Abstract
We summarize different studies describing mechanisms through which bacteria in a biofilm mode of growth resist mechanical and chemical challenges. Acknowledging previous microscopic work describing voids and channels in biofilms that govern a biofilms response to such challenges, we advocate a more quantitative approach that builds on the relation between structure and composition of materials with their viscoelastic properties. Biofilms possess features of both viscoelastic solids and liquids, like skin or blood, and stress relaxation of biofilms has been found to be a corollary of their structure and composition, including the EPS matrix and bacterial interactions. Review of the literature on viscoelastic properties of biofilms in ancient and modern environments as well as of infectious biofilms reveals that the viscoelastic properties of a biofilm relate with antimicrobial penetration in a biofilm. In addition, also the removal of biofilm from surfaces appears governed by the viscoelasticity of a biofilm. Herewith, it is established that the viscoelasticity of biofilms, as a corollary of structure and composition, performs a role in their protection against mechanical and chemical challenges. Pathways are discussed to make biofilms more susceptible to antimicrobials by intervening with their viscoelasticity, as a quantifiable expression of their structure and composition.
Keywords: biofilm, structure, extracellular polymeric substances (EPS), antimicrobial penetration, detachment, viscoelasticity
Recalcitrance of biofilms against mechanical and chemical challenges has been looked at for ages from a microbiological perspective, but an approach based on viscoelastic properties of biofilms yields new insights in this recalcitrance.
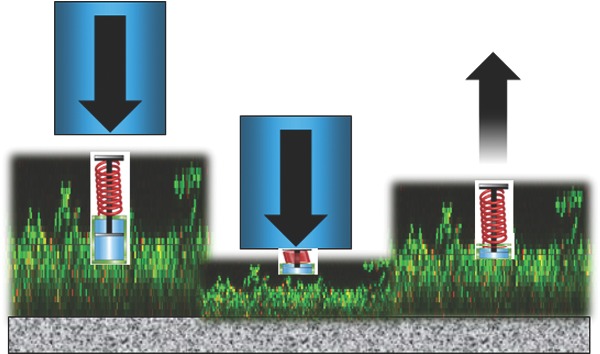
Recalcitrance of biofilms against mechanical and chemical challenges has been looked at for ages from a microbiological perspective, but an approach based on viscoelastic properties of biofilms yields new insights in this recalcitrance.
INTRODUCTION
Biofilms appear in many environmental settings and industrial processes where they can appear either beneficial or detrimental (Bos, Van der Mei and Busscher 1999). Biofilms are beneficial, for instance, to degrade environmentally hazardous substances in soil, but detrimental on food and slaughterhouse equipment. In many biotechnological processes, it is attempted to maintain biofilms in order to stimulate efficient degradation of chemicals. In the medical arena, it is currently estimated that over 60% of all human infections treated by physicians are due to biofilms (Fux, Costerton and Stewart 2005), examples being oral biofilms (‘dental plaque’) and biofilms involved in a variety of pathological conditions like for instance osteomyelitis, chronic otitis media, the infected diabetic foot, chronic bacterial prostatitis or in biomaterial-associated infections (Costerton, Stewart and Greenberg 1999; Busscher et al., 2012). Accordingly, research is focused mostly on how to prevent and control formation of an infectious, pathogenic biofilm, and on how to keep the commensal microflora of the skin, urinary and intestinal tract or oral cavity intact and free of potential pathogens (Reid et al., 2011).
In their biofilm mode of growth, bacteria adhering to a substratum surface and co-adhering with each other (Kolenbrander et al., 2010) embed themselves in a matrix of extracellular polymeric substances (EPS). This matrix not only yields bacterial phenotypes that can be different from their planktonic counterparts, but also offers physical protection against mechanical and chemical challenges (Flemming and Wingender 2010).
Biofilms and mechanical challenges
Biofilm formation starts with the adhesion of bacteria to a substratum surface that can either be of biological or synthetic origin. This layer of initially adhering bacteria provides a link connecting other bacteria that either grow or adhere on top of it to the substratum surface (Bos, Van der Mei and Busscher 1999). Biofilms can be mechanically challenged during growth, for instance by water pressure in marine environments, industrial pipelines or membrane filtration, in the oral cavity during fluid flow arising from powered toothbrushing and tongue movement, from pulsatile blood flow in intravascular catheters or from the movement of tissues, fluid and biomaterial components in an orthopedic joint prosthesis. When mechanical challenges occur (Fig. 1A) and detachment forces acting on a biofilm exceed the forces acting between different organisms in a biofilm, the biofilm is overloaded and failure occurs in the biofilm (‘cohesive failure’). Alternatively, when detachment forces operate exceeding the forces by which the initially adhering organisms connect with a substratum surface, the entire biofilm dislodges from the substratum surface (‘adhesive failure’) (Towler et al., 2003). Often biofilms go through cycles of fluctuating mechanical challenges, and cohesive or adhesive failure along with growth occur accordingly.
Figure 1.
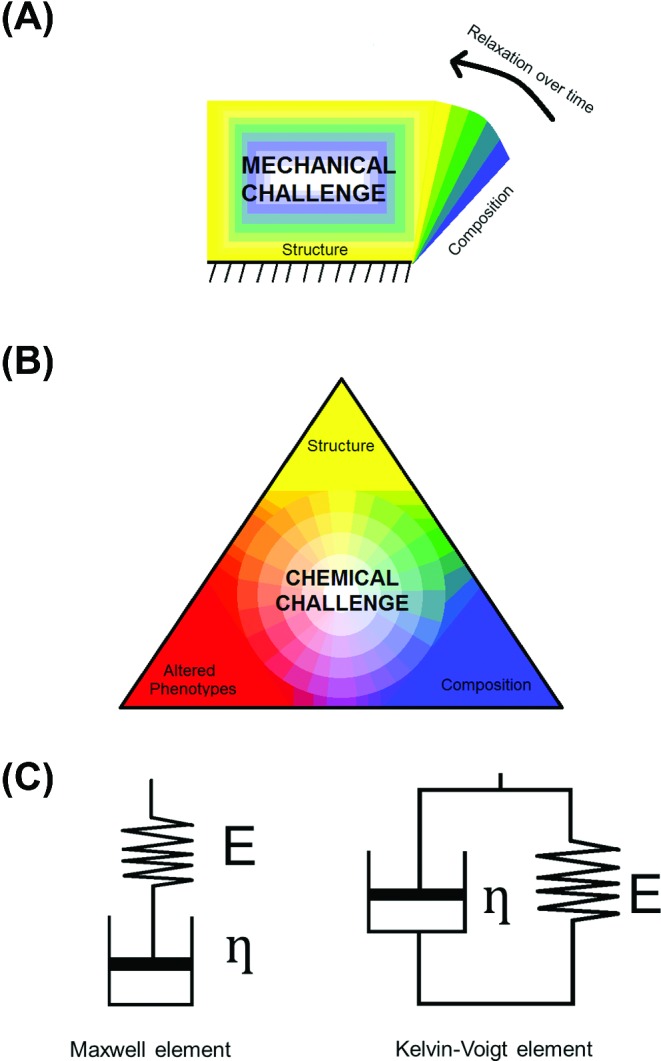
Key-properties of biofilms governing biofilm recalcitrance toward mechanical and chemical challenges. (A) Structure and composition govern biofilm resistance to environmental detachment and deformation forces. Resistance to deformation can be a time-dependent process yielding relaxation to an original shape over time. (B) Structure and composition govern the resistance of biofilms against chemical challenge in combination with altered phenotypes that are intrinsically more resistant to antimicrobials. (C) Composition and structure are jointly reflected in the viscoelasticity of a biofilm. Elasticity is generally presented as a spring with spring constant E, while the viscosity η (or its inverse, the fluidity φ) is shown as a dashpot. Springs and dashpots can be arranged in series (named ‘Maxwell’ element) or in parallel (called ‘Kelvin–Voigt’ element). Springs react immediately to an applied force, while dashpots dampen the speed of reaction. Usually, biological materials like biofilms cannot be represented by a single combination of springs and dashpots.
Biofilms and chemical challenges
The biofilm mode of growth protects individual bacteria from a variety of environmental challenges, including chemically diverse biocides (Mah and O'Toole 2001), host immune responses and antimicrobials (Vu et al., 2009). Poor antimicrobial penetration is the major obstacle for treating biofilm infections with antibiotics and this has been known since Van Leeuwenhoek (Van Leeuwenhoek 1684) reported in the 17th century that ‘the vinegar with which I washt my teeth, kill'd only those animals which were on the outside of the scurf, but did not pass thro the whole substance of it’. Although it has become clear in the meantime that the protection offered by biofilms to its inhabitants against chemical challenges consists of multiple mechanisms working in tandem, the exact mechanism is still not fully understood (Ito et al., 2009). In Fig. 1B, we identify three key properties of biofilms that govern the mechanisms through which biofilms become recalcitrant to antimicrobials (Thomas and Nakaishi 2006; Aslam 2008; Lazar and Chifiriuc 2010; Eastman et al., 2011; Stewart 2012). Biofilm structure determines many transport processes within a biofilm and can lead to micro-environments with specific pH or nutrient availability (Koo, Falsetta and Klein 2013). Penetration of antimicrobials and nutrients into a biofilm depends on the degree of channelization of the biofilm and the presence of a suitable medium for molecular transport through the biofilm. Usually transport of antimicrobials and nutrients is limited, based on whether the composition of the EPS matrix and the bacterial cell surfaces adsorb these compounds. Importantly, biofilm structure is dynamic, adapting in both space and time to its environmental conditions (Donlan 2002), amongst which are pH, temperature, fluid shear, nutrient availability and host defenses. As a result of nutrient deprivation, bacterial phenotypes in a biofilm can alter, leading to formation of persister cells (Lopez, Vlamakis and Kolter 2010; Poole 2012) that can remain dormant without causing disease for prolonged periods of time. Moreover, many antimicrobial agents target macromolecule synthesis inside bacteria (Dodds, Grobe and Stewart 2000) during active metabolism. Thus, the presence of a slow metabolism contributes to antimicrobial recalcitrance of biofilms in general, and persister cells can tolerate higher concentrations of antimicrobials than nearby recalcitrant biofilm organisms (Spoering and Lewis 2001; Keren et al., 2004). Consequently, whereas planktonic organisms have ample access to nutrients and, by the same token, are highly susceptible to antimicrobials, structural and compositional features of a biofilm form the major impediments for nutrient deprivation, the development of altered phenotypes and antimicrobial recalcitrance.
Aim of this review
In this review, we advocate that through a relation between structure and composition of biofilms with their viscoelasticity (Fig. 1C), viscoelasticity of biofilms can be recognized as a reflection of their structure and composition, including the EPS matrix and bacterial interactions. Using stress relaxation analysis, macroscopic physical properties of a biofilm can be derived that facilitate explanation of the resistance of biofilms in ancient and modern environments as well as of infectious biofilms to mechanical and chemical challenges on a more quantitative basis than can be obtained by microscopic means. Finally, this review identifies new pathways for the treatment of infectious bacterial biofilms by interfering with their viscoelastic properties.
VISCOELASTICTY OF NATURAL SUBSTANCES
To understand the implications of viscoelasticity as a contributing factor to the resistance of biofilms to mechanical and chemical challenges, we first give a comprehensive description of the concept of viscoelasticity (for an excellent introduction to biomechanics including the viscoelasticity of materials see Vincent (2012)).
‘Viscoelasticity is a material property in which a material exhibits both viscous and elastic characteristics. Viscous materials deform irreversibly over time to relieve stress, while elastic materials deform instantaneously to relieve stress and come back to their original state once stress is removed. A viscoelastic material will deform under stress, while returning over time to a state similar to, but not necessarily identical to the pre-deformed state, when stress is removed. Whereas elasticity is usually the result of atomic or molecular stretching, viscosity is the result of “atomic or molecular flow” (Ratner et al., 2004b)’.
Elasticity is generally presented as a spring with spring constant E, while the viscosity η (or its inverse, the fluidity φ) is shown as a dashpot. A spring reacts to an applied force with an immediate change in length, depending on the magnitude of the force applied, but this is different for a dashpot. A dashpot behaves like a piston that is free to move up and down a cylindrical vessel filled with a fluid, provided the fluid can flow through the piston-cylinder gap. Thus, the speed at which the piston can move heavily depends on the viscosity of the fluid. Spring and dashpot can either be placed in series or in parallel (see Fig. 1C). Note that biological materials usually cannot be represented by a single combination of a spring and dashpot. Table 1 provides examples of values for the elasticity and viscosity of some known, biological and synthetic materials, including biofilms.
Table 1.
Viscoelasticity of different biological and synthetic materials, including the elasticity or bulk modulus E and the viscosity η at room temperature (20°C), unless stated otherwise.
| Material | Elasticity 109 Pa | Viscosity 10−3 Pa.s | Reference |
|---|---|---|---|
| Titanium | 106–108 | – | Boyer and Collings (2007) |
| Aluminum | 68–70 | – | ASM International (1990) |
| Silicone rubber | 0.001–0.05 | – | http://www.azom.com |
| Hyaluronic acid-based tissue-engineering scaffolds | 10−4 | 107 | Borzacchiello et al. (2007) |
| Skin | 0.015–0.15 | – | Edwards and Marks (1995) |
| Human cortical bone | 15–30 | – | Ratner et al. (2004a) |
| Dental enamel | 80 | – | Ferracane (2001) |
| Hair | 7 | – | Lee and Kwon (2013) |
| Water | – | 1 | |
| Saliva (37°C) | – | 1.3–2.0 | Rantonen and Meurman (1998) |
| Blood (37°C) | 3–4 | Elert (2014) | |
| Urine (37°C) | 0.8 | Inman et al. (2013) | |
| Pseudomonas biofilm, EPS only (shear mode) | 10−10 | 102 | Wloka et al. (2004) |
| Pseudomonas entire biofilms (shear mode) | 10−5 | Körstgens et al. (2001) | |
| Miscellaneous biofilms (shear mode) | 10−10–10−4 | 103–1013 | Shaw et al. (2004) |
| Environmental and industrial biofilms (tensile mode) | 10−8 | Stoodley et al. (1999a) | |
| Oral biofilms (compressive mode) | 10−8–10−7 | Paramonova et al. (2009) |
VISCOELASTICITY AND BIOFILM STRUCTURE AND COMPOSITION
Microscopic studies on biofilm structure and composition
Microscopic techniques, and particularly confocal laser scanning microscopy (CLSM), have yielded a general description of biofilm structure and to a limited degree its microbiological and biochemical composition. Whereas early electron microscopic techniques enabled imaging of organisms in a biofilm, the desiccation required by high-vacuum conditions has long obscured the role of the EPS matrix that appeared as a dark rim of condensed matter on the bacterial cell surfaces in a biofilm (Stewart and Costerton 2001). Improved preservation methods such as cryopreservation prevent condensation of the EPS matrix (Matias et al., 2003; Schaudinn et al., 2009). Recent development of stages and methodologies allows sequential sections to be made and imaged. These sections can be reconstructed into 3D volumes, providing highly detailed insights into biofilm structure. Focused ion beam scanning electron microscopy (FIB-SEM), for instance, uses ion-sputtering of biofilm mass with repetitive imaging to observe the organisms within biofilms at high resolution (Wallace, Arey and Mahaffee 2011), and 2D images selected from a 3D image stack can be utilized to visualize cell wall damage inside a biofilm due to antimicrobial attack (see Fig. 2). Still, due to requirements of some degree of preservation, these electron microscopy techniques are often susceptible to corruption of the natural state of biofilms (Wu et al., 2014). Environmental SEM has the advantage that it does not require dehydration or fixation, but it cannot see inside the highly hydrated EPS to reveal internal structure (Danilatos and Postle 1982; Bridier, Meylheuc and Briandet 2013).
Figure 2.
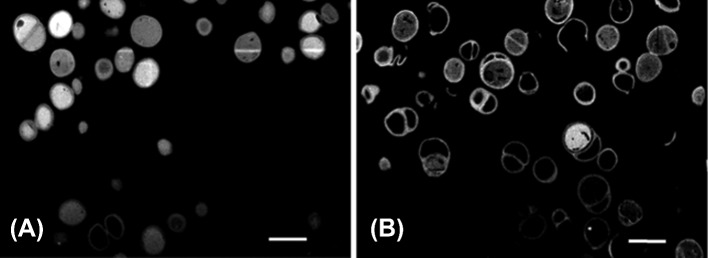
2D FIB-SEM cross sections from a 3D image stack of OsO4-stained, FIB-sectioned S. epidermidis ATCC 35984 biofilms prior to and after exposure to quaternary-ammonium solutions, demonstrating holes in the bacterial cell wall due to the interdigitization of the hydrophobic tail of the quaternary ammonium molecules (unpublished data). (A) Control (exposure to tryptone soya broth), (B) after exposure to 1 x MBC of a quaternary-ammonium solution (Ethoquad C/25) [Cocoalkyl methyl (polyoxyethylene) ammonium chloride], the scale bar denotes 1 μm.
CLSM has become the most employed microscopy technique to study biofilms. Many key components of biofilm structure have been determined using CLSM in association with chemically binding fluorophores, including 3D architecture (Salek, Jones and Martinuzzi 2009; Uppuluri, Chaturvedi and Lopez-Ribot 2009; Robinson 2011), mushroom-shaped colonies (Entcheva-Dimitrov and Spormann 2004; Venugopalan et al., 2005), stratification (Lawrence et al., 1991), establishment of water channels (Donlan 2002; Stewart 2012), presence of EPS (Sanford et al., 1996; Lawrence et al., 2003; Li, Bai and Liu 2008), bacterial cell surface damage and viability (Hope, Clements and Wilson 2002) as well as biofilm failure under compression (Cense et al., 2006). The establishment of water channels, however, has never been directly proven by CLSM methods, but rather indirectly indicated by the absence of stain uptake as by bacteria and matrix components. Chemically reactive lectins provide information about the molecular composition of sugars within a biofilm (Allison 2003). Alternatively, fluorescent in situ hybridization (better known by its abbreviation ‘FISH’) differentially stains individual cells of different bacterial species, providing information about the location of different species within multispecies biofilms (Weber et al., 2007). CLSM also has the advantage of allowing real-time imaging of localized processes of attachment, growth and dispersal as well as metabolic activity and flow through the biofilm. A limiting factor in the use of CLSM is that the penetration ability of the fluorophores is typically confined to 20 to 40 μm (Vroom et al., 1999), and it is difficult to see much deeper within a biofilm structure. Moreover, the penetration capability of the laser itself limits the depth of view within a biofilm structure. Two-photon laser systems have helped to increase the penetration depth of the CLSM imaging, yet they still require the use of fluorophores that may have harmful effects to the biofilms (Mclean, Ona and Majors 2008).
These drawbacks of CLSM have stimulated the use of other microscopic techniques that image biofilm structure but do not rely on staining. Optical coherence tomography, for instance, relies on the scattering of light rather than on fluorescent staining and is used to accurately describe voids and water channels (Wagner et al., 2010). Water channels have been indirectly indicated by CLSM methods, assuming that they correspond with voids, but CLSM easily overestimates the presence of voids, because they also appear due to lack of stain penetration. Optical coherence tomography, not relying on stain penetration, has demonstrated that heterotrophic biofilms had a porosity under 70%, when 98% porosity was found using CLSM (Wagner et al., 2010).
In summary, advances in microscopic imaging techniques have yielded structural information on voids and water channels in biofilms and their compositional heterogeneity. However, in essence, these advances still reflect a similar conclusion as drawn in the 17th century, when Antonie van Leeuwenhoek reported on the poor penetration of vinegar into oral biofilm using his first, self-made microscopes (Van Leeuwenhoek 1684). This calls for an urgent need for more quantitative, observer-independent techniques to characterize biofilms in terms of structure and composition. One such alternative technique centers on biofilm viscoelasticity.
Measurement of the viscoelasticity of biofilms
Measurement of the viscoelasticity of a biofilm either involves measuring deformation under constant stress (‘creep’) (Lau et al., 2009) in either a compressive, shear or tensile mode or measuring the stress required to maintain a constant deformation (‘stress relaxation’) (Cense et al., 2006), as summarized in Fig. 3A and B. Constant mechanical stress or deformation (often reported as ‘strain’) can, amongst other methods, be applied by uniaxial compression (Körstgens et al., 2001), in which a macroscopic plunger mechanically stresses or deforms the biofilm and strain or stress relaxation is recorded as a function of time. Since relaxation results from multiple re-arrangement processes in a deformed biofilm, the mathematical description of relaxation possesses multiple elements. Although creep is the increase of strain under an imposed load and stress relaxation is the dissipation of stress under an imposed strain (Gupta et al., 2010), their measurement can be analyzed using similar mathematical models, which result in similar time constants of their response processes (Poznanski, Pawlowski and Fikus 1992). Generally, due to its multi-component nature, stress relaxation of biofilms can be described by three to four Maxwell or Kelvin–Voigt elements (see Fig. 1C) with each element containing a dashpot, responsible of a single time-dependent re-arrangement process, combined with a spring constant, representing the immediate elastic response. Note that in a Kelvin–Voigt element, dashpot and spring are placed in parallel, causing dampening of the immediate response as immediate relaxation has to occur against the action of the dashpot. In a Maxwell model (Fig. 3C), multiple Maxwell elements are placed in parallel, while in a Burgers model a Kelvin–Voigt element is placed between the spring and dashpot of a Maxwell element (Fig. 3D). However, the more elements one includes, the better the fit but the more uncertainty in the values of the parameters derived will occur. The creep response commonly found in biofilms may require a minimum of four elements (Jones et al., 2011).
Figure 3.
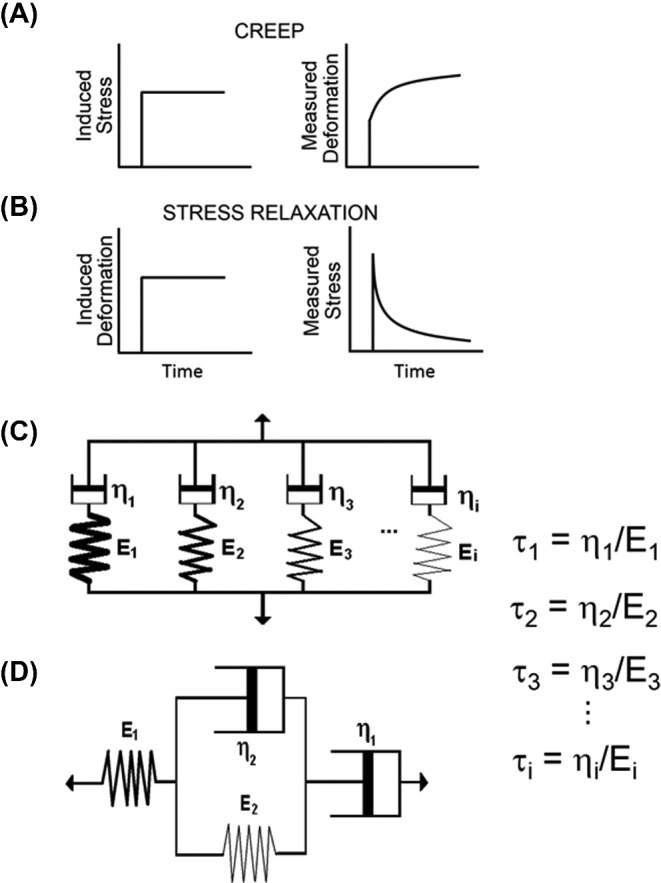
Viscoelastic measurements of ‘creep’ and ‘stress relaxation’. (A) During creep measurements, a constant stress is induced while the deformation of the biofilm is recorded over time. (B) During stress relaxation measurements, a constant deformation is induced and the stress required to maintain that deformation is recorded over time. (C) In a Maxwell model, response to an induced stress or deformation is mathematically modeled using multiple Maxwell elements in parallel, each with their characteristic time constants representing the viscous part of the response (‘the dashpots’, ηn) and an immediate elastic response component (‘the springs’, En). Characteristics time constants of each individual response process follow from τn as indicated in the graph. (D) In a Burger's model, this response is mathematically modeled using a Kelvin–Voigt element separated by a spring and dashpot, that together represent a Maxwell element.
Using microbead atomic force microscopy, relaxation processes in a biofilm can also be monitored on a more microscopic level (Lau et al., 2009). Often, the microbead is comprised of a silica particle with a diameter typically in the order of up to 50 μm. Stress deformation of Pseudomonas biofilms using microbead atomic force microscopy yielded relaxation times of less than 1 s, with a reduced viscoelastic response in more matured biofilms of Pseudomonas strains deficient in lipopolysaccharides. Individual bonds between single bacteria are also viscoelastic in nature and bacterial probe atomic force microscopy has revealed relaxation times for a variety of Gram-negative bacterial strains between 0.8 and 2.6 s based on a three-element standard solid model (Vadillo-Rodriguez and Dutcher 2011). Relaxation times of Gram-positive staphylococcal bonds were slightly higher between 1 and 6 s and shorter in absence of an EPS matrix around the bacteria (Chen et al., 2014).
While indentation and rheometry measure bulk properties of biofilms, microscopy-based techniques can be utilized to measure local viscoelastic properties within a biofilm and in different biofilm structures. Digital image correlation of 2D bright field microscope images of biofilms grown under flow revealed that biofilm deforms differently in response to elevations in fluid shear in the attached head as compared to in the streamer tail (Mathias and Stoodley 2009). More recently microrheometry using CLSM to track the motion of magnetic beads in response to an oscillating magnetic field (Galy et al., 2012) or the natural brownian motion of beads within the biofilm (Chew et al., 2014) have been used to map the viscoelastic properties of biofilms at specific locations in 3D. Galy et al. (2012) found that some parts of a biofilm behave like elastic solids, while others behave more like viscoelastic liquids and the base of the biofilm was stiffer than the upper layers, illustrating the importance of mechanical heterogeneity. It was also reported that the local mechanical properties were not related to local cell density, suggesting that the EPS was the principal determinant. Chew et al. (2014) were able to link the mechanical properties of different polymers expressed in Pseudomonas aeruginosa biofilms with streamer formation. Optical coherence tomography also has good potential for measuring 3D biofilm structural responses to applied fluid shear stresses (Wagner et al., 2010). The difficulty in simulating in situ biofilm rheological behavior in the laboratory using pure protein and polysaccharide gels suggests that the rheological complexity is a result of structural and compositional heterogeneity within a biofilm. The measurement of relaxation processes at a more microscopic level combined with local staining of specific EPS components offers the challenging possibility of modeling the macroscopic viscoelasticity of a biofilm in terms of its EPS response and the response of individually interacting bacteria.
Association between viscoelastic properties with structure and composition of biofilms
Bacteria in a biofilm constitute the heaviest masses; thus, their re-arrangement upon an induced deformation will be slow, and the relative importance of the slowest Maxwell element in relaxation has been intuitively associated with bacterial re-arrangement in a biofilm. Recently, the relatively slow re-arrangement of P. aeruginosa was confirmed by direct microscopic observation of relaxation processes, showing that bacteria could not find new, stable positions within 100 s upon an imposed deformation (Peterson et al., 2014), while EPS displaced by deformation moved toward deeper layers within 20 s. In the so-called ‘fluctuating binding point model’ (Körstgens et al., 2001), this implies that binding points in a biofilm, existing prior to the application of deformation, can be more readily re-established between EPS components than between bacteria or between bacteria with EPS components. Water, on the other hand, has the lowest viscosity in a biofilm, and therefore the fastest Maxwell element has been associated with the flow of water through a compressed biofilm. This leaves an association between the behavior of EPS with intermediate Maxwell element(s) (Peterson et al., 2012; He et al., 2013). Principal component analyses of Maxwell elements describing the stress relaxation exhibited by biofilms with different matrix chemistries have pointed out that in general three principal components suffice to describe stress relaxation of biofilms (Peterson et al., 2013). The fastest principal component was associated with the outflow of water and soluble polysaccharides. A second principal component was associated with the EPS matrix as a whole, with a distinct impact of the presence of eDNA included in a third component, possessing a narrowly confined time constant range within the range of the second principal component. The presence of bacteria themselves as the heaviest masses is included in the first principal component with an inverse impact of bacterial prevalence with respect to the presence of water and soluble polysaccharides, i.e. the free space in a biofilm available for bacterial re-arrangement processes (Peterson et al., 2013). Importantly, herewith there exists a relation between quantifiable viscoelastic properties of a biofilm that relates with their structure and composition that goes beyond the possibilities of microscopic imaging techniques.
VISCOELASTICITY OF BIOFILMS AS A MEANS OF SURVIVAL IN ANCIENT AND MODERN ENVIRONMENTS
In their natural environment, most biofilms are exposed to external forces applied either in a compressive, tensile or shear mode. Whereas microscopic techniques in general yield semi-quantitative descriptions of structure and composition of a biofilm at best, viscoelasticity is determined by an interplay of structure and composition as outlined above, and can be quantitatively assessed over larger areas of a biofilm than microscopically possible.
Biofilms are typical examples of a multi-component biological material. EPS alone is a multi-component substance (Whitchurch et al., 2002; Flemming and Wingender 2010; Koo, Falsetta and Klein 2013), and, as a consequence, biofilms react in a time-dependent manner with a combination of distinct elastic and viscous responses to external stress. The elastic part of the response is immediate and followed by viscous relaxation (Klapper et al., 2002; Stoodley et al., 2002) as represented by the springs and dashpots in Fig. 1C, respectively. More specifically, a number of studies suggest that biofilms behave as viscoelastic liquids (Stoodley et al., 1999a; Körstgens et al., 2001; Towler et al., 2003; Shaw et al., 2004), i.e. they flow under sustained load, and even though values of the elastic and viscous moduli vary by many orders of magnitude, viscoelasticity is a common feature of many different types of medical, oral and environmental biofilms (Shaw et al., 2004) (see also Table 1). Intriguingly, Shaw et al. (2004) noticed that despite wide variations in elasticity and viscosity of biofilms, their overall characteristic relaxation time constant was about 18 min over a wide collection of biofilms derived from fresh water and hot spring isolates, Staphylococcus aureus, P. aeruginosa and Streptococcus mutans strains. Considering that bacterial doubling times in regularly growing biofilms are of the same order of magnitude (Gottenbos et al., 1999), 18 min may represent a possible survival significance as the period over which a biofilm can respond to transient, external stresses (Shaw et al., 2004).
There is evidence that the ability of biofilms to flow along surfaces when exposed to elevated shear of an overlying water stream may allow bacteria to yield to the flow, yet remain attached to the surface. Time lapse images in flow cells captured the flow and the ability of the biofilm to rapidly change ripple and streamer morphology when the water flow was changed (Stoodley et al., 1999b). Ripple structures have also been observed in modern streams (Battin et al., 2003) as well as ancient fossils (Noffke et al., 2013a; Noffke, Decho and Stoodley 2013b; Thomas et al., 2013). Energy dissipation through such viscoelastic behavior can also explain the large pressure drops and drag associated with biofilms in systems as diverse as sulfate-reducing bacterial biofilms in industrial pipelines (Dunsmore et al., 2002) as well as biofouling of ship hulls (Schultz et al., 2011).
SURVIVAL OF INFECTIOUS BIOFILMS UNDER MECHANICAL AND CHEMICAL CHALLENGE
In addition to survival in natural environments, biofilm viscoelasticity, as a corollary of structure and composition, also has implications for the way pathogenic biofilms deal with mechanical and chemical challenges, particularly when biofilms are associated with biomaterials implants and devices, as will be illustrated in the following sections.
Survival of biofilms on intravascular catheters in the human blood stream
Catheter-associated blood stream infections are common after long-term catheterization of patients and result from catheter-associated biofilms. Biofilms on intravascular catheters have to withstand the fluctuating shear from the pulsatile blood flow, using their viscoelastic properties to adjust to local changes in fluid flows while maintaining their structural integrity. In an initial attempt to withstand increasing shear forces, biofilms extend in the direction of flow through re-arrangement into so-called ‘streamers’, as shown in Fig. 4A (Rusconi et al., 2010, 2011). The viscoelasticity of biofilms allows these streamers the possibility to extend and therewith organisms residing in the biofilm gain time to adapt to the new flow conditions. Recently, it was hypothesized that viscoelastic streamer formation by S. aureus in intravenous catheter may block these catheters (Kim et al., 2014). However, it is also possible that the formation and breaking of viscoelastic EPS tethers allow S. aureus biofilms to roll along catheter surfaces (Rupp, Fux and Stoodley 2005), much like selectin-mediated leukocyte rolling during extravasation (Sundd, Pospieszalska and Ley 2013). Recent evidence has suggested that biofilms might be involved in the formation of atherosclerotic plaques and be involved in their detachment (Lanter, Sauer and Davies 2014). How and when biofilms in the blood stream fail is arguably the most important biofilm property in an intravascular catheter application (Guelon, Mathias and Stoodley 2011).
Figure 4.
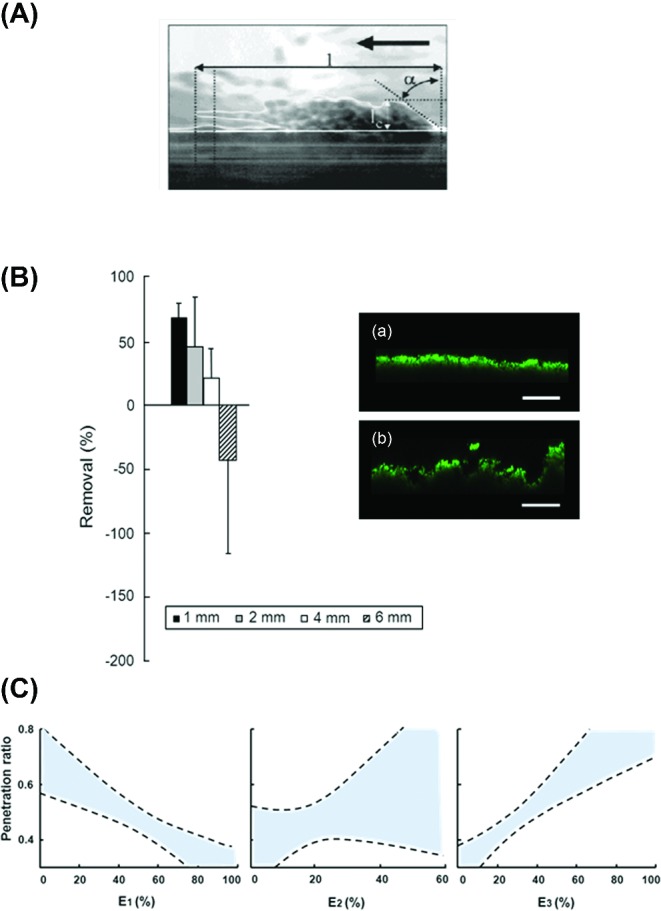
Role of viscoelasticity of biofilms in their survival under mechanical and chemical challenges. (A) Side view of a streamer in a biofilm growing under an applied wall shear stress. The edge of the streamer has been outlined for clarity and the direction of fluid flow is indicated by the thick arrow [(Stoodley et al., 1999a), with permission of the publisher]. (B) Biofilm removal or expansion (negative removal) for different distances between a biofilm and the bristle tips of a powered toothbrush [adapted from Busscher et al., 2010) with permission of the publisher], together with CLSM images (unpublished) of biofilms prior to (a) and after (b) non-contact brushing showing volumetric expansion (scale bar indicates 75 μm). (C) Penetration ratio of chlorhexidine as a generalized function of the relative importance of the three Maxwell elements E1, E2 and E3, denoting the fast, intermediate and slow relaxation components, respectively. Dashed lines represent 95% confidence intervals.
Biofilms on endotracheal tubes
Endotracheal tubes are used to intubate patients for breathing and deliver pulsatile flow during a positive pressure inspired delivery and neutral pressure expired release. The gas is humidified and a moist layer containing respiratory secretions forms on the inner lumen of the tube which rapidly becomes colonized with bacteria from the oral cavity, upper airway and nosocomial hospital pathogens (Vandecandelaere et al., 2013). Bacteria from biofilms on endotracheal tubes can enter the airway to cause ventilator-associated pneumonia through spreading growth, detachment into the gas stream (Inglis et al., 1989). However, Inglis (1993) also made the salient observation that endotracheal tube biofilms had formed wave-like structures and hypothesized that these might be caused by biofilm flow along the surface, contributing to ventilator-associated pneumonia. As discussed previously, time lapse imaging has shown that biofilms can form ripples and flow as viscoelastic liquids induced by the overlying liquid flow (Stoodley et al., 1999b) with implications for dissemination of pathogens within or from medical devices.
Mechanical removal of biofilms in the oral cavity
In the oral cavity as well as in the blood stream, biofilms are mechanically challenged by naturally occurring fluid flows, but also by tongue movements and daily toothbrushing. Toothbrushing removes oral biofilm through direct contact with toothbrush filaments, but powered toothbrushes also do so in a non-contact mode (Verkaik et al., 2010), provided the energy output of the brush is high enough (Busscher et al., 2010). The energy output of powered toothbrushes varies according to distance from the edge of the toothbrush filaments and navigates the complex geometry of tooth surfaces. Thus, removal of oral biofilms via non-contact mode brushing is susceptible to a wide distribution of shear forces (Rmaile et al., 2014). The viscoelasticity of oral biofilms under non-contact brushing causes the biofilm to expand rather than to directly disperse prior to disruption (Busscher et al., 2010). Moreover, if toothbrushing is arrested before biofilm detachment occurs, biofilms are left in an expanded state (see Fig. 4B) (He et al., 2014).
Antimicrobial penetration in oral biofilms
Penetration of chlorhexidine into oral biofilms increased with increasing relative importance of the slow and decreasing importance of the fast relaxation element (see Fig. 4C) (He et al., 2013). Involvement of slow relaxation elements suggests that biofilm structures allowing extensive bacterial re-arrangement after deformation are more open, allowing better antimicrobial penetration. Involvement of fast relaxation elements suggests that water dilutes the antimicrobial upon penetration to an ineffective concentration in deeper layers of the biofilm (He et al., 2013). Note that these results could also have been expressed in one principal component, since the more open structure allowing bacterial re-arrangement inversely relates to the presence of water-filled channels (Peterson et al., 2013). The flow of water through biofilms is an important consideration in terms of antimicrobial penetration to target bacteria in a biofilm. Generally, flow will be primarily from convection (fast) and diffusion (slow). Early work with fluorescent microspheres and nano-injection of fluorescent dyes established that an overlying flow induced convective flow in the channels (Stoodley, De Beer and Lewandowski 1994), while in clusters alone there was only diffusion. Larger molecules such as human IgG antibody could not penetrate the EPS matrix (DeBeer, Stoodley and Lewandowski 1997). Nevertheless, submerged biofilms themselves are highly compressible (Peterson et al., 2012), and since water is incompressible there must be a dewatering effect to explain the biofilm volume decrease. Under compression, it is likely that water is squeezed out of the channels, and, while it has not been directly shown whether compression induces convection through the EPS matrix of the cell clusters, modeling of the viscoelastic response predicts that there is (He et al., 2013). Squeezing out of water through channels in compressed biofilms may also constitute the reason as to why biofilm elasticity and viscosity vary when derived in different modes of stress application. In many experimental systems, biofilms are usually ‘unconfined’ and stress is applied locally over a relatively small surface area, allowing water to flow away from the stressed region to its surrounding as is also possible in natural conditions.
VISCOELASTICITY OF BIOFILMS: A POSSIBLE TARGET FOR THE TREATMENT OF INFECTION
In the medical arena, the pathogenicity of bacteria is usually related to conventional definitions of virulence describing virulence factors as molecules that are essential for a pathogen to cause disease. Such factors include molecules that enable the pathogen to colonize the host by attachment to and invasion in host tissue, or to evade the host immune response. However, with the growing realization that biofilms rather than planktonically present organisms represent the more pathogenic state of bacterial growth, additional factors come into play. In a biofilm, bacterial adhesion to a substratum surface causes a phenotypic change that makes removal upon a mechanical challenge more difficult (Vuopio-Varkila and Schoolnik 1991; Gonzalez-Valencia, Munoz and Torres 1991; Pal et al., 1992; Kallman et al., 1993). Mechanical strategies used by biofilms to persist in the environment which allow part to remain attached, coalesce and flow over surfaces, while other parts detach in response to external mechanical forces, are being seen as playing an important role for persistence of infection (Lieleg et al., 2011; Stewart 2014). In addition, structure and composition of the EPS matrix of oral biofilms as reflected in the viscoelastic properties of a biofilm, have been related with bacterial activity in a biofilm (Koo, Falsetta and Klein 2013). The biofilm mode of growth is also recognized to increase recalcitrance to antimicrobials as a result of diffusion limitation through the biofilm (Arciola, Campoccia and Montanaro 2002; Oelschlaeger, Dobrindt and Hacker 2002). Survival requires structural and compositional re-arrangements to evade these challenges on an appropriate timescale, as represented in the viscoelasticity of a biofilm (Shaw et al., 2004).
The realization that viscoelasticity is a quantifiable reflection of the structure and composition impacting its survival under mechanical or chemical challenge opens new pathways for the control of biofilms in disease and understanding of ill-understood phenomena like the increased susceptibility of biofilms to antibiotics in combination with pulsating waves of energy (Rediske et al., 1998). The CLSM images in Fig. 4B clearly demonstrated that biofilms expand upon the transfer of energy by non-contact, powered toothbrushing (Busscher et al., 2010; He et al., 2014). This effect was accompanied by an increase in the slow and a decrease in the fast Maxwell relaxation of these biofilms. These changes facilitated increased penetration of oral antimicrobials (He et al., 2014). Non-contact, powered toothbrushing is usually done at frequencies between 260 and 750 Hz (Blanco et al., 1997). These frequencies might be aligned in the future with the characteristic time constants or resonance frequencies of the different relaxation processes occurring upon mechanically challenging a biofilm, to increase bacterial detachment. Obviously, the implications of this suggestion reach beyond oral health care, and pertain to all situations where it is desirable or necessary to remove biofilms by mechanical means.
Pulsating waves of energy have been shown to amplify antimicrobial efficacy against biofilms, and is generally referred to as the ‘bio-acoustic effect’ (Pitt et al., 1994; Shen et al., 2010). Gentamicin has been shown to have enhanced killing in P. aeruginosa and Escherichia coli biofilms in vitro and in vivo in rabbits after ultrasound treatment with frequencies ranging from 30 to 500 kHz and energies ranging from 10 to 3000 mW cm−2 (Qian, Stoodley and Pitt 1996; Rediske et al., 2000; Carmen et al., 2004, 2005). Although biofilms of Gram-positive bacteria were initially thought to be resistant to enhanced killing from ultrasonic waves (Pitt et al., 1994), with enough energy input (3000 mW cm−2) Gram-positive bacteria were also found to be susceptible (Rediske et al., 1998). Reducing the frequency of the wave to 2–3 Hz increased efficacy of antimicrobials in biofilms of S. aureus and single and mixed species oral biofilms (Gerdesmeyer et al., 2005; Novak et al., 2008; Muller et al., 2011). Since, despite the lower frequencies used in non-contact, powered toothbrushing (Blanco et al., 1997), there is an analogy between non-contact powered toothbrushing with the bio-acoustic effect, it is likely that ultrasound will change the structure of biofilms to cause better penetration of antimicrobials into a biofilm and therewith increased killing. Viscoelastic measurements have not yet been done however on ultrasonically treated biofilms, but such measurements may shed more light on the mechanisms of the bio-acoustic effect. Interestingly, low frequencies were more efficient in increasing the killing efficacy of antimicrobials than higher ones (Qian, Sagers and Pitt 1999). The characteristic time constants derived from the stress relaxation of deformed biofilms, ranging from <5 s, 5–100 s and >100 s for the fast, intermediate and slow elements, respectively, suggest that the main effects of pulsating energy waves are through effects on the fast principal Maxwell element and thus associated with the presence of water in the biofilm. At the low frequencies associated with more efficient killing, the pulsating energy waves are closer to cutoff between the frequencies of the fast and intermediate Maxwell elements. Taken together, these considerations point to a new pathway to increase the efficacy of pulsating waves of energy by fine-tuning the frequencies of these treatments to the resonance frequencies of the different constituents in biofilm matrices, which will have a major impact on both their mechanical stability and antimicrobial penetration through a direct effect on the viscoelasticity of biofilms.
Changes in viscoelastic properties of biofilms that enhance antimicrobial efficacy can not only be achieved through pulsating waves of energy but also chemically (Jones et al., 2011). After exposure to detergents, S. aureus biofilms grown on stainless steel had increased expression of the slowest Maxwell element, reflecting bacterial re-arrangement. Pseudomonas aeruginosa biofilms on stainless steel were affected by N-acetyl-L-cysteine, as both the fast and intermediate Maxwell elements were reduced by more than 50%, while the slowest Maxwell element nearly doubled. Pseudomonas aeruginosa biofilms on membrane filters were similarly affected by both N-acetyl-L-cysteine and DNaseI, reducing both the fastest and intermediate principal elements (Peterson et al., 2013). Thus, both the pre-treatment and post-treatment of biofilms by chemicals directly affects their viscoelastic properties and changes them into a direction favorable for subsequent antimicrobial treatment.
CONCLUSIONS
Bacterial biofilms provide added protection against environmental detachment forces, antimicrobials and the host immune system, increasing the chance of survival under mechanical and chemical attack. This review identifies the viscoelasticity of biofilms as a corollary of the structure and composition of biofilms. The viscoelasticity of biofilms therewith becomes a quantifiable property of biofilms, pivotal for the way biofilms deal with mechanical and chemical challenges. This identification opens new pathways to prevent or control bacterial biofilms by intervening with their viscoelastic properties that may be particularly relevant in the medical arena where over 60% of all human infections are due to biofilms.
Acknowledgments
YH would like to thank the China Scholarship Council and the W.J. Kolff institute for financial support. Opinions and assertions contained herein are those of the authors and are not construed as necessarily representing views of the funding organization or their respective employers. HJB is also director of a consulting company, SASA BV. AJvW is co-owner of Laboral, a company in oral diagnostics and he has minor stock ownership in Dentaid Benelux.
FUNDING
This study was funded by the authors’ institutions. In addition, YH received a scholarship from the China Scholarship Council.
Conflict of interest statement. None declared.
REFERENCES
- Allison DG. The biofilm matrix. Biofouling. 2003;19:139–50. doi: 10.1080/0892701031000072190. [DOI] [PubMed] [Google Scholar]
- Arciola CR, Campoccia D, Montanaro L. Detection of biofilm-forming strains of Staphylococcus epidermidis and S. aureus. Expert Rev Mol Diagn. 2002;2:478–84. doi: 10.1586/14737159.2.5.478. [DOI] [PubMed] [Google Scholar]
- Aslam S. Effect of antibacterials on biofilms. Am J Infect Control. 2008;36:S175.e9–11. doi: 10.1016/j.ajic.2008.10.002. [DOI] [PubMed] [Google Scholar]
- ASM International. ASM Handbook. 10th edn. Vol. 2. Metals Park, OH, USA: ASM International; 1990. Properties & Selection—Nonferrous Alloys & Special-Purpose Materials. [Google Scholar]
- Battin TJ, Kaplan LA, Newbold JD, et al. Effects of current velocity on the nascent architecture of stream microbial biofilms. Appl Environ Microb. 2003;69:5443–52. doi: 10.1128/AEM.69.9.5443-5452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco VL, Cobb CM, Williams KB, et al. In vitro effect of the Sensonic toothbrush on Treponema denticola. J Clin Periodontol. 1997;24:318–23. doi: 10.1111/j.1600-051x.1997.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Borzacchiello A, Mayol L, Ramires PA, et al. Structural and rheological characterization of hyaluronic acid-based scaffolds for adipose tissue engineering. Biomaterials. 2007;28:4399–408. doi: 10.1016/j.biomaterials.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Bos R, Van der Mei HC, Busscher HJ. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol Rev. 1999;23:179–230. doi: 10.1111/j.1574-6976.1999.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Boyer R, Collings EW. In Materials Properties Handbook: Titanium Alloys. 4th edn. Materials Park, OH, USA: ASM International; 2007. [Google Scholar]
- Bridier A, Meylheuc T, Briandet R. Realistic representation of Bacillus subtilis biofilms architecture using combined microscopy (CLSM, ESEM and FESEM) Micron. 2013;48:65–9. doi: 10.1016/j.micron.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Busscher HJ, Jager D, Finger G, et al. Energy transfer, volumetric expansion, and removal of oral biofilms by non-contact brushing. Eur J Oral Sci. 2010;118:177–82. doi: 10.1111/j.1600-0722.2010.00723.x. [DOI] [PubMed] [Google Scholar]
- Busscher HJ, Van der Mei HC, Subbiahdoss G, et al. Biomaterial-associated infection: locating the finish line in the race for the surface. Sci Transl Med. 2012;4:153rv10. doi: 10.1126/scitranslmed.3004528. [DOI] [PubMed] [Google Scholar]
- Carmen JC, Nelson JL, Beckstead BL, et al. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J Infect Chemother. 2004;10:193–9. doi: 10.1007/s10156-004-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmen JC, Roeder BL, Nelson JL, et al. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am J Infect Control. 2005;33:78–82. doi: 10.1016/j.ajic.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cense AW, Peeters EA, Gottenbos B, et al. Mechanical properties and failure of Streptococcus mutans biofilms, studied using a microindentation device. J Microbiol Meth. 2006;67:463–72. doi: 10.1016/j.mimet.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Chen Y, Van der Mei HC, Busscher HJ, et al. Viscous nature of the bond between adhering bacteria and substratum surfaces probed by atomic force microscopy. Langmuir. 2014;30:3165–9. doi: 10.1021/la404874x. [DOI] [PubMed] [Google Scholar]
- Chew SC, Kundukad B, Seviour T, et al. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccarides. mBio. 2014;5:e01536–14. doi: 10.1128/mBio.01536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Danilatos GD, Postle R. The environmental scanning electron microscope and its applications. Scan Electron Micros. 1982;(Pt 1):1–16. [PubMed] [Google Scholar]
- DeBeer D, Stoodley P, Lewandowski Z. Measurement of local diffusion coefficients in biofilms by micro-injection and confocal microscopy. Biotechnol Bioeng. 1997;53:151–8. doi: 10.1002/(SICI)1097-0290(19970120)53:2<151::AID-BIT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dodds MG, Grobe KJ, Stewart PS. Modeling biofilm antimicrobial resistance. Biotechnol Bioeng. 2000;68:456–65. [PubMed] [Google Scholar]
- Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–90. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmore BC, Jacobsen A, Hall-Stoodley L, et al. The influence of fluid shear on the structure and material properties of sulphate-reducing bacterial biofilms. J Ind Microbiol Biot. 2002;29:347–53. doi: 10.1038/sj.jim.7000302. [DOI] [PubMed] [Google Scholar]
- Eastman JM, Harmon LJ, La HJ, et al. The onion model, a simple neutral model for the evolution of diversity in bacterial biofilms. J Evolution Biol. 2011;24:2496–504. doi: 10.1111/j.1420-9101.2011.02377.x. [DOI] [PubMed] [Google Scholar]
- Edwards C, Marks R. Evaluation of biomechanical properties of human skin. Clin Dermatol. 1995;13:375–80. doi: 10.1016/0738-081x(95)00078-t. [DOI] [PubMed] [Google Scholar]
- Elert G. The Physics Hypertextbook. 2014. Viscosity. http://physics.info. [Google Scholar]
- Entcheva-Dimitrov P, Spormann AM. Dynamics and control of biofilms of the oligotrophic bacterium Caulobacter crescentus. J Bacteriol. 2004;186:8254–66. doi: 10.1128/JB.186.24.8254-8266.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracane JL. 2nd ed. Philadelphia, PA, USA: Lippencott, Williams and Wilkins; 2001. Materials in Dentistry: Principles and applications. [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–33. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Fux CA, Costerton JW, Stewart PS, et al. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Galy O, Latour-Lambert P, Zrelli K, et al. Mapping of bacterial biofilm local mechanics by magnetic microparticle actuation. Biophys J. 2012;103:1400–8. doi: 10.1016/j.bpj.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdesmeyer L, Von Eiff C, Horn C, et al. Antibacterial effects of extracorporeal shock waves. Ultrasound Med Biol. 2005;31:115–9. doi: 10.1016/j.ultrasmedbio.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Valencia G, Munoz O, Torres JF. Toxigenicity and adherence in Clostridium difficile strains isolated from patients with and without diarrhoea. Arch Invest Med (Mex) 1991;22:189–96. [PubMed] [Google Scholar]
- Gottenbos B, Van der Mei HC, Busscher HJ, et al. Initial adhesion and surface growth of Pseudomonas aeruginosa on negatively and positively charged poly(methacrylates) J Mater Sci Mater Med. 1999;10:853–5. doi: 10.1023/a:1008989416939. [DOI] [PubMed] [Google Scholar]
- Guelon T, Mathias JD, Stoodley P. Advances in biofilm. In: Flemming HC, Wingender J, Szewzyk U, editors. Mechanics Biofilm Highlights. 5th edn. Berlin, Heidelberg: Springer; 2011. pp. 111–39. [Google Scholar]
- Gupta HS, Seto J, Krauss S, et al. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J Struct Biol. 2010;169:183–91. doi: 10.1016/j.jsb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- He Y, Peterson BW, Jongsma MA, et al. Stress relaxation analysis facilitates a quantitative approach towards antimicrobial penetration into biofilms. PLoS One. 2013;8:e63750. doi: 10.1371/journal.pone.0063750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Peterson BW, Ren Y, et al. Antimicrobial penetration in a dual-species oral biofilm after noncontact brushing: an in vitro study. Clin Oral Invest. 2014;18:1103–9. doi: 10.1007/s00784-013-1097-x. [DOI] [PubMed] [Google Scholar]
- Hope CK, Clements D, Wilson M. Determining the spatial distribution of viable and nonviable bacteria in hydrated microcosm dental plaques by viability profiling. J Appl Microbiol. 2002;93:448–55. doi: 10.1046/j.1365-2672.2002.01703.x. [DOI] [PubMed] [Google Scholar]
- Inglis TJ. Evidence for dynamic phenomena in residual tracheal tube biofilm. Brit J Anaesth. 1993;70:22–4. doi: 10.1093/bja/70.1.22. [DOI] [PubMed] [Google Scholar]
- Inglis TJ, Millar MR, Jones JG, et al. Tracheal tube biofilm as a source of the lung. J Clin Microbiol. 1989;27:2014–8. doi: 10.1128/jcm.27.9.2014-2018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman BA, Etienne W, Rubin R, et al. The impact of temperature and urinary constituents on urine viscosity and its relevance to bladder hyperthermia treatment. Int J Hyperther. 2013;29:206–10. doi: 10.3109/02656736.2013.775355. [DOI] [PubMed] [Google Scholar]
- Ito A, Taniuchi A, May T, et al. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl Environ Microb. 2009;75:4093–100. doi: 10.1128/AEM.02949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WL, Sutton MP, McKittrick L, et al. Chemical and antimicrobial treatments change the viscoelastic properties of bacterial biofilms. Biofouling. 2011;27:207–15. doi: 10.1080/08927014.2011.554977. [DOI] [PubMed] [Google Scholar]
- Kallman J, Schollin J, Hakansson S, et al. Adherence of group B streptococci to human endothelial cells in vitro. APMIS. 1993;101:403–8. doi: 10.1111/j.1699-0463.1993.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Keren I, Kaldalu N, Spoering A, et al. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230:13–8. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- Kim MK, Drescher K, Pak OS, et al. Filaments in curved streamlines: rapid formation of Staphylococcus aureus biofilm streamers. New J Phys. 2014;16:065024.422a. doi: 10.1088/1367-2630/16/6/065024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper I, Rupp CJ, Cargo R, et al. Viscoelastic fluid description of bacterial biofilm material properties. Biotechnol Bioeng. 2002;80:289–96. doi: 10.1002/bit.10376. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ, Jr, Periasamy S, et al. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–80. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013;92:1065–73. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körstgens V, Flemming HC, Wingender J, et al. Uniaxial compression measurement device for investigation of the mechanical stability of biofilms. J Microbiol Meth. 2001;46:9–17. doi: 10.1016/s0167-7012(01)00248-2. [DOI] [PubMed] [Google Scholar]
- Lanter BB, Sauer K, Davies DG. Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. mBio. 2014;5:e01206–14. doi: 10.1128/mBio.01206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PC, Dutcher JR, Beveridge TJ, et al. Absolute quantitation of bacterial biofilm adhesion and viscoelasticity by microbead force spectroscopy. Biophys J. 2009;96:2935–48. doi: 10.1016/j.bpj.2008.12.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JR, Korber DR, Hoyle BD, et al. Optical sectioning of microbial biofilms. J Bacteriol. 1991;173:6558–67. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JR, Swerhone GD, Leppard GG, et al. Scanning transmission X-ray, laser scanning, and transmission electron microscopy mapping of the exopolymeric matrix of microbial biofilms. Appl Environ Microb. 2003;69:5543–4. doi: 10.1128/AEM.69.9.5543-5554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar V, Chifiriuc MC. Medical significance and new therapeutical strategies for biofilm associated infections. Roum Arch Microbiol Immunol. 2010;69:125–38. [PubMed] [Google Scholar]
- Lee J, Kwon HJ. Measurement of stress-strain behaviour of human hair fibres using optical techniques. Int J Cosmetic Sci. 2013;35:238–43. doi: 10.1111/ics.12031. [DOI] [PubMed] [Google Scholar]
- Li T, Bai R, Liu J. Distribution and composition of extracellular polymeric substances in membrane-aerated biofilm. J Biotechnol. 2008;135:52–7. doi: 10.1016/j.jbiotec.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Lieleg O, Caldara M, Baumgärtel R, et al. Mechanical robustness of Pseudomonas aeruginosa biofilms. Soft Matter. 2011;7:3307–14. doi: 10.1039/c0sm01467b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–9. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Mathias JD, Stoodley P. Applying the digital image correlation method to estimate the mechanical properties of bacterial biofilms subjected to a wall shear stress. Biofouling. 2009;25:695–703. doi: 10.1080/08927010903104984. [DOI] [PubMed] [Google Scholar]
- Matias VR, Al-Amoudi A, Dubochet J, et al. Cryo-transmission electron microscopy of frozen-hydrated sections of Escherichia coli and Pseudomonas aeruginosa. J Bacteriol. 2003;185:6112–8. doi: 10.1128/JB.185.20.6112-6118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclean JS, Ona ON, Majors PD. Correlated biofilm imaging, transport and metabolism measurements via combined nuclear magnetic resonance and confocal microscopy. ISME J. 2008;2:121–31. doi: 10.1038/ismej.2007.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Guggenheim B, Attin T, et al. Potential of shock waves to remove calculus and biofilm. Clin Oral Invest. 2011;15:959–90. doi: 10.1007/s00784-010-0462-2. [DOI] [PubMed] [Google Scholar]
- Noffke N, Christian D, Wacey D, et al. Microbially induced sedimentary structures recording an ancient ecosystem in the ca. 3.48 billion-year-old Dresser Formation, Pilbara, Western Australia. Astrobiology. 2013a;13:1103–24. doi: 10.1089/ast.2013.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noffke N, Decho AW, Stoodley P. Slime through time: the fossil record of prokaryote evolution. Palaios. 2013b;28:1–5. [Google Scholar]
- Novak KF, Govindaswami M, Ebersole JL, et al. Effects of low-energy shock waves on oral bacteria. J Dent Res. 2008;87:928–31. doi: 10.1177/154405910808701009. [DOI] [PubMed] [Google Scholar]
- Oelschlaeger TA, Dobrindt U, Hacker J. Virulence factors of uropathogens. Curr Opin Urol. 2002;12:33–8. doi: 10.1097/00042307-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Pal A, Ramamurthy T, Ghosh AR, et al. Virulence traits of Aeromonas strains in relation to species and source of isolation. Zbl Bakt. 1992;276:418–28. doi: 10.1016/s0934-8840(11)80549-9. [DOI] [PubMed] [Google Scholar]
- Paramonova E, Kalmykowa OJ, Van der Mei HC, et al. Impact of hydrodynamics on oral biofilm strength. J Dent Res. 2009;88:922–6. doi: 10.1177/0022034509344569. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Busscher HJ, Sharma PK, et al. Environmental and centrifugal factors influencing the visco-elastic properties of oral biofilms in vitro. Biofouling. 2012;28:913–20. doi: 10.1080/08927014.2012.721515. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Busscher HJ, Sharma PK, et al. Visualization of microbiological processes underlying stress relaxation in Pseudomonas aeruginosa biofilms. Microsc Microanal. 2014;20:912–5. doi: 10.1017/S1431927614000361. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Van der Mei HC, Sjollema J, et al. A distinguishable role of eDNA in the viscoelastic properties of biofilms. mBio. 2013;4:e00497–13. doi: 10.1128/mBio.00497-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt WG, McBride MO, Lunceford JK, et al. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrob Agents Ch. 1994;38:2577–82. doi: 10.1128/aac.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K. Stress responses as determinants of antimicrobial resistance in Gram-negative bacteria. Trends Microbiol. 2012;20:227–34. doi: 10.1016/j.tim.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Poznanski J, Pawlowski P, Fikus M. Bioelectrorheological model of the cell. 3. Viscoelastic shear deformation of the membrane. Biophys J. 1992;61:612–20. doi: 10.1016/S0006-3495(92)81866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Sagers RD, Pitt WG. Investigation of the mechanism of the bioacoustic effect. J Biomed Mater Res. 1999;44:198–205. doi: 10.1002/(sici)1097-4636(199902)44:2<198::aid-jbm10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Qian Z, Stoodley P, Pitt WG. Effect of low-intensity ultrasound upon biofilm structure from confocal scanning laser microscopy observation. Biomaterials. 1996;17:1975–80. doi: 10.1016/0142-9612(96)00022-1. [DOI] [PubMed] [Google Scholar]
- Rantonen PJ, Meurman JH. Viscosity of whole saliva. Acta Odontol Scand. 1998;56:210–4. doi: 10.1080/00016359850142817. [DOI] [PubMed] [Google Scholar]
- Ratner BD, Hoffman AS, Schoen FJ, et al. San Diego, CA, USA: Elsevier, Academic Press; 2004a. Biomaterials Science: An introduction to materials in medicine. 2nd ed. [Google Scholar]
- Ratner BD, Hoffman AS, Schoen FJ, et al. San Diego, CA, USA: Elsevier, Academic Press; 2004b. pp. 28–31. Bulk properties of materials by Cooke FW. In: Biomaterials Science: An introduction to materials in medicine. [Google Scholar]
- Rediske AM, Hymas WC, Wilkinson R, et al. Ultrasonic enhancement of antibiotic action on several species of bacteria. J Gen Appl Microbiol. 1998;44:283–8. doi: 10.2323/jgam.44.283. [DOI] [PubMed] [Google Scholar]
- Rediske AM, Roeder BL, Nelson JL, et al. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob Agents Ch. 2000;44:771–2. doi: 10.1128/aac.44.3.771-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- Rmaile A, Carugo D, Capretto L, et al. Removal of interproximal dental biofilms by high-velocity water microdrops. J Dent Res. 2014;93:68–73. doi: 10.1177/0022034513510945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C. Mass transfer of therapeutics through natural human plaque biofilms: a model for therapeutic delivery to pathological bacterial biofilms. Arch Oral Biol. 2011;56:829–36. doi: 10.1016/j.archoralbio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Rupp CJ, Fux CA, Stoodley P. Viscoelasticity of Staphylococcus aureus biofilms in response to fluid shear allows resistance to detachment and facilitates rolling migration. Appl Environ Microb. 2005;71:2175–8. doi: 10.1128/AEM.71.4.2175-2178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi R, Lecuyer S, Autrusson N, et al. Secondary flow as a mechanism for the formation of biofilm streamers. Biophys J. 2011;100:1392–9. doi: 10.1016/j.bpj.2011.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi R, Lecuyer S, Guglielmini L, et al. Laminar flow around corners triggers the formation of biofilm streamers. J R Soc Interface. 2010;7:1293–9. doi: 10.1098/rsif.2010.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek MM, Jones SM, Martinuzzi RJ. The influence of flow cell geometry related shear stresses on the distribution, structure and susceptibility of Pseudomonas aeruginosa 01 biofilms. Biofouling. 2009;25:711–25. doi: 10.1080/08927010903114603. [DOI] [PubMed] [Google Scholar]
- Sanford BA, De Feijter AW, Wade MH, et al. A dual fluorescence technique for visualization of Staphylococcus epidermidis biofilm using scanning confocal laser microscopy. J Ind Microbiol. 1996;16:48–56. doi: 10.1007/BF01569921. [DOI] [PubMed] [Google Scholar]
- Schaudinn C, Carr G, Gorur A, et al. Imaging of endodontic biofilms by combined microscopy (FISH/CLSM - SEM) J Microsc. 2009;235:124–7. doi: 10.1111/j.1365-2818.2009.03201.x. [DOI] [PubMed] [Google Scholar]
- Schultz MP, Bendick JA, Holm ER, et al. Economic impact of biofouling on a naval surface ship. Biofouling. 2011;27:87–98. doi: 10.1080/08927014.2010.542809. [DOI] [PubMed] [Google Scholar]
- Shaw T, Winston M, Rupp CJ, et al. Commonality of elastic relaxation times in biofilms. Phys Rev Lett. 2004;93:098102. doi: 10.1103/PhysRevLett.93.098102. [DOI] [PubMed] [Google Scholar]
- Shen Y, Stojicic S, Qian W, et al. The synergistic antimicrobial effect by mechanical agitation and two chlorhexidine preparations on biofilm bacteria. J Endodont. 2010;36:100–4. doi: 10.1016/j.joen.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183:6746–51. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS. Mini-review: convection around biofilms. Biofouling. 2012;28:187–98. doi: 10.1080/08927014.2012.662641. [DOI] [PubMed] [Google Scholar]
- Stewart PS. Biophysics of biofilm infection. Pathog Dis. 2014;70:212–8. doi: 10.1111/2049-632X.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Cargo R, Rupp CJ, et al. Biofilm material properties as related to shear-induced deformation and detachment phenomena. J Ind Microbiol Biot. 2002;29:361–7. doi: 10.1038/sj.jim.7000282. [DOI] [PubMed] [Google Scholar]
- Stoodley P, De Beer D, Lewandowski Z. Liquid flow in biofilm systems. Appl Environ Microb. 1994;60:2711–6. doi: 10.1128/aem.60.8.2711-2716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Lewandowski Z, Boyle JD, et al. Structural deformation of bacterial biofilms caused by short-term fluctuations in fluid shear: an in situ investigation of biofilm rheology. Biotechnol Bioeng. 1999a;65:83–90. [PubMed] [Google Scholar]
- Stoodley P, Lewandowski Z, Boyle JD, et al. The formation of migratory ripples in a mixed species bacterial biofilm growing in turbulent flow. Environ Microbiol. 1999b;1:447–55. doi: 10.1046/j.1462-2920.1999.00055.x. [DOI] [PubMed] [Google Scholar]
- Sundd P, Pospieszalska MK, Ley K. Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings. Mol Immunol. 2013;55:59–69. doi: 10.1016/j.molimm.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JG, Nakaishi LA. Managing the complexity of a dynamic biofilm. J Am Dent Assoc. 2006;137(Supplement):10S–5S. doi: 10.14219/jada.archive.2006.0409. [DOI] [PubMed] [Google Scholar]
- Thomas K, Herminghaus S, Porada H, et al. Formation of Kinneyia via shear-induced instabilities in microbial mats. Philos T Roy Soc A. 2013;371:20120362. doi: 10.1098/rsta.2012.0362. [DOI] [PubMed] [Google Scholar]
- Towler BW, Rupp CJ, Cunningham AB, et al. Viscoelastic properties of a mixed culture biofilm from rheometer creep analysis. Biofouling. 2003;19:279–85. doi: 10.1080/0892701031000152470. [DOI] [PubMed] [Google Scholar]
- Uppuluri P, Chaturvedi AK, Lopez-Ribot JL. Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia. 2009;168:101–9. doi: 10.1007/s11046-009-9205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadillo-Rodriguez V, Dutcher JR. Viscoelasticity of the bacterial cell envelop. Soft Matter. 2011;7:4101–10. [Google Scholar]
- Van Leeuwenhoek AP. Containing some microscopical observations, about animals in the scrurf of the teeth. Philos T R Soc Lond. 1684;14:568–74. [Google Scholar]
- Vandecandelaere I, Matthijs N, Nelis HJ, et al. The presence of antibiotic-resistant nosocomial pathogens in endotracheal tube biofilms and corresponding surveillance cultures. Pathog Dis. 2013;69:142–8. doi: 10.1111/2049-632X.12100. [DOI] [PubMed] [Google Scholar]
- Venugopalan VP, Kuehn M, Hausner M, et al. Architecture of a nascent Sphingomonas sp. biofilm under varied hydrodynamic conditions. Appl Environ Microb. 2005;71:2677–86. doi: 10.1128/AEM.71.5.2677-2686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkaik MJ, Busscher HJ, Rustema-Abbing M, et al. Oral biofilm models for mechanical plaque removal. Clin Oral Invest. 2010;14:403–9. doi: 10.1007/s00784-009-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. Structural Biomaterials. 3rd edn. Princeton, NJ, USA: Princeton University Press; 2012. [Google Scholar]
- Vroom JM, De Grauw KJ, Gerritsen HC, et al. Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy. Appl Environ Microb. 1999;65:3502–11. doi: 10.1128/aem.65.8.3502-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu B, Chen M, Crawford RJ, et al. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules. 2009;14:2535–54. doi: 10.3390/molecules14072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuopio-Varkila J, Schoolnik GK. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J Exp Med. 1991;174:1167–77. doi: 10.1084/jem.174.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Taherzadeh D, Haisch C, et al. Investigation of the mesoscale structure and volumetric features of biofilms using optical coherence tomography. Biotechnol Bioeng. 2010;107:844–53. doi: 10.1002/bit.22864. [DOI] [PubMed] [Google Scholar]
- Wallace PK, Arey B, Mahaffee WF. Subsurface examination of a foliar biofilm using scanning electron- and focused-ion-beam microscopy. Micron. 2011;42:579–85. doi: 10.1016/j.micron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Weber SD, Ludwig W, Schleifer KH, et al. Microbial composition and structure of aerobic granular sewage biofilms. Appl Environ Microb. 2007;73:6233–40. doi: 10.1128/AEM.01002-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, et al. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Wloka M, Rehage H, Flemming HC, et al. Rheological properties of viscoelastic biofilm extracellular polymeric substances and comparison to the behavior of calcium alginate gels. Colloid Polym Sci. 2004;282:1067–76. [Google Scholar]
- Wu Y, Liang J, Rensing K, et al. Extracellular matrix reorganization during cryo preparation for scanning electron microscope imaging of Staphylococcus aureus biofilms. Microsc Microanal. 2014;5:1348–55. doi: 10.1017/S143192761401277X. [DOI] [PubMed] [Google Scholar]


