Review of regulation of mTOR signaling in T cells, and roles of mTOR and metabolic pathways in conventional and regulatory T cells.
Keywords: metabolism, Tregs, T cell differentiation, lipid, glycolysis
Abstract
mTOR signaling links bioenergetic and biosynthetic metabolism to immune responses. mTOR is activated by diverse upstream stimuli, including immune signals, growth factors, and nutrients. Recent studies highlight crucial roles of mTOR signaling in immune functions mediated by conventional T cells and Tregs. In this review, we discuss the regulation of mTOR signaling in T cells and the functional impacts of mTOR and metabolic pathways on T cell-mediated immune responses, with a particular focus on the differentiation and function of Tregs.
Introduction
Over the last 2 decades, the function of mTOR in basic cellular biology and more recently, immunology has been widely investigated. mTOR is an atypical serine/threonine kinase related to the PI3Ks and integrates signals from surface receptors, environmental cues, and nutrients to exert a variety of cellular functions. mTOR interacts with several scaffolding proteins to form mTORC1 and mTORC2, which contain the obligate adaptors, Raptor and Rictor, respectively (Fig. 1A). mTOR signaling regulates gene expression and protein translation, driving cell growth, proliferation, and survival. The mTORC1-driven phosphorylation of the ribosomal S6 protein and the translational regulator 4EBP-1 are linked to these processes [1, 2]. Additionally, mTOR signaling activates c-MYC, HIF-1α, and SREBP transcriptional activity to control cell metabolism [3] (Fig. 1B). As it is a key regulator of metabolism and other cellular functions, abnormal mTOR signaling is linked to cancer, obesity, type-2 diabetes, and neurodegeneration [2].
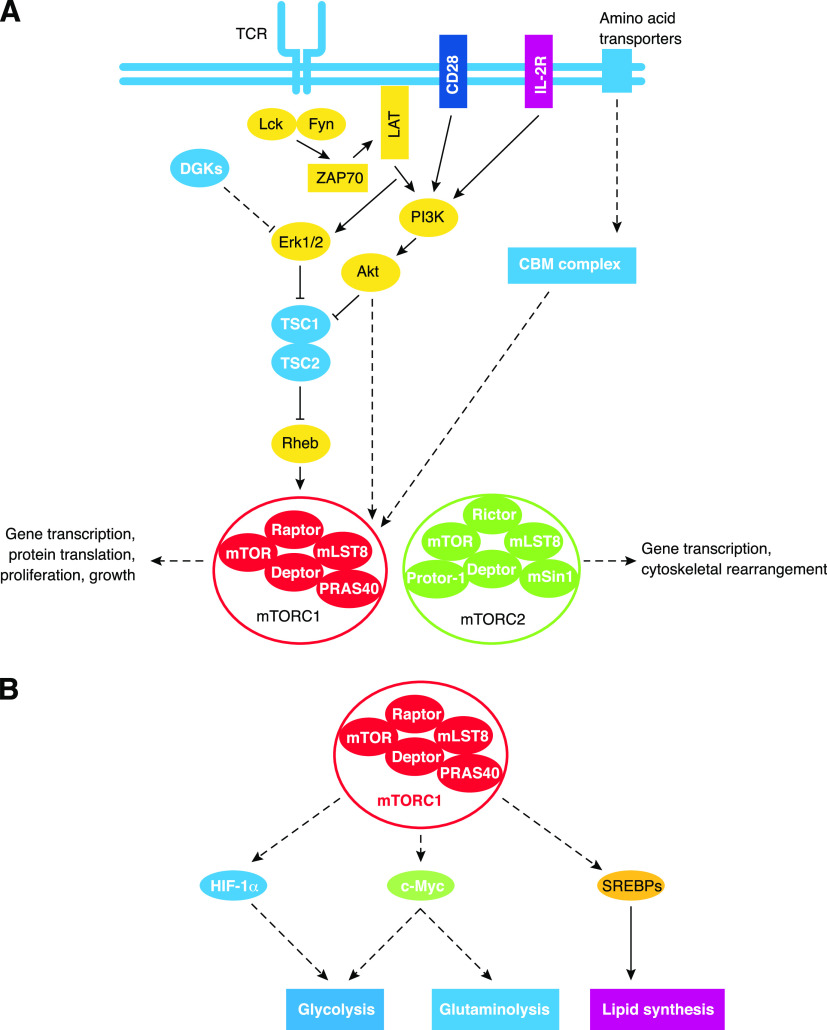
Figure 1. Overview of mTOR signaling and metabolic regulation.
(A) Activators and inhibitors of mTORC1 and mTORC2 signaling in T cells. mTORC1 is activated by surface receptor signaling, environment cues, and nutrients. TCR, CD28, and IL-2R signaling to the PI3K-Akt pathway activates mTORC1 via multiple mechanisms. These include inhibiting the activity of TSC2, promoting stabilized Raptor-mTOR interactions, and removing steric hindrance mediated by PRAS40. TCR and CD28-induced Erk1/2 activation may also facilitate mTORC1 activation, and this process is likely antagonized by DGKs. Amino acid uptake enhances mTORC1 activation via the CBM complex by unknown mechanisms. Collectively, mTORC1 signaling promotes many functional outputs. The upstream regulators of mTORC2 activation remain poorly understood, but mTORC2 signaling drives cytoskeletal rearrangements and alters gene transcription. (B) Control of metabolic programs by mTORC1, which regulates metabolism via several transcriptional factors. mTORC1 activates HIF-1α and c-MYC to promote glycolysis. Additionally, c-MYC stimulates glutaminolysis. mTORC1 also triggers lipid synthesis by activating SREBPs. mLST8, mammalian lethal with SEC13 protein 8; Deptor, disheveled, Egl-10, and pleckstrin domain-containing mTOR-interacting protein, Protor-1, protein observed with Rictor-1; mSin1, mammalian stress-activated protein kinase-interacting protein 1.
Recent work demonstrates that mTOR signaling is a critical regulator of T cell biology [1, 4]. Conventional T cells, which are comprised of naïve, effector, and memory CD4+ or CD8+ T cells, mediate antigen-specific immune responses to pathogens. iNKT cells are a nonconventional T cell population with diverse functions [5]. Dysregulation of conventional and non-conventional T cell responses promotes autoimmune and other immune-mediated disorders [5, 6]. Tregs curtail excessive immune reactions and are classified into 3 groups according to the newest nomenclature: tTregs, pTregs, or iTregs [6, 7]. In this review, we delineate how mTOR signaling functionally regulates metabolism to influence T cell biology, with a particular focus on its impacts on Tregs . First, we discuss how upstream signaling pathways tune mTOR activation. Next, the role of mTOR in thymocyte development is discussed. Third, we summarize the roles of mTOR in T cell homeostasis and functional activation. We then describe how mTOR and metabolic signaling cooperate to influence multiple aspects of Treg biology. Finally, we discuss the implications for targeting mTOR or metabolic pathways for disease therapeutics.
REGULATION OF mTOR ACTIVITY IN T CELLS
mTOR is activated by 3 major instructive signals in T cells: immunologic signals, growth factors, and nutrient and metabolic cues [1]. Below, we discuss the molecular events driving mTOR activation downstream of various receptor systems.
Overview of canonical signal transduction pathways in T cells
TCR stimulation is critical for the generation of antigen-specific, adaptive immune responses. The TCR recognizes specific antigenic peptides expressed in the context of peptide-MHC molecules that are presented by professional APCs. TCR signal transduction is initiated by the tyrosine kinases, Lck/Fyn and ZAP70. Subsequently, LAT-containing complexes promote PLC-γ1, Erk1/2, and PI3K activation [8]. PLC-γ1 promotes Ca2+ flux, which indirectly drives NFAT transcriptional function, and DAG production, which promotes protein kinase C-θ and Ras-Mek1/2-Erk1/2 activity. These kinase pathways indirectly activate NF-κB and AP-1 transcriptional function, respectively. PI3K phosphorylates membrane-associated PIP2 to generate PIP3, which recruits the serine/threonine kinases, PDK1 and Akt, to the plasma membrane. Here, the rate-limiting, activating phosphorylation of Akt by PDK1 occurs. PI3K signaling is antagonized by PTEN, which converts PIP3 back into PIP2. The costimulatory receptor CD28 binds to CD80/86 on APCs, and this interaction promotes naïve T cell proliferation and differentiation [8]. CD28 costimulation augments TCR-induced PI3K signaling and drives NF-κB transcriptional function by recruiting PDK1, which promotes assembly of the CBM complex [8, 9]. Cytokines also intersect with TCR and costimulatory signals to shape T cell responses via the Jak-STAT and PI3K pathways. Below, we discuss how these and other receptor-induced pathways influence mTOR activation.
TCR and CD28 drive mTOR activation via the PI3K pathway
Compared with mTORC2, upstream signaling pathways controlling mTORC1 activity are better understood. PI3K signaling, induced by TCR and CD28 costimulation, activates mTORC1 via several mechanisms. First, Akt phosphorylates and inactivates the TSC1/2 complex, which functions as a GAP for the Rheb GTPase. This event allows Rheb to exist in a GTP-bound state and activate mTORC1 signaling via the RAG/Ragulator complex [2]. Akt has also been demonstrated to promote mTORC1 activation in TSC1-independent manners, including by directly phosphorylating the mTORC1 inhibitor PRAS40 and by driving Raptor phosphorylation to stabilize mTOR-Raptor association [10, 11]. Finally, PDK1 regulates mTORC1 activity linked to effector CD8+ T cell proliferation in a PI3K-dependent, Akt-independent manner [12, 13].
Adaptor proteins regulate mTOR activation in response to antigen and costimulatory signals
Adaptor proteins promote mTORC1 activation upon TCR and CD28 ligation. The chaperone protein Hsp90 binds to Raptor and prevents its protein degradation after TCR stimulation [14], although the molecular mechanisms are currently unknown. The Hsp90-Raptor interaction prevents T cell anergy [14], a state of hyporesponsiveness that occurs when T cells receive TCR signals in the absence of costimulatory receptor ligation [8]. Recently, Hamilton et al. [15] found that the CARMA1-MALT1 complex is required for the optimal activation of mTORC1 in response to TCR and CD28 stimulation. This process appears to be independent of the classic CBM complex that induces NF-κB transcriptional activity, as Bcl10 deletion does not impair mTOR activation [15]. In contrast, Bcl10 is required to drive maximal TCR and CD28-induced mTORC1 activation in the presence of certain amino acids [16]. We further discuss how amino acid uptake is linked to mTOR activation and the functions of T cells in later sections of this review.
Amino acids tune mTOR activation in T cells
Amino acids also influence mTOR activation in T cells in the absence and presence of TCR and costimulatory/cytokine receptor signaling [16, 17]. In other cell lineages, amino acids promote mTORC1 activation at the lysosomes by influencing the GTPase activity of Rheb. The GAP activity of TSC2 inactivates Rheb in the absence of PI3K signaling [18, 19]. Indeed, TSC1-deficient T cells have high basal levels of mTORC1 signaling [20]. As Rheb-deficient T cells have only a transient loss of mTORC1 activity [21], other mechanisms exist to facilitate mTORC1 activation in T cells. Leu and Gln transport are facilitated by ASCT2, and Gln uptake by ASCT2 enhances TCR and CD28-induced mTORC1 activation, which depends on the CBM complex [16]. The related amino acid transporter, Slc7a5, also activates mTORC1 by promoting Leu uptake, among other neutral amino acids [17]. Future studies will continue to elucidate the precise mechanisms by which amino acids activate mTORC1 within T cells.
TCR and CD28 stimulation triggers inhibitory pathways to dampen mTOR activation
Additional factors negatively regulate mTOR signaling induced by TCR activation. The Ras-Mek1/2-Erk1/2 signaling cascade positively regulates mTORC1 activation [2], and the activation of this pathway is supported by the lipid molecule DAG [8]. Therefore, inhibitors of DAG signaling, including the DGKs, can antagonize mTORC1 activation [22, 23]. Consistent with this idea, DGKα and DGKζ double-deficient T cells have enhanced mTORC1 and Ras-Mek1/2-Erk1/2 signaling [24]. The alteration of the structure of amino acids also inhibits mTORC1 activation. BCATc is activated upon TCR stimulation, which increases Leu transamination and subsequently diminishes the intracellular concentrations of Leu [25]. BCATc-deficient CD4+ T cells have increased phosphorylation of S6 and 4EBP-1 and have higher rates of glycolysis [25]. Thus, BCATc is an inhibitor of mTORC1 downstream of the TCR.
Cytokines induce mTOR activation in T cells
Cytokines activate mTOR. IL-7 signals via IL-7R to promote T cell development and homeostasis [26]. In contrast with the rapid activation of mTOR by the TCR, IL-7 induces delayed and sustained PI3K-AKT signaling, and IL-7-induced mTOR activation is STAT5-dependent [27, 28]. IL-15 is another homeostatic cytokine that regulates memory T cell formation [26, 29], but IL-15-driven PI3K-mTOR activation in naïve T cells is not required for memory T cell formation [30].
IL-2 promotes T cell proliferation, Treg development, and Treg functional activation [26]. IL-2R signaling drives these functions by activating the Jak3-STAT5 and PI3K-Akt-mTORC1 pathways, triggering transcriptional and metabolic reprogramming [1, 26]. Recent studies also link the tyrosine kinase, inducible Tec kinase, to IL-2-induced mTOR activation, but the mechanisms are not completely understood [31]. In Tregs, IL-2R signaling augments TCR-induced mTOR activation [32].
Additional cytokines, such as IL-12, IL-4, and IL-1, influence the effector fate decisions of T cells [33]. In activated CD8+ T cells, IL-12 triggers the STAT4-dependent activation of mTOR [34]. IL-4 and IL-1 promote cell-cycle progression by activating mTOR in Th2 and Th17 cells, respectively [35, 36]. We describe how mTOR is linked to effector and memory CD4+ and CD8+ differentiation in more detail below.
mTOR activity is regulated by various growth factors in T cells
Many growth factors positively regulate mTOR activation. Leptin, an adipocyte-derived hormone, drives T cell proliferation and cytokine production [37]. Of note, the transcriptional signatures between rapamycin-treated effector T cells and those after leptin blockade are very similar [38]. Likewise, S1P is a natural lysophospholipid that signals primarily through S1PR1 in T cells and promotes thymocyte egress into the periphery and trafficking to the peripheral lymph nodes [39–41]. S1PR1 signaling is dispensable for immediate mTOR activation but sustains PI3K-Akt-mTOR activity during the differentiation of naive T cells into effector T cells [42]. We discuss in later sections of this review how S1PR1 and leptin receptor signaling contribute to effector T cell differentiation and Treg differentiation and function.
mTOR CONTROLS T CELL DEVELOPMENT
mTOR signaling influences conventional T cell development
Thymocytes are classified into distinct maturational stages. The earliest stage is the CD4−CD8− DN stage, which is divided further into 4 major substages (DN1–DN4). The next stage is the CD4+CD8+ DP stage, from which mature CD4+ or CD8+ single-positive cells develop. The development of early DN2 stage progenitors requires Raptor-mTORC1 function in vivo [43], whereas loss of Raptor at later stages of thymopoiesis does not impact T cell development [21, 43]. Deficiency of Raptor results in cell-cycle abnormalities in early T cell progenitors that are associated with the instability of the cyclin D2/D3-cyclin-dependent kinase 6 complexes [43]. Similar to Raptor deficiency, loss of Rictor leads to a reduction of DP cells, but this is a consequence of impaired DN1 and DN4 cell generation [43, 44]. Mechanistically, the deficiency of Rictor disrupts the NOTCH-driven proliferation and differentiation of pre-T cells and also diminishes the expression of multiple receptors involved in thymocyte development [44, 45]. Thus, mTORC1 and mTORC2 regulate conventional T cell development, albeit via different mechanisms.
iNKT cell development is controlled by mTOR signaling
iNKT cells are a nonconventional T cell population expressing a restricted TCR repertoire relative to conventional T cells. These cells develop from DP cells in response to selection antigens presented by CD1d, and their different developmental stages are tracked by surface molecules (CD24, CD44, and NK1.1) and transcription factors (PLZF, T-bet, GATA-binding protein 3, and ROR-γt) [5]. The cytokine-producing capacity of iNKT cells is also correlated with the expression of these molecules, as IL-4-producing NKT-2 cells, IL-17-producing NKT-17 cells, or IFN-γ-producing NKT-1 cells are enriched at different developmental stages [5, 46].
mTOR signaling is critical for the development of iNKT cells. Hyperactivation of mTORC1 in the absence of TSC1 arrests iNKT cell development at the CD24−CD44+NK1.1− Stage 2 [20, 47]. Moreover, TSC1-deficient iNKT cells produce more IL-17 but less IFN-γ, indicating a role of TSC1 in dictating the fate decisions between the NKT-17 and NKT-1 lineages [47]. These data are also consistent with the observation that NKT-17 cells and NKT-1 cells are enriched in Stage 2 and Stage 3 (CD24−CD44+NK1.1+), respectively [46]. Moreover, Raptor is critical for the development of iNKT cells [48–50]. The transition from Stage 2 to Stage 3 is blocked in Raptor-deficient iNKT cells [49], leading to a loss of NKT-1 cells. Mechanistically, Raptor regulates iNKT development by controlling the function and cellular localization of PLZF [48]. mTORC2 has also been shown to regulate iNKT cell proliferation and/or survival to drive NKT-2 and NKT-17 cell development, but this is independent of PLZF regulation [50, 51]. These collective results indicate that mTORC1 and mTORC2 also control iNKT cell development in different manners.
PERIPHERAL T CELL RESPONSES ARE LINKED TO mTOR-DRIVEN CHANGES IN METABOLISM
Overview of metabolic reprogramming in T cells
T cell fate decisions are intimately linked to changes in cellular metabolism. Resting naïve or memory T cells and Tregs generate energy via the TCA cycle and fatty acid β-oxidation, which promote mitochondrial oxidative phosphorylation. Therefore, these cells may use a variety of nutrient sources, including glucose, amino acids, and lipids, to promote homeostatic proliferation and survival. By contrast, activated T cells are highly anabolic and have a striking increase in glycolysis [52–54]. This conversion to glycolysis is accompanied by a dramatic increase in glucose uptake, cell size, and robust proliferation. Cholesterol and lipid synthesis are also critical for Treg and memory T cell maintenance and function [32, 55]. Throughout the remainder of this review, we discuss how mTOR signaling and metabolic reprogramming are linked to T cell fate decisions and Treg biology.
Hyperactivation of mTORC1 abrogates metabolic processes that maintain T cell quiescence
Mature CD4+ and CD8+ T cells found in the peripheral tissues are quiescent, a state characterized by small cell size, lack of proliferation, and the use of mitochondrial, oxidative phosphorylation to fuel the bioenergetic needs [55]. The threshold of mTOR signaling is a critical factor for maintaining quiescence and peripheral T cell homeostasis. In support of this conclusion, TSC1 regulates peripheral T cell quiescence and homeostasis [20, 56, 57]. TSC1-deficient T cells, which have elevated mTORC1 but reduced mTORC2 activation, lose quiescence, as indicated by the spontaneous entry into the cell cycle [20]. Tsc1−/− T cells are also more sensitive to undergoing apoptosis, as excessive TCR signaling drives increased expression of the proapoptotic protein, Bim [20, 57]. The phosphatase PTEN and serine/threonine kinase LKB1 also suppress mTOR signaling [1, 2]. Pten−/− and Lkb1−/− T cells have defects under homeostasis, including increased proliferation and apoptosis [58–60]. Mitochondrial respiration-driven reactive oxygen species production and glycolysis are enhanced in the absence of TSC1 and LKB1, respectively [20, 57, 59], indicating that defects in cellular metabolism profoundly influence quiescence and T cell homeostasis.
mTOR controls glucose metabolism via c-MYC to induce T cell proliferation
After receiving TCR and costimulatory receptor cues, T cells undergo rapid, clonal expansion. Raptor-mTORC1 drives activation-induced cell-cycle entry from quiescence [21]. However, Raptor-mTORC1 signaling appears to be dispensable for continuous proliferation [21]. As a potential consequence of reduced mTOR activation, Slc7a5−/− T cells have profound defects in proliferation and effector cell function [17]. Likewise, the functional activation and differentiation of Asct2−/− T cells are also impaired [16], demonstrating the importance of amino acids in regulating mTORC1 activation that drives T cell responses.
As we mentioned above, activated T cells primarily use glycolytic metabolism [52–54], which is supported by mTORC1 activation at multiple levels. The increased expression of glucose transporters, including Glut1, on activated T cells augments intracellular glucose concentrations and supports glycolysis [61]. Indeed, in vitro-stimulated murine and human T cells express high levels of Glut1 [62]. Overexpression of Glut1 increases effector T cell frequencies, which promote inflammatory disease development [63, 64]. TCR and CD28-induced Akt signaling is important for Glut1-mediated glucose transport [63]. Moreover, Glut1 up-regulation may be linked to mTORC1 activation induced by amino acids, as reduced Leu uptake in Asct2−/− is correlated with lower levels of Glut1 expression [16].
mTOR signaling also promotes glycolysis via the oncogene c-MYC, a crucial regulator of metabolic reprogramming in T cells [52]. Quiescent T cells express low levels of c-MYC, which is controlled, in part, by the Krüppel-like transcription factor 2 [65]. c-MYC expression increases after TCR stimulation in a mTOR-dependent manner, where it alters the expression of rate-limiting glycolytic enzymes [52]. Additionally, c-MYC controls the Gln metabolism that facilitates T cell proliferation [52]. Mechanistically, c-MYC transcriptional activity inhibits expression of the microRNAs miR23a/b, which regulate the expression of Gln-metabolizing enzymes [66]. Recently, c-MYC was shown to induce the transcriptional activity activating enhancer binding protein 4, subsequently sustaining the activation of antigen-specific CD8+ T cells [67]. These data highlight the importance of c-MYC in T cell functional activation.
mTOR controls HIF-1α expression and impacts CD4+ T cell effector differentiation via metabolic reprogramming
In addition to c-MYC, mTOR signaling induces HIF-1α expression, which regulates glycolysis [52, 55]. The deletion of this protein does not impair T cell proliferation [12, 52]. However, in activated human T cells, HIF-1α functionally blocks TCR-induced apoptosis to enhance cell survival [68]. CD4+ T cells also differentiate into effector T cell lineages, including Th1, Th2, and Th17 cells [69]. mTOR signaling drives T cell differentiation, in part, by regulating cellular metabolism [21, 70–73] (Fig. 2). For instance, Rptor−/− CD4+ T cells have defects in c-MYC and SREBP-dependent metabolic reprogramming, which impairs their functional activation and differentiation into Th2 cells [21]. Whereas the roles that mTORC1 and mTORC2 serve in Th1 and Th2 differentiation remain controversial [21, 70–72], the mTOR-HIF-1α axis promotes Th17 differentiation by inducing ROR-γt expression or localization and glycolysis [70, 71, 73–75]. As this review mainly focuses on how mTOR-mediated metabolic reprogramming influences Treg biology, we encourage our readers to explore other reviews that describe how mTOR influences effector CD4+ T cell differentiation [1, 4].
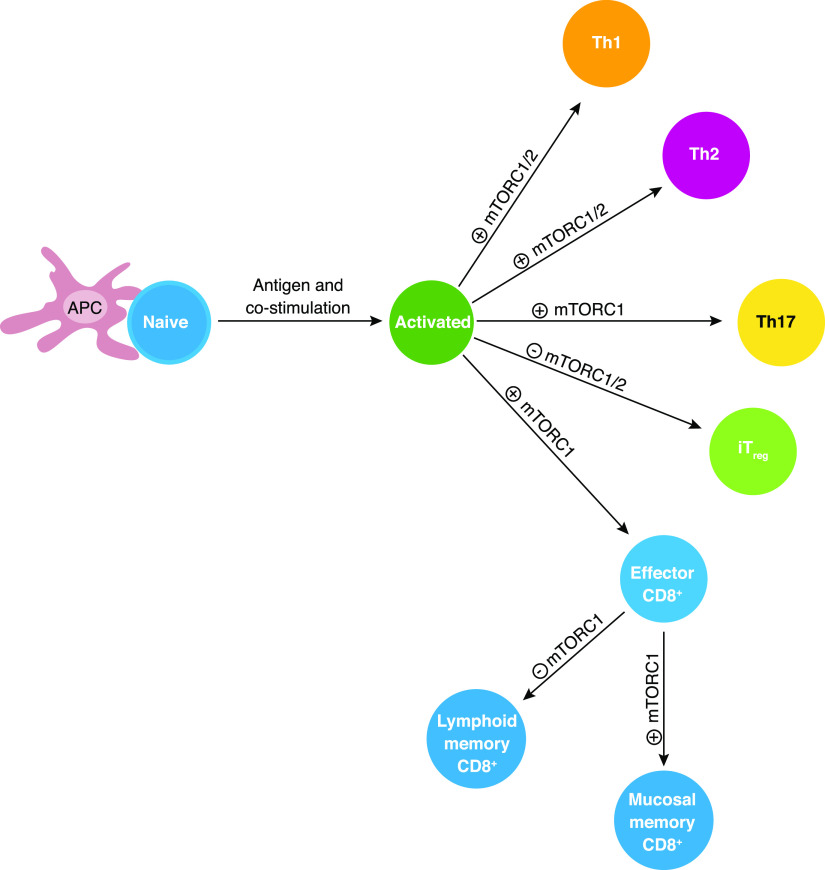
Figure 2. Roles of mTOR in conventional T cell differentiation.
Following antigen stimulation, mTOR signaling positively regulates the differentiation of Th1, Th2, and Th17 effector cells, as well as CD8+ cytotoxic effector cells and mucosal-associated CD8+ memory T cells. By contrast, mTOR signaling inhibits memory CD8+ T cells and iTreg differentiation.
Metabolic reprogramming via mTOR controls CD8+ T cell differentiation
CD8+ T cell responses involve dynamic phases of activation, expansion, contraction, and memory formation, which are regulated by cytokines and transcription factor networks [33, 76, 77]. Memory CD8+ T cells are long lived and rapidly respond to antigen re-exposure [76]. The homeostatic proliferation of naïve CD8+ T cells requires IL-7 but not IL-15 signaling to mTOR [30]. Furthermore, IL-7-induced homeostatic proliferation drives T-bet transcriptional activity and promotes the functional maturation of CD8+ T cells in lymphopenic environments [30]. mTORC1 is also a critical regulator of effector CD8+ T cell responses, in part, as it regulates HIF-1α expression [12, 21, 78]. This transcription factor enhances perforin expression to support effector CD8+ T cell function [12]. However, HIF-1α also negatively regulates TCR-induced cytokine production in CD8+ T cells [79]. Mechanistically, this phenomenon may be mediated by lactate, a glycolytic byproduct. Recent studies suggest that exogenous lactate alters CD4+ and CD8+ T cell functions [80–82]. HIF-1α regulates glycolytic programs driving lactate production in CD8+ T cells [12]. Therefore, future studies could investigate if T cell-derived lactate contributes to HIF-1α-mediated suppression of CD8+ T cell function in different diseases.
mTOR signaling suppresses memory CD8+ T cell differentiation, as rapamycin treatment and Raptor deficiency enhance memory CD8+ T cell formation in vivo [30, 83–86]. This phenotype is linked to the IL-12-driven expression of the transcription factor, Eomesodermin [34], which favors memory CD8+ T cell differentiation [33]. Moreover, IL-15-induced mTORC1 signaling also influences glycolytic and oxidative phosphorylation programs, which regulate effector versus memory CD8+ T cell differentiation [55, 87, 88]. However, it should be noted that mTOR signaling differentially regulates memory T cell formation in peripheral lymphoid tissues versus mucosal sites by controlling CD8+ T cell trafficking [89]. We encourage our readers to explore how mTOR signaling is coupled to T cell trafficking in other reviews [1, 4]. Future studies will determine if metabolic signaling also influences the fate decisions of CD8+ T cells within nonlymphoid tissues.
mTOR signaling controls lipid metabolism via SREBPs
SREBPs control lipid signaling associated with membrane turnover [90]. The functional activation of SREBPs in response to antigen and costimulatory signals is regulated by mTORC1 activation [21, 90]. In a PI3K-mTOR-dependent fashion, these proteins modulate the expression of lipid biosynthesis-related genes [91]. mTORC1 stimulates SREBP-induced lipogenesis through S6K-dependent or S6K-independent pathways [3, 92], whereas mTORC2 stimulates lipogenesis via AKT-dependent activation of SREBP-1c [93, 94]. It is important to note that SREBPs not only regulate lipogenesis but also influence nucleotide biosynthesis via the pentose phosphate pathway [3, 55]. We discuss how lipid biosynthesis impacts Treg function in the next section of this review.
mTOR AND METABOLIC REPROGRAMMING CONTROL MULTIPLE ASPECTS OF Treg BIOLOGY
Treg development in the thymus and periphery is regulated by mTOR-mediated control of metabolism
Tregs arise in the thymus or from naïve T cells in response to antigen, costimulation, or cytokine signaling. Several studies have addressed how mTOR signaling influences Treg development and differentiation. The deletion of many mTOR-associated genes, including Mtor, Raptor, and Tsc1, does not profoundly diminish Treg cellularity in vivo [20, 70, 95], although it remains unclear if tTreg or pTreg generation is affected when these proteins are deleted. In contrast to these in vivo systems, mTOR signaling controls iTreg differentiation. In the absence of TSC1, iTreg differentiation is retained [95]. By contrast, rapamycin treatment increases the de novo expression or influences the stability of Foxp3 expression, driving enhanced iTreg differentiation [70, 96]. Consistent with these findings, Mtor−/− T cells spontaneously develop into iTregs, as they are hyper-responsive to TGF-β stimulation [70]. The Rheb-mTORC1 and Rictor-mTORC2 signaling axes serve functionally redundant roles as inhibitors of iTreg differentiation [71]. Finally, the specific deletion of Pten within Tregs also increases Treg cellularity, and this may be a result of elevated mTORC2 activation in these cells [97, 98].
Additional studies have linked mTOR-induced metabolic pathways to Treg development and differentiation. It was demonstrated recently that the generation of tTregs and iTregs is independent of Glut1 [62], but other glucose transporters may control these processes. Interestingly, glycolysis suppresses iTreg differentiation. Treatment of CD4+ T cells with the glycolytic inhibitor 2-deoxy-d-glucose potentiates iTreg differentiation while simultaneously suppressing Th17 cell differentiation [74, 75]. By contrast, glycolytic programs are increased in PTEN-deficient Tregs, which likely contribute to the enhancement in their cellularity [97, 98]. HIF-1α suppresses iTreg differentiation in favor of Th17 cell differentiation by controlling glycolysis under Th17 differentiation conditions and by inhibiting Foxp3 expression [74, 75, 99]. Thus, mTOR signaling and metabolic reprogramming regulate Treg cellularity.
mTORC1 signaling supports Treg function via cholesterol and lipid synthesis
Whereas mTOR suppression enhances iTreg differentiation, the functional activation of Tregs requires mTORC1 function [32]. Mice bearing Raptor-deficient Tregs spontaneously develop autoimmune disease as a consequence of impaired Treg-mediated suppression. Despite the redundant roles of mTORC1 and mTORC2 for the inhibition of iTreg differentiation, Rictor deficiency does not abrogate Treg cell function, and the simultaneous deletion of Raptor and Rictor in Tregs partially restores Treg suppression function [32]. Thus, it appears that the mTORCs serve different roles in Treg differentiation and function. However, it is also feasible that the requirement for mTOR signaling differs between tTregs and pTregs, as iTregs generated in the absence of mTOR activity retain their functional capacity in vitro [70].
Several groups have investigated the roles of mTOR-mediated metabolic reprogramming in regulating Treg differentiation and function. It was recently demonstrated that the suppressive function of Glut1-deficient Tregs is normal [62]. However, elevated levels of glycolysis are associated with Treg instability that drives autoimmunity in vivo [97, 98]. Moreover, HIF-1α-deficient Tregs have impaired suppressive function and have diminished capacities to ameliorate T cell-mediated colitis [99]. Thus, glycolytic metabolism appears to modulate Treg function, although this possibility awaits further investigation.
A crucial role of mTORC1-induced cholesterol and lipid metabolism in Treg function was identified recently. In the absence of Raptor, expression of enzymes related to cholesterol and lipid biosynthesis is reduced in Tregs [32]. The functional consequences of these alterations are concomitant reductions in Treg proliferation and CTLA-4 or ICOS expression. These changes impede Treg function in vitro and in vivo [32]. Statins, which suppress cholesterol and lipid synthesis, similarly attenuate wild-type Treg responses [32]. Thus, mTORC1 supports cholesterol and lipid biosynthesis to regulate Treg function.
Upstream activators of mTORC1 influence Treg cellularity and function
As multiple cellular inputs regulate mTOR in Tregs (Fig. 3), studies have also addressed how these signals control Treg development, differentiation, and function. The duration of TCR signaling influences Foxp3 expression in a PI3K-Akt-mTOR-dependent manner, as transient signaling promotes, and chronic signaling suppresses Foxp3 expression [100]. Consistent with this idea, the expression of catalytically active Akt in thymocytes selectively impairs Treg development [101]. IL-2- and TGF-β-induced Foxp3 induction is also impaired when activated CD4+ T cells express constitutively active Akt [101]. Thus, antigens and cytokines affect Treg development and differentiation via Akt-mTOR-dependent mechanisms.
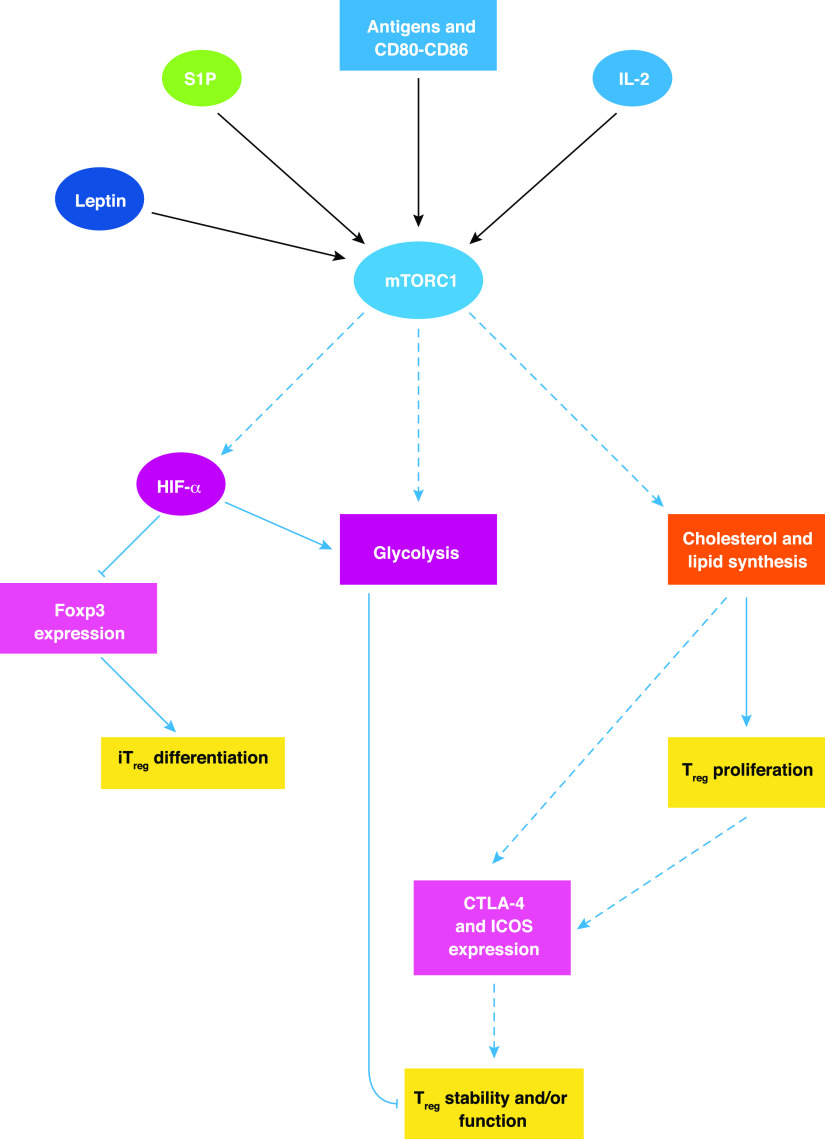
Figure 3. Role of mTOR in Treg differentiation and function.
In Tregs, mTOR activity is modulated by many upstream inputs from surface receptors (including TCR, CD28, IL-2R, leptin receptor, and S1PR1). Loss of mTORC1 function in Tregs leads to a dramatic reduction in lipid biosynthesis via the mevalonate pathway, which is required for Treg-suppressive activity. mTOR can also regulate Treg biology by controlling glycolysis and HIF-1α function. A summary of such regulation is described in this figure, with blue arrows indicating positive regulation and blue, flat-ended lines depicting negative regulation.
Our group has found that S1P over-rides Treg-mediated immune suppression through AKT-mTOR. S1P delivers an intrinsic, negative signal through its receptor, S1PR1, to restrain the thymic generation, peripheral maintenance, and suppressive activity of Tregs [42, 102]. Mechanistically, S1P-induced mTOR activation inhibits TGF-β signaling to Smad3 [42, 102]. Leptin also controls mTOR signaling within Tregs to suppress their proliferation and to support a maximal Treg-suppressive function [103, 104]. We discuss how mTOR signaling is linked to Treg proliferation in more detail below. Thus, the extent of mTOR activity is modulated by multiple signals in Tregs, and the tuning of mTOR activation is critical for proper Treg generation. How S1PR1 or leptin receptor signaling impacts metabolic programs in Tregs is unknown.
Tregs express a variety of molecules that modulate amino acid availability within a microenvironment. Indeed, CTLA-4 binds to CD80/86 on DC and induces IDO, an enzyme that converts tryptophan into various metabolic byproducts. [6, 105, 106]. Tregs have also been demonstrated to induce arginase expression in DCs to limit arginine availability [6]. By driving APC to express enzymes that catabolize amino acids, Tregs prevent T cell activation and enforce Foxp3 expression in CD4+ T cells to promote iTreg differentiation [105, 106]. Future work should explore how the manipulation of amino acid availability contributes to immune dysregulation in metabolic disorders, including cancer and obesity.
The threshold of mTOR signaling tunes Treg proliferation, stability, and function
Although Tregs proliferate in vivo, these cells are refractory to conventional T cell-activating stimuli in vitro. Recent work indicates that this is associated with high levels of mTORC1 signaling within these cells [32, 103]. Transient and chronic rapamycin treatments have been used to determine the role of mTOR in Treg proliferation. When performed before TCR stimulation, transient rapamycin treatment without exogenous IL-2 drives Treg proliferation and clustering [103]. Mechanistically, transient rapamycin treatment augments IL-2 production by Tregs, which drive Treg proliferation [103]. Tregs transiently treated with rapamycin retain Foxp3 expression and express higher levels of the Treg effector molecules, CD39, CTLA-4, and glucocorticoid-induced TNFR [103]. However, chronic rapamycin treatment suppresses Treg proliferation in the absence of IL-2 but supports Treg expansion in the presence of supraphysiological concentrations of IL-2 [107, 108]. Thus, the duration of rapamycin treatment can influence Treg proliferation and may impact Treg function. Interestingly, leptin receptor signaling restrains the proliferation of untreated and rapamycin-treated Tregs [103, 104], indicating that the leptin receptor-mTOR axis sets the threshold for Treg responsiveness to receptor-mediated cues driving proliferation.
The negative regulators of mTOR also control Treg function. PTEN-deficient Tregs are unstable; they lose Foxp3 expression and gain Th1 effector-like functions that drive autoimmune development [97, 98]. Fatty acid β-oxidation promotes mitochondrial respiration to support Treg homeostasis [55]. PTEN-deficient Tregs have elevated levels of glycolysis and reduced mitochondrial fitness, which likely mediates their instability [97, 98]. The conditional deletion of TSC1 in Tregs does not have drastic effects on CD4+ or CD8+ T cell homeostasis [95], unlike Raptor or PTEN deficiency in these cells [27, 97, 98]. However, TSC1-deficient Tregs acquire Th17-like effector functions under homeostasis and inflammation. This phenotypic switch is partially regulated by the hyperactivation of S6K-S6 signaling and the reduction in FoxO1/3a function [95]. TSC1 deficiency may also result in the loss of mitochondrial fitness in Tregs, as was reported in conventional T cells [20, 57], which requires additional investigation.
mTOR, Tregs, AND DISEASE THERAPEUTICS
Increased numbers of Tregs in breast, colorectal, and ovarian cancers are indicative of a poor prognosis [109]. Infectious pathogens can also increase the activity of Tregs [110, 111]. However, Tregs are best known for their suppression of autoimmune disorders and transplant rejection. It was recently demonstrated that alterations in IL-2R-driven Treg proliferation are linked to autoimmunity in humans [112]. This phenotype is correlated with elevated mTOR signaling in autoimmune patient-derived Tregs relative to those from healthy controls, which cannot be attenuated by leptin blockade. As a functional consequence of these alterations, Foxp3 expression is reduced, and the suppressive function of these Tregs is impaired in vitro [112]. Therefore, abnormal mTOR signaling in Tregs is connected to human autoimmunity.
Tregs mediate transplant tolerance in humans and animal models [113]. The combination of transient rapamycin treatment and the transfer of alloantigen-specific Tregs provides long-term, organ graft survival [114]. Furthermore, Tregs prevent the rejection of skin grafts in multiple models, but a challenge to the use of Tregs in this context is that IL-17-producing Tregs can promote graft rejection [115]. Furthermore, it is difficult to obtain sufficient numbers of long-lived Tregs that can prevent allograft rejection [113]. As mTOR signaling impacts iTreg differentiation and function, Treg therapies may be improved by coadministration of rapamycin or other mTOR inhibitors. However, further investigation into how next-generation mTOR inhibitors influence Treg expansion or function is needed before this therapeutic strategy is adopted. Additional insight into the role of mTOR in Treg metabolism may make it possible to design more targeted, innovative therapies to modulate Treg differentiation or function.
CONCLUSIONS AND FUTURE DIRECTIONS
Studies in recent years have revealed how mTOR signaling and metabolism control T cell fate decisions. Although multiple studies have addressed the role of mTOR signaling in metabolic reprogramming within T cell populations, we do not completely understand how the cross-talk between mTOR and metabolism controls Treg responses. Future research will determine how the mTOR-mediated control of c-MYC and SREBP, among other molecules, influences Treg function and stability, which will provide more insight into how glycolytic and lipid metabolism controls these processes. Additional studies may also address how mTOR signaling influences metabolic reprogramming to support the function of specific effector Treg populations. These studies may identify novel therapeutic targets to multiple conditions related to dysregulated mTOR activation.
In addition to residing in lymphoid tissues, Tregs are found in adipose tissue. These cells appear to control immune suppression during starvation and inflammation triggered by obesity and therefore, may contribute to the development of metabolic diseases [116, 117]. To maintain Foxp3 expression, adipose tissue-associated Tregs require expression of peroxisome proliferator-activated receptor γ, a lipid-activated transcription factor [118]. Given that adipose tissue contains high levels of fatty acids, cholesterol, and leptin, future studies should explore how the interplay among these factors influences Treg functions under homeostasis and in different metabolic diseases. These studies may identify novel mechanisms by which these diseases could be ameliorated.
ACKNOWLEDGMENTS
Funding support is provided by The Hartwell Foundation Biomedical Research Fellowship (to N.M.C.), U.S. National Institutes of Health (AI101407, AI105887, CA176624, and NS064599), National Multiple Sclerosis Society, Crohn's & Colitis Foundation of America, and American Lebanese Syrian Associated Charities (to H.C.). The authors acknowledge the researchers who have greatly contributed to this field but whose work was not cited as a result of space limitations.
Glossary
- 4EBP-1
4E binding protein 1
- ASCT2
ASC amino acid transporter 2
- BCATc
branched-chain aminotransferase c
- Bcl10
B cell lymphoma 10
- CARMA1
caspase recruitment domain containing membrane-associated protein 1
- CBM
Carma1/Bcl10/MALT1
- DAG
diacylglycerol
- DC
dendritic cells
- DGK
diacylglycerol kinase
- DN
double-negative
- DP
double-positive
- Fox/O1/3a/p3
forkhead box O1/3a/p3
- GAP
GTPase-activating protein
- Gln
glutamine
- Glut1
glucose transporter 1
- HIF-1α
hypoxia-inducible factor 1α
- Hsp90
heat shock protein 90
- iNKT
invariant NKT
- iTreg
in vitro-induced regulatory T cell
- LAT
linker for activation of T cell
- Leu
leucine
- LKB1
liver kinase B1
- MALT1
mucosa-associated lymphoid tissue lymphoma translocation protein 1
- mTOR
mechanistic target of rapamycin
- mTORC1/2
mechanistic target of rapamycin complexes 1/2
- PDK1
phosphatidylinositol-dependent kinase 1
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PLC-γ1
phospholipase C-γ1
- PLZF
promyelocytic leukemia zinc-finger
- PRAS40
proline-rich Akt substrate of 40 kDa
- PTEN
phosphatase and tensin homolog
- pTreg
peripherally derived regulatory T cell
- Raptor
regulatory-associated protein of mechanistic target of rapamycin
- Rheb
Ras homolog enriched in brain
- Rictor
rapamycin-insensitive companion of mechanistic target of rapamycin
- ROR-γt
retinoic acid receptor-related orphan receptor γt
- S1P
sphingosine 1-phosphate
- S6K
ribosomal S6 kinase
- Slc7a5
solute carrier family 7 member 5
- SREBP
sterol regulatory element-binding protein
- T-bet
T-box transcription factor Tbx21
- Treg
regulatory T cell
- TSC1/2
tuberous sclerosis 1/2
- tTreg
thymus-derived regulatory T cell
REFERENCES
- 1.Chi, H. (2012) Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12, 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laplante, M., Sabatini, D. M. (2012) mTOR signaling in growth control and disease. Cell 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Düvel, K., Yecies, J. L., Menon, S., Raman, P., Lipovsky, A. I., Souza, A. L., Triantafellow, E., Ma, Q., Gorski, R., Cleaver, S., Vander Heiden, M. G., MacKeigan, J. P., Finan, P. M., Clish, C. B., Murphy, L. O., Manning, B. D. (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell, J. D., Pollizzi, K. N., Heikamp, E. B., Horton, M. R. (2012) Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30, 39–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan, P. J., Brigl, M., Brenner, M. B. (2013) Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13, 101–117. [DOI] [PubMed] [Google Scholar]
- 6.Josefowicz, S. Z., Lu, L. F., Rudensky, A. Y. (2012) Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbas, A. K., Benoist, C., Bluestone, J. A., Campbell, D. J., Ghosh, S., Hori, S., Jiang, S., Kuchroo, V. K., Mathis, D., Roncarolo, M. G., Rudensky, A., Sakaguchi, S., Shevach, E. M., Vignali, D. A., Ziegler, S. F. (2013) Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 14, 307–308. [DOI] [PubMed] [Google Scholar]
- 8.Brownlie, R. J., Zamoyska, R. (2013) T Cell receptor signalling networks: branched, diversified and bounded. Nat. Rev. Immunol. 13, 257–269. [DOI] [PubMed] [Google Scholar]
- 9.Park, S. G., Schulze-Luehrman, J., Hayden, M. S., Hashimoto, N., Ogawa, W., Kasuga, M., Ghosh, S. (2009) The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat. Immunol. 10, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan, H. C., Ebbs, A., Pasparakis, M., Van Dyke, T., Basseres, D. S., Baldwin, A. S. (2014) Akt-dependent activation of mTORC1 complex involves phosphorylation of mTOR (mammalian target of rapamycin) by IκB kinase α (IKKα). J. Biol. Chem. 289, 25227–25240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vander Haar, E., Lee, S. I., Bandhakavi, S., Griffin, T. J., Kim, D. H. (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9, 316–323. [DOI] [PubMed] [Google Scholar]
- 12.Finlay, D. K., Rosenzweig, E., Sinclair, L. V., Feijoo-Carnero, C., Hukelmann, J. L., Rolf, J., Panteleyev, A. A., Okkenhaug, K., Cantrell, D. A. (2012) PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macintyre, A. N., Finlay, D., Preston, G., Sinclair, L. V., Waugh, C. M., Tamas, P., Feijoo, C., Okkenhaug, K., Cantrell, D. A. (2011) Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 34, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgoffe, G. M., Kole, T. P., Cotter, R. J., Powell, J. D. (2009) Enhanced interaction between Hsp90 and raptor regulates mTOR signaling upon T cell activation. Mol. Immunol. 46, 2694–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton, K. S., Phong, B., Corey, C., Cheng, J., Gorentla, B., Zhong, X., Shiva, S., Kane, L. P. (2014) T Cell receptor-dependent activation of mTOR signaling in T cells is mediated by Carma1 and MALT1, but not Bcl10. Sci. Signal. 7, ra55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakaya, M., Xiao, Y., Zhou, X., Chang, J. H., Chang, M., Cheng, X., Blonska, M., Lin, X., Sun, S. C. (2014) Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinclair, L. V., Rolf, J., Emslie, E., Shi, Y. B., Taylor, P. M., Cantrell, D. A. (2013) Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efeyan, A., Zoncu, R., Sabatini, D. M. (2012) Amino acids and mTORC1: from lysosomes to disease. Trends Mol. Med. 18, 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jewell, J. L., Russell, R. C., Guan, K. L. (2013) Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 14, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, K., Neale, G., Green, D. R., He, W., Chi, H. (2011) The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol. 12, 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang, K., Shrestha, S., Zeng, H., Karmaus, P. W., Neale, G., Vogel, P., Guertin, D. A., Lamb, R. F., Chi, H. (2013) T Cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 39, 1043–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong, X. P., Guo, R., Zhou, H., Liu, C., Wan, C. K. (2008) Diacylglycerol kinases in immune cell function and self-tolerance. Immunol. Rev. 224, 249–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mérida, I., Avila-Flores, A., Merino, E. (2008) Diacylglycerol kinases: at the hub of cell signalling. Biochem. J. 409, 1–18. [DOI] [PubMed] [Google Scholar]
- 24.Gorentla, B. K., Wan, C. K., Zhong, X. P. (2011) Negative regulation of mTOR activation by diacylglycerol kinases. Blood 117, 4022–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananieva, E. A., Patel, C. H., Drake, C. H., Powell, J. D., Hutson, S. M. (2014) Cytosolic branched chain aminotransferase (BCATc) regulates mTORC1 signaling and glycolytic metabolism in CD4+ T cells. J. Biol. Chem. 289, 18793–18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rochman, Y., Spolski, R., Leonard, W. J. (2009) New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 9, 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathmell, J. C., Farkash, E. A., Gao, W., Thompson, C. B. (2001) IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167, 6869–6876. [DOI] [PubMed] [Google Scholar]
- 28.Wofford, J. A., Wieman, H. L., Jacobs, S. R., Zhao, Y., Rathmell, J. C. (2008) IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111, 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy, M. K., Glaccum, M., Brown, S. N., Butz, E. A., Viney, J. L., Embers, M., Matsuki, N., Charrier, K., Sedger, L., Willis, C. R., Brasel, K., Morrissey, P. J., Stocking, K., Schuh, J. C., Joyce, S., Peschon, J. J. (2000) Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, Q., Rao, R. R., Araki, K., Pollizzi, K., Odunsi, K., Powell, J. D., Shrikant, P. A. (2011) A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity 34, 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Rodriguez, J., Wohlfert, E. A., Handon, R., Meylan, F., Wu, J. Z., Anderson, S. M., Kirby, M. R., Belkaid, Y., Schwartzberg, P. L. (2014) Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J. Exp. Med. 211, 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng, H., Yang, K., Cloer, C., Neale, G., Vogel, P., Chi, H. (2013) mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaech, S. M., Cui, W. (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao, R. R., Li, Q., Odunsi, K., Shrikant, P. A. (2010) The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 32, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephenson, L. M., Park, D. S., Mora, A. L., Goenka, S., Boothby, M. (2005) Sequence motifs in IL-4R alpha mediating cell-cycle progression of primary lymphocytes. J. Immunol. 175, 5178–5185. [DOI] [PubMed] [Google Scholar]
- 36.Gulen, M. F., Kang, Z., Bulek, K., Youzhong, W., Kim, T. W., Chen, Y., Altuntas, C. Z., Sass Bak-Jensen, K., McGeachy, M. J., Do, J. S., Xiao, H., Delgoffe, G. M., Min, B., Powell, J. D., Tuohy, V. K., Cua, D. J., Li, X. (2010) The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity 32, 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lord, G. M., Matarese, G., Howard, J. K., Baker, R. J., Bloom, S. R., Lechler, R. I. (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901. [DOI] [PubMed] [Google Scholar]
- 38.Procaccini, C., De Rosa, V., Galgani, M., Carbone, F., Cassano, S., Greco, D., Qian, K., Auvinen, P., Calì, G., Stallone, G., Formisano, L., La Cava, A., Matarese, G. (2012) Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J. Immunol. 189, 2941–2953. [DOI] [PubMed] [Google Scholar]
- 39.Matloubian, M., Lo, C. G., Cinamon, G., Lesneski, M. J., Xu, Y., Brinkmann, V., Allende, M. L., Proia, R. L., Cyster, J. G. (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360. [DOI] [PubMed] [Google Scholar]
- 40.Allende, M. L., Dreier, J. L., Mandala, S., Proia, R. L. (2004) Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279, 15396–15401. [DOI] [PubMed] [Google Scholar]
- 41.Chi, H., Flavell, R. A. (2005) Cutting edge: regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J. Immunol. 174, 2485–2488. [DOI] [PubMed] [Google Scholar]
- 42.Liu, G., Yang, K., Burns, S., Shrestha, S., Chi, H. (2010) The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat. Immunol. 11, 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoshii, T., Kasada, A., Hatakeyama, T., Ohtani, M., Tadokoro, Y., Naka, K., Ikenoue, T., Ikawa, T., Kawamoto, H., Fehling, H. J., Araki, K., Yamamura, K., Matsuda, S., Hirao, A. (2014) Loss of mTOR complex 1 induces developmental blockage in early T-lymphopoiesis and eradicates T-cell acute lymphoblastic leukemia cells. Proc. Natl. Acad. Sci. USA 111, 3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, K., Nam, K. T., Cho, S. H., Gudapati, P., Hwang, Y., Park, D. S., Potter, R., Chen, J., Volanakis, E., Boothby, M. (2012) Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J. Exp. Med. 209, 713–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou, P. C., Oh, W. J., Wu, C. C., Moloughney, J., Rüegg, M. A., Hall, M. N., Jacinto, E., Werlen, G. (2014) Mammalian target of rapamycin complex 2 modulates αβTCR processing and surface expression during thymocyte development. J. Immunol. 193, 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, Y. J., Holzapfel, K. L., Zhu, J., Jameson, S. C., Hogquist, K. A. (2013) Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14, 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, J., Yang, J., Yang, K., Wang, H., Gorentla, B., Shin, J., Qiu, Y., Que, L. G., Foster, W. M., Xia, Z., Chi, H., Zhong, X. P. (2014) iNKT cells require TSC1 for terminal maturation and effector lineage fate decisions. J. Clin. Invest. 124, 1685–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin, J., Wang, S., Deng, W., Wu, J., Gao, J., Zhong, X. P. (2014) Mechanistic target of rapamycin complex 1 is critical for invariant natural killer T-cell development and effector function. Proc. Natl. Acad. Sci. USA 111, E776–E783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, L., Tschumi, B. O., Corgnac, S., Rüegg, M. A., Hall, M. N., Mach, J. P., Romero, P., Donda, A. (2014) Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J. Immunol. 193, 1759–1765. [DOI] [PubMed] [Google Scholar]
- 50.Wei, J., Yang, K., Chi, H. (2014) Cutting edge: discrete functions of mTOR signaling in invariant NKT cell development and NKT17 fate decision. J. Immunol. 193, 4297–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prevot, N., Pyaram, K., Bischoff, E., Sen, J. M., Powell, J. D., Chang, C. H. (2015) Mammalian target of rapamycin complex 2 regulates invariant NK T cell development and function independent of promyelocytic leukemia zinc-finger. J. Immunol. 194, 223–230.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, R., Dillon, C. P., Shi, L. Z., Milasta, S., Carter, R., Finkelstein, D., McCormick, L. L., Fitzgerald, P., Chi, H., Munger, J., Green, D. R. (2011) The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearce, E. L., Poffenberger, M. C., Chang, C. H., Jones, R. G. (2013) Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacIver, N. J., Michalek, R. D., Rathmell, J. C. (2013) Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, R., Green, D. R. (2012) Metabolic checkpoints in activated T cells. Nat. Immunol. 13, 907–915. [DOI] [PubMed] [Google Scholar]
- 56.Wu, Q., Liu, Y., Chen, C., Ikenoue, T., Qiao, Y., Li, C. S., Li, W., Guan, K. L., Liu, Y., Zheng, P. (2011) The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. J. Immunol. 187, 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien, T. F., Gorentla, B. K., Xie, D., Srivatsan, S., McLeod, I. X., He, Y. W., Zhong, X. P. (2011) Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur. J. Immunol. 41, 3361–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu, X., Karnell, J. L., Yin, B., Zhang, R., Zhang, J., Li, P., Choi, Y., Maltzman, J. S., Pear, W. S., Bassing, C. H., Turka, L. A. (2010) Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J. Clin. Invest. 120, 2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacIver, N. J., Blagih, J., Saucillo, D. C., Tonelli, L., Griss, T., Rathmell, J. C., Jones, R. G. (2011) The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J. Immunol. 187, 4187–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamás, P., Macintyre, A., Finlay, D., Clarke, R., Feijoo-Carnero, C., Ashworth, A., Cantrell, D. (2010) LKB1 is essential for the proliferation of T-cell progenitors and mature peripheral T cells. Eur. J. Immunol. 40, 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thorens, B., Mueckler, M. (2010) Glucose transporters in the 21st century. Am. J. Physiol. Endocrinol. Metab. 298, E141–E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macintyre, A. N., Gerriets, V. A., Nichols, A. G., Michalek, R. D., Rudolph, M. C., Deoliveira, D., Anderson, S. M., Abel, E. D., Chen, B. J., Hale, L. P., Rathmell, J. C. (2014) The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobs, S. R., Herman, C. E., Maciver, N. J., Wofford, J. A., Wieman, H. L., Hammen, J. J., Rathmell, J. C. (2008) Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 180, 4476–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michalek, R. D., Gerriets, V. A., Jacobs, S. R., Macintyre, A. N., MacIver, N. J., Mason, E. F., Sullivan, S. A., Nichols, A. G., Rathmell, J. C. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckley, A. F., Kuo, C. T., Leiden, J. M. (2001) Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nat. Immunol. 2, 698–704. [DOI] [PubMed] [Google Scholar]
- 66.Wise, D. R., DeBerardinis, R. J., Mancuso, A., Sayed, N., Zhang, X. Y., Pfeiffer, H. K., Nissim, I., Daikhin, E., Yudkoff, M., McMahon, S. B., Thompson, C. B. (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 105, 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chou, C., Pinto, A. K., Curtis, J. D., Persaud, S. P., Cella, M., Lin, C. C., Edelson, B. T., Allen, P. M., Colonna, M., Pearce, E. L., Diamond, M. S., Egawa, T. (2014) c-Myc-induced transcription factor AP4 is required for host protection mediated by CD8 T cells. Nat. Immunol. 15, 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makino, Y., Nakamura, H., Ikeda, E., Ohnuma, K., Yamauchi, K., Yabe, Y., Poellinger, L., Okada, Y., Morimoto, C., Tanaka, H. (2003) Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. J. Immunol. 171, 6534–6540. [DOI] [PubMed] [Google Scholar]
- 69.Zhu, J., Yamane, H., Paul, W. E. (2010) Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delgoffe, G. M., Kole, T. P., Zheng, Y., Zarek, P. E., Matthews, K. L., Xiao, B., Worley, P. F., Kozma, S. C., Powell, J. D. (2009) The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delgoffe, G. M., Pollizzi, K. N., Waickman, A. T., Heikamp, E., Meyers, D. J., Horton, M. R., Xiao, B., Worley, P. F., Powell, J. D. (2011) The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee, K., Gudapati, P., Dragovic, S., Spencer, C., Joyce, S., Killeen, N., Magnuson, M. A., Boothby, M. (2010) Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurebayashi, Y., Nagal, S., Ikejiri, A., Ohtani, M., Ichiyama, K., Baba, Y., Egami, S., Hoshii, T., Hirao, A., Matsuda, S., Koyasu, S. (2012) PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORγ. Cell Rep 1, 360–373. [DOI] [PubMed] [Google Scholar]
- 74.Dang, E. V., Barbi, J., Yang, H. Y., Jinasena, D., Yu, H., Zheng, Y., Bordman, Z., Fu, J., Kim, Y., Yen, H. R., Luo, W., Zeller, K., Shimoda, L., Topalian, S. L., Semenza, G. L., Dang, C. V., Pardoll, D. M., Pan, F. (2011) Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146, 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi, L. Z., Wang, R., Huang, G., Vogel, P., Neale, G., Green, D. R., Chi, H. (2011) HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masopust, D., Ahmed, R. (2004) Reflections on CD8 T-cell activation and memory. Immunol. Res. 29, 151–160. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, N., Bevan, M. J. (2011) CD8(+) T cells: foot soldiers of the immune system. Immunity 35, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doedens, A. L., Phan, A. T., Stradner, M. H., Fujimoto, J. K., Nguyen, J. V., Yang, E., Johnson, R. S., Goldrath, A. W. (2013) Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat. Immunol. 14, 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lukashev, D., Klebanov, B., Kojima, H., Grinberg, A., Ohta, A., Berenfeld, L., Wenger, R. H., Ohta, A., Sitkovsky, M. (2006) Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J. Immunol. 177, 4962–4965. [DOI] [PubMed] [Google Scholar]
- 80.Fischer, K., Hoffmann, P., Voelkl, S., Meidenbauer, N., Ammer, J., Edinger, M., Gottfried, E., Schwarz, S., Rothe, G., Hoves, S., Renner, K., Timischl, B., Mackensen, A., Kunz-Schughart, L., Andreesen, R., Krause, S. W., Kreutz, M. (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819. [DOI] [PubMed] [Google Scholar]
- 81.Shime, H., Yabu, M., Akazawa, T., Kodama, K., Matsumoto, M., Seya, T., Inoue, N. (2008) Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J. Immunol. 180, 7175–7183. [DOI] [PubMed] [Google Scholar]
- 82.Yabu, M., Shime, H., Hara, H., Saito, T., Matsumoto, M., Seya, T., Akazawa, T., Inoue, N. (2011) IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. Int. Immunol. 23, 29–41. [DOI] [PubMed] [Google Scholar]
- 83.Araki, K., Turner, A. P., Shaffer, V. O., Gangappa, S., Keller, S. A., Bachmann, M. F., Larsen, C. P., Ahmed, R. (2009) mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berezhnoy, A., Castro, I., Levay, A., Malek, T. R., Gilboa, E. (2014) Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J. Clin. Invest. 124, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pearce, E. L., Walsh, M. C., Cejas, P. J., Harms, G. M., Shen, H., Wang, L. S., Jones, R. G., Choi, Y. (2009) Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turner, A. P., Shaffer, V. O., Araki, K., Martens, C., Turner, P. L., Gangappa, S., Ford, M. L., Ahmed, R., Kirk, A. D., Larsen, C. P. (2011) Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am. J. Transplant. 11, 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shrestha, S., Yang, K., Wei, J., Karmaus, P. W., Neale, G., Chi, H. (2014) Tsc1 promotes the differentiation of memory CD8+ T cells via orchestrating the transcriptional and metabolic programs. Proc. Natl. Acad. Sci. USA 111, 14858–14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sukumar, M., Liu, J., Ji, Y., Subramanian, M., Crompton, J. G., Yu, Z., Roychoudhuri, R., Palmer, D. C., Muranski, P., Karoly, E. D., Mohney, R. P., Klebanoff, C. A., Lal, A., Finkel, T., Restifo, N. P., Gattinoni, L. (2013) Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 123, 4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sowell, R. T., Rogozinska, M., Nelson, C. E., Vezys, V., Marzo, A. L. (2014) Cutting edge: generation of effector cells that localize to mucosal tissues and form resident memory CD8 T cells is controlled by mTOR. J. Immunol. 193, 2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimobayashi, M., Hall, M. N. (2014) Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 15, 155–162. [DOI] [PubMed] [Google Scholar]
- 91.Kidani, Y., Elsaesser, H., Hock, M. B., Vergnes, L., Williams, K. J., Argus, J. P., Marbois, B. N., Komisopoulou, E., Wilson, E. B., Osborne, T. F., Graeber, T. G., Reue, K., Brooks, D. G., Bensinger, S. J. (2013) Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 14, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Porstmann, T., Santos, C. R., Griffiths, B., Cully, M., Wu, M., Leevers, S., Griffiths, J. R., Chung, Y. L., Schulze, A. (2008) SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hagiwara, A., Cornu, M., Cybulski, N., Polak, P., Betz, C., Trapani, F., Terracciano, L., Heim, M. H., Rüegg, M. A., Hall, M. N. (2012) Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 15, 725–738. [DOI] [PubMed] [Google Scholar]
- 94.Yuan, M., Pino, E., Wu, L., Kacergis, M., Soukas, A. A. (2012) Identification of Akt-independent regulation of hepatic lipogenesis by mammalian target of rapamycin (mTOR) complex 2. J. Biol. Chem. 287, 29579–29588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park, Y., Jin, H. S., Lopez, J., Elly, C., Kim, G., Murai, M., Kronenberg, M., Liu, Y. C. (2013) TSC1 regulates the balance between effector and regulatory T cells. J. Clin. Invest. 123, 5165–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valmori, D., Tosello, V., Souleimanian, N. E., Godefroy, E., Scotto, L., Wang, Y., Ayyoub, M. (2006) Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. 177, 944–949. [DOI] [PubMed] [Google Scholar]
- 97.Shrestha, S., Yang, K., Guy, C., Vogel, P., Neale, G., Chi, H. (2015) Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 16, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huynh, A., DuPage, M., Priyadharshini, B., Sage, P. T., Quiros, J., Borges, C. M., Townamchai, N., Gerriets, V. A., Rathmell, J. C., Sharpe, A. H., Bluestone, J. A., Turka, L. A. (2015) Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 16, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clambey, E. T., McNamee, E. N., Westrich, J. A., Glover, L. E., Campbell, E. L., Jedlicka, P., de Zoeten, E. F., Cambier, J. C., Stenmark, K. R., Colgan, S. P., Eltzschig, H. K. (2012) Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. USA 109, E2784–E2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sauer, S., Bruno, L., Hertweck, A., Finlay, D., Leleu, M., Spivakov, M., Knight, Z. A., Cobb, B. S., Cantrell, D., O’Connor, E., Shokat, K. M., Fisher, A. G., Merkenschlager, M. (2008) T Cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA 105, 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haxhinasto, S., Mathis, D., Benoist, C. (2008) The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu, G., Burns, S., Huang, G., Boyd, K., Proia, R. L., Flavell, R. A., Chi, H. (2009) The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 10, 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Procaccini, C., De Rosa, V., Galgani, M., Abanni, L., Calì, G., Porcellini, A., Carbone, F., Fontana, S., Horvath, T. L., La Cava, A., Matarese, G. (2010) An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 33, 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Rosa, V., Procaccini, C., Calì, G., Pirozzi, G., Fontana, S., Zappacosta, S., La Cava, A., Matarese, G. (2007) A key role of leptin in the control of regulatory T cell proliferation. Immunity 26, 241–255. [DOI] [PubMed] [Google Scholar]
- 105.Cobbold, S. P., Adams, E., Farquhar, C. A., Nolan, K. F., Howie, D., Lui, K. O., Fairchild, P. J., Mellor, A. L., Ron, D., Waldmann, H. (2009) Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl. Acad. Sci. USA 106, 12055–12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fallarino, F., Grohmann, U., Hwang, K. W., Orabona, C., Vacca, C., Bianchi, R., Belladonna, M. L., Fioretti, M. C., Alegre, M. L., Puccetti, P. (2003) Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4, 1206–1212. [DOI] [PubMed] [Google Scholar]
- 107.Battaglia, M., Stabilini, A., Roncarolo, M. G. (2005) Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–4748. [DOI] [PubMed] [Google Scholar]
- 108.Strauss, L., Czystowska, M., Szajnik, M., Mandapathil, M., Whiteside, T. L. (2009) Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS ONE 4, e5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dranoff, G. (2005) The therapeutic implications of intratumoral regulatory T cells. Clin. Cancer Res. 11, 8226–8229. [DOI] [PubMed] [Google Scholar]
- 110.Shafiani, S., Dinh, C., Ertelt, J. M., Moguche, A. O., Siddiqui, I., Smigiel, K. S., Sharma, P., Campbell, D. J., Way, S. S., Urdahl, K. B. (2013) Pathogen-specific Treg cells expand early during Mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity 38, 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McSorley, H. J., Harcus, Y. M., Murray, J., Taylor, M. D., Maizels, R. M. (2008) Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J. Immunol. 181, 6456–6466. [DOI] [PubMed] [Google Scholar]
- 112.Carbone, F., De Rosa, V., Carrieri, P. B., Montella, S., Bruzzese, D., Porcellini, A., Procaccini, C., La Cava, A., Matarese, G. (2014) Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 20, 69–74. [DOI] [PubMed] [Google Scholar]
- 113.Edozie, F. C., Nova-Lamperti, E. A., Povoleri, G. A., Scottà, C., John, S., Lombardi, G., Afzali, B. (2014) Regulatory T-cell therapy in the induction of transplant tolerance: the issue of subpopulations. Transplantation 98, 370–379. [DOI] [PubMed] [Google Scholar]
- 114.Raimondi, G., Sumpter, T. L., Matta, B. M., Pillai, M., Corbitt, N., Vodovotz, Y., Wang, Z., Thomson, A. W. (2010) Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J. Immunol. 184, 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Issa, F., Wood, K. J. (2014) The potential role for regulatory T-cell therapy in vascularized composite allograft transplantation. Curr. Opin. Organ Transplant. 19, 558–565. [DOI] [PubMed] [Google Scholar]
- 116.Lehtimäki, S., Lahesmaa, R. (2013) Regulatory T cells control immune responses through their non-redundant tissue specific features. Front. Immunol. 4, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng, H., Chi, H. (2013) mTOR and lymphocyte metabolism. Curr. Opin. Immunol. 25, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cipolletta, D., Feuerer, M., Li, A., Kamei, N., Lee, J., Shoelson, S. E., Benoist, C., Mathis, D. (2012) PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]