Abstract
Adult animals continue to modify their behavior throughout life, a process that is highly influenced by past experiences. To shape behavior, specific mechanisms of neural plasticity to learn, remember, and recall information are required. One of the most robust examples of adult plasticity in the brain occurs in the dentate gyrus (DG) of the hippocampus, through the process of adult neurogenesis. Adult neurogenesis is strongly upregulated by external factors such as voluntary wheel running (RUN) and environmental enrichment (EE); however, the functional differences between these two factors remain unclear. Although both manipulations result in increased neurogenesis, RUN dramatically increases the proliferation of newborn cells and EE promotes their survival. We hypothesize that the method by which these newborn neurons are induced influences their functional role. Furthermore, we examine how EE-induced neurons may be primed to encode and recognize features of novel environments due to their previous enrichment experience. Here, we gave mice a challenging contextual fear-conditioning (FC) procedure to tease out the behavioral differences between RUN-induced neurogenesis and EE-induced neurogenesis. Despite the robust increases in neurogenesis seen in the RUN mice, we found that only EE mice were able to discriminate between similar contexts in this task, indicating that EE mice might use a different cognitive strategy when processing contextual information. Furthermore, we showed that this improvement was dependent on EE-induced neurogenesis, suggesting a fundamental functional difference between RUN-induced neurogenesis and EE-induced neurogenesis.
Keywords: environment, neurogenesis, irradiation, fear-conditioning, exercise
INTRODUCTION
Adult neurogenesis is required to discriminate between closely related and distinct information (pattern separation), an ability that can be enhanced by increasing neurogenesis (Aimone, 2006; Zhao, 2008; Clelland, 2009; Creer, 2010; Sahay, 2011). Increasing adult neurogenesis through RUN and EE can impact not only the numbers of newborn cells but also their differentiation, survival, and individual neuronal characteristics such as morphology and intrinsic electrophysiological properties (Kempermann, 1997; van Praag, 1999a,b; Gould, 1999; Kempermann, 2002; Schoenfeld, 2012). Despite the robust increases in neurogenesis observed with the two manipulations, it is clear that they work through distinct mechanisms, suggesting a possible functional difference between RUN-induced neurogenesis and EE-induced neurogenesis (Olson, 2006). The contribution of RUN to neurogenesis and hippocampus-dependent behavior has been well documented (Vivar, 2013); however, less is understood about EE. EE was originally shown to dramatically impact neurogenesis and behavior, but it was later determined that a large part of this effect was attributable to the presence of the running wheel inside the enrichment cages (Kobilo, 2011; Mustroph, 2012). The behavioral effects of EE alone are less clear and possibly non-existent; however, this remains an area of controversy (Meshi, 2006). While previous studies may differ about the functional contribution of EE, there is no doubt that EE increases neurogenesis, albeit to a lesser extent than RUN.
The majority of adult newborn neurons undergo apoptosis within the first 2 weeks after birth (Kempermann, 2003; Sierra, 2010). EE increases hippocampal neurogenesis by increasing newborn cell survival and is particularly effective with newborn neurons during the early stages of maturation (Van Praag, 1999a; Tashiro, 2007). In addition, newborn neurons whose survival is promoted through EE appear to retain specific information about the EE. Re-exposure to the same EE preferentially reactivates the same population of neurons, suggesting that EE-induced newborn neurons encode specific information related to the environment (Tashiro, 2007). Further supporting this finding, a computational model predicts that, due to the continuous nature of adult neurogenesis, the constant supply of new neurons allows the animal to encode new environments experienced over time (Aimone, 2009).
Recent studies have investigated the relationship between the activation of dentate granule cells (DGCs) and encoding contexts in a FC procedure. In a context discrimination procedure, DGCs activated selectively to a previously experienced and to a novel environment, suggesting that separate populations encoded similar but distinct environments (Deng, 2013). Interestingly, context-shock association could be formed by the artificial activation of DGCs. These DGCs associated the shock with the context they were artificially activated in as well as with the shock context in which they were spontaneously activated (Ramirez, 2013).
We hypothesize that, during the exploration of an environment, EE-induced newborn neurons encode both general and specific features of the environment that aid the animals in making behavioral judgments. In this study we provide behavioral evidence demonstrating that animals that experience EE use a fundamentally different cognitive strategy than either control (CON) animals or RUN animals in a contextual FC procedure. This study suggests that not only the quantity but also the quality of the new neurons generated impacts their functional contribution.
MATERIALS AND METHODS
Animals
Female C57BL/6 mice (8-weeks old) were purchased from Harlan (San Diego) and housed under standard 12-h light/dark cycles with free access to food and water. BrdU was injected i.p. at 50 mg/kg, eight times over 2 days. All animal procedures were performed in accordance with the animal guidelines at The Salk Institute for Biological Studies and UCLA.
Immunohistochemistry
Mice were sacrificed and brain sections were prepared as previously reported (Deng, 2013). Primary antibodies used were: rat anti-BrdU (1:250, AbD Serotec CAT# OBT0030CX), mouse anti-NeuN (1:100, Millipore (CHEMICON/Upstate/Linco) CAT# MAB377), and goat anti-DCX (1:250, Santa Cruz Biotechnology CAT# sc-8066). All secondary antibodies were from Jackson ImmunoResearch (Donkey anti-rat IgG Cy3 CAT# 712-165-153, donkey anti-mouse IgG Alexa Fluor 647 CAT# 715-605-151, donkey anti-goat IgG Alexa Fluor 488 CAT# 705-545-147) at 1:250 dilutions. DAPI (Sigma–Aldrich CAT# D9542) was used to stain individual cells.
Confocal Images
Representative immunohistochemistry images were taken using a 20× objective, two by three tiled 1012 × 1012 pixels, that we acquired on a Zeiss LSM 710 Laser Scanning Confocal Microscope. Acquisition and post-acquisition processing were performed using Zen 2012. BrdU-and DCX-positive cells were quantified using a 20× objective and an upright epifluorescence microscope (E800; Nikon, Tokyo, Japan). The experimenter was blind to group conditions for all quantifications.
EE Cages
Enrichment cages were custom-made with Plexi-glass, 90 cm × 90 cm × 30 cm, and contained food, water, plastic tunnels, and igloos (no running wheels). EE animals were housed continuously in these cages for 2 weeks (five animals per cage) (Fig. 1C). No new animals were introduced during the enrichment experience to eliminate any possible effects of social enrichment.
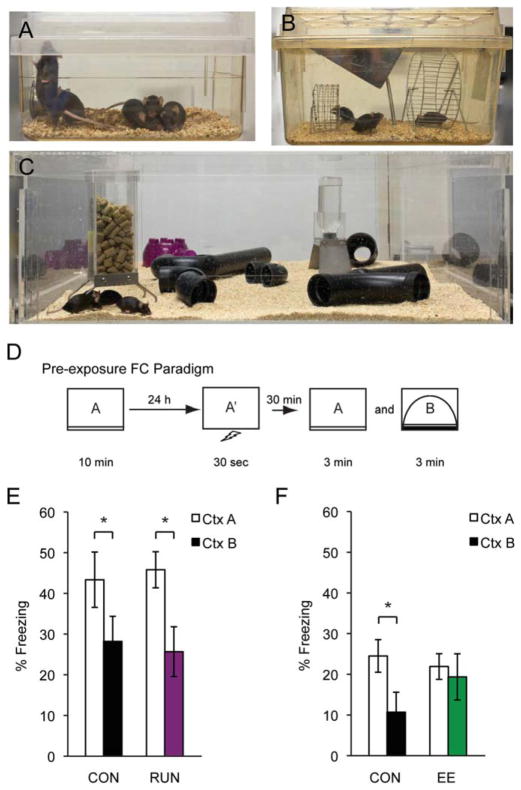
FIGURE 1.
Housing conditions used: (A) CON cage, (B) RUN cage, and (C) EE cage, (D) Pre-exposure FC procedure. (E) Freezing data between CON (n = 10) and RUN (n = 12) during a 30-min discrimination trial. (F) Freezing data between CON (n = 9) and EE (n = 10) during a 30-min discrimination trial. All data are presented as mean ± SEM, *P < 0.05.
Running Cages
RUN animals were housed in a rat cage (three animals per cage) where they had free access to two running wheels (Fig. 1B). RUN animals were housed continuously in these cages for 3 weeks.
FC Contexts
The operant box and software were obtained from Med Associates. A total of four contexts (Ctx) were used, similar to the ones described in Deng (2013). Briefly, animals were trained in CtxA and CtxA′, and tested in CtxA and CtxB or CtxA and CtxC. CtxA differed from CtxA′ in that a plastic board was placed over the wired grid to prevent the generalization of fear response (McHugh, 2007). Animals only received the shock in CtxA′ and CtxA was used for the remaining exposures and testing. CtxB was the same operant box as CtxA and CtxA′ except colored posters were placed on the inside of the box and a piece of plastic was placed inside the chamber to change the shape of the back wall. CtxC was an open field chamber completely different than CtxA and CtxB and located in a different room.
Experience Dependent Discrimination (EDD) Procedure
On day 1, mice were placed in CtxA′ and received a two-second foot shock (0.7 mA) 15 seconds after entry, during the 30-s shock trial (Fig. 2C). Thirty minutes later, mice were given a three-minute exposure to CtxA. On day 2, animals were given a discrimination trial where they were placed either in CtxA for 3 min or CtxB for 3 min in a counter-balanced order. Freezing behaviors in CtxA and CtxB were scored and displayed as a percentage (freezing time/total time). Freezing in CtxA and CtxB was scored using video freeze software (Med Associates), and behavior in CtxC was recorded by a video camera and scored manually by experimenters blinded to mouse groups. Experimenters also hand scored freezing in CtxA and CtxB to ensure that computer scoring and hand scoring were comparable.
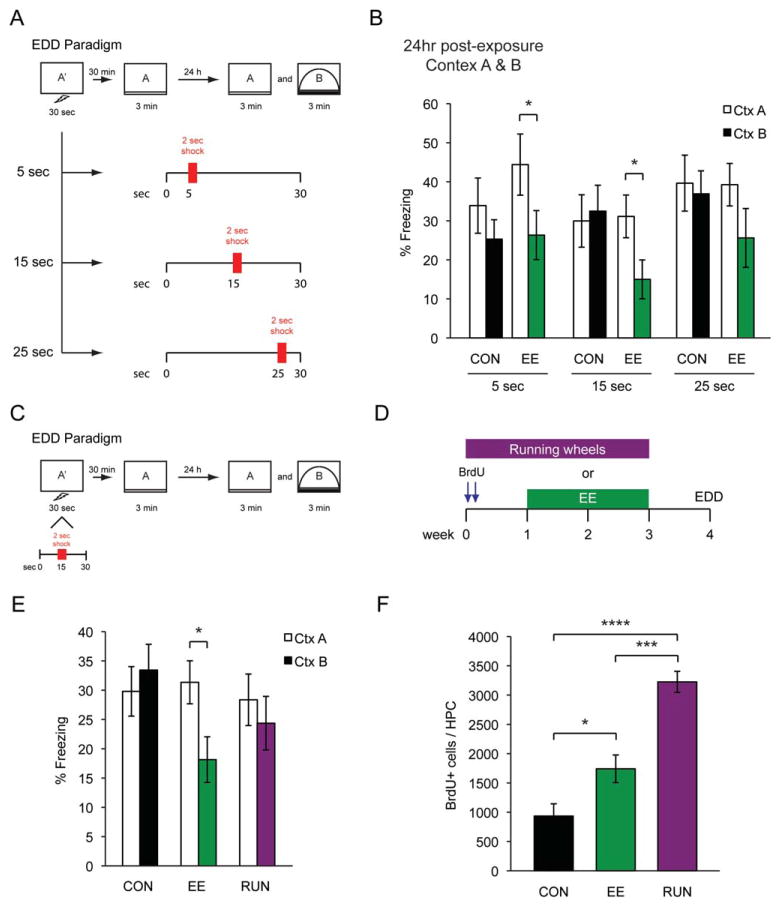
FIGURE 2.
(A) Schematic outlining three different shock trials. (B) Freezing data for 5-s (CON n = 8, EE n = 8), 15-s (CON n = 8, EE n = 8), and 25-s (CON n = 8, EE n = 8) shocks of both CON and EE groups during a 24-h discrimination trial. (C) Experience-dependent discrimination (EDD) procedure. (D) Experimental timeline used to RUN and EE groups. (E) Freezing data of CON (n = 16), EE (n = 16), and RUN (n = 12) groups during a 24-h discrimination trial. (F) BrdU quantification of CON, EE, and RUN groups. All data are presented as mean ± SEM, *P < 0.05, ***P < 0.001 ****P < 0.0001.
Focal X-ray Irradiation of the Hippocampus
Targeted, low-dose X-ray irradiation (IRR) was used to disrupt the neural progenitor cell population in the hippocampus. Mice were anesthetized with ketamine/xylazine (10 mg kg−1) and placed in a stereotaxic frame with their heads protected by a lead shield except for a 3.22 × 11 mm2 opening directly above the hippocampus. The equipment used for this procedure was a Gulmay RS320 X-irradiator operated at 300 kVp and 20 mA located in the UCLA Department of Radiation Oncology’s Division of Molecular and Cellular Oncology. A dose of 1.8 Gy per min at a source to skin distance of 30 cm was administered for two minutes and 47 s for a total of 5 Gy. Mice were allowed to recover from anesthesia in their home cage. This same procedure was conducted three times on three separate days separated by 24 h.
Discrimination Index
A discrimination index (%freezing A − %freezing B)/(total freezing) was calculated in the IRR experiment (Fig. 4E), eliminating the need for a three-way ANOVA (context, enrichment, and irradiation).
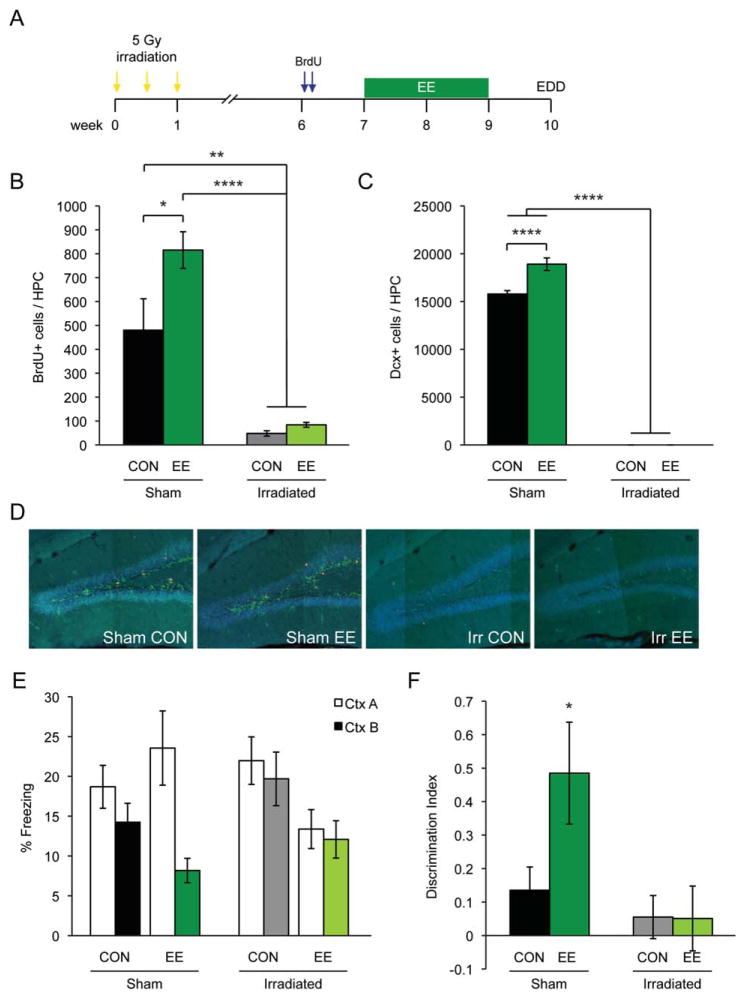
FIGURE 4.
(A) Experimental timeline for irradiation experiment (IRR). (B) BrdU and (C) DCX quantification of SHAM CON, SHAM EE, IRR CON, and IRR EE groups. (D) Representative confocal images. Blue, dapi; Red, BrdU; Green, DCX. (E) Freezing data of SHAM CON (n = 12), SHAM EE (n = 12), IRR CON (n = 12), and IRR EE (n = 12) during a 24-h discrimination trial. (F) Freezing data of Figure 4e presented using a discrimination index. All data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ****P < 0.0001.
RESULTS
Pre-exposure Rescues Immediate Shock Deficit in CON and RUN Animals but not EE Animals
During FC, animals need time to explore the context prior to the shock to generate an association between the context and shock. If animals are shocked immediately after entering the context, they will fail to make the association and exhibit minimal freezing in the context, which is known as the immediate shock deficit (ISD) (Fanselow, 1986). The ISD can be attenuated by pre-exposing animals to the context 24 h before the shock trial (Fanselow, 1990). To tease out the behavioral differences between RUN and EE animals, we modified the ISD procedure and added a discrimination test as described in Deng (2013), allowing us to vary the degree of difficulty with the presence or absence of the pre-exposure and post-exposure trials.
In the pre-exposure FC procedure (Fig. 1D), CON (Fig. 1A) animals were pre-exposed to the shock context (CtxA) and were able to discriminate between the shock context (CtxA) and a similar but different context (CtxB) (Figs. 1E,F). To determine if increased neurogenesis enhanced context discrimination, RUN and EE animals were tested in the pre-exposure FC procedure (Figs. 1B,C). Similar to CON, RUN animals displayed significantly more freezing to CtxA than to CtxB, illustrating their ability to discriminate between similar contexts (CON n = 10, RUN n = 12; Significant main effect of context, two-way ANOVA: F(1,20) = 13.69, P = 0.0014; Bonferroni posthoc: CON (P < 0.05), RUN (P < 0.05)) (Fig. 1E). However, contrary to expectations, there was no enhancement of discrimination in RUN animals when compared to CON animals. Surprisingly, EE animals failed to discriminate between the contexts in the pre-exposure FC procedure. There was no difference in percent freezing in CtxA and CtxB, suggesting a possible deficit in contextual discrimination in EE animals (CON n = 9, EE n = 10; Significant main interaction, two-way ANOVA: F(1,17) = 5.412, P = 0.0326; Bonferroni posthoc: CON (P < 0.05) (Fig. 1F). Alternatively, it is possible that EE may enhance the generalization across CtxA and CtxB, given their overall similarity. The absence of an enhancement in RUN animals and the failure of EE animals to discriminate suggested the need for a more difficult and sensitive task to tease out these discrepancies.
A Short Post-exposure Rescues ISD in EE Animals but not CON and RUN Animals
To develop a more sensitive task, we enhanced the difficulty of the task by removing the pre-exposure and adding a post-exposure, essentially making it a version of the ISD procedure with a discrimination trial. Animals were given a 30-s shock trial, followed 30 min later by a 3-min post-exposure and 24 h later by a context discrimination test between CtxA and CtxB (Fig. 2A). Consistent with previous studies, a certain amount of exploratory time before the foot shock was essential for the successful formation of the context-shock association by CON animals (Figs. 2A,B). To determine the minimum amount of time required for contextual conditioning, the time interval between the placement and shock delivery was varied from 5 to 25 s (Fig. 2A). With a pre-exploratory shock time of 5 or 15 s, EE animals were able to discriminate between CtxA and CtxB (5 s (CON n = 8, EE n = 8), 15 s (CON n = 8, EE n = 8), 25 s (CON n = 8, EE n = 8). Significant main effect of context, two-way ANOVA: F(1,42) = 8.202, P = 0.0065; Bonferroni posthoc: 5 and 15 s (P < 0.05); CON animals were not (Fig. 2B). We did not observe any difference in freezing time between CON and EE animals during the post-exposure to CtxA (data not shown). For the remainder of the experiments, we used the 15-s pre-exploratory shock time and we refer to this task as the experience-dependent discrimination (EDD) task (Fig. 2C).
To determine if the EDD task was sensitive enough to detect cognitive improvements in animals with increased levels of neurogenesis, CON, RUN, and EE groups were tested in this task (Fig. 2D). Consistent with our previous observation, EE animals, with an almost two-fold increase in neurogenesis, were able to discriminate CtxA from CtxB [CON n = 16, EE n = 16, RUN n = 12; although there was no significant main effect, two-way ANOVA: F(2,41) = 3.094, P = 0.056; Bonferroni posthoc revealed: EE group (P < 0.05)] (Figs. 2E,F). In contrast, RUN mice, despite a 3.5-fold increase in neurogenesis (one-way ANOVA: F(2,14) = 37.70, P < 0.0001), failed to discriminate between the two contexts, similar to the CON mice (Figs. 2E,F). These data suggested that enhanced levels of neurogenesis, as demonstrated by RUN animals, were not sufficient to enhance performance on the EDD task and that other unknown factors specifically related to the enrichment experience might be responsible for the improved performance of EE animals on the EDD task. Furthermore, this result revealed a potential underlying functional difference between RUN-induced neurogenesis and EE-induced neurogenesis.
A Post-exposure to the Conditioned Context is Necessary for EE Animals to Discriminate in the EDD Task
To determine if this phenomenon was dependent on the similarity of the contexts, two very different contexts (CtxA and CtxC) were used for the discrimination trial (Fig. 3C). Both CON and EE animals were able to discriminate between two very different contexts (CtxA and CtxC) (CON n = 12, EE n = 12; Significant main effect of context, two-way ANOVA: F(1,17) = 10.28, P = 0.0052; Bonferroni posthoc: CON and EE (P < 0.05)) (Fig. 3B). Thus, EE appeared to specifically enhance the discrimination of the similar contexts in the EDD task.
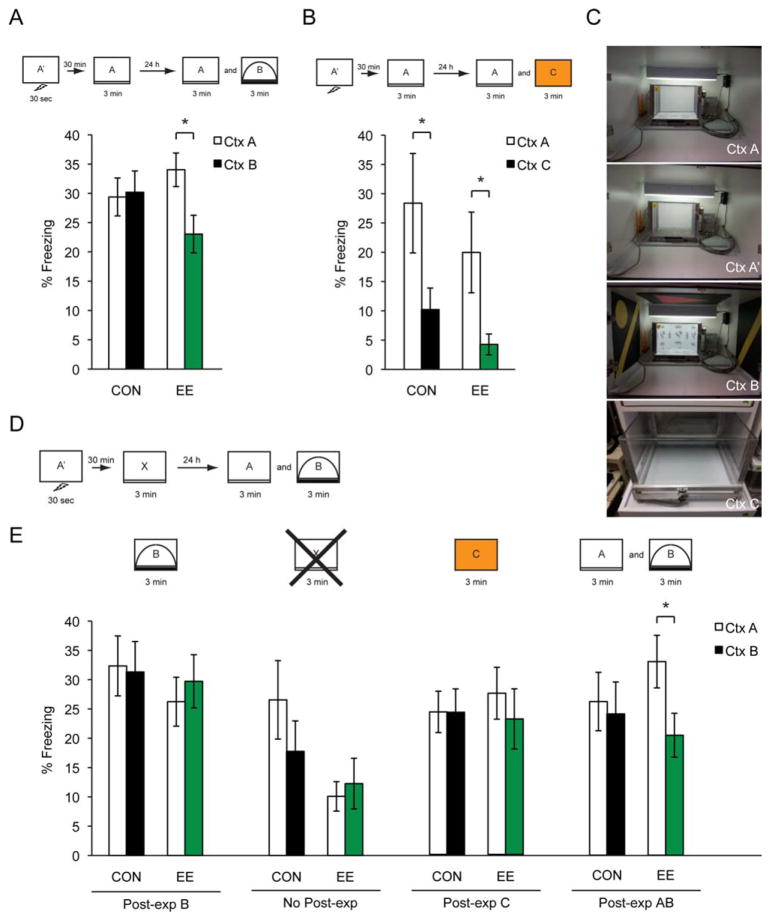
FIGURE 3.
(A) Freezing data of CON (n = 16) and EE (n = 16) animals during a 24-h discrimination trial, with a post-exposure to CtxtA. (B) Freezing data of CON (n = 12) and EE (n = 12) groups during a 24-h discrimination trial between CtxA and CtxC. (C) Operant chamber pictures of different context setups: CtxA, CtxA′, CtxB, and CtxC. (D) EDD procedure. The “x” represents the box that is substituted for the subsequent post-exposures. (E) Box “B” (CON n = 12, EE n = 12); Box “No Post-exp” (CON n = 12, EE n = 12); Box “C” (CON n = 12, EE n = 12); Boxes ‘A and B’ (CON n = 12, EE n = 12). All data are presented as mean ± SEM, *P < 0.05.
After discovering the EE animals’ unexpected ability to discriminate in the EDD task with a limited amount of time, it remained unclear whether the EE animals were actually learning the environment in the 15 s prior to the shock or if they required a post-exposure to complete the contextual representation. To determine which features of the post-exposure were critical, the EDD task was modified to use similar or distinct contexts for the post-exposure. These post-exposure manipulations were as follows: CtxA, CtxB, none, CtxC, and CtxA & B (Figs. 3A,E). By manipulating the presence and context of the post-exposure, we found that the EE animals required a post-exposure specifically to the shock trial context (CtxA) in order to discriminate 24 h later (Box ‘A’ (CON n = 16, EE n = 16; Significant interaction, two-way ANOVA: F(1,30) = 6.766, P = 0.0143; Bonferroni posthoc: EE (P < 0.05)); Box ‘B’ (CON n = 12, EE n = 12; two-way ANOVA: F(1,21) = 0.06979, P = 0.7942); Box “No Post-exp” (CON n = 12, EE n = 12; two-way ANOVA: F(1,14) = 0.6209, P = 0.4438); Box ‘C’ (CON n = 12, EE n = 12; two-way ANOVA: F(1,22) = 0.322, P = 0.5762); Boxes ‘A and B’ (CON n = 12, EE n = 12; Significant main effect of context, two-way ANOVA: F(1,22) = 4.767, P = 0.04; Bonferroni posthoc: EE (P < 0.05) (Figs. 3A,E). Eliminating the post-exposure impaired the EE animals’ ability to discriminate; this was also true when the post-exposure was a similar context (CtxB) or a completely different context (CtxC) (Fig. 3E). These data suggested that the EE animals learned features of the environment during the pre-exploratory 15 s of the shock trial but that this memory was not robust enough and required a post-exposure to substantiate the representation of the context. Regardless of the actual reasons for why the pre-exposure FC procedure and the EDD produced different behavioral outcomes, the behavioral task was successful in indicating that EE and RUN have very different functional consequences for cognition.
Adult Hippocampal Neurogenesis is Necessary for EE Animals to Discriminate in the EDD Task
To further dissect the potential role of neurogenesis in the EDD task, we asked if EE-induced neurogenesis was necessary for discrimination in the EDD task. We used focal irradiation to ablate adult hippocampal neurogenesis (Fig. 4). BrdU and DCX histological counts demonstrated the significant increase in neurogenesis in sham enriched (SHAM EE) animals compared to sham controls (SHAM CON) (BrdU (one-way ANOVA: F(3,30) = 21.04, P < 0.0001); DCX (one-way ANOVA: F(3,23) = 726.7, P < 0.0001) (Figs. 4B–D). As expected, irradiation almost completely ablated BrdU and DCX in both irradiated controls (IRR CON) and irradiated enriched (IRR EE) mice. Confirming our previous results, SHAM EE mice, but not SHAM CON animals, were able to discriminate between CtxA and CtxB in the EDD task [SHAM CON n = 12, SHAM EE n = 12, IRR CON n = 12, IRR EE n = 12; Significant interaction and a significant main effect of context, two-way ANOVA: F(1,44) = 6.363, P = 0.0153; Bonferroni posthoc: SHAM EE (P < 0.05)] (Fig. 4E). Similar to SHAM CON animals, IRR CON animals were unable to discriminate between the two contexts. Interestingly, IRR EE animals did not discriminate between CtxA and CtxB, indicating that EE-induced adult neurogenesis was critical for contextual discrimination in the EDD task.
DISCUSSION
We have developed a novel behavioral test that effectively illustrates a behavioral phenotype that is specific to EE exposure. Using an ISD procedure, which provides insufficient time to learn a context, we demonstrated that exposure to EE aids the animal in its ability to discriminate in an ISD procedure previously thought to be too challenging for these animals. This behavior specifically emerges as a result of EE-induced neurogenesis and not RUN-induced neurogenesis. Given the size of the EE cages, it is likely that EE mice have increased activity levels that may contribute to neurogenesis levels. However, the functional contribution of these neurons (from increased activity) in EE mice may be negligible, based on the behavioral differences observed between RUN and EE mice. The unique behaviors presented in this study suggest a number of interesting functional contributions by EE-induced neurons.
The deficit in freezing with immediate shock is typically viewed as a failure to form a representation of the context in the brief time allotted (Fanselow, 1986). Pre-exposure is thought to overcome this deficit because it allows formation of the contextual representation before the shock trial. During the ISD procedure, the animal only needs to reactivate this representation, and the very brief time between placement and shock is sufficient for such reactivation (Fanselow, 1990; Barrientos, 2002). From this theoretical perspective, the benefit of post-exposure that occurred in the EE mice is unexpected. One possibility is that the learning with post-exposure is a form of second-order conditioning, where a simple contextual feature becomes associated with shock during the immediate shock session and then this feature reinforces the other features of the context during post-exposure. Importantly, EE-induced neurogenesis appears to be necessary for this higher-order learning ability.
The fact that the discriminative abilities of the EE mice are impaired with pre-exposure but improved with post-exposure suggests that the EE mice used fundamentally different cognitive strategies than not only the CON mice but also the mice with RUN-induced neurogenesis. While the total quantity of adult neurogenesis is an important factor in many instances, the method and circumstances in which these new neurons are induced is just as critical.
It is important to point out that, when dealing with EE, the majority of studies use EE and exercise concurrently, often putting running wheels within the enriched environment. Only a handful of studies have made the distinction between environment and exercise (van Praag, 1999b, Fabel, 2009, Kobilo, 2011, Mustroph, 2012) and discovered that many of the benefits of EE can be attributed to the running wheel placed inside these environments (Kobilo, 2011, Mustroph, 2012). Our study reveals that the environmental experience alone may contribute to behavior in an environment-specific manner.
It remains to be determined whether the newborn neurons whose survival is dependent on EE benefit the animal because of the quality of information encoded by the individual neurons or because of an underlying characteristic(s) of the network/circuit that is promoted by the EE experience. Regardless, these results clearly show a unique behavioral phenotype of EE and illustrate how past experiences may meaningfully affect learning and memory function in subsequent behavior.
Acknowledgments
Grant sponsor: Mathers Foundation; Grant number: MH090258-04; James S. McDonnell Foundation; Ellison Medical Foundation; JPB Foundation; UCLA Office of Research Administration; Grant number: 444040-FA-52522.
The authors thank M.L. Gage for editorial comments and J.B. Aimone and S. Parylak for discussions on the manuscript.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, O’Reilly C, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002;134:299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Mayford M, Gage FH. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. Elife. 2013;2:e00312. doi: 10.7554/eLife.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:50. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned fear-induced opiate analgesia: A competing motivatinoal state theory of stress analgesia. Ann N Y Acad Sci. 1986;467:40–54. doi: 10.1111/j.1749-6632.1986.tb14617.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold indcution of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57Bl/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- Sahay AD, Wilson DA, Hen R. Pattern separation: A common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TH, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999b;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H. All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2013;15:189–210. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]