Abstract
Identifying the processes by which people remember to execute an intention at an appropriate moment (prospective memory) remains a fundamental theoretical challenge. One account is that top-down attentional control is required to maintain activation of the intention, self-initiate intention retrieval, or support monitoring. A diverging account suggests bottom-up spontaneous retrieval can be triggered by cues that have been associated with the intention; sustained attentional processes are not required. We used a specialized experimental design and fMRI methods to selectively marshal and identify each process. Results revealed a clear dissociation. One prospective memory task recruited sustained activity in attentional control areas, such as anterior prefrontal cortex; the other engaged purely transient activity in parietal and ventral brain regions associated with attentional capture, target detection, and episodic retrieval. These patterns provide critical evidence that there are two neural routes to prospective memory, with each route emerging under different circumstances.
Keywords: Memory, Cognitive Neuroscience
In planning to go to the grocery store later that day, Thelma intended to take reusable bags to use for packing her groceries. As preparation, she placed the bags in a basket beside her front door. Upon returning home from work, she gathered her shopping list, fed the dog, and was thinking about some unresolved business as she left the house to drive to the grocery. When paying for the groceries, she realized that she had forgotten to bring her reusable bags, as she had intended to do.
This common everyday example illustrates the failure of a particular memory function, termed prospective memory, that is ubiquitous in everyday life. Prospective memory (PM) involves remembering to execute an intention at the appropriate point in the future. In the present study we examine a current debate regarding the neural and cognitive processes that support prospective memory. A standard account of prospective memory is that it requires sustained, top-down attentional control: processes that serve to maintain activation of the intention while carrying out other ongoing activities (e.g., Burgess, Quayle, & Frith, 2001), self-initiate periodic retrieval of the intention (Craik, 1986), and/or support monitoring for the environmental event(s) that signal appropriate execution of the intention (Smith, 2003). On this account, Thelma failed to remember her intention to take her reusable bags to the grocery because she did not sustain the control processes that support prospective remembering (i.e., she was distracted by the ongoing demands of the day).
A contrasting perspective, known as the multiprocess theory, suggests that a second mechanism can support PM retrieval (McDaniel & Einstein, 2000). The second potential mechanism, bottom-up spontaneous retrieval, does not require monitoring or other sustained attentional processes, but instead is a transient process that is triggered by stimulus cues with strong associations to the PM intention (McDaniel, Guynn, Einstein, & Breneiser, 2004). An important, but unresolved theoretical issue concerns whether prospective memory can in fact be supported by a spontaneous retrieval route that does not require sustained attentional control (e.g., see Smith, 2003). Returning to the vignette, the idea is that upon leaving the house, Thelma’s intention to take the grocery bags might have been spontaneously retrieved had she been attending fully to the basket or had perhaps strongly linked exiting the front door with her intention to take her reusable bags.
Viewed from the theoretical perspectives just described, PM emerges as a compelling paradigm for encapsulating general issues of planned versus stimulus-driven behavior, or alternatively, proactive versus reactive control (Braver, 2012; Bugg, McDaniel, & Einstein, in press). Consequently, the extent to which prospective remembering primarily requires sustained (proactive) control versus transient (spontaneous, reactive) control bears critically not only on understanding PM (and its failure), but may also help to inform more general issues of goal-driven behavior. Unfortunately, behavioral research methods, such as estimating the cost of a PM task to ongoing performance, have not been able to convincingly adjudicate between the above views, leaving the current debate unresolved in the literature (see Einstein & McDaniel, 2010; Smith, 2010).
In the present experiment, we introduce a novel approach to illuminate these theoretical processes in PM. Using powerful fMRI methods, we contrasted two PM conditions that were completely identical except in one subtle way. In both conditions, participants busily engaged in an ongoing task (semantic classification), but were additionally instructed that if they ever encountered a particular target event, they should try to remember to perform the PM task (press another button). The two PM conditions differed only in terms of the stimulus cue that designates the PM trial; in one, the PM trial is indicated by a particular word (e.g., table), and in the other, the PM trial is indicated by a particular syllable (e.g., “tor”, as in tornado, actor, history). Following the literature, we refer to the word cue task as focal PM because processing the word (and its meaning) is focal to the ongoing task of semantic processing. Conversely, we refer to the syllable cue task as nonfocal PM because identifying a particular syllable is not focal to the ongoing task of semantic processing (Einstein et al., 2005).
The fMRI analysis was specifically optimized to dissociate sustained versus transient neural activity dynamics through the use of a mixed block/ event-related design. This design enables separate identification and categorization of brain regions showing sustained patterns of activity (i.e., stably maintained across trials during specific task blocks), from those that are transient (i.e., event-related; active only on specific task trials). Prior fMRI studies of PM have consistently observed neural activation patterns associated with sustained attentional control, with the anterior prefrontal cortex (aPFC) being the primary system involved, along with other components of the frontoparietal attention system (Burgess, Gonen-Yaacovi, & Volle, 2011). However, this prior literature has been limited in that the fMRI methods have not typically enabled a direct assessment of whether the activity dynamics in these regions is sustained versus transient (see Reynolds, West, & Braver, 2009, for an exception). Moreover, the experimental designs have uniformly employed target events that are nonfocal to the demands of the ongoing task (Burgess, Quayle, & Frith, 2001; Reynolds et al., 2009;,for a possible exception see(Gilbert, Gollwitzer, Cohen, Oettingen, & Burgess, 2009), precluding a comparison of activation patterns and dynamics under nonfocal versus focal PM. Thus, the current study design and methods represent a considerable advance over the prior literature.
The multiprocess theory makes a number of strong predictions regarding brain activity patterns in focal and nonfocal PM (McDaniel & Einstein, 2011). First, we predicted that the neural signature of top-down attentional control – sustained activity in aPFC and other frontoparietal regions – should only be observed during nonfocal conditions. Second, we hypothesized that such top-down control would be necessary during nonfocal PM to preactivate the system to notice the PM target event and successfully retrieve the stored intention. We tested this hypothesis by examining functional connectivity patterns between aPFC and retrieval-related regions (e.g., parietal cortex; Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Wagner, Shannon, Kahn, & Buckner, 2005) that were selective to successful PM trials, predicting that these would be higher under nonfocal conditions. During focal PM conditions, a different pattern of activity dynamics and connectivity was expected. Here transient, reactive control is sufficient, because processing of the stimulus cue as part of the ongoing task, should by itself capture attention and spontaneously trigger PM retrieval. Accordingly, a third prediction is that the focal PM condition would not produce sustained activation, but instead would be associated with increased transient activity, selective to PM trials, in a widely-distributed set of brain regions that support bottom-up processes (e.g., attentional capture, target detection, episodic retrieval). Finally a last prediction is that focal PM trials would be associated with a unique pattern of aPFC functional connectivity, reflecting a bottom-up rather than top-down mode of PM intention retrieval and implementation.
Method
Participants
Forty-five younger adults (age range 18–37) participated, with 25 randomly assigned to the Nonfocal PM condition, and 20 to the Focal PM condition. Participants were right-handed, native English speakers with normal or corrected-to-normal vision that had no history of neurological or psychiatric disorders or history of illicit drug use (see Supplementary Materials available online for additional details).
Procedure
Participants in both Focal and Nonfocal conditions performed an ongoing semantic classification task. The task required judgments of whether visually presented target words (appearing in lowercase) matched the semantic category indicated by an immediately preceding cue word (e.g., COLOR – green = match; FURNITURE – grape = nonmatch, indicated by a button-press response with the index or middle finger of right hand). Control and PM blocks were administered in both conditions. In the PM but not the Control blocks participants were given the additional task of trying to remember to make a response (pressing a third button with their right ring finger) when the PM target appeared. The only difference between the Focal and Nonfocal PM blocks was the specification of the PM target (Focal: a particular word [e.g., table]; Nonfocal: a particular syllable [e.g., tor]).
Participants first received practice to familiarize them with the ongoing task. The experimental session, performed while participants underwent fMRI scans, consisted of 10 scanning runs (8.5 minutes duration), 3 Control and 7 PM (with order counterbalanced across participants). Each scanning run was composed of alternating task blocks (3 @ ~2 min duration each) and resting fixation blocks (4 @ ~30 sec duration each), with 25 trials per task block (total trials: 225 in Control blocks, 525 in PM blocks). Twenty PM trials were randomly interspersed amongst the 525 ongoing PM block trials (i.e., each block contained 0, 1, or 2 PM trials).
To facilitate identification of event-related brain activation, we jittered the interval between category cue and target using an exponential distribution (range: 2,500–20,500 ms). We varied the category-target interval rather than the inter-trial interval (held constant at 1500 ms) to ensure that participants would be focused on actively maintaining the category cue rather than rehearsing the PM goal during the unfilled jitter intervals. We treated the short interval between the target and next trial’s category cue as a single-event for purposes of event-related modeling.
fMRI Data Analysis
Details on fMRI data acquisition and preprocessing are available in Supplemental Materials. A general linear model (GLM) approach (Friston, Frith, Frackowiak, & Turner, 1995) was used in combination with a mixed block/event-related design (Reynolds, et al., 2009; Visscher et al., 2003). This design enables simultaneous and independent estimation of brain activation responses that are sustained (i.e., stably increased across trials during task blocks) from those that are transient (i.e., event-related). Sustained task-related activity was estimated separately for PM (PM-Sus) and Control (CTL-Sus) blocks. Event-related (transient) activity was estimated for PM trials and ongoing trials, yielding three separate event types (PM, Ong-PM, Ong-CTL). These estimates were restricted to correct trials only because there were too few error trials to provide reliable estimates.
We had a strong a-priori hypothesis that sustained activity would be selectively increased during the Nonfocal condition in brain regions supporting top-down attentional control and monitoring. As such, we focused our analysis of sustained activity on a canonical set of frontoparietal regions-of-interest (ROIs) that have been identified through meta-analyses of working memory and cognitive control tasks (Owen, McMillan, Laird, & Bullmore, 2005; Wager & Smith, 2003). Additionally, we also included an aPFC ROI that has been associated with sustained attentional monitoring in previous PM studies (Gilbert et al., 2006). We tested each of these ROIs to determine whether they showed increased sustained activity in the PM relative to Control blocks, and whether this activity was additionally selective to the Nonfocal condition. We also conducted a whole-brain analysis testing for the presence of any additional regions showing the reverse effect, i.e., sustained activity selectively increased in the Focal condition.
Because we had less strong hypotheses regarding transient, PM-related activation, these analyses were conducted in an exploratory, whole-brain fashion (using appropriate family-wise error corrections when testing for statistical significance). Regions were identified that showed activation on PM trials relative to ongoing trials (in both PM and Control blocks) in both the Focal and Nonfocal conditions. Identified regions were then further tested for either overlap (conjunction) or differential activation across the two conditions. We conducted an analysis of this type for the aPFC ROI as well, because of our theoretical interest in sustained versus transient activity within this region.
A final analysis tested for PM-related changes in brain functional connectivity selective to either the Focal or Nonfocal condition, using the Psychophysiological Interaction (PPI) approach (for details see Supplementary Materials). The aPFC region was used as an ROI seed in the PPI analysis. Regions were identified that showed significant increases in functional connectivity with aPFC on PM trials for which participants remembered to respond (correct PM trials), and were also selective to the Focal or Nonfocal condition. Target regions identified through this analysis were then further examined in terms of their activation profile on the different task trial types.
Results
Behavioral
Participants in both the Focal and Nonfocal conditions performed the ongoing task at a high-level of accuracy in PM (>90% for both Focal and Nonfocal) and Control (i.e., non-PM) blocks (92%). PM performance was lower in the Nonfocal (M=.74) compared to Focal condition (M=.89; t(43)=3.33, p<.05), consistent with the assumption that identifying the Nonfocal PM target required processing that overlapped less with the ongoing task than did identifying a Focal PM target. Moreover, replicating prior findings (Einstein et al., 2005), monitoring costs (slower ongoing task RTs in PM relative to Control blocks) were present for the Nonfocal task (RT: 71 msec; t(24)=8.52, p<.001) and were significantly greater than that observed in the Focal condition (RT: 40 msec; Condition × Block interaction, F(1,43)=5.96, p<.05, ηp2 =.122). This pattern supports the interpretation that the Nonfocal condition placed greater demands on sustained attentional monitoring processes. However, the behavioral data were not definitive on this point, again replicating prior findings (Scullin, McDaniel, Shelton, & Lee, 2010, Experiment 3), in that significant RT monitoring costs were also observed in the Focal condition (t(19) = 4.15, p = .001). To more directly address the question of whether Focal and Nonfocal PM reflected different mechanisms of task performance we turned to the neuroimaging data.
Sustained Brain Activation
Our primary hypothesis was that the two PM conditions would demonstrate differential sustained activity in brain regions reflecting attentional monitoring. Tests for evidence of sustained activation conducted separately in the two conditions showed a strikingly different pattern of findings. During Nonfocal PM, sustained activation was found in a number of a priori defined regions-of-interest (ROIs) that make up the canonical frontoparietal cognitive control network (see Figure 1; Table S2 in Supplemental Materials), as well as in the left aPFC region that has been most consistently associated with attentional monitoring activity in the prior PM neuroimaging literature (see Figure 2). In contrast, during the Focal condition there was no evidence of sustained activation anywhere in the brain, even when a liberal statistical threshold was employed. We formally confirmed a significant Condition (Focal/Nonfocal) × Block (PM/Control) interaction (p < .05) in all identified Nonfocal ROIs.1
Figure 1.
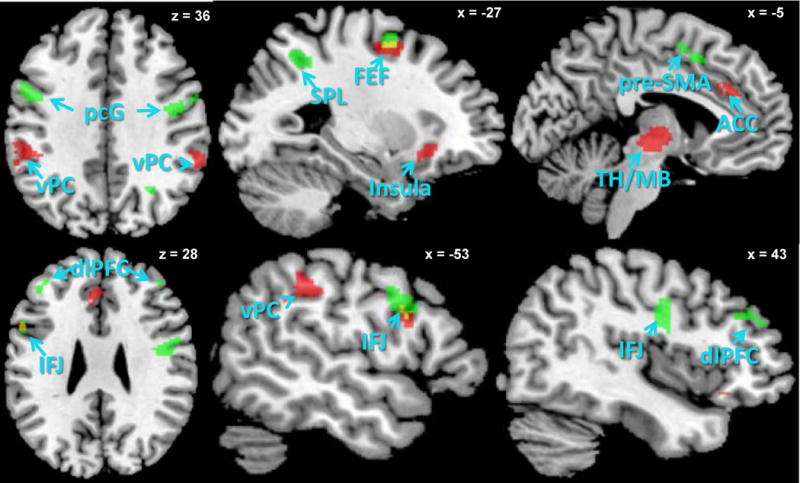
Green: sustained activations selective to the Nonfocal condition; Red: overlap of transient activations on both Focal and Nonfocal PM trials; Yellow: overlap regions showing both Nonfocal sustained and transient activations (in both conditions).
Figure 2.
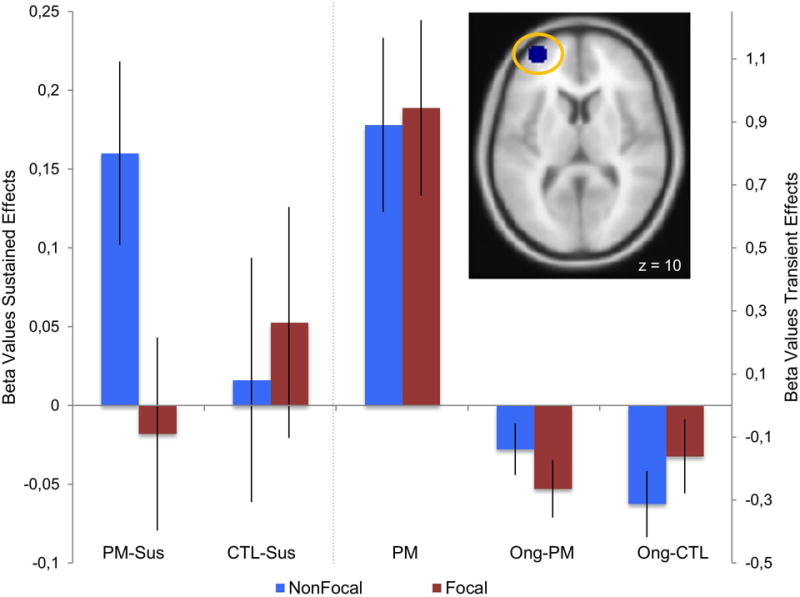
Sustained and transient effects in left aPFC ROI (8mm sphere at −34, 56, 9) for Focal and Nonfocal PM. PM Sus: sustained effect in PM block, CTL Sus: sustained effect in control block; PM: transient effect on PM trials (correct trials only); Ong-PM: transient effect for category decision task trials (ongoing trials) during the PM block; Ong- CTL: transient effect for ongoing trials during the control block.
Transient Brain Activation
We next turned to the transient (event-related) activation that was increased on correct PM trials relative to ongoing trials (in both PM and Control blocks). Here, a very different pattern emerged. A widely-distributed pattern of activation was found (Figure 1; Table S3), including dorsal frontoparietal regions associated with top-down attentional control, that were also identified in the Nonfocal sustained analysis (e.g., superior frontal cortex near to the frontal eye fields). However, we also observed transient activation in ventral brain regions typically engaged by bottom-up shifts of attention and detection of salient target stimuli (e.g., ventral parietal cortex and the cingulo-opercular “salience” network; Cabeza et al., 2008; Corbetta & Shulman, 2002; Seeley et al., 2007). This pattern of transient activity was equally prominent in the Focal as well as Nonfocal conditions, as confirmed via an overlap analysis (Table S3). The analysis did not reveal any regions that exhibited differential patterns of PM transient activation, across the two conditions (via an interaction contrast with statistical significance thresholded to correct for whole-brain family-wise error). Thus, transient PM related activity (on correct PM trials) was present and equivalent in both Focal and Nonfocal PM, whereas sustained activity was observed only in the Nonfocal condition.
Functional Connectivity
Our initial analyses suggested that the Focal and Nonfocal conditions were not strongly differentiated in terms of transient activity on PM trials. Indeed, this pattern extended to the left aPFC region, which exhibited selective sustained activation in the Nonfocal condition, but also showed a significant transient increase on correct PM trials, that was equivalent in both Focal and Nonfocal conditions (see Figure 2). Nevertheless, we hypothesized that aPFC might play distinct functional roles in the two conditions, through interactions with other brain regions that trigger the retrieval and implementation of PM intentions in either a top-down (i.e., initiated from sustained attentional monitoring) or a bottom-up (i.e., triggered by attentional capture from a salient stimulus event) manner. Thus, we conducted a PPI analysis to test whether aPFC showed differential functional connectivity with other brain regions in Focal versus Nonfocal conditions during correct PM trials. It is important to note that examining transient connectivity changes on correct PM trials (rather than sustained or block-related connectivity) provides a stricter test for dissociable effects, given that aPFC activity was equivalent across the two conditions.
The PPI analysis revealed a double dissociation, in which aPFC exhibited stronger connectivity with the precuneus on correct Nonfocal PM trials, and with the right middle temporal gyrus on correct Focal PM trials. This double dissociation was confirmed through the presence of a significant Region (precuneus/middle temporal) × Condition interaction (F(1,41)=19.28, p< 0.001, ηp2 =.320, see Figure 3). We examined these two target regions identified by PPI, to also determine their pattern of transient activation in PM and ongoing (non-PM) trials in both conditions. Interestingly, in both regions it was found that activation on PM trials was selectively stronger in the Focal compared to the Nonfocal condition (ps < .05). It is worth pointing out that these effects were only detectible in ROI-based analyses (i.e., they did not meet the threshold for significance in the initial whole-brain voxelwise analysis) due to the enhanced statistical power afforded by this analysis.
Figure 3.
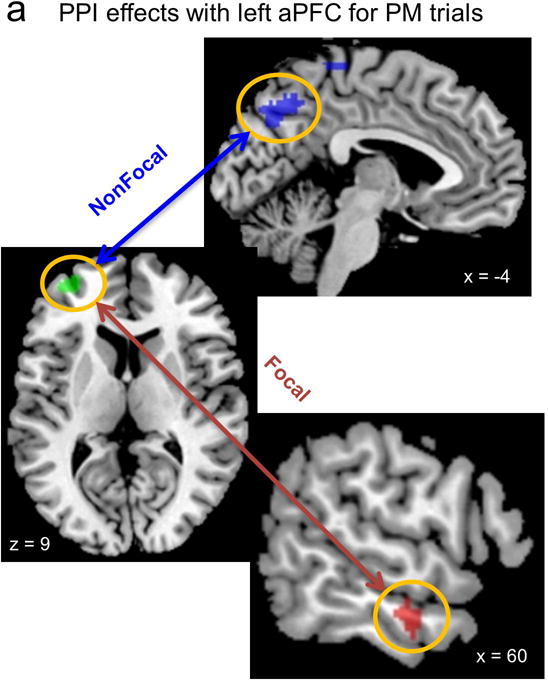
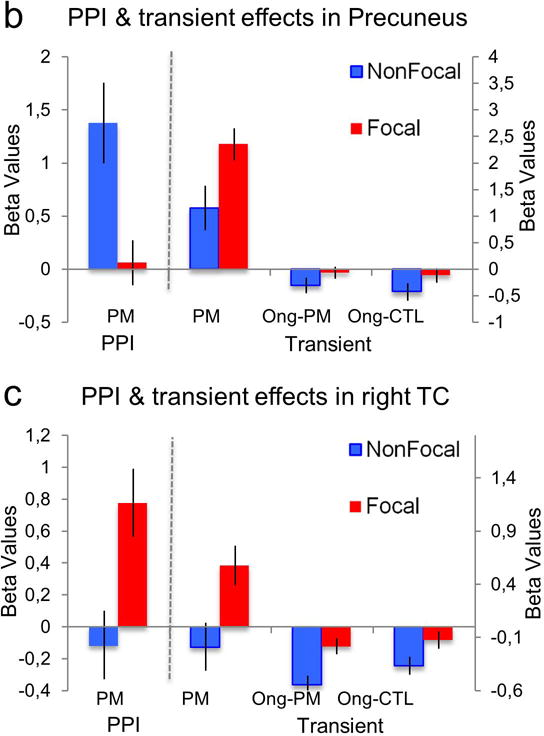
A. Psychophysiological Interaction (PPI) effects between right middle temporal gyrus (62 −12 −16) and precuneus (−2 −72 40) and the left aPFC. B. The precuneus shows stronger left aPFC connectivity in the Nonfocal condition, but greater PM transient activity in the Focal condition. C. The right temporal cortex (TC) shows stronger left aPFC connectivity in the Focal condition, and also stronger PM transient activity in this condition as well. PM: transient effect on PM trials (correct trials only); Ong-PM: transient effect for category decision task trials (ongoing trials) during the PM block; Ong- CTL: transient effect for ongoing trials during the control block.
Discussion
There has been an on-going theoretical debate regarding the cognitive processes that support prospective remembering, which has proven difficult to resolve via purely behavioral measures. One account holds that people must sustain attentional control processes to support PM (as suggested in the opening vignette). The other account is that environmental stimuli can often spontaneously prompt PM retrieval (e.g., a cue linked to the intention during encoding), thereby obviating the need for sustaining attentional control processes. The present fMRI study provides critical new evidence suggesting that both routes can lead to successful prospective memory, but under different circumstances.
The central finding was that subtle shifts in the PM task–shifts motivated by previous theoretical analyses (e.g., McDaniel & Einstein, 2000, 2011)—led to dramatic shifts in the neural systems, activation dynamics, and connectivity patterns associated with task performance. First, consider the Nonfocal PM condition. Our interpretation is that when PM trials are signaled by nonfocal cues, sustained attentional control is recruited to enable recognition of the cue as a PM target. In line with this interpretation, activation in the frontoparietal control network was observed, including the aPFC region that has been most consistently associated with top-down attentional control in PM tasks (Burgess et al., 2011). Here, we definitively demonstrate that the PM-related activity is sustained, replicating a prior finding by Reynolds et al. (2009), but additionally showing that such sustained activity patterns are highly specific to nonfocal PM (rather than a general property of all PM tasks). Additionally, on nonfocal PM trials, aPFC showed a particular change in connectivity that may have enabled more efficient detection of the target cue, and retrieval of the associated PM intention, via top-down biasing. Specifically, the aPFC showed selectively increased connectivity (PPI effect) with the precuneus, a medial parietal region that may link together retrospective and prospective memory (Buckner & Carroll, 2007; Cavanna & Trimble, 2006; Wagner, et al., 2005) Although the PPI effect does not indicate the directionality of the connectivity increase, it is consistent with the idea that PM retrieval was primed by sustained attentional control subserved by the aPFC. One might think of sustained activation in aPFC as placing the system in a “retrieval mode” (Guynn, 2003; Lepage, Ghaffar, Nyberg, & Tulving, 2000), such that the nonfocal cue on PM trials would be appropriately interpreted with respect to the PM retrieval goals. Moreover, this retrieval mode would presumably attenuate the transient activation of this retrieval system when encountering the cue, because the system is already primed and so requires less bottom-up activation to be fully engaged. Converging with this theoretical analysis, the precuneus showed reduced transient activation in response to nonfocal PM trials (see Figure 3).
The most novel findings of the study were observed in the Focal PM condition, and suggest that the processes engaged during Nonfocal PM are only one route by which successful PM retrieval can occur. Under Focal PM conditions, there was no sustained activity in the aPFC, frontoparietal control network, or elsewhere in the brain, suggesting an absence of top-down attentional monitoring; nevertheless, very high levels of PM performance were obtained. In contrast, transient activation on correct PM trials was equally strong in most regions (relative to Nonfocal PM). The similar transient activation pattern for Focal and Nonfocal PM is consistent with the idea that processes in addition to intention retrieval are necessary to complete execution of the PM task on a PM trial, processes that may include disengagement and/or interruption of ongoing activity, as well as coordination of the PM response (Marsh, Hicks, & Watson, 2002; McDaniel & Einstein, 2011). Moreover, this pattern also likely reflects the fact that some retrieval processes are presumably involved on both Focal and Nonfocal PM trials (McDaniel & Einstein, 2000).
An important further finding, however, was that transient activation was significantly increased on correct Focal PM trials (relative to Nonfocal) in two additional regions (the precuneus and right middle temporal gyrus, as indicated by the ROI analysis; Figure 3). This double dissociation between sustained and transient activity across nonfocal and focal tasks supports the proposal that two distinct types of processing (retrieval) strategies can support PM. This interpretation is additionally supported by the widely-distributed pattern of increased transient activity observed on correct focal PM trials, centered on parietal and ventral brain regions – not only middle temporal gyrus and precuneus, but also ventral parietal cortex and the cingulo-opercular network– brain areas that are widely thought to be involved with the detection of salient target events and bottom-up shifts of attention (Cabeza et al., 2008; Seeley et al., 2007).
We suggest that in Focal PM conditions, the retrieval of the PM intention is primarily a bottom-up phenomenon initiated by detection of salient target cues. The increased activity observed in precuneus during focal PM trials supports the idea that the episodic retrieval processes engaged during these trials may be spontaneous, that is, occurring in the absence of sustained aPFC activation (see also Beck, Ruge, Walser, & Goschke, 2012). Although we do not have a strong interpretation regarding the connectivity pattern observed between right middle temporal gyrus and aPFC during focal PM, we speculate that it may reflect a bottom-up retrieval process initiated by the temporal cortex, that may enable the suspension of on-going processing, shifting focus towards the retrieval-related significance of the cue. Although the role of the right middle temporal gyrus is not typically emphasized in memory and attention studies, it is a reliable component of brain networks engaged by target detection / novelty tasks (Linden et al., 1999), response inhibition (Swick, Ashley, & Turken, 2011) and episodic/autobiographical memory studies (Burianova, McIntosh, & Grady, 2010; Svoboda, McKinnon, & Levine, 2006). Thus, further research will be needed to more clearly understand the functional interaction between these two regions.
A residual question arising from the current results relates to why apparent monitoring costs were observed during focal PM (albeit reduced, relative to nonfocal PM), in the absence of sustained activation patterns. We posit that focal PM tasks can be supported by spontaneous retrieval, because full processing of the focal event is stimulated by the ongoing activity and such processing could in principle support a reflexive retrieval process (of the prospective memory intention; McDaniel & Einstein, 2000, 2011). In contrast, during nonfocal PM, target events are not fully processed as a consequence of the ongoing activity. Accordingly, sustained, controlled processing must be engaged to detect the nonfocal cue as a PM target, with subsequent processes required to support retrieval of the intended activity. However, it is possible that the current focal PM condition, like many (if not all) real-world PM situations, might involve a mixture of spontaneous retrieval with intermittent monitoring and other non-functional cognitive processes engaged by the PM demands. These processes could lead to some cost (i.e., slower responses) during ongoing performance, as has been observed in prior studies with focal PM tasks (e.g., Einstein et al., 2005; Meier & Rey-Mermet, 2012; Scullin et al., 2010). Nevertheless, if such monitoring or other processes were intermittent rather than stable, they would not be reflected in a reliable pattern of sustained activity that could be detected with the current fMRI analysis.
The current study also bears on the general issue in the memory literature of whether retrieval processes require a sustained, explicit “retrieval mode” that enables the processing of current events in relation to stored memories (e.g., by reinstantiating the encoding context; Lepage et al., 2000). This issue has been difficult to adjudicate in retrospective memory experiments because the memory instructions would always potentially switch the system to a retrieval mode. In the present paradigm, there is no requirement that the individual make a memory decision on every trial; in fact, the ongoing task does not require a memory decision. In the focal PM condition, PM retrieval was very successful, yet there was no neural evidence that the system was in a different sustained state than during trials in which there was no memory task. These patterns thus provide support to the claim that retrieval of episodic information (e.g., a previously formed intention) need not require activation of a memory retrieval mode.
In closing, we note that there is functional and adaptive value to having several routes to PM retrieval (Einstein & McDaniel, 2008). PM is ubiquitous as an important daily memory activity, as illustrated in the opening vignette. Given the resource demands and somewhat fragile nature of sustained attentional control over time (e.g., Muraven & Baumeister, 2000), having to relying on this proactive control system for the myriad of PM tasks faced daily would likely be overwhelming. A complementary route to PM retrieval that is spontaneous/reactive would help support PM when resources are not available for maintaining top-down control, or when distractions disrupt the ability to maintain intentions over time (as in the opening vignette). Recognizing the existence of this alternative PM route offers practical implications. For example, to support bottom-up retrieval of the intention to take reusable bags to the grocery, the bags could be placed in a location that will be fully attended when leaving the house (e.g., under the car keys). Knowledge about how a fragile attentionally-controlled route to PM retrieval can be side-tracked might promote utilization of the less-vulnerable bottom-up route, which could aid in “rescuing” retrieval of an intention that might otherwise be lost.
Supplementary Material
Acknowledgments
We are grateful to Bruna Martins for her assistance in testing participants and to Michael Coles for initial input on accommodating the prospective memory paradigm to fMRI. Pamela LaMontagne is now at the Washington University School of Medicine.
Funding
This research and preparation of the article were supported by NIH/NIA Grant No. RC1AG036258.
Footnotes
Authorship
M.A.M. and T.S.B. developed the study concept. M.A.M., T.S.B., and M.K.S. were the primary contributors to the study design, with extensive pilot testing to develop a tractable paradigm for fMRI performed by M.K.S. Testing and data collection were performed by P.L., and P.L. and S.M.B. performed the data analysis and interpretation under the supervision of T.S.B. M.A.M., T.S.B., P.L., and S.M.B. drafted the paper, S.M.B. developed the Supplementary Materials, and M.K.S. provided critical revisions. All authors approved the final version of the paper for submission.
A potential alternative interpretation of the increased sustained activity in the Nonfocal condition is that it reflects increased task-difficulty and poorer performance, and thus differential performance monitoring, rather than PM processes per se. As described fully in the Supplementary Materials, we conducted an analysis that involved 15 participants in each condition who were matched on PM performance. All of the obtained effects were retained in this performance-matched subset, suggesting that this alternative interpretation is unlikely.
References
- Beck SM, Ruge H, Walser M, Goschke T. The Functional Neuroanatomy of Spontaneous Retrieval and Strategic Monitoring of Delayed Intentions. Department of Psychology, Technische Universität Dresden; Dresden, Germany: 2012. Manuscript under review. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Science. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Science. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bugg JM, McDaniel MA, Einstein GO. Event-Based Prospective Remembering: An Integration of Prospective Memory and Cognitive Control Theories. In: Reisberg D, editor. The Oxford Handbook of Cognitive Psychology. (in press) [Google Scholar]
- Burgess PW, Gonen-Yaacovi G, Volle E. Functional neuroimaging studies of prospective memory: what have we learnt so far? Neuropsychologia. 2011;49(8):2246–2257. doi: 10.1016/j.neuropsychologia.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39(6):545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49(1):865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Klix F, Hagendorf H, editors. Human memory and cognitive capabilities: Mechanisms and performances. Amsterdam: North-Holland; 1986. pp. 409–422. [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory and metamemory: The skilled use of basic attentional and memory processes. In: Benjamin AS, Ross B, editors. The Psychology of Learning and Motivation, Vol. 48. Skill and Strategy in Memory Use. San Diego, CA: Elsevier; 2008. pp. 145–173. [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory and what costs do not reveal about retrieval processes: A commentary on Smith, Hunt, McVay, and McConnell (2007) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1082–1088. doi: 10.1037/a0019184. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: General. 2005;134:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2(2):166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Gollwitzer PM, Cohen AL, Oettingen G, Burgess PW. Separable Brain Systems Supporting Cued Versus Self-Initiated Realization of Delayed Intentions. Journal of Experimental Psychology: Learning Memory and Cognition. 2009;35(4):905–915. doi: 10.1037/a0015535. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of Cognitive Neuroscience. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Guynn MJ. A two-process model of strategic monitoring in event-based prospective memory: Activation/retrieval mode and checking. International Journal of Psychology. 2003;38:245–256. [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proceedings of the National Academy of Sciences. 2000;97(1):506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cerebral Cortex. 1999;9(8):815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Watson V. The dynamics of intention retrieval and coordination of action in event-based prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:652–659. doi: 10.1037//0278-7393.28.4.652. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- McDaniel MA, Einstein GO. The neuropsychology of prospective memory in normal aging: A componential approach. Neuropsychologia. 2011;49:2147–2155. doi: 10.1016/j.neuropsychologia.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Guynn MJ, Einstein GO, Breneiser J. Cue-focused and reflexive-associative processes in prospective memory retrieval. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2004;30:605–614. doi: 10.1037/0278-7393.30.3.605. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier B, Rey-Mermet A. Beyond monitoring: after-effects of responding to prospective memory targets. Consciousness and Cognition. 2012;21:1644–1653. doi: 10.1016/j.concog.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin. 2000;126(2):247. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver T. Distinct Neural Circuits Support Transient and Sustained Processes in Prospective Memory and Working Memory. Cerebral Cortex. 2009;19(5):1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Shelton JT, Lee JH. Focal/nonfocal cue effects in prospective memory: Monitoring difficulty or different retrieval processes? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:736–749. doi: 10.1037/a0018971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning Memory & Cognition. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE. What costs do reveal and moving beyond the cost debate: Reply to Einstein and McDaniel (2010) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1089–1095. doi: 10.1037/a0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, et al. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19(4):1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Science. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


