Introduction
Neuropeptide Y (NPY) has a multitude of functions in peripheral organs, so it is no surprise that the same appears to be the case in the brain. One of the reasons why NPY potentially contributes to so many functions may be due to its plasticity, meaning the ability to change its level of expression and pattern of expression. This makes NPY a potentially useful tool to modulate the behavior of an organism in response to the challenges of the external environment. Thus, NPY mRNA, protein, and receptors can change dramatically not only in their level of expression but also their location in different types of neurons and neuronal processes. This occurs in response to perturbations of the environment, and also in pathological conditions, suggesting a role not only in the normal condition, but also in disease.
This plasticity is well exemplified in the dentate gyrus of rat hippocampus, and this review will focus on NPY in the dentate gyrus to illustrate the striking plasticity of NPY in the brain. We will specifically address the changes that occur after seizures because the changes in NPY and its receptors after seizures are robust and dramatic. Furthermore, they could have important functional implications. Thus, the excitability of neurons in the hippocampus change after seizures, and these changes may be initiated, or at the very least influenced, by the alterations in NPY and its receptors. In addition, there are additional functional implications for dentate granule cell neurogenesis, because NPY appears to be a key regulator of dentate granule cell neurogenesis. This chapter first explains the pattern of expression of NPY and its receptors in the normal rat dentate gyrus, and then describes the changes in expression after seizures. Functional implications are subsequently considered, including effects on excitability, as well as neurogenesis.
Overview of the cell types and circuitry in the normal rat dentate gyrus
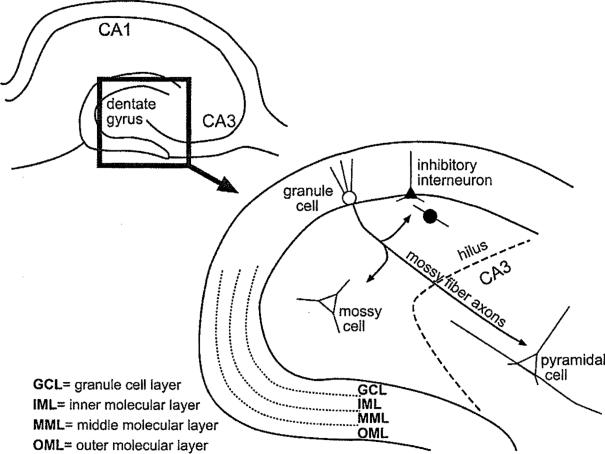
The adult rat dentate gyrus consists of a dense layer of granule cells, and many other types of neurons and glia that are dispersed throughout all strata (Fig. 1). The somata of granule cells are tightly packed, and form the so-called granule cell layer (also referred to as stratum granulosum). Granule cells are oriented in a stereotypical fashion, with a spiny arbor of dendrites that begins at the cell body and extends unilaterally to the hippocampal fissure (Fig. 1). The area where the dendrites are located also contains scattered non-granule cells, and is referred to as the molecular layer (stratum moleculare). It is divided into three sublayers: the inner, middle and outer molecular layers (Fig. 1). These layers contain numerous afferents from nuclei extrinsic to the hippocampus, and axons from neurons that lie within the dentate gyrus such as various GABAergic neurons (inhibitory interneurons) and the glutamatergic “mossy” cells. The middle and outer molecular layer are the primary sites of termination for the major cortical input to the dentate gyrus from the medial and lateral entorhinal cortex, respectively. A hallmark of the extrinsic afferents is the specificity for each particular sublayer, with axons from one system rarely crossing the borders to adjacent substrata.
Figure 1.
Schematic of the adult rat dentate gyrus. A transverse section through the rat dentate gyrus in the horizontal plane illustrates its location in the hippocampus. The inset diagrams the locations of the different cell types and lamination of the dentate gyrus.
On the opposite pole of the granule cells from their dendrites lie the granule cell axons or “mossy fibers.” These axons are a major component of the large region just beneath the granule cell layer, called the hilar region or hilus (Fig. 1). The mossy fibers primarily target the proximal apical dendrites of CA3 pyramidal cells of hippocampus, but also form a complex array of collaterals that terminate on hilar cells. These hilar cells can be divided into the mossy cells, which innervate the inner third of the ipsilateral and contralateral granule cell layer, and also have local collaterals. There also are hilar cells that are GABAergic, and these neurons are highly diverse in morphology and projection. The somata and axons of GABAergic neurons are located throughout the dentate gyrus.
All of the cells in the dentate gyrus, both glutamatergic and GABAergic, contain secondary chemicals and proteins that are thought to be neuromodulators. Different cell types are often selective for the expression of distinct neuromodulators. For example, granule cells contain several peptides such as enkephalin, the calcium binding protein calbindin D28K, as well as other substances such as zinc, but these are only weakly expressed, if at all, in other types of dentate gyrus neurons [1–3]. Mossy cells preferentially express calcitonin gene-regulated peptide (CGRP; [4]). GABAergic neurons contain a variety of unique substances that are not normally expressed in other dentate gyrus neurons, such as somatostatin, cholecystokinin, the calcium binding protein parvalbumin, and vasoactive intestinal polypeptide, to name a few [5].
Normal localization of NPY and its receptors in the rat dentate gyrus
The normal expression pattern of NPY protein and its receptors in the rat dentate gyrus are shown in Figures 2 and 3. NPY is normally expressed very specifically in GABAergic neurons and not other cell types [6, 7]. Fiber systems that innervate the dentate gyrus do not appear to express NPY.
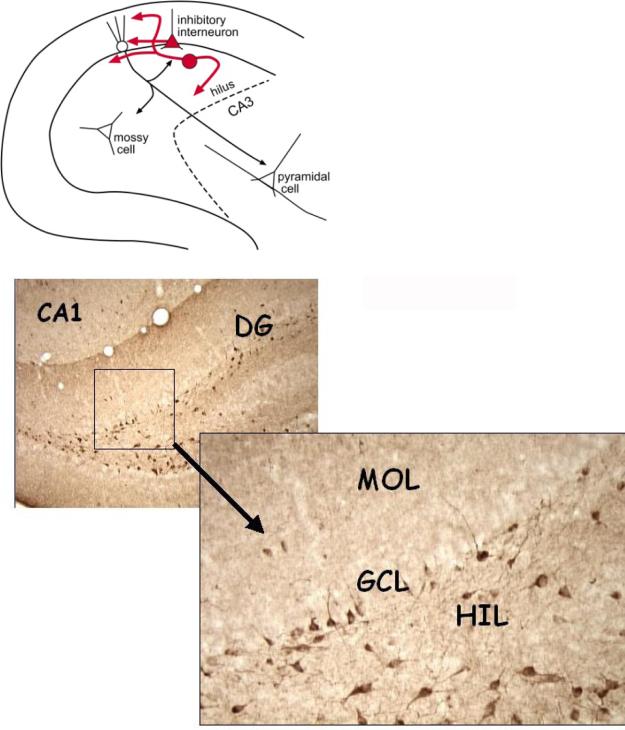
Figure 2.
Normal NPY expression in the adult dentate gyrus. TOP: A schematic shows the normal distribution of NPY protein is in various inhibitory neurons. BOTTOM: A micrograph showing expression of NPY in a normal male adult rat. The neurons that are NPY-immunoreactive have somata mainly in the granule cell layer (GCL) and hilus (HIL), and co-express GABA. DG = dentate gyrus. MOL = molecular layer.
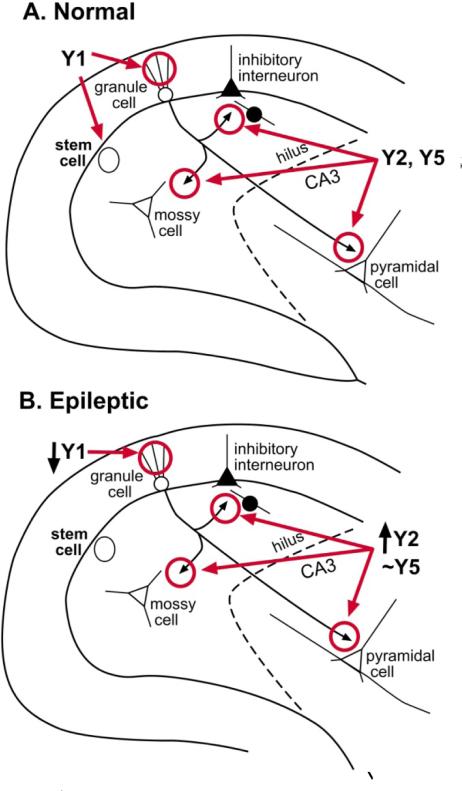
Figure 3.
The distribution of NPY receptors in the normal and epileptic rat dentate gyrus. NPY receptor distribution of a normal adult rat (A) and an adult rat that has had severe continuous seizures (status epilepticus; B) are shown. The distributions appears similar regardless of the methods to induce status, and seizures last for 1 or more hours. Methods that are commonly used to induce status include the convulsant pilocarpine or kainic acid, or electrical stimulation. After status epilepticus, spontaneous repetitive seizures develop within weeks, and persist for the life of the animal (i.e., epileptogenesis occurs). For further explanation and references, see text.
Only a subset of the GABAergic neurons in the dentate gyrus express NPY. Many of these cells co-localize NPY with other peptides, including somatostatin and cholecystokinin, but this is not always the case. Thus, immunocyto-chemistry reveals some neurons that are NPY-imrnunoreactive but lack somatostatin-immunoreactivity, whereas in others the two peptides are co-localized. Depending on the antibodies, laboratory, and other variables, the percentages of GABAergic neurons that are NPY-ergic can vary substantially. However, there are some general rules that can be concluded. First, most of the cell bodies are located in the hilus and in the granule cell layer [7]. In addition, these neurons appear mostly to be “local circuit neurons” because their axon typically arborizes in the hippocampal lamella where the cell body is located. There is only one report of NPY-immunoreactive neurons that have a contralateral projection, and they were estimated to be only 2% of the total population of NPY containing neurons [6]. The axon contributes to the innervation of granule cells, hilar cells, and also contributes to fibers innervating processes in the outer two-thirds of the molecular layer [6–8]. Most NPY-immunore-active terminals appose molecular layer dendrite, including dendrites of granule cells as well as other non-granular cells [6, 8]. NPY-immunoreactive terminals do appear to appose other terminals in the outer molecular layer [8], but there does not appear to be a strong influence of exogenous NPY on the perforant path input to the molecular layer [9, 10]. NPY terminals also appose astrocytic processes [11], suggesting an interaction with glia [12].
Of the five NPY receptors known to be expressed in the brain, Y3 and Y4 are relatively weakly expressed in the hippocampus. In the adult rat dentate gyrus, Y3 mRNA is not detectable in the normal adult rat, and Y4 mRNA levels are low [13]. In contrast, Y1, Y2, and Y5 mRNA is robust [13]. mRNA for Y1, Y2, and especially Y5 receptors appear to be expressed primarily on hilar neurons and in granule cells [13]. Receptor protein appears to have a different expression pattern, with Y2 and Y5 receptors primarily on mossy fibers and and Y1 receptors primarily in the molecular layer ([14]; Fig. 3A). The localization of Y2 and Y5 receptors to mossy fibers suggest a presynaptic action of NPY. This is also suggested by electrophysiological studies, which have shown that NPY influences mossy fiber transmission by a presynaptic mechanism [10]. Both Y2 and Y5 receptors have been implicated in these effects [15, 16].
The role of the molecular layer Y1 receptor is less clear. Studies of Y1 receptor agonists reveal inhibitory effects on Ca2+ entry into dendrites of granule cells [16]. Other studies suggest a potentially proconvulsant effect [17]. Studies in mouse slices indicate that Y1 receptors regulate t GIRK ((define)) potassium channel [18]. To date, no studies have examine the hypothesis that transmission from the entorhinal cortex is modulated by Y1 receptors, although such actions are suggested by the evidence that boutons of NPY-immunoreactive neurons appear to innervate non-NPY-immunoreactive boutons in the outer two-thirds of the molecular layer [8]. However, these could be other boutons besides those of the perforant path, because NPY itself seems to do little to perforant path transmission (see above). Interestingly, another potential role of Y1 receptors is regulation of neurogenesis, because granule cell progenitors express Y1 receptors, and neuroproliferation increases in response to NPY (see below).
NPY and dentate gyrus neurogenesis
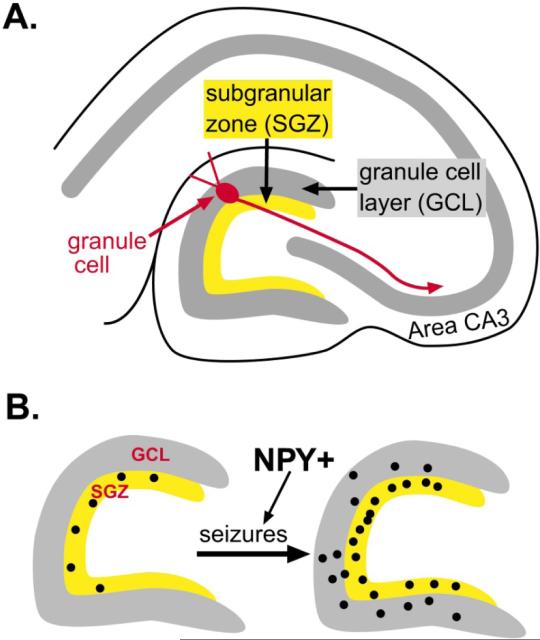
Neurogenesis occurs primarily in three locations in the adult brain, the olfactory bulb, the subventricular zone, and the dentate gyrus. In the dentate gyrus, there is a 50–100 μm layer in the hilus just beneath the granule cell layer called the subgranular zone (SGZ), where it is thought that the precursors of granule cells are located (Fig. 4). These progenitors appear to spontaneously divide at a slow rate throughout life, and their progeny develop into neurons (neurogenesis). Primarily the new neurons become granule cells, but there are reports that other neuronal types, such as GABAergic neurons, may also develop [19]. In addition, these precursors may develop into non-neuronal cells. Indeed, their development may arise entirely from radial glia, dividing into one or more daughter cells that become neurons, as well as additional cells that ultimately become mature astrocytes [20, 21].
Figure 4.
Seizure-induced neurogenesis in the rat dentate gyrus. A. A schematic illustrates the area of the dentate gyrus where progenitors are located in the adult rat, the subgranular zone. B. A schematic illustrates the increase in progenitors, labeled by the mitotic marker bromodeoxyuridine (BrdU), after seizures. As discussed in the text, NPY appears to facilitate this process.
The regulation of adult neurogenesis has become a topic of substantial interest because several studies have implicated dentate gyrus neurogenesis in important functions, such as learning [22, 23]. Thus, it is possible that new neurons must be continually supplied to the dentate gyrus granule cell layer to maintain the ability of the hippocampus to mediate or modulate learning and memory. In addition, it has been observed that many factors that influence behavior also influence neurogenesis in the dentate gyrus, such as the hormones estrogen and prolactin, exercise, administration of growth factors, etc. [24 ]. Increased neurogenesis in the adult dentate gyrus has typically been associated with beneficial effects, and decreased neurogenesis with the opposite, such as psychiatric disorders and learning deficits. For example, decreased neurogenesis in the dentate gyrus has been suggested to underlie depression [25]. Indeed, stress and glucocorticoids, which are elevated in depressed individuals, decrease neurogenesis in laboratory animals [26, 27]. Neurological disorders such as Alzheimer's disease, autism, or schizophrenia may be a result of altered neurogenesis in the adult brain also [28–31].
Interestingly, it has been shown that various types of insults to the brain, as well as seizures, lead to a rapid increase in the rate of dentate gyrus neurogenesis (Fig. 4). Remarkably, this period of increased neurogenesis can be long lived, lasting weeks in the case of some of the most severe seizures, such as status epilepticus [32–34]. This is remarkable because status epilepticus typically leads to substantial neuronal cell death. This phenomenon raises the possibility that neurogenesis may increase when the organism is threatened, injured, or damage occurs. In other words, a regulatory mechanism is present that provides compensation for the damage, and part of that mechanism includes increasing dentate gyrus neurogenesis. This is consistent with the general consensus that injury and seizures may be followed by a period when the brain begins to express proteins similar to those that are produced during development, a so-called “recapitulation of development” that is an effort by the brain to re-grow and hence repair itself. Interestingly, the increase in neurogenesis after status epilepticus may not necessarily be beneficial, because some of the new neurons appear to migrate to abnormal locations and disrupt the normal circuitry of the hippocampus [34, 35].
A role for NPY in dentate gyrus neurogenesis has only recently been identified. Initial studies hinted for such a role by showing that NPY was implicated in neurogenesis in the olfactory bulb [36]. This led to studies in the dentate gyrus, and it has now been shown that NPY facilitates dentate gyrus neurogenesis also (see below, and chapter in this volume by Gray et al.). These studies have ident11e at Y1 receptors are present on the progenitors of the dentate gyrus that are located in the SGZ. This may explain part of the role of the Y1 receptor. It also suggests a potential “division of labor” among NPY receptors: Y2 and Y5 may primarily influence synaptic transmission, whereas Y1 may be dedicated to other types of functions, such as neurogenesis. The actions of Y1 receptors on the regulation of calcium entry may actually work in part to modulate neurogenesis and proliferation indirectly, since it is likely that intracellular calcium will influence the cell cycle and associated events [37, 38].
NPY protein and receptor expression after seizures
It was first shown in the 1990s that NPY expression in the rat dentate gyrus dramatically increases after seizures. Subsequent studies from many different laboratories showed the reproducibility and robust nature of these changes [39–41], and the upregulation of NPY in the dentate gyrus has become an accepted marker of seizure activity.
One of the reasons why NPY expression after seizures has been studied so much is that it changes in a very interesting and yet robust manner, depending on the degree and duration of seizures. After acute seizures (lasting minutes), there is an elevation of expression in many non-granule cells, particularly those in the hilus and granule cell layer (Fig. 5). Acute studies have used, for example, pentylenetetrazol-induced seizures [42], electroconvulsive shock [43], and kindled seizures [44]. After more severe seizures, such as status epilepticus following electrical stimulation, kainic acid or pilocarpine administration [39], this acute upregulation also occurs, but in addition some of the NPY cells die due to excitotoxicity [45].
Figure 5.
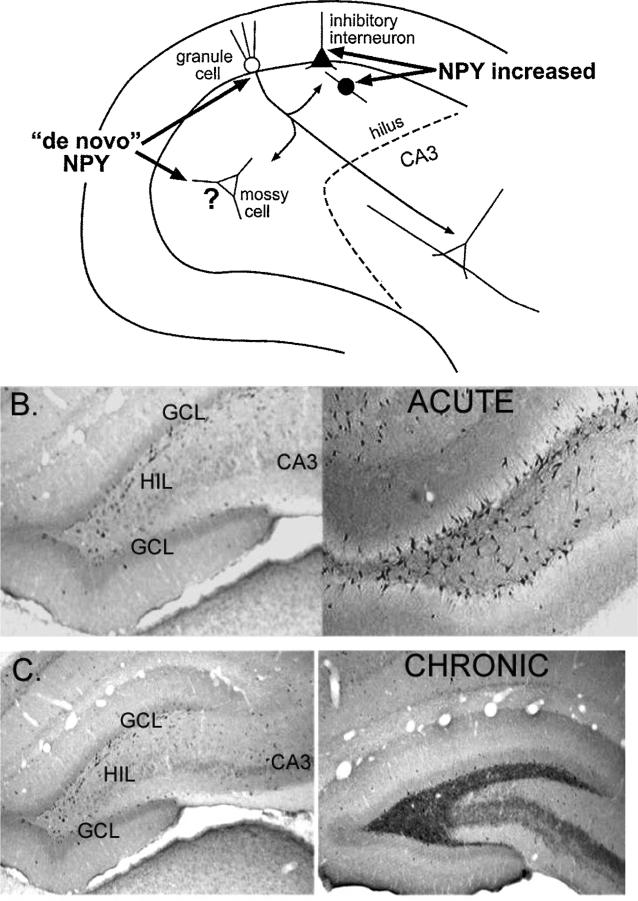
Changes in NPY expression after seizures. A. A diagram is used to illustrate where NPY protein increases in the adult rat dentate gyrus after seizures. It appears to increase in inhibitory neurons, and also develop in neurons such as granule cells and mossy cells that do not normally express the protein (de novo expression). B. The increase in NPY protein in the dentate gyrus is shown using an antibody to NPY. Left: Saline control. Right: 1 day after status epilepticus. C. Increased NPY in mossy fibers is illustrated after chronic seizures. Left: Saline control. Right: 2 months after status epilepticus. Spontaneous seizures were observed in the animal that had status epilepticus.
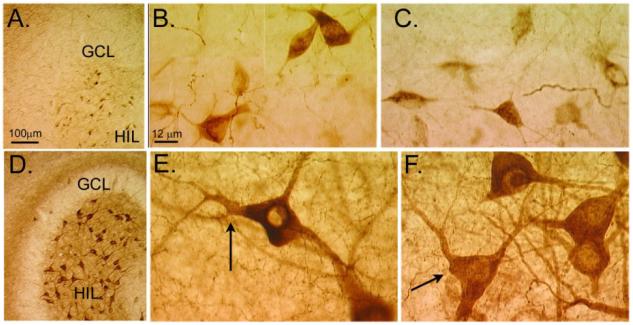
After status epilepticus, it appears that NPY expression can also develop in some of the hilar neurons that normally do not express the protein (Fig. 6). This can be appreciated by comparing the immunoreactivity of sections from animals that had status and those that did not (Fig. 6). The normal, small hilar cells that are NPY-immunoreactive appear to be lost after status, but the hilus does not appear devoid of cells as a result. Instead, other cells that are quite large appear to express NPY. These large NPY-immunoreactive cells are not apparent in the normal tissue, so it is likely that they have developed de novo expression. They appear to be either large GABAergic neurons or the glutamatergic mossy cells. The latter is surprising, because mossy cells are not thought to express NPY normally or after seizures. However, this may be due to the fact that most periods of status epilepticus induce substantial mossy cell loss. We have found that if status epilepticus is abbreviated by diazepam administration after 1 h, mossy cells can survive [45]. When our tissue is examined for NPY expression, cells with the morphology of mossy cells are immunoreactive (Fig. 6). Thus, they are large, multipolar, and have large-diameter primary dendrites. Furthermore, the area where the primary dendrites join the soma is uneven or “ruffled”, rather than smooth. One would expect this characteristic of mossy cells, which have complexes of large spines (thorny excrescences) on their primary dendrites, especially at the junction with the cell body. Other hilar neurons do not have thorny excrescences, and the initial portion of their primary dendrites is relatively smooth.
Figure 6.
De novo expression of NPY in hilar cells after seizures. NPY immunoreactivity in the den-tate gyrus of a pilocarpine-treated rat that had no behavioral seizures (A-C) compared to a pilocarpine-treated rat that had I h of status epilepticus and subsequently had spontaneous seizures (D-F). Both animals were killed 2 months after pilocarpine treatment. A-C. The normal pattern of NPY immunoreactivity includes NPY expression in many hilar cells and fibers, as well as fibers in the outer molecular layer (A). B and C show NPY-immunoreactive hilar cells at higher magnification. GCL = granule cell layer; HIL = hilus. D-F. NPY immunoreactivity in the epileptic rat shows increased immunoreactivity in hilar cells and in fibers (D; same magnification as A). In addition, a novel band of staining in the inner molecular layer is present, a reflection of mossy fiber sprouting. E and F show higher magnification of hilar cells that are NPY-immunoreactive. Note the large size of these cells and irregular, large primary dendrites (arrows) relative to the normal NPY-immunoreactive cells shown in E and F (magnification is the same in B, C, E, F). It is assumed from these changes in immunoreactivity that the cells in the normal hilus die due to seizure-induced neuronal death, and the residual surviving cells, including some mossy cells, develop NPY immunoreactivity.
When seizures occur chronically, for example in animals that have status epilepticus, and then are examined months later, NPY expression is also abnormal, but the changes are distinct from the pattern observed after acute seizures (Fig. 5C) [46–48]. There continues to be an increase in NPY expression in non-granule cells, but in addition, NPY is apparent in the granule cells and their axons [42, 49]. NPY immunolabeling becomes distributed throughout the mossy fiber axon plexus. Another change that occurs in many GABAergic neurons that survive seizures is sprouting of their axons [50]. This sprouting also appears to occur for the NPY-immunoreactive GABAergic neurons that survive seizures [51]. It may allow compensation for the loss of some of the original NPY-containing neurons.
Another interesting change in expression of NPY after seizures relates to the presence of NPY in mossy fibers, and the fact that many chronically seizing animals develop collateralization of mossy fibers into the inner molecular layer. This “mossy fiber sprouting” may serve to increase recurrent excitation, because the new collaterals innervate granule cells [52–55]. But the new collaterals also innervate GABAergic neurons, which would potentially negate any increase in recurrent excitation [56, 57]. What is significant for the present discussion is that the parent and sprouted mossy fibers are indistinguishable in NPY immunoreactivity; the new fibers appear identical in NPY expression as the parent axons. Thus, epileptic animals demonstrate a novel band of NPY immunoreactive fibers in the inner molecular layer that is not present in normal rats (see Fig. 6).
There are substantial changes in NPY receptor expression after seizures (Fig. 3B), although no evidence of a distinct pattern of expression, as appears to be the case for NPY protein. After acute and chronic seizures, Y2 receptors increase expression in mossy fibers, but do not appear to change elsewhere [57]. The data for Y5 receptors are less clear [59], but it appears that they also change in mossy fibers [60]. Regarding Y1 receptors, it appears that molecular layer expression of Y1 receptors diminishes after seizures [61].
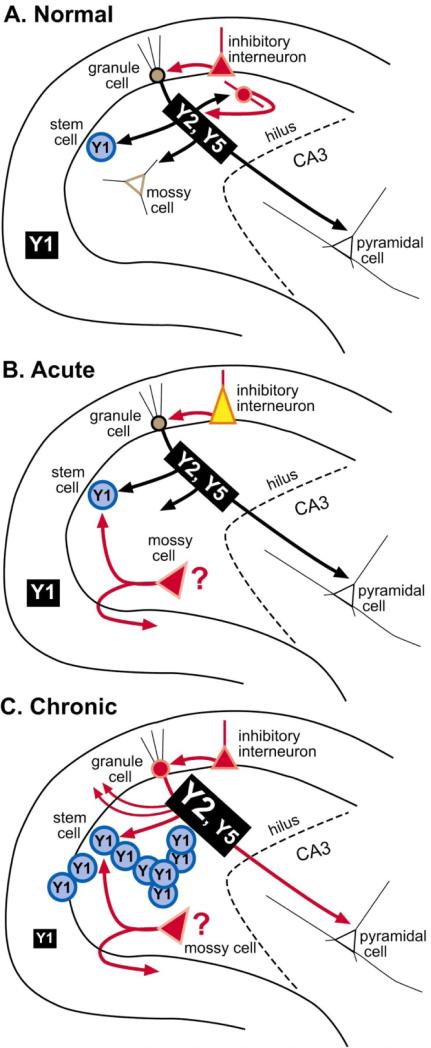
In summary, acute seizures increase NPY expression of many non-granule cells. There also may be cell death of NPY-expressing neurons, especially if seizure activity is severe. After chronic seizures, additional NPY expression develops in the mossy fiber axons of dentate granule cells, and possibly surviving mossy cells. Regarding receptors, Y2/Y5 receptors mainly increase and Y1 receptors appear to decrease. These changes are summarized in Figure 7.
Figure 7.
Summary of NPY plasticity in the rat dentate gyrus after seizures. A summary of the changes that occur in protein and receptor expression after acute seizures and further changes after chronic seizures. A. Normal condition B. After acute seizures, NPY protein increases in inhibitory neurons, although some may also be lost due to seizure-induced neuronal death, and some may sprout collaterals, making the new NPY intemeurons network potentially novel. In addition, some hilar cells that do not normally express NPY protein may begin to do so, such as surviving mossy cells. C. After chronic seizures, granule cells and their axons, the mossy fibers, express NPY and sprout into the inner molecular layer. Y2, and possibly Y5 receptors, increase in mossy fibers, and Y1 receptors in the molecular layer appear to decrease. In addition, seizures increase the proliferation of granule cells from progenitors in the subgranular zone which express the Y1 receptor; this is likely to occur during a window between day 3–4 and 30 after status epilepticus, at least in the case of pilocarpine-induced status epilepticus [32, 34]. This coincides with the period when spontaneous seizures are beginning to occur, suggesting a role for seizure-induced neurogenesis in epileptogenesis [33].
Functional implications of seizure-induced changes in NPY and its receptors
Synaptic transmission
Why might the changes in NPY, and its receptors, occur in the epileptic brain? The increased expression in non-granule cells, as well as mossy fiber NPY expression, is an inherent compensatory reaction that serves to decrease the possibility of subsequent seizure activity. This follows logically from studies showing that NPY depresses mossy fiber transmission. This would blunt any ability of seizures to propagate through the major pathway that seizures take through the hippocampus, the trisynaptic circuit. Depression of excitatory mossy fiber transmission might also be neuroprotective because the transmitter is glutamate, and the targets of mossy fibers (hilar cells, pyramidal cells) are quite vulnerable to seizure-induced damage [51, 62].
Consistent with the hypothesis that the changes in NPY protein after seizures may suppress further seizures, there is an increase in the receptors that mediate actions of NPY to depress synaptic transmission of the mossy fibers. Taken together, the increased mossy fiber NPY, coupled with the increased Y2/Y5 receptors, should serve to dampen excitability very effectively in the epileptic rat dentate gyrus.
However, the anticonvulsant and neuroprotective effects discussed above assume that NPY receptor-mediated actions are equivalent in the normal and epileptic brain. One cannot necessarily assume they are identical, and in fact there appears to be little effect of NPY on perforant path-evoked transmission to granule cells in normal rat brain [9, 10], but a potent inhibitory effect in epileptic human brain [63].
There are at least a few other reasons to suspect that the increase in NPY and NPY receptors after seizures may not necessarily have the same effects on synaptic transmission as it does in the normal brain. One of the factors that could be complicating is that other peptides and receptors of the mossy fibers change after seizures. They may interact with NPY in a way that does not occur normally. Metabotropic glutamate receptors are a prime candidate, because they normally modulate mossy fiber transmission, these receptors change after seizures, and the influence NPY [64]. Another complication is that the expression of GABA increases in mossy fibers after seizures [65]. It appears that this pool of GABA can be released and inhibit the target cells of mossy fibers [66], although the net effect is not clear, because GABA and glutamate are released at similar times from mossy fiber boutons. Nevertheless, if NPY depresses mossy fiber transmission, it may not only depress glutamatergic transmission (as has been assumed) but also GABAergic components of mossy fiber transmission. Indeed, in thalamus, NPY does depress GABA release [67]. If this is the case, NPY might actually have a partially disinhibitory effect by blocking the GABAergic component of mossy fiber transmission. This potential disinhibition could be greater after seizures, because under these conditions the GABAergic component of mossy fiber transmission appears to increase greatly.
Neurogenesis
One of the aspects of seizures that is perhaps as robust as the induction of NPY expression is the ability to increase neurogenesis of granule cells [33, 35]. This may not be a coincidence: it is possible that the increase in NPY mediates the increase in neurogenesis of granule cells. Thus, NPY may serve to protect the epileptic brain from further seizures by modulating synaptic transmission (see above), and at the same time contribute to its repair by promoting the genesis of new granule cells.
What is the evidence for the hypothesis that increased NPY after seizures mediates seizure-induced neurogenesis? First of all, experimental seizure models which have demonstrated that seizures increase neurogenesis are models that have also shown an increase in NPY. Second, the increase in NPY is rapid, and occurs before neurogenesis begins. Such timing would be necessary if neurogenesis is dependent on NPY. Third, NPY facilitates neuroproliferation in the olfactory bulb [36], providing a precedent.
Much more direct evidence has been obtained recently by studying the influence of NPY exposure on cultures of granule cell precursor [68]. NPY facilitated neuroproliferation, and did so at very low concentrations, similar to what would be expected to occur in situ. In addition, mice that lacked NPY had a lower basal level of neurogenesis [69]. Interestingly, the effect appeared to be due to the Y1 receptor, based on pharmacology [68]. In addition, cultures from mice that lack the Y1 receptor failed to demonstrate an effect of NPY on neurogenesis [70]. Furthermore, the precursors appear to be immunoreactive to a Y1 receptor antibody [68]. Thus, NPY appears to modulate neurogenesis and does so by acting at Y1 receptors.
How NPY modulates neurogenesis in vivo is still somewhat unclear. One question is how NPY from NPYergic neurons is made available to precursors in the SGZ. This is an issue because most of the axon projection of NPY containing cells is thought to project far from the SGZ, in the outer molecular layer. Only a small number of collaterals are thought to terminate in the SGZ. If the role of NPY is to modulate neurogenesis, why would the axons not terminate entirely in the SGZ? Perhaps the answer is that Y1 receptors have two functions in the dentate gyrus, one to modulate excitability by actions of ion channels (in the molecular layer and hilar neurons) and the other to influence progenitor division (in the SGZ). The innervation of the molecular layer would allow NPY neurons to influence dendritic function, and the innervation of the SGZ would allow them to influence neurogenesis.
Another puzzle is that the Y1 receptors that appear to mediate the actions of NPY on neurogenesis exist primarily in the molecular layer, and furthermore, they decrease after seizures. This is not logical if a function of NPY is to contribute to seizure-induced neurogenesis in the SGZ. One would expect that NPY receptors, and specifically Y1 receptors, would be necessary in the SGZ. However, it is likely that the Y1 receptors are located in the SGZ, but are sparse enough not to generate a large signal, at least relative to other lamella. This is because physiological evidence indicates they are present [17]. And some Y1 binding is apparent in hilus, although it may be associated with mossy fibers [71]. And perhaps the SGZ Y1 receptors do not decrease after seizures, although those in the molecular layer do. Indeed, there was a transient increase in Y1 receptor binding in the dentate gyrus molecular layer after seizures, but this was not significant [61]. Higher resolution techniques will be needed to clarify these issues. It is important to add that it is not yet clear how NPY-containing cells would influence SGZ progenitors specifically, because synapses have not been revealed, and release onto progenitors has not yet been examined. One possibility is that there is diffusion from the processes of NPY-expressing neurons, analogous to the diffusion of dynorphin from granule cell dendrites to its target receptors in the molecular layer [72]. Although long range diffusion would be unlikely in light of the ability of proteases to cleave NPY extracellularly, there have been suggestions that NPY could potentially function over long distances nevertheless [73].
Summary
In summary, NPY is clearly an important peptide in the adult rat dentate gyrus because it has the potential to influence synaptic transmission and neurogenesis. It may even have other functions, as yet undiscovered, mediated by glia or vasculature [74]. The remarkable plasticity of NPY puts it in a position to allow dentate gyrus function to be modified in a changing environment. The importance of this plasticity in the context of epilepsy cannot be emphasized enough. It could help explain a range of observations about epilepsy that currently is poorly understood. For example, rapid increases in NPY could mediate postictal depression, the period of depression that can last for several hours after generalized seizures. It may mediate the “priming effect,” which is a reduction in seizure threshold following an initial period of seizures [75–77]. Finally, it could contribute to the resistance of dentate granule cells to degeneration after seizures [78]. However, despite the focus in this review on seizure-induced changes, the changes described here also appear to occur after other types of manipulations [79–82], which considerably broadens the scope of NPY's role in the brain.
Acknowledgements
This work was supported by the Human Frontiers Science Program and NIH grant NS 37562. We thank Catherine Snodgrass and Annmarie Curcio for secretarial assistance.
References
- 1.Abe H, Watanabe M, Yamakuni T, Kuwano R, Takahashi Y, Kondo H. Localization of gene expression of calbindin in the brain of adult rats. Neurosci Lett. 1992;138:211–215. doi: 10.1016/0304-3940(92)90917-v. [DOI] [PubMed] [Google Scholar]
- 2.Frederickson CJ, Danscher G. Zinc-containing neurons in hippocampus and related CNS structures. Prog Brain Res. 1990;83:71–84. doi: 10.1016/s0079-6123(08)61242-x. [DOI] [PubMed] [Google Scholar]
- 3.Gall C, Brecha N, Karten HJ, Chang KJ. Localization ofenkephalin-like immunoreactivity to identified axonal and neuronal populations of the rat hippocampus. J Comp Neurol. 1981;198:335–350. doi: 10.1002/cne.901980211. [DOI] [PubMed] [Google Scholar]
- 4.Freund TF, Hajos N, Acsady L, Gores TJ, Katona I. Mossy cells of the rat dentate gyrus are immunoreactive for calcitonin gene-related peptide (CGRP). Eur J Neurosci. 1997;9:1815–1830. doi: 10.1111/j.1460-9568.1997.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 5.Freund TF, Bazsaki G. Intemeurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Deller T, Leranth C. Synaptic connections of neuropeptide Y (NPY) immunoreactive neurons in the hilar area of the rat hippocampus. J Camp Neurol. 1990;300:433–447. doi: 10.1002/cne.903000312. [DOI] [PubMed] [Google Scholar]
- 7.Kohler C, Eriksson L, Davies S, Chan-Palay V. Neuropeptide Y innervation of the hippocampal region in the rat and monkey brain. J Comp Neurol. 1986;244:384. doi: 10.1002/cne.902440310. [DOI] [PubMed] [Google Scholar]
- 8.Milner TA, Veznedaroglu E. Ultrastructural localization of neuropeptide Y-like immunore-activity in the rat hippocampal formation. Hippocampus. 1992;2:107–126. doi: 10.1002/hipo.450020204. [DOI] [PubMed] [Google Scholar]
- 9.Haas HL, Hermann A, Greene RW, Chan-Palay V. Action and location of neuropeptide tyrosine (Y) on hippocampal neurons of the rat in slice preparations. J Camp Neuro. 1987;257:208–215. doi: 10.1002/cne.902570207. [DOI] [PubMed] [Google Scholar]
- 10.Klapstein GJ, Colmers WF. On the sites of presynaptic inhibition by neuropeptide Yin rat hippocampus in vitro. Hippocampus. 1993;3:103–111. doi: 10.1002/hipo.450030111. [DOI] [PubMed] [Google Scholar]
- 11.Pickel VM, Chan J, Veznedaroglu E, Milner TA. Neuropeptide Y and dynorphin-immunore-active large dense-core vesicles are strategically localized for presynaptic modulation in the hippocampal formation and substantia nigra. Synapse. 1995;19:160–169. doi: 10.1002/syn.890190303. [DOI] [PubMed] [Google Scholar]
- 12.Ubink R, Calza L, Hokfelt T. “Neuro”-peptides in glia: focus on NPY and galanin. Trends Neurosci. 2003;26:604–609. doi: 10.1016/j.tins.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Parker RMC, Herzog H. Regional distribution ofY-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 14.Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hokfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 15.Guo H, Castro PA, Palmiter RD, Baraban SC. Y5 receptors mediate neuropeptide Y actions at excitatory synapses in area CA3 of the mouse hippocampus. J Neurophysiol. 2002;87:558–566. doi: 10.1152/jn.00532.2001. [DOI] [PubMed] [Google Scholar]
- 16.McQuiston AR, Petrozzino JJ, Connor JA, Colmers WF. Neuropeptide Y1 receptors inhibit N-type calcium currents and reduce transient calcium increases in rat dentate granule cells. Neurosci. 1996;16:1422–1429. doi: 10.1523/JNEUROSCI.16-04-01422.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gariboldi M, Conti M, Cavaleri D, Samanin R, Vezzani A. Anticonvulsant properties of BIBP3226, a non-peptide selective antagonist at neuropeptide Y Y1 receptors. Eur J Neurosci. 1998;10:757–759. doi: 10.1046/j.1460-9568.1998.00061.x. [DOI] [PubMed] [Google Scholar]
- 18.Paredes MF, Greenwood J, Baraban SC. Neuropeptide Y modulates a G protein-coupled inwardly rectifying potassium current in the mouse hippocampus. Neurosci Lett. 2003;340:9–12. doi: 10.1016/s0304-3940(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 19.Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multi potent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 21.Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhauser C, Gray WP. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult den-tate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18:2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- 22.Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: A possible role in learning. Trends Cogn Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 23.Prickaerts J, Koopmans G, Blokland A, Schecpens A. Learning and adult neurogenesis: survival with or without proliferation? Neurobiol Learn Mem. 2004;81:1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- 25.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 27.Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Dong WK, Greenough WT. Plasticity of nonneuronal brain tissue: roles in developmental disorders. Ment Retard Disabil Res Rev. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- 29.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neuro-genesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostatis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 30.Keilhoff G, Bernstein HG, Becker A, Grecksch G, Wolf G. Increased neurogenesis in a rat ketamine model of schizophrenia. Biol Psychiatry. 2004;56:317–322. doi: 10.1016/j.biopsych.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126:305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 32.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scharfman HE. Functional implications of seizure-induced neurogenesis. Adv Exp Med Biol. 2004;548:192–212. doi: 10.1007/978-1-4757-6376-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parent JM, Lowenstein DH. Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog Brain Res. 2002;135:121–131. doi: 10.1016/S0079-6123(02)35012-X. [DOI] [PubMed] [Google Scholar]
- 36.Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- 37.Moreau M, Leclerc C. The choice between epidermal and neural fate: a matter of calcium. Int J Dev Biol. 2004;48:75–84. doi: 10.1387/ijdb.15272372. [DOI] [PubMed] [Google Scholar]
- 38.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Sperk G, Marksteiner J, Gurber B, Bellmann R, Mahata M, Ortler M. Functional changes in neuropeptide Y- and somatostatin-containing neurons induced by limbic seizures in the rat. Neuroscience. 1992;50:831–846. doi: 10.1016/0306-4522(92)90207-i. [DOI] [PubMed] [Google Scholar]
- 40.Vezzani A, Monhemius R, Tutka P, Milani R, Samanin R. Functional activation of somatostatin- and neuropeptide Y-containing neurons in the entorhinal cortex of chronically epileptic rats. Neuroscience. 1996;75:551–557. doi: 10.1016/0306-4522(96)00261-8. [DOI] [PubMed] [Google Scholar]
- 41.Vezzani A, Ravizza T, Moneta D, Conti M, Borroni A, Rizzi M, Samanin R, Maj R. Brain-derived neurotrophic factor immunoreactivity in the limbic system of rats after acute seizures and during spontaneous convulsions: temporal evolution of changes as compared to neuropeptide Y. Neuroscience. 1999;90:1445–1461. doi: 10.1016/s0306-4522(98)00553-3. [DOI] [PubMed] [Google Scholar]
- 42.Marksteiner J, Ortler M, Bellman R, Sperk G. Neuropeptide Y biosynthesis is markedly induced in mossy fibers during temporal lobe epilepsy of the rat. Neurosci Lett. 1990;112:143–148. doi: 10.1016/0304-3940(90)90193-d. [DOI] [PubMed] [Google Scholar]
- 43.Madsen TM, Greisen MH, Nielsen SM, Bolwig TG, Mikkelsen JD. Electroconvulsive stimuli enhance both neuropeptide Y receptor Y1 and Y2 messenger RNA expression and levels of binding in the rat hippocampus. Neuroscience. 2000;98:33–39. doi: 10.1016/s0306-4522(00)00078-6. [DOI] [PubMed] [Google Scholar]
- 44.Rizzi M, Monno A, Samanin R, Sperk G, Vezzani A. Electrical kindling of the hippocampus is associated with functional activation of neuropeptide Y-containing neurons. Eur J Neurosci. 1993;5:1534–1538. doi: 10.1111/j.1460-9568.1993.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell J, Cook I, Hervey V. Effect of seizures on hippocampal petidergic neurons. Neuropath Appl Neurobiol. 1997;23:299–306. [PubMed] [Google Scholar]
- 46.Scharfman HE, Smith KL, Goodman JH, Sollas AL. Survival of dentate hilar mossy cells after pilocarpine-induced seizures and their synchronized burst discharges with area CA3 pyram-idal cells. Neuroscience. 2001;104:741–759. doi: 10.1016/s0306-4522(01)00132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lurton D, Cavalheiro EA. Neuropeptide-Y immunoreactivity in the pilocarpine model of temporal lobe epilepsy. Exp Brain Res. 1997;116:186–190. doi: 10.1007/pl00005739. [DOI] [PubMed] [Google Scholar]
- 48.Scharfman HE, Sollas AL, Smith KL, Jackson MB, Goodman JH. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J Camp Neural. 2002;454:424–439. doi: 10.1002/cne.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarthy JB, Walker M, Pierce J, Camp P, White JD. Biosynthesis and metabolis of native and oxidized neuropeptide Y in the hippocampal mossy fiber system. J Neurochem. 1998:1950–1963. doi: 10.1046/j.1471-4159.1998.70051950.x. [DOI] [PubMed] [Google Scholar]
- 50.Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 1990;109:180–190. doi: 10.1016/0014-4886(90)90072-z. [DOI] [PubMed] [Google Scholar]
- 51.Vezzani A, Sperk G. Overexpression of NPY and Y2 receptors in epileptic brain tissue: an endogenous neuroprotective mechanism in temporal lobe epilepsy? Neuropeptides. 2004;38:245–252. doi: 10.1016/j.npep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Camp Neural. 1997;385:385–404. [PubMed] [Google Scholar]
- 53.Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- 54.Scharfman HE, Sollas AL, Berger RE, Goodman JH. Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J Neurophysio. 2003;90:2536–2547. doi: 10.1152/jn.00251.2003. [DOI] [PubMed] [Google Scholar]
- 55.Sutula TP, Golarai G, Cavazos J. Assessing the functional significance of mossy fiber sprouting. Epilepsy Res Suppl. 1992;7:251–259. [PubMed] [Google Scholar]
- 56.Kotti T, Riekkinen PJS, Miettinen R. Characterization of target cells for aberrant mossy fiber collaterals in the dentate gyrus of epileptic rat. Exp Neurol. 1997;46:323–330. doi: 10.1006/exnr.1997.6553. [DOI] [PubMed] [Google Scholar]
- 57.Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- 58.Schwarzer C, Kofler N, Sperk G. Up-regulation ofneuropeptide Y-Y2 receptors in an animal model of temporal lobe epilepsy. Mol Pharmacal. 1998b;53:6–13. doi: 10.1124/mol.53.1.6. [DOI] [PubMed] [Google Scholar]
- 59.Bregola G, Dumont Y, Fournier A, Zucchini S, Quirion R, Simonato M. Decreased levels of neuropeptide Y(5) receptor binding sites in two experimental models of epilepsy. Neuroscience. 2000;98:697–703. doi: 10.1016/s0306-4522(00)00162-7. [DOI] [PubMed] [Google Scholar]
- 60.Vezzani A, Moneta D, Mule F, Ravizza T, Gobbi M, French-Mullen J. Plastic changes in neuropeptide Y receptor subtypes in experimental models of limbic seizures. Epilepsia. 2000;41(Suppl 6):S115–S121. doi: 10.1111/j.1528-1157.2000.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 61.Kofler N, Kirchmair E, Schwarzer C, Sperk G. Altered expression of NPY-Y1 receptors in kainic acid induced epilepsy in rats. Neurosci Lett. 1997;230:129–132. doi: 10.1016/s0304-3940(97)00492-8. [DOI] [PubMed] [Google Scholar]
- 62.Silva AP, Pinheiro PS, Carvalho AP, Carvalho CM, Jakobsen B, Zimmer J, Malva JO. Activation of neuropeptide Y receptors is neuroprotective against excitotoxicity in organotypic hippocampal slice cultures. FASEB J. 2003;17:1118–1120. doi: 10.1096/fj.02-0885fje. [DOI] [PubMed] [Google Scholar]
- 63.Patrylo PR, VanDenPol AN, Spencer DD, Williamson A. NPY inhibits glutamatergic excitation in the epileptic human dentate gyrus. J Neurophysiol. 1999;82:478–483. doi: 10.1152/jn.1999.82.1.478. [DOI] [PubMed] [Google Scholar]
- 64.Schwarzer C, Kirchmair E, Sperk G. Metabotropic glutamate receptors mediate activation of NPY-Y2 receptor expression in the rat dentate gyrus. Neuro Report. 1998a;9:2347–2351. doi: 10.1097/00001756-199807130-00036. [DOI] [PubMed] [Google Scholar]
- 65.Schwarzer C, Marksteiner J, Kroesen S, Kohl C, Sperk G, Winkler H. Secretoneurin: A marker in rat Hippocampal Pathways. J Camp Neurol. 1997;377:29–40. [PubMed] [Google Scholar]
- 66.Gutierrez R. The GABAergic phenotype of the “glutamatergic” granule cells of the dentate rus. Prog Neurobiol. 2003;71:337–358. doi: 10.1016/j.pneurobio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Sun QQ, Akk G, Huguenard JR, Prince DA. Differential regulation of GABA release and neuronal excitability mediated by neuropeptide Y1 and Y2 receptors in rat thalamic neurons. J Physiol. 2001;53l:81–94. doi: 10.1111/j.1469-7793.2001.0081j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howell OW, Scharfman HE, Herzog H, Sundstrom LE, Beck-Sickinger A, Gray WP. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86:646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 69.Sosunov AA, Howell OW, McKhann G, Scharfman HE, Gray WP. Program No 9409 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2004. Neuropeptide Y is important for seizure-induced precursor cell proliferation in the adult dentate gyrus. [Google Scholar]
- 70.Gray WP, Scharfman HE, Herzog H, Beck-Sickinger AG, Goodman JH, Howell OW. Program No 9408 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2004. Neuropeptide Y modulates neurogenesis in the postnatal and adult dentate gyrus. Online. [Google Scholar]
- 71.Gobbi M. Cellular localization of neuropeptide-Y receptors in the rat hippocampus: long-term effects of limbic seizures. Neuro Report. 1996;17:1475–1480. doi: 10.1097/00001756-199606170-00006. [DOI] [PubMed] [Google Scholar]
- 72.Chavkin C. Dynorphins are endogenous opioid peptides released from granule cells to act neurohumorly and inhibit excitatory neurotransmission in the hippocampus. Prog Brain Res. 2000;125:363–367. doi: 10.1016/S0079-6123(00)25025-5. [DOI] [PubMed] [Google Scholar]
- 73.Fuxe K, Agnati L, Aguirre J, Bjelke B, Tinner B, Merlo-Pich E, Eneroth P. On the existence of volume transmission in the central neuropeptide Y neuronal systems. Studies on transmitter receptor mismatches and on biological effects of neuropeptide Y fragments. In: Fuxe K, Agnati L, editors. Advances in Neuroscience, Volume Transmission in the Brain, Novel Mechanisms for Neural Transmission. Vol. 1. Raven; New York: 1991. pp. 105–130. [Google Scholar]
- 74.Abounader R, Hamel E. Associations between neuropeptide Y nerve terminals and intraparenchymal microvessels in rat and human cerebral cortex. J Camp Neurol. 1997;388:444–453. [PubMed] [Google Scholar]
- 75.Kelly ME, Mcintyre DC. Hippocampal kindling protects several structures from the neuronal damage resulting from kainic acid-induced status epilepticus. Brain Res. 1994;634:245–256. doi: 10.1016/0006-8993(94)91927-5. [DOI] [PubMed] [Google Scholar]
- 76.Kondratyev A, Sahibzada N, Gale K. Electroconvulsive shock exposure prevents neuronal apoptosis after kainic acid-evoked status epilepticus. Mol Brain Res. 2001;91:1–13. doi: 10.1016/s0169-328x(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X, Cui SS, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, Honer WG, Corcoran ME. Relations between brain pathology and temporal lobe epilepsy. J Neurosci. 2002;22:6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sloviter RS. Hippocampal pathology and pathophysiology in temporal lobe epilepsy. Neurologia. 1996;11:29–32. [PubMed] [Google Scholar]
- 79.Bayer LE, Milner TA. Transient increases in neuropeptide Y-like immunoreactivity in den-tate hilar neurons following fimbria/fornix transection. J Neurosci Res. 1993;34:434–441. doi: 10.1002/jnr.490340408. [DOI] [PubMed] [Google Scholar]
- 80.Bison S, Crews F. Alcohol withdrawal increases neuropeptide Y immunoreactivity in rat brain. Alcohol Clin Exp Res. 2003;27:1173–1183. doi: 10.1097/01.ALC.0000075827.74538.FE. [DOI] [PubMed] [Google Scholar]
- 81.Conrad CD, McEwen BS. Acute stress increases neuropeptide Y mRNA within the arcuate nucleus and hilus of the dentate gyrus. Mol Brain Res. 2000;79:102–109. doi: 10.1016/s0169-328x(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 82.Diez M, Koistinaho J, Dearmond SJ, Groth D, Prusiner SB, Hokfelt T. Marked decrease of neurooeotide Y Y2 receptor binding sites in the hippocampus. J Comp Neurol. 1997;257:208–215. doi: 10.1073/pnas.94.24.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]