, the co-agonist, is required for efficient activation of NMDA receptor. Third, at resting membrane potential the NMDA receptor channels are blocked by extracellular magnesium and open only on simultaneous depolarization and agonist binding.55 Native NMDA receptors are composed of NR1, NR2 (A, B, C, and D), and NR3 (A and B) subunits. Co-expression studies have demonstrated that formation of functional NMDA receptor channels requires a combination of NR1, an essential channel-forming subunit, and at least one of the NR2 subunits.56
NMDA receptor activation has long been reported to be associated with visceral hyperalgesia.57 Due to slow inactivation, NMDA receptors produce LTP which is reported to generate chronic pain.58 NMDA receptors are found on enteric extrinsic afferents from colon and bladder; these afferents release calcitonin gene-related peptide and substance P once the receptor is activated.59 NMDA receptors within the rostral ventromedial medulla are important in modulating pain.60 Miranda et al61 reported that spinal NMDA receptors play an important role in the development of hyperalgesia following painful events early in life. The study demonstrated that subpopulations of spinal neurons (short latency-sustained neurons) are sensitized as a result of neonatal somatic pain and the function of these neurons is predominantly influenced by NMDA receptors. In the CNS, hippocampal N-methyl D-aspartate receptor subtype 2B (NR2B)-NMDA receptors were found to be responsible for the facilitation of CA1 LTP via tyrosine phosphorylation, which leads to visceral hypersensitivity.62 In this study, the tyrosine kinase inhibitor (genistein) was found to significantly restrict the induction of LTP via inhibition of tyrosine phosphorylation of NR2B subunit, resulting in the inhibitory effect on LTP maintenance. A similar finding on the role of tyrosine phosphorylation of NR2B subunit was also reported to play a crucial role in central sensitization of chronic visceral pain.63 Zhuo64 suggested increased expression of NR2B subunit of NMDA receptor and glutamate receptor 2 (GluR2) of AMPA receptor might be involved in the key mechanisms for long-term synaptic plastic changes responsible for visceral hypersensitivity in rats. Further, activation of NMDA receptors has been shown to be critical to the manifestation of wind-up in spinal neurons recorded and plays a major role in amplification of a nociceptive input in post-inflammatory conditions.65 In clinical studies, Verne et al66 reported on the involvement of NMDA receptor in the development of somatic hypersensitivity in IBS patients suggesting a role for these receptors in convergence-facilitation mechanism observed in a subset of IBS patients who display enhanced pain sensitivity to rectal and cutaneous heat stimulation.
Kainate receptors
Kainate receptors are ionotropic receptors that respond to the neurotransmitter glutamate. Despite the key role played by glutamate in transmission of nociception, the role of kainate receptors on nociceptive transmission of the viscera has not been investigated While postsynaptic kainate receptors are involved in excitatory neurotransmission, presynaptic kainate receptors have been implicated in inhibitory neurotransmission by modulating release of the inhibitory neurotransmitter GABA through a presynaptic mechanism. There are 5 types of kainate receptor subunits, GluR5, GluR6, GluR7, KA1, and KA2 which are similar to AMPA and NMDA receptor subunits and can be arranged in different ways to form a tetramer.67 The ionotropic kainate receptor subunit, GluR5 (GluK1), is expressed in many regions of nervous system related to sensory transmission. Recent study has also shown that GLUR5 receptor in the spinal cord regulates neurotransmitter release from the GABAergic and glycinergic interneurons in the spinal cord.68 In naïve rats, intrathecal administration of GLUR5-selective antagonist, LY382884 was found to attenuate capsaicin- and carrageenan-induced mechanical allodynia in rats.69 This suggests that under normal conditions, there is a role of these receptors in spinal nociceptive transmission. Spinal administration of MSVIII-19 (a selective ligand for the GluK1 receptor) reverses hypersensitivity in several models of pain in mice, supporting the clinical potential of GluK1 antagonists for the management of pain.70 Similarly, kainate receptor subunit GluR6 was reported to play an important role in the visceral pain.71 Administration of GluR6 antisense oligonucleotides (ODNs) can suppress the expression of GluR6 in rat spinal cord, and subsequently found to alleviate the formalin-induced pain. The results of this study suggest that kainate subunit GluR6 plays a pivotal role in the visceral pain of rectum induced by formalin injection, and it may serve as a potential target in designing new therapy for visceral inflammatory pain.
AMPA receptors
AMPA receptor is a non-NMDA-type ionotropic transmembrane receptor for glutamate that mediates fast synaptic transmission in the CNS. AMPA receptors are composed of various combinations of 4 subunits (GluR1- GluR4), and only AMPA receptors that lack the GluR2 subunit are permeable to Ca2+.72 AMPA receptors are reported to be involved in neuropathic pain conditions; however information on their role in visceral pain is limited. Studies performed by Chen et al62 provide direct evidence that peripheral nerve injury induces postsynaptic GluA1 accumulation in cingulate cortical neurons and inhibits postsynaptic GluA1 accumulation. Banerjee et al73 reported that esophageal acid exposure in rats significantly increased expression of GluA1, pGluA1Ser831, and phosphorylated CaMKIIThr286, in the cortical membrane preparations. Electrophysiology studies showed that microinjection of IEM-1460 (open-channel blocker of AMPA receptors.) near the recording site significantly attenuated acid-induced sensitization of cortical neurons suggesting a role for AMPA receptors in acid reflux induced esophageal pain.
Metabotropic Glutamate Receptor
Metabotropic glutamate (mGlu) receptors are G-protein coupled receptors that have been subdivided into 3 groups, based on sequence similarity, pharmacology and intracellular signaling mechanisms. Group I mGlu receptors (mGlu1 and mGlu5) are coupled to PLC and intracellular calcium signaling, while group II (mGlu2 and mGlu3) and group III receptors (mGlu4, mGlu6, mGlu7, and mGlu8) are negatively coupled to adenylyl cyclase.74–76 Lindström et al77 first reported that the mGluR5 antagonists (MPEP and MTEP) inhibit colorectal distension-evoked viscero-motor responses and cardiovascular changes in conscious rats suggesting that mGluR5 participates in mediating mechanically evoked visceral nociception in the gastrointestinal tract. Crock et al78 demonstrated that demonstrated that activation of metabotropic glutamate receptor 5 (mGluR5) in the central nucleus of the amygdala (CeA) induces bladder pain sensitization by increasing CeA output. Optogenetic activation of the CeA neurons expressing mGluR5 receptor was found to produce a robust increase in the visceral pain response indicating the involvement of mGluR5 receptors in visceral pain. The CeA-localized effects on responses to bladder distention were associated with changes in extracellular signal-regulated kinases 1/2 (ERK1/2) phosphorylation in the spinal cord demonstrating that mGluR5 activation leads to increased CeA output that drives bladder pain sensitization. Liu et al79 showed that Activation of mGluR7 in the nucleus tractus solitarii had anti-nociceptive effects, whereas activation of mGluR8 in the nucleus tractus solitarii had pro-nociceptive effects on cardiac-evoked muscle hyperalgesia and this facilitatory effect was found to be dependent on vagal afferents.
Opioid Receptors
Opioid receptors are a group of G protein-coupled receptors with opioids as ligands. They are activated both by endogenously produced opioid peptides and by exogenously administered opioid drugs, such as morphine, which are not only among the most effective analgesics known but also highly addictive drugs of abuse.80 To date, 4 types of opioid receptors have been identified: μ (mu for morphine), κ (kappa for ketocyclazocine), δ (delta for deferens given that it was originally discovered in the vas deferens of mice).80 The use of opioid analgesics has a long history, dating back several millennia. In spite of extensive research and wide spread use of opiods for the treatment of various forms of pain, controversies still linger. The mu, delta and kappa opioid receptors are all involved in nociception and are thus obvious candidates for a targeted drug development in the clinical treatment of both acute and chronic pain.
Kappa and mu opioid receptors are found on visceral afferents.81–83 Distribution along the gastrointestinal tract indicates that endogenous opiate peptides have a modulating function for both gastrointestinal motility and secretory function. Su et al84 documented that responses of mechanosensitive pelvic nerve afferent fibers innervating the colon are inhibited by kappa-opioid receptor agonists having varying affinities for putative kappa-opioid receptor subtypes. Su et al85 also reported that kappa, but not mu or delta opioid receptor agonists attenuated the responses of bladder sensitive pelvic nerve afferent fibers to urinary bladder distension suggesting a role for these receptors in modulating bladder pain. Sengupta et al86 reported a peripheral upregulation of kappa-opioid receptors in rats following in colonic inflammation produced by intracolonic instillation of trinitrobenzene sulfonic acid. Clinical studies with human volunteers found that oxycodone which is thought to be a kappa and mu receptor agonist significantly blocked visceral pain better than morphine which has little kappa receptor activity.87 Recently, Fichna et al88 showed that the activation of the endogenous nociceptin/orphanin (N/OFQergic) system by using a new potent non-peptide nociception orphanin peptide agonist, SCH 221510, displays a significant antinociceptive effect in 2 mouse models of visceral hypersensitivity mimicking IBS pathology. Moreover, the same group has previously reported an antinociceptive effect of the same drug when orally administered in a mouse model of trinitrobenzene sulfonic acid-induced colitis.89 In vitro afferent recordings from mouse splanchnic high-threshold nociceptors from mice with colonic inflammation we found that asimadoline (peripherally restricted selective KOR agonist) dose-dependently inhibited colonic nociceptors. Furthermore, asimadoline also dose dependently inhibited colonic nociceptors from chronic visceral hypersensitivity, an effect that was prevented by the prior application of a KOR antagonist. Overall, these data indicate KOR expression is functionally upregulated during inflammation and chronic visceral hypersensitivity.90
Serotonin Receptors
Serotonin (5-HT) is an important neurotransmitter in the brain-gut interaction, with 80% of the total body 5-HT located in the gastrointestinal (GI) tract.91 Approximately 95% of the human body’s serotonin is produced and stored in enterochromaffin cells in the intestinal epithelium. However, small amounts of 5-HT are also present in serotonergic neurons of the enteric nervous system where 5-HT takes part in the slow and fast neurotransmission.92–94 Serotonin exerts its biological activity through interaction with different receptors, currently classified into 7 groups on the basis of their structure, transduction mechanism and pharmacological profile: 5-HT1–7.95,96 Most of these receptors are expressed in the GI tract, and their stimulation plays different roles (either inhibitory or excitatory) in the control of intestinal motility and secretion. The 5-HT3 receptor is coupled to an ion channel, whereas 5-HT 1,2,4,5,6,7 receptors are coupled to G proteins.97 Although the 5-HT receptors are extensively investigated, conflicting results exist regarding the role of these receptors in somatic and visceral nociceptive processing.
Mickle et al98 reported that the 5-HT1A receptor agonist DPAT produces pronociceptive effects, primarily via the activation of presynaptic 5-HT1A receptors in GABAergic neuron to restrict GABA release and thereby disinhibiting the excitatory glutamatergic neurons in the spinal cord. The 5-HT4 receptor agonist tegaserod has also been reported to modulate visceral pain. Tegaserod produces analgesia via activation of supraspinal 5-HT4 receptors which triggers the release of opioids at supraspinal site, which in turn activates the descending noradrenergic pathways to the spinal cord to produce analgesia.99 Systemically and centrally administered alosetron (5-HT3 antagonist), reversed the mechanical somatic hypersensitivity and prevented the development of visceral hyperalgesia, suggesting a centrally mediated effect.100
Cannabinoid Receptors
The cannabinoid (CB) receptors found in mammals are CB1 and CB2, both members of the superfamily of G protein-coupled receptors. CB1 receptors are found primarily in neurons of the brain and GI tract extrinsic and intrinsic nervous system.101 CB2 receptors have been identified through immunohistochemical studies in most neurons of the ileum enteric nervous system of mice and in peripheral immune cells.102,103 There is a variety of published data to show that cannabinoid receptor agonists have an antinociceptive effect in inflammatory and visceral hyper-algesia in animal models, however these observations are not convincingly supported by human studies.
Feng et al104 reported on the role of endocannabinoids in 5-HT mediated modulation of visceral nociception in a rat model suggesting that vagal 5-HT3 receptor-mediated duodenal anandamide release contributes to acute luminal 5-HT-induced antinociception via CB1 signaling. In contrast, decreased anandamide is associated with hyperalgesia upon chronic 5-HT treatment. Similarly, taranabant, an inverse agonist of CB1 receptor was reported to reduce abdominal pain and increases intestinal transit in mice.88 While several animal studies have been reported on the analgesic effect of CB receptor agonists, some clinical studies argue against centrally acting CB agonists as tool to decrease visceral hypersensitivity. In a case controlled study involving ten IBS patients and 12 healthy volunteers, mixed CB1/CB2 receptor agonist delta-9-tetrahydrocannabinol (dronabinol) did not alter baseline rectal perception to distension compared to placebo in healthy or IBS patients. Similarly, after sigmoid stimulation there were no significant differences between placebo and delta-9-tetrahydrocannabinol in sensory thresholds of discomfort indicating non-involvement of these receptors in visceral sensation.105 These reports necessitate the need to further investigate the role of CB receptors in somatic and visceral pain conditions.
Transient Receptor Potential Ion Channels
The transient receptor potential (TRP) family of ion channels is TRPV1, TRPV2, TRPV3, TRPV4, TRPM 8, and TRPA1. These channels are, in general, thermoreceptors found on poorly myelinated and nonmyelinated afferents arising from the dorsal root ganglia, nodose ganglia, and the CNS.106–110 These channels are calcium permeable, nonselective channels with 4 identical subunits and a central pore. TRPA1 is highly expressed on primary afferents.111
TRPV1 has been demonstrated to be upregulated in upper gastrointestinal disorders such as gastroesophageal reflux disease and pancreatitis.112,113 TRPV1 expression was found to be upregulated not only in those with inflammatory bowel disease in remission but also with irritable bowel type symptoms.114 TRPA1 is upregulated in experimental colitis and deletion of TRPA1 expression in a mouse gene knock-out model reduced colitis-induced mechanical hypersensitivity.115 Charrua et al116 also reported on the synergistic effect of TRPV1 and TRPV4 receptors although they are expressed in different bladder afferent populations. While low doses of TRPV4 antagonist (RN1734) and TRPV1 antagonist (SB366791) had no effect on bladder activity, co-administration of the 2 completely reversed bladder hyper-activity induced by lipopolysaccharide. The synergistic activity of antagonists for these receptors in very low doses may offer the opportunity to treat lower urinary tract symptoms while minimizing the potential side-effects of each drug. Jurik et al117 reported that constitutive genetic deletion of TRPV1 or peripheral TRPV1 deletion reduced acetic acid-evoked abdominal constrictions, without affecting referred abdominal hyperalgesia or allodynia in an acute pancreatitis induced visceral pain model. Additionally, intracerebral TRPV1 antagonism by SB 366791 significantly reduced chemical and inflammatory spontaneous abdominal nocifensive responses, as observed by reduced expressions of nociceptive facial grimacing. In addition to the established role of cerebral TRPV1 in anxiety, fear, or emotional stress, this study demonstrates for the first time that TRPV1 in the brain modulates visceral nociception by interfering with the affective component of abdominal pain. The transient receptor potential canonical subfamily 4 (TRPC4) ion channel, which is involved in the tissue-specific and stimulus-dependent regulation of intracellular Ca2+ signaling also plays a role in visceral pain. Westlund et al118 reported that rats with TRPC4-knockout mutation displayed tolerance to visceral pain induced by colonic mustard oilexposure, but not somatic or neuropathic pain stimuli. Behavioral studies showed that ML-204 (TRPC4 antagonist) inhibited visceral pain-related behavior in a dose-dependent manner without noticeable adverse effects. These data provide evidence that TRPC4 is required for detection and/or transmission of colonic mustard oil visceral pain sensation.
Conclusion
It is now widely accepted that chronic visceral pain is mediated by unique peripheral and central mechanisms.119,120 This article, based on a literature review, focused on the involvement of the major ionotropic and metabotropic receptors in the pathophysiology of chronic visceral pain. Considerable advances in research have provided an insight into many of the molecular mechanisms involved and animal studies using clinically relevant models of visceral pain along with in vitro studies are expanding the field of knowledge. In summary, various ionotropic and metabotropic are widely reported to be involved in visceral pain modulation and is likely to involve a number of interactive components and pathways. The involvement of these molecular components in visceral hypersensitivity in experimental models and patients provides a deeper insight and complexities involved and the challenges associated with the treatment of visceral pain. Many of the potential targets discussed here need to be translated into clinical practice with further good clinical trials.
Footnotes
Conflicts of interest: None.
Financial support: The study was supported by an NIH (Grant No. R01DK099201-01).
References
- 1.Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20(suppl 7):31–39. doi: 10.1111/j.1365-2036.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 2.Patrizi F, Freedman SD, Pascual-Leone A, Fregni F. Novel therapeutic approaches to the treatment of chronic abdominal visceral pain. Scientific World Journal. 2006;6:472–490. doi: 10.1100/tsw.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collett B. Visceral pain: the importance of pain management services. Br J Pain. 2013;7:6–7. doi: 10.1177/2049463713480138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilleri M, Williams DE. Economic burden of irritable bowel syndrome. Proposed strategies to control expenditures. Pharmaco-economics. 2000;17:331–338. doi: 10.2165/00019053-200017040-00003. [DOI] [PubMed] [Google Scholar]
- 5.Farmer AD, Aziz Q. Visceral pain hypersensitivity in functional gastrointestinal disorders. Br Med Bull. 2009;91:123–136. doi: 10.1093/bmb/ldp026. [DOI] [PubMed] [Google Scholar]
- 6.Docherty MJ, Jones RC, 3rd, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (NY) 2011;7:592–601. [PMC free article] [PubMed] [Google Scholar]
- 7.Konturek SJ, Pawlik WW, Dajani EZ. Brain-gut axis in gastrointestinal system: introductory remarks. J Physiol Pharmacol. 2003;54(suppl 4):3–7. [Google Scholar]
- 8.Farmer AD, Aziz Q. Gut pain & visceral hypersensitivity. Br J Pain. 2013;7:39–47. doi: 10.1177/2049463713479229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 10.Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/S0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- 11.Ren K, Anseloni V, Zou SP, et al. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587(Pt 12):2927–2935. doi: 10.1113/jphysiol.2008.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda A, Mickle A, Schmidt J, et al. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscero-visceral convergence. Neurogastroenterol Motil. 2011;23:683, e281. doi: 10.1111/j.1365-2982.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broderick DF. Neuroimaging in neuropsychiatry. Psychiatr Clin North Am. 2005;28:549–566. doi: 10.1016/j.psc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Akbar A, Walters JR, Ghosh S. Review article: visceral hyper-sensitivity in irritable bowel syndrome: molecular mechanisms and therapeutic agents. Aliment Pharmacol Ther. 2009;30:423–435. doi: 10.1111/j.1365-2036.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- 17.Wesselmann U, Baranowski AP, Börjesson M, et al. Emerging therapies and novel approaches to visceral pain. Drug Discov Today Ther Strateg. 2009;6:89–95. doi: 10.1016/j.ddstr.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis MP. Drug management of visceral pain: concepts from basic research. Pain Res Treat. 2012;2012:265605. doi: 10.1155/2012/265605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervero F. Visceral pain - Central sensitisation. Gut. 2000;47(suppl 4):iv56–iv57. doi: 10.1136/gut.47.suppl_4.iv56. discussion iv58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283:1710–1714. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- 21.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 22.de Carvalho Rocha HA, Dantas BP, Rolim TL, Costa BA, de Medeiros AC. Main ion channels and receptors associated with visceral hypersensitivity in irritable bowel syndrome. Ann Gastroenterol. 2014;27:200–206. [PMC free article] [PubMed] [Google Scholar]
- 23.Chebib M, Johnston GA. GABA-activated ligand gated ion channels: Medicinal chemistry and molecular biology. J Med Chem. 2000;43:1427–1447. doi: 10.1021/jm9904349. [DOI] [PubMed] [Google Scholar]
- 24.Bormann J. The ‘ABC’ of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/S0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 25.Johnston GA. GABAA receptor channel pharmacology. Curr Pharm Des. 2005;11:1867–1885. doi: 10.2174/1381612054021024. [DOI] [PubMed] [Google Scholar]
- 26.Gassmann M, Bettler B. Regulation of neuronal GABAB receptor functions by subunit composition. Nat Rev Neurosci. 2012;13:380–394. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
- 27.Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, 4th, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/S1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- 29.Mirza NR, Munro G. The role of GABAA receptor subtypes as analgesic targets. Drug News Perspect. 2010;23:351–360. doi: 10.1358/dnp.2010.23.6.1489909. [DOI] [PubMed] [Google Scholar]
- 30.Munro G, Hansen RR, Mirza NR. GABAA receptor modulation: potential to deliver novel pain medicines? Eur J Pharmacol. 2013;716:17–23. doi: 10.1016/j.ejphar.2013.01.070. [DOI] [PubMed] [Google Scholar]
- 31.Sengupta JN, Pochiraju S, Kannampalli P, et al. MicroRNA-mediated GABAAα-1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. Pain. 2013;154:59–70. doi: 10.1016/j.pain.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brink TS, Hellman KM, Lambert AM, Mason P. Raphe magnus neurons help protect reactions to visceral pain from interruption by cutaneous pain. J Neurophysiol. 2006;96:3423–3432. doi: 10.1152/jn.00793.2006. [DOI] [PubMed] [Google Scholar]
- 33.Partosoedarso ER, Young RL, Blackshaw LA. GABAB receptors on vagal afferent pathways: peripheral and central inhibition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G658–G668. doi: 10.1152/ajpgi.2001.280.4.G658. [DOI] [PubMed] [Google Scholar]
- 34.Smid SD, Young RL, Cooper NJ, Blackshaw LA. GABABR expressed on vagal afferent neurones inhibit gastric mechanosensitivity in ferret proximal stomach. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1494–G1501. doi: 10.1152/ajpgi.2001.281.6.G1494. [DOI] [PubMed] [Google Scholar]
- 35.Saulino M. The use of intrathecal baclofen in pain management. Pain Manag. 2012;2:603–608. doi: 10.2217/pmt.12.60. [DOI] [PubMed] [Google Scholar]
- 36.Bussières N, El Manira A. GABAB receptor activation inhibits N- and P/Q-type calcium channels in cultured lamprey sensory neurons. Brain Res. 1999;847:175–185. doi: 10.1016/S0006-8993(99)02002-8. [DOI] [PubMed] [Google Scholar]
- 37.Filippov AK, Couve A, Pangalos MN, Walsh FS, Brown DA, Moss SJ. Heteromeric assembly of GABABR1 and GABABR2 receptor subunits inhibits Ca2+ current in sympathetic neurons. J Neurosci. 2000;20:2867–2874. doi: 10.1523/JNEUROSCI.20-08-02867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Lambert NA. GABAB receptors couple to potassium and calcium channels on identified lateral perforant pathway projection neurons. J Neurophysiol. 2000;83:1073–1078. doi: 10.1152/jn.2000.83.2.1073. [DOI] [PubMed] [Google Scholar]
- 39.Sengupta JN, Medda BK, Shaker R. Effect of GABAB receptor agonist on distension-sensitive pelvic nerve afferent fibers innervating rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1343–G1351. doi: 10.1152/ajpgi.00124.2002. [DOI] [PubMed] [Google Scholar]
- 40.Brusberg M, Ravnefjord A, Martinsson R, Larsson H, Martinez V, Lindström E. The GABAB receptor agonist, baclofen, and the positive allosteric modulator, CGP7930, inhibit visceral pain-related responses to colorectal distension in rats. Neuropharmacology. 2009;56:362–367. doi: 10.1016/j.neuropharm.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Hara K, Saito Y, Kirihara Y, Yamada Y, Sakura S, Kosaka Y. The interaction of antinociceptive effects of morphine and GABA receptor agonists within the rat spinal cord. Anesth Analg. 1999;89:422–427. doi: 10.1097/00000539-199908000-00032. [DOI] [PubMed] [Google Scholar]
- 42.Lindström E, Brusberg M, Ravnefjord A, et al. Oral baclofen reduces visceral pain-related pseudo-affective responses to colorectal distension in rats: relation between plasma exposure and efficacy. Scand J Gastroenterol. 2011;46:652–662. doi: 10.3109/00365521.2011.560677. [DOI] [PubMed] [Google Scholar]
- 43.Birder LA, de Groat WC. Contribution of C-fiber afferent nerves and autonomic pathways in the urinary bladder to spinal c-fos expression induced by bladder irritation. Somatosens Mot Res. 1998;15:5–12. doi: 10.1080/08990229870916. [DOI] [PubMed] [Google Scholar]
- 44.Lu Y, Westlund KN. Effects of baclofen on colon inflammation-induced Fos, CGRP and SP expression in spinal cord and brainstem. Brain Res. 2001;889:118–130. doi: 10.1016/S0006-8993(00)03124-3. [DOI] [PubMed] [Google Scholar]
- 45.Hara K, Saito Y, Kirihara Y, Sakura S. The interaction between gamma-aminobutyric acid agonists and diltiazem in visceral anti-nociception in rats. Anesth Analg. 2004;98:1380–1384. doi: 10.1213/01.ANE.0000107935.84035.48. [DOI] [PubMed] [Google Scholar]
- 46.Ong J, Kerr DI. Clinical potential of GABAB receptor modulators. CNS Drug Rev. 2005;11:317–334. doi: 10.1111/j.1527-3458.2005.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalinichev M, Palea S, Haddouk H, et al. ADX71441, a novel, potent and selective positive allosteric modulator of the GABAB receptor, shows efficacy in rodent models of overactive bladder. Br J Pharmacol. 2014;171:995–1006. doi: 10.1111/bph.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalinichev M, Donovan-Rodriguez T, Girard F, et al. Evaluation of peripheral versus central effects of GABAB receptor activation using a novel, positive allosteric modulator of the GABAB receptor ADX71943, a pharmacological tool compound with a fully peripheral activity profile. Br J Pharmacol. 2014;171:4941–4954. doi: 10.1111/bph.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Lio A, Benke D, Besson M, et al. HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul J, Yévenes GE, Benke D, et al. Antihyperalgesia by α2-GABAA receptors occurs via a genuine spinal action and does not involve supraspinal sites. Neuropsychopharmacology. 2014;39:477–487. doi: 10.1038/npp.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee B, Medda BK, Zheng Y, et al. Alterations in N-methyl-D-aspartate receptor subunits in primary sensory neurons following acid-induced esophagitis in cats. Am J Physiol Gastrointest Liver Physiol. 2009;296:G66–G77. doi: 10.1152/ajpgi.90419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traynelis SF, Wollmuth LP, McBain CJ, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/S0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 55.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 56.Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–37. doi: 10.1016/0028-3908(95)00109-J. [DOI] [PubMed] [Google Scholar]
- 57.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 58.Randic M, Jiang MC, Rusin KI, Cerne R, Kolaj M. Interactions between excitatory amino acids and tachykinins and long-term changes of synaptic responses in the rat spinal dorsal horn. Regul Pept. 1993;46:418–420. doi: 10.1016/0167-0115(93)90106-I. [DOI] [PubMed] [Google Scholar]
- 59.McRoberts JA, Coutinho SV, Marvizón JC, et al. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- 60.Da Silva LF, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain. 2010;151:155–161. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miranda A, Mickle A, Bruckert M, Kannampalli P, Banerjee B, Sengupta JN. NMDA receptor mediates chronic visceral pain induced by neonatal noxious somatic stimulation. Eur J Pharmacol. 2014;744:28–35. doi: 10.1016/j.ejphar.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen T, Wang W, Dong YL, et al. Postsynaptic insertion of AMPA receptor onto cortical pyramidal neurons in the anterior cingulate cortex after peripheral nerve injury. Mol Brain. 2014;7:76–88. doi: 10.1186/s13041-014-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo XQ, Cai QY, Chen Y, et al. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord contributes to chronic visceral pain in rats. Brain Res. 2014;1542:167–175. doi: 10.1016/j.brainres.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130146. doi: 10.1098/rstb.2013.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verne GN, Price DD, Callam CS, Zhang B, Peck J, Zhou Q. Viscerosomatic facilitation in a subset of IBS patients, an effect mediated by N-methyl-D-aspartate receptors. J Pain. 2012;13:901–909. doi: 10.1016/j.jpain.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 68.Xu H, Wu LJ, Zhao MG, et al. Presynaptic regulation of the inhibitory transmission by GluR5-containing kainate receptors in spinal substantia gelatinosa. Mol Pain. 2006;2:29. doi: 10.1186/1744-8069-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones CK, Alt A, Ogden AM, et al. Antiallodynic and anti-hyperalgesic effects of selective competitive GLUK5 (GluR5) ionotropic glutamate receptor antagonists in the capsaicin and carrageenan models in rats. J Pharmacol Exp Ther. 2006;319:396–404. doi: 10.1124/jpet.106.105601. [DOI] [PubMed] [Google Scholar]
- 70.Qiu CS, Lash-Van Wyhe L, Sasaki M, Sakai R, Swanson GT, Gereau RW., 4th Antinociceptive effects of MSVIII-19, a functional antagonist of the GluK1 kainate receptor. Pain. 2011;152:1052–1060. doi: 10.1016/j.pain.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang WG, Zhang LC, Peng ZD, Zeng YM. Intrathecal injection of GluR6 antisense oligodeoxynucleotides alleviates acute inflammatory pain of rectum in rats. Neurosci Bull. 2009;25:319–323. doi: 10.1007/s12264-009-0326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 73.Banerjee B, Medda BK, Pochiraju S, et al. AMPA receptor subunits expression and phosphorylation in cingulate cortex in rats following esophageal acid exposure. Neurogastroenterol Motil. 2013;25:973, e776. doi: 10.1111/nmo.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 75.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salt TE, Jones HE, Copeland CS, Sillito AM. Function of mGlu1 receptors in the modulation of nociceptive processing in the thalamus. Neuropharmacology. 2014;79:405–411. doi: 10.1016/j.neuropharm.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindström E, Brusberg M, Hughes PA, et al. Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain. 2008;137:295–305. doi: 10.1016/j.pain.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Crock LW, Kolber BJ, Morgan CD, et al. Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J Neurosci. 2012;32:14217–14226. doi: 10.1523/JNEUROSCI.1473-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu XH, Han M, Zhu JX, et al. Metabotropic glutamate subtype 7 and 8 receptors oppositely modulate cardiac nociception in the rat nucleus tractus solitarius. Neuroscience. 2012;220:322–329. doi: 10.1016/j.neuroscience.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 80.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 81.Ding YQ, Li JL, Lü BZ, Wang D, Zhang ML, Li JS. Co-localization of mu-opioid receptor-like immunoreactivity with substance P-LI, calcitonin gene-related peptide-LI and nitric oxide synthase-LI in vagal and glossopharyngeal afferent neurons of the rat. Brain Res. 1998;792:149–153. doi: 10.1016/S0006-8993(98)00205-4. [DOI] [PubMed] [Google Scholar]
- 82.Aicher SA, Goldberg A, Sharma S, Pickel VM. μ-opioid receptors present in vagal afferents and their dendritic targets in the medial nucleus tractus solitaries. J Comp Neurol. 2000;422:181–190. doi: 10.1002/(SICI)1096-9861(20000626)422:2<181::AID-CNE3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 83.Rau KK, Caudle RM, Cooper BY, Johnson RD. Diverse immunocytochemical expression of opioid receptors in electro-physiologically defined cells of rat dorsal root ganglia. J Chem Neuroanat. 2005;29:255–264. doi: 10.1016/j.jchemneu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Su X, Sengupta JN, Gebhart GF. Effects of kappa opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol. 1997;78:1003–1012. doi: 10.1152/jn.1997.78.2.1003. [DOI] [PubMed] [Google Scholar]
- 85.Su X, Sengupta JN, Gebhart GF. Effects of opioids on mechano-sensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1997;77:1566–1580. doi: 10.1152/jn.1997.77.3.1566. [DOI] [PubMed] [Google Scholar]
- 86.Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain. 1999;79:175–185. doi: 10.1016/S0304-3959(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 87.Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. A comparative study of oxycodone and morphine in amulti-modal, tissue-differentiated experimental pain model. Pain. 2006;123:28–36. doi: 10.1016/j.pain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 88.Fichna J, Sibaev A, Sałaga M, Sobczak M, Storr M. The cannabinoid-1 receptor inverse agonist taranabant reduces abdominal pain and increases intestinal transit in mice. Neurogastroenterol Motil. 2013;25:e550–e559. doi: 10.1111/nmo.12158. [DOI] [PubMed] [Google Scholar]
- 89.Sobczak M, Cami-Kobeci G, Salaga M, Husbands SM, Fichna J. Novel mixed NOP/MOP agonist BU08070 alleviates pain and inhibits gastrointestinal motility in mouse models mimicking diarrhea-predominant irritable bowel syndrome symptoms. Eur J Pharmacol. 2014;736:63–69. doi: 10.1016/j.ejphar.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes PA, Castro J, Harrington AM, et al. Increased κ-opioid receptor expression and function during chronic visceral hypersensitivity. Gut. 2014;63:1199–1200. doi: 10.1136/gutjnl-2013-306240. [DOI] [PubMed] [Google Scholar]
- 91.Greenwood-van Meerveld B. Importance of 5-hydroxytryptamine receptors on intestinal afferents in the regulation of visceral sensitivity. Neurogastroenterol Motil. 2007;19(suppl 2):13–18. doi: 10.1111/j.1365-2982.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- 92.Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13(suppl 2):15–30. doi: 10.1046/j.1365-2036.1999.00002.x-i2. [DOI] [PubMed] [Google Scholar]
- 93.Gershon MD. Review article: serotonin receptors and transporters - roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 94.Gershon MD, Tack J. The serotonin signalling system: From basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 95.Varnäs K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tonini M. 5-Hydroxytryptamine effects in the gut: the 3, 4 and 7 receptors. Neurogastroenterol Motil. 2005;17:637–642. doi: 10.1111/j.1365-2982.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- 97.Stasi C, Bellini M, Bassotti G, Blandizzi C, Milani S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech Coloproctol. 2014;18:613–621. doi: 10.1007/s10151-013-1106-8. [DOI] [PubMed] [Google Scholar]
- 98.Mickle A, Kannampalli P, Bruckert M, Miranda A, Banerjee B, Sengupta JN. Pronociceptive effect of 5-HT1A receptor agonist on visceral pain involves spinal N-methyl-D-aspartate (NMDA) receptor. Neuroscience. 2012;219:243–254. doi: 10.1016/j.neuroscience.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sengupta JN, Mickle A, Kannampalli P, et al. Visceral analgesic effect of 5-HT4 receptor agonist in rats involves the rostroventral medulla (RVM) Neuropharmacology. 2014;79:345–358. doi: 10.1016/j.neuropharm.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miranda A, Peles S, McLean PG, Sengupta JN. Effects of the 5-HT3 receptor antagonist, alosetron, in a rat model of somatic and visceral hyperalgesia. Pain. 2006;126:54–63. doi: 10.1016/j.pain.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 101.Storr MA, Yüce B, Andrews CN, Sharkey KA. The role of the endocannabinoid system in the pathophysiology and treatment of irritable bowel syndrome. Neurogastroenterol Motil. 2008;20:857–868. doi: 10.1111/j.1365-2982.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 102.Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duncan M, Mouihate A, Mackie K, et al. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol. 2008;295:G78–G87. doi: 10.1152/ajpgi.90285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng CC, Yan XJ, Chen X, et al. Vagal anandamide signaling via cannabinoid receptor 1 contributes to luminal 5-HT modulation of visceral nociception in rats. Pain. 2014;155:1591–1604. doi: 10.1016/j.pain.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Klooker TK, Leliefeld KE, Van Den Wijngaard RM, Boeckxstaens GE. The cannabinoid receptor agonist delta-9-tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol Motil. 2011;23:30–35. e2. doi: 10.1111/j.1365-2982.2010.01587.x. [DOI] [PubMed] [Google Scholar]
- 106.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neliropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 107.Valtschanoff JG, Rustioni A, Guo A, Hwang SJ. Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. J Comp Neurol. 2001;436:225–235. doi: 10.1002/cne.1063. [DOI] [PubMed] [Google Scholar]
- 108.Hwang SJ, Valtschanoff JG. Vanilloid receptor VR1-positive afferents are distributed differently at different levels of the rat lumbar spinal cord. Neurosci Lett. 2003;349:41–44. doi: 10.1016/S0304-3940(03)00750-X. [DOI] [PubMed] [Google Scholar]
- 109.Roberts LA, Connor M. TRPV1 antagonists as a potential treatment for hyperalgesia. Recent Pat CNS Drug Discov. 2006;1:65–76. doi: 10.2174/157488906775245309. [DOI] [PubMed] [Google Scholar]
- 110.Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol. 2006;4:1–15. doi: 10.2174/157015906775202995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lapointe TK, Altier C. The role of TRPA1 in visceral inflammation and pain. Channels. 2011;5:525–529. doi: 10.4161/chan.5.6.18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DJ, Anand P. Increased capsaicin receptor TRPV1 nerve fibers in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 113.Adam B, Liebregts T, Gschossmann JM, et al. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain. 2006;123:179–186. doi: 10.1016/j.pain.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 114.Akbar A, Yiangou Y, Facer P, et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59:767–774. doi: 10.1136/gut.2009.194449. [DOI] [PubMed] [Google Scholar]
- 115.Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G81–G91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Charrua A, Cruz CD, Jansen D, Rozenberg B, Heesakkers J, Cruz F. Co-administration of transient receptor potential vanilloid 4 (TRPV4) and TRPV1 antagonists potentiate the effect of each drug in a rat model of cystitis. BJU Int. 2015;115:452–460. doi: 10.1111/bju.12861. [DOI] [PubMed] [Google Scholar]
- 117.Jurik A, Ressle A, Schmid RM, Wotjak CT, Thoeringer CK. Supraspinal TRPV1 modulates the emotional expression of abdominal pain. Pain. 2014;155:2153–2160. doi: 10.1016/j.pain.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 118.Westlund KN, Zhang LP, Ma F, et al. A rat knockout model implicates TRPC4 in visceral pain sensation. Neuroscience. 2014;262:165–175. doi: 10.1016/j.neuroscience.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cervero F. Visceral pain: mechanisms of peripheral and central sensitization. Ann Med. 1995;27:235–239. doi: 10.3109/07853899509031965. [DOI] [PubMed] [Google Scholar]
- 120.Moshiree B, Zhou Q, Price DD, Verne GN. Central sensitisation in visceral pain disorders. Gut. 2006;55:905–908. doi: 10.1136/gut.2005.078287. [DOI] [PMC free article] [PubMed] [Google Scholar]