Abstract
Objectives
To evaluate survival after detection of recurrent cervical cancer by FDG-PET in symptomatic versus asymptomatic patients.
Methods
This is a prospective registry study of 103 patients treated with definitive chemoradiation for advanced cervical cancer who demonstrated no abnormal FDG uptake (a complete metabolic response, CMR) on their 3-month posttherapy FDG-PET. Their median age was 48 years (range 26-84). The clinical stages were Ib in 38, IIa in 1, IIb in 39, and IIIb in 25. All patients underwent subsequent surveillance FDG-PET. Patients were grouped according to symptom status at the time of the surveillance FDG-PET. Recurrence sites and survival data were analyzed.
Results
The median time from the 3-month posttherapy FDG-PET to the first surveillance FDG-PET was 13 months. 25 patients (25/103; 24%) were symptomatic at the time of surveillance FDG-PET and 21 of these had FDG-PET findings indicative of recurrence. 78 patients (78/103; 76%) were asymptomatic and 9 of these had tumor recurrence detected by PET. All recurrences were confirmed by biopsy or radiologic progression. The recurrences in the 21 symptomatic patients were loco regional in 4 and distant in 17. The 9 asymptomatic patients had isolated loco regional disease in 8 and distant disease in 1. All patients received treatment for recurrence. The three year cause-specific survival for symptomatic recurrences was 19% versus 59% for asymptomatic recurrences (p=0.09).
Conclusions
Surveillance FDG-PET can detect asymptomatic recurrent disease that is potentially amenable to salvage therapy. Prospective investigation of surveillance PET is warranted.
Keywords: FDG/PET, Cervix Cancer, Radiation, Recurrence
Introduction
Cervical cancer is the third most common gynecologic malignancy in the United States, with 11,070 new cases and 3,870 deaths in 2008 [1]. Worldwide, cervical cancer is the third most common cancer of women. It accounts for approximately 150,000 deaths annually. Most patients developing cervical cancer reside in developing countries [2]. Despite advances in screening and prevention, cervical cancer remains a significant source of morbidity and mortality. Almost half of all cases occur in women less than 50 years of age and, therefore, cervical cancer is a major cause of average life years lost per person.
Approximately one third of patients with advanced stage disease will develop recurrent or progressive cervical cancer which usually occurs within the first two years after treatment. 70% of patients with a recurrence will have distant disease [3, 4]. Survival after cervical cancer recurrence is poor. Earlier detection of recurrent disease may lead to improved outcomes in these patients [3, 5, 6]. Patients with symptoms suggestive of recurrent tumor have a significantly higher chance of having recurrent disease compared to their asymptomatic counterparts [7, 8].
Positron emission tomography (PET) with the glucose analogue F-18 fluorodeoxyglucose (FDG) has emerged as an important oncologic imaging method that is now widely used for diagnosis, initial staging, and restaging of many types of cancer [9]. In patients with cervical cancer, FDG-PET is more sensitive than CT at detecting lymph node metastasis and is a better predictor of survival. [10, 11] It is now also being used to plan radiotherapy and to evaluate therapeutic response [9]. Complete metabolic response on PET after completion of chemo radiotherapy has been shown to be a robust indicator of long-term survival in both retrospective and prospective studies [12, 13].
It has been reported that patients who have early detection and treatment of asymptomatic recurrent cervical cancer demonstrate improved survival compared to patients who present with symptomatic recurrent disease. [3, 5, 6]. The utility of FDG-PET in the detection of clinically suspected recurrent cervical cancer has been described. However, the role of PET in the detection of asymptomatic recurrent disease has not been well described. Therefore we evaluated the utility of FDG-PET, after initial treatment, in symptomatic and asymptomatic patients with cervical cancer who had a complete metabolic response to therapy on their posttherapy FDG-PET.
Patients and Methods
Patients
The data for this study were obtained from a prospective tumor registry that was approved by the Washington University Human Research Protection Office. Patients were treated with definitive chemoradiation for advanced-stage (Ib1-IIIb) cervical cancer of all histological subtypes between November 2000 and October 2006. All patients underwent pretreatment FDG-PET for initial staging and a posttherapy FDG-PET three months after completion of therapy. There were 248 patients (Figure 1) who had a complete metabolic response on their 3-month posttherapy FDG-PET. Of these 248 patients, 103 patients constitute this study because they underwent one or more subsequent FDG-PET scans for follow-up of their disease (145 patients had no further imaging studies performed).
Figure 1.
Schema of the study population.
The characteristics of the 103 patients in the study cohort are shown in Table 1. Median patient age at diagnosis was 48 years (range 26-84). There were 15 patients with International Federation of Gynecology and Obstetrics stage Ib1 disease, 23 with stage Ib2, 1 with IIa, 39 with stage IIb, and 25 with stage IIIb. The majority of tumors were squamous cell cancers. The PET lymph node status at diagnosis was negative in 49, 44 patients had positive pelvic nodes, and 10 patients had positive pelvic and para-aortic nodes. The mean time from completion of therapy to the initial posttherapy FDG-PET was 12.8 weeks (range 6-45 weeks).
Table 1.
| Patient Characteristics at Initial Diagnosis | Asymptomatic* (n=78) | Symptomatic* (n=25) |
|---|---|---|
| Median age (range) | 50 (27-84) | 46 (26-64) |
| Stage | ||
| Ib1 | 15 (19%) | 0 (0%) |
| Ib2 | 17 (13%) | 6 (24%) |
| IIa | 1 (1%) | 0 (0%) |
| IIb | 28 (36%) | 11 (44%) |
| IIIb | 25 (32%) | 8 (32%) |
| Histology | ||
| Squamous | 70 (90%) | 23 (92%) |
| Adenocarcinoma | 5 (6%) | 2 (4%) |
| Other | 3 (4%) | 0 (0%) |
| Pre treatment lymph nodes positive | ||
| Aortic | 9 (12%) | 1 (4%) |
| Pelvic | 31 (40%) | 13 (52%) |
| None | 38 (49%) | 11 (44%) |
| Duration of follow up (months) | 13 | 8 |
Symptom status refers to first surveillance FDG-PET
PET Imaging
Before November 2002 a conventional PET scanner was used for FDG-PET and the images were routinely interpreted as described by Grigsby et al [10]. Thereafter, a hybrid PET/CT scanner was used as described by Wright et al [14]. If the patient's blood glucose exceeded 200 mg/dL, the study was deferred. Combined PET/CT images were interpreted separately as well as in a fused mode in routine clinical fashion. Pre-treatment lymph node status was assessed on pretreatment FDG-PET. Recurrences detected by FDG-PET were defined as either areas of abnormal FDG uptake in the initially treated area that had been negative on the initial posttherapy study, or new foci of abnormal FDG uptake. All recurrences noted on FDG-PET were confirmed by biopsy or by radiologic evidence of disease progression in rare instances when biopsy was either not feasible or the patient declined.
Treatment
Definitive chemoradiation therapy consisted of external pelvic irradiation (or pelvic and para-aortic irradiation for patients with para-aortic nodal disease on pre-treatment PET), intracavitary brachytherapy and concurrently administered Cisplatin therapy (40mg/m2 weekly for 6 cycles).
All patients were treated for recurrent disease as determined by the treating physicians. Treatment for recurrent disease was individualized and consisted of chemotherapy, surgery, irradiation, or combinations of these therapies. (Table 2).
Table 2.
| Pt # | Initial FIGO stage | Initial FDG-PET lymph node status | Site of recurrence | Time from completion of treatment to PET recurrence (months) | Treatment for recurrence | Time from recurrence to last follow-up or death (months) | Status |
|---|---|---|---|---|---|---|---|
| Asymptomatic Detected on first surveillance FDG-PET | |||||||
| 1 | Ib1 | None | Lung | 30 | RT, chemo | 21 | Dead |
| 2 | IIb | Para-aortic | Mediastinum, liver | 20 | Chemo | 17 | Alive |
| 3 | Ib2 | Pelvic | Para-aortic nodes | 13 | RT, chemo | 51 | Alive |
| 4 | IIIb | None | Pelvic,para-aortic nodes, mediastinum | Chemo | 5 | Dead | |
| 5 | IIb | Pelvic | Supraclavicular nodes, mediastinum | 14 | Chemo | 20 | Alive |
| 6 | IIIb | None | Pelvic nodes | 16 | Chemo | 20 | Alive |
| 7 | IIIb | Pelvic | Supraclavicular nodes | 8 | RT, chemo | 9 | Alive |
| 8 | IIIb | Pelvic | Cervix | 33 | Surgery | 10 | Alive |
| 9 | IIIb | None | Para-aortic nodes | 14 | Surgery, RT, chemo | 33 | Alive |
| Asymptomatic Detected on second surveillance FDG-PET | |||||||
| 1 | IIIb | None | Pelvic, Para-aortic nodes, mediastinum | 18 | RT, chemo | 28 | Alive |
| 2 | IIIb | Pelvic | Para-aortic nodes | 17 | Surgery | 36 | Alive |
| 3 | IIIb | Pelvic | Pelvic nodes, para-aortic nodes, lung | 25 | Chemo | 17 | Alive |
| 4 | IIIb | Para-aortic | Bone, mediastinal, supraclavicular nodes | 37 | Chemo | 5 | Alive |
RT = radiation therapy; Chemo = chemotherapy
Follow-up
Patients underwent clinical assessment six weeks after completion of therapy, and were then followed every three months throughout the first year, every 4 months during the second year, and every six months for years 3 through 5. Follow-up chest radiography or CT was not routinely performed. Surveillance FDG-PET imaging was performed at the discretion of the treating physician and was prompted by symptoms suggestive of recurrent cancer (bleeding, pain) in 25 patients or for routine surveillance in 78 asymptomatic patients (Figure 1). Follow-up cytology was obtained at the discretion of the patient's primary gynecologic oncologist. None of the study patients had cytology consistent with carcinoma during the period of this study.
Statistical Analysis
The primary study endpoints were progression-free and overall survival measured from the time of the initial 3-month posttherapy PET. Statistical analysis was performed using the Stat-view software (version 5.0.1, SAS institute Inc, Cary, North Carolina). The Kaplan-Meier (product-limit) method was used to derive estimates of survival.[15] Tests of equivalence of estimates of survival were performed by the generalized Wilcoxon log-rank test.[16]
Results
First Surveillance FDG-PET Scan
The median time from the 3-month posttherapy FDG-PET to the first subsequent follow-up FDG-PET study was 13 months. This median time was 13 months for patients who were asymptomatic compared to 8 months for those patients who were symptomatic. Table 2 shows the radiographic findings of the first surveillance FDG-PET study grouped by patient symptom status. Of the 103 patients, 78 were asymptomatic and had follow-up PET for routine surveillance (76%). FDG-PET findings indicative of recurrence were present in 9 of these 78 patients (12%). There were 25 patients who had FDG-PET imaging prompted by symptoms suggesting recurrence. Of these 25, 21 (84%) had findings suggestive of recurrence by PET and were confirmed by tissue biopsy.
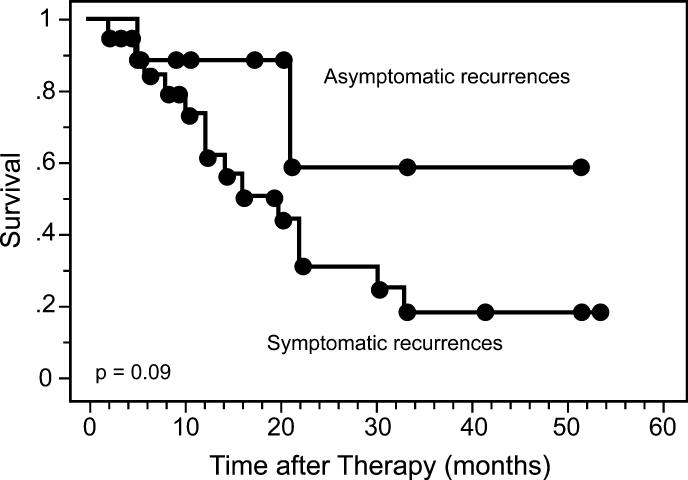
Four of the nine asymptomatic recurrences detected by FDG-PET were isolated to either the cervix, pelvic, or para-aortic nodes and, thus, represented potentially curable disease (Table 2). All 9 patients were treated for their recurrence and seven of nine were still alive at last follow-up. Patients with symptomatic disease had multiple sites of metastatic disease. Most of these patients were treated with chemotherapy or irradiation to palliate the symptoms of their disease. Patients with asymptomatic recurrences had longer survival than the patients with symptomatic recurrences. Figure 2 demonstrates the cause-specific survival for these patients. The three-year survival for patients with symptomatic recurrences was 19% compared with 59% for patients with asymptomatic recurrences (p=0.09).
Figure 2.
Cause-specific survival for patients with symptomatic (n=21) versus asymptomatic (n=9) recurrences on their first surveillance FDG-PET scan.
Second Surveillance FDG-PET Scan
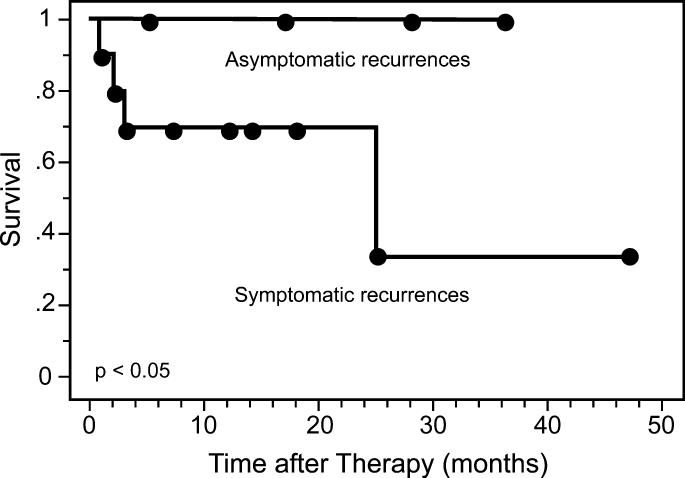
There were 38 patients whose first surveillance FDG-PET scan was negative for recurrent disease who subsequently underwent a second surveillance FDG-PET study. The median time from the first surveillance FDG-PET to the second was 6.5 months. Ten patients underwent FDG-PET scans prompted by symptoms (26%) and 28 (74%) were obtained for routine surveillance in asymptomatic patients. All of the patients with symptoms had evidence of tumor recurrence by FDG-PET. There were 28 asymptomatic patients of whom 24 had negative PET findings and 4 had evidence of recurrent tumor. Three of the four patients with asymptomatic recurrences had isolated sites disease recurrence. All four underwent therapy and are still alive at last follow-up. The cause-specific survivals in these patients are demonstrated in Figure 3. The 3-year cause-specific survivals were 100% for the patients with asymptomatic recurrences compared to 35% for those with symptomatic recurrences (p<0.05).
Figure 3.
Cause-specific survival for patients with symptomatic (n=10) versus asymptomatic (n=4) recurrences on their second surveillance FDG-PET scan.
Discussion
Early diagnosis of recurrent cervical cancer may allow for therapy that significantly alters outcome, especially in asymptomatic patients with local recurrences. The five-year survival for patients treated with chemoradiation for isolated, asymptomatic para-aortic lymph node recurrences has been reported to be as high as 100% [17]. In our study group, patients who were symptomatic were much more likely to have recurrent disease than asymptomatic patients. Only 12% of asymptomatic patients were found to have recurrence on first follow-up FDG-PET compared to 84% of those who were symptomatic.
Patients with recurrent disease detected before the development of symptoms may have improved survival compared to patients that present with symptomatic recurrences. Bodurka et al. demonstrated in a retrospective cohort of 1,096 patients treated for stage Ib cervical cancer that symptom status at the time of recurrence was a significant predictor of survival. In their study, 19 asymptomatic patients had recurrent disease detected by pelvic examination. The median survival after recurrence was 42 months compared to 11 months in the 114 patients with a symptomatic recurrence. No difference was found in the disease-free interval between the two groups. Additionally, cytological screening did not detect a single asymptomatic recurrence in their study [5].
In our study there were 13 patients with asymptomatic recurrences detected by FDG-PET and 7 had disease isolated to the cervix, pelvic, and/or para-aortic lymph nodes. Patients with asymptomatic recurrences also had improved survival compared to those with symptomatic recurrences. This survival advantage persisted in the subset of patient who underwent a second surveillance FDG-PET study.
Pelvic exenteration is often used to treat central pelvic recurrences when there are no distant metastases. The use of FDG-PET may allow for earlier detection of local recurrences which are amenable to treatment by pelvic exenteration, while sparing those patients with disease elsewhere from the morbidity of the procedure [3, 5, 6].
Chung and colleagues retrospectively reviewed whole-body FDG-PET results for 121 patients who had a complete clinical remission after primary treatment. Recurrence was diagnosed in 76 patients, 20 of whom were asymptomatic. Pelvic exenteration was performed in symptomatic and asymptomatic patients if there was no evidence of extra-pelvic disease on the FDG-PET scan. Of the 20 asymptomatic patients with recurrent cervical cancer, PET detected the recurrence in 17. Eight of the 17 patients with PET-detected asymptomatic recurrences were treated with curative intent and 5 of these patients (5/8) were cured of their disease [6].
Unger and associates reported the results of patients undergoing follow-up FDG-PET. Recurrent disease was present in 31% of 26 patients undergoing PET for surveillance compared to 67% of 21 symptomatic patients. Time from treatment to detection of recurrence was 5 months shorter in symptomatic compared to asymptomatic patients. The sensitivity of FDG-PET for detecting recurrence was 80% in asymptomatic patients and 100% in symptomatic patients. The specificity was 100% in asymptomatic patients and 86% in symptomatic patients [8].
Follow-up strategies in cervical cancer vary greatly, largely because of the absence of good evidence to support any particular strategy in an era driven by evidence-based medicine and cost effectiveness. Accordingly, no ideal follow-up strategy for patients with cervical cancer has been established [18]. A retrospective review by Morice and colleagues of 583 patients with stage I-II cervical cancer treated with combined surgery and radiation therapy found clinical examination to be the most effective method of following patients. Routine cytology, ultrasonography, or chest radiography did not lend any survival advantage in the screening of asymptomatic patients. Unlike others, they found similar survival between 38 symptomatic and 7 asymptomatic patients with recurrent disease [19].
Zola and associates, in a multicenter retrospective analysis, described 327 patients with recurrent cervical cancer from 8 Italian institutions followed clinically with cervical cytology and with ultrasonography, magnetic resonance imaging, or CT. Ninety-seven patients (29.7%) were symptomatic, 66 (20.2%) reported symptoms when asked, and 164 (50.1%) were asymptomatic. Survival was significantly longer in asymptomatic patients with recurrent disease (median overall survival 109 months versus 37 months), though the median disease-free interval was shorter (24 months versus 36 months). Only 5 patients (3%) had recurrence detected by cytology. Cox multivariate analysis showed symptom status at time of recurrence detection was a significant predictor of overall survival. More of the asymptomatic patients had vaginal vault recurrences (12.8% versus 7.6%), while more of the symptomatic patients had distant recurrences (15.3% versus 8.9%) which may have contributed to the survival difference demonstrated in this study [20].
At our institution, we now routinely use FDG-PET/CT in the posttherapy evaluation and follow-up of patients with cervical cancer. We routinely perform surveillance as follows: clinical examination and evaluation as indicated by symptoms or every three months for the first two years after therapy, every four months in the third year, and then every six months for years four and five. We are now performing FDG-PET/CT 3 months post therapy, every six months for three years, annually for the next two years, and then clinically as indicated.
There are several limitations to our study. Lead-time bias, in which earlier diagnosis of recurrence allows for patients to live with known disease longer without contributing to any real survival advantage, could be contributing to the apparently longer survival of patients with asymptomatic, PET-detected recurrent disease. Additionally, it is possible that patients with more slowly growing tumors are more likely to have recurrence detected by FDG-PET and this length-time bias allows for earlier detection of recurrent disease in asymptomatic patients. Whether this difference is a reflection of inherent tumor biology or not is difficult to prove.
Our findings indicate that FDG-PET frequently demonstrates recurrence in patients with symptoms suggestive of recurrent cervical cancer. In addition, our findings demonstrate that asymptomatic recurrences can be detected by FDG-PET and patients have improved survival compared to those with symptomatic recurrences. This work suggests that prospective validation of surveillance FDG/PET is warranted to assess whether its use can lead to improved survival of patients with asymptomatic recurrent cervical cancer.
Précis.
The findings of this study demonstrate that FDG-PET can detect asymptomatic recurrences from cervical cancer and that early detection may result in improved survival.
Footnotes
Presented at the Annual Meeting on Women's Cancer, March 9-12, 2008; Tampa, FL.
Conflict of Interest Statement:
BAS reported stock ownership of Radiology Corporation of America, being a medical advisory board member, and receiving lecture honorarium from PETNET Pharmaceuticals, Inc., GE Healthcare, Eastern Isotopes, Inc., and Cardinal Health, Inc. All other authors have no conflicts of interest to declare.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. l2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res. l2002;89:285–93. doi: 10.1016/s0168-1702(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 3.Grigsby PW. The role of FDG-PET/CT imaging after radiation therapy. Gynecol Oncol. l2007;107:S27–9. doi: 10.1016/j.ygyno.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Ryu SY, Kim MH, Choi SC, Choi CW, Lee KH. Detection of early recurrence with 18FFDG PET in patients with cervical cancer. Journal of Nuclear Medicine. l2003;44:347–352. [PubMed] [Google Scholar]
- 5.Bodurka-Bevers D, Morris M, Eifel P, Levenback C, Bevers M, Lucas K, Wharton T. Posttherapy surveillance of women with cervical cancer: an outcomes analysis. Gynecologic Oncology. l2000;78:187–193. doi: 10.1006/gyno.2000.5860. [DOI] [PubMed] [Google Scholar]
- 6.Chung HH, Kim SK, Kim TH, Lee S, Kang KW, Kim JY, Park SY. Clinical impact of FDGPET imaging in post-therapy surveillance of uterine cervical cancer: from diagnosis to prognosis. Gynecol Oncol. l2006;103:165–70. doi: 10.1016/j.ygyno.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Havrilesky LJ, Wong TZ, Secord AA, Berchuck A, Clarke-Pearson DL, Jones EL. The role of PET scanning in the detection of recurrent cervical cancer. Gynecologic Oncology. l2003;90:186–190. doi: 10.1016/s0090-8258(03)00256-7. [DOI] [PubMed] [Google Scholar]
- 8.Unger JB, Ivy JJ, Connor P, Charrier A, Ramaswamy MR, Ampil FL, Monsour RP. Detection of recurrent cervical cancer by whole-body FDG PET scan in asymptomatic and symptomatic women. Gynecol Oncol. l2004;94:212–216. doi: 10.1016/j.ygyno.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. l2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 10.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. l2001;19:3745–9. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara Y, Eisbruch A, Kosuda S, Recker BE, Kison PV, Wahl RL. Evaluation of FDG PET in patients with cervical cancer. J Nucl Med. l1999;40:1125–31. [PubMed] [Google Scholar]
- 12.Grigsby PW, Siegel BA, Dehdashti F, Rader J, Zoberi I. Posttherapy [18F] fluorodeoxyglucose positron emission tomography in carcinoma of the cervix: response and outcome. J Clin Oncol. l2004;22:2167–71. doi: 10.1200/JCO.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. l2007;298:2289–95. doi: 10.1001/jama.298.19.2289. [DOI] [PubMed] [Google Scholar]
- 14.Wright JD, Dehdashti F, Herzog TJ, Mutch DG, Huettner PC, Rader JS, Gibb RK, Powell MA, Gao F, Siegel BA, Grigsby PW. Preoperative lymph node staging of early-stage cervical carcinoma by [18F]-fluoro-2-deoxy-D-glucose-positron emission tomography. Cancer. l2005;104:2484–91. doi: 10.1002/cncr.21527. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric statistics from incomplete observations. J. Am. Stat. Assoc. l1958;53:901–913. [Google Scholar]
- 16.Breslow N. A generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika. l1970;57:579–594. [Google Scholar]
- 17.Singh AK, Grigsby PW, Dehdashti F, Herzog TJ, Siegel BA. FDG-PET lymph node staging and survival of patients with FIGO stage IIIB cervical carcinoma. International Journal of Radiation Oncology, Biology, Physics. l2003;56:489–493. doi: 10.1016/s0360-3016(02)04521-2. [DOI] [PubMed] [Google Scholar]
- 18.Zola P, Fuso L, Mazzola S, Gadducci A, Landoni F, Maggino T, Sartori E. Follow-up strategies in gynecological oncology: searching appropriateness. Int J Gynecol Cancer. l2007;17:1186–93. doi: 10.1111/j.1525-1438.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 19.Morice P, Deyrolle C, Rey A, Atallah D, Pautier P, Camatte S, Thoury A, Lhomme C, Haie-Meder C, Castaigne D. Value of routine follow-up procedures for patients with stage I/II cervical cancer treated with combined surgery-radiation therapy. Ann Oncol. l2004;15:218–23. doi: 10.1093/annonc/mdh050. [DOI] [PubMed] [Google Scholar]
- 20.Zola P, Fuso L, Mazzola S, Piovano E, Perotto S, Gadducci A, Galletto L, Landoni F, Maggino T, Raspagliesi F, Sartori E, Scambia G. Could follow-up different modalities play a role in asymptomatic cervical cancer relapses diagnosis? An Italian multicenter retrospective analysis. Gynecol Oncol. l2007;107:S150–4. doi: 10.1016/j.ygyno.2007.07.028. [DOI] [PubMed] [Google Scholar]