Abstract
The present studies determined whether clinically relevant phosphodiesterase 5 (PDE5) inhibitors interacted with a clinically relevant NSAID, celecoxib, to kill tumor cells. Celecoxib and PDE5 inhibitors interacted in a greater than additive fashion to kill multiple tumor cell types. Celecoxib and sildenafil killed ex vivo primary human glioma cells as well as their associated activated microglia. Knock down of PDE5 recapitulated the effects of PDE5 inhibitor treatment; the nitric oxide synthase inhibitor L-NAME suppressed drug combination toxicity. The effects of celecoxib were COX2 independent. Over-expression of c-FLIP-s or knock down of CD95/FADD significantly reduced killing by the drug combination. CD95 activation was dependent on nitric oxide and ceramide signaling. CD95 signaling activated the JNK pathway and inhibition of JNK suppressed cell killing. The drug combination inactivated mTOR and increased the levels of autophagy and knock down of Beclin1 or ATG5 strongly suppressed killing by the drug combination. The drug combination caused an ER stress response; knock down of IRE1α/XBP1 enhanced killing whereas knock down of eIF2α/ATF4/CHOP suppressed killing. Sildenafil and celecoxib treatment suppressed the growth of mammary tumors in vivo. Collectively our data demonstrate that clinically achievable concentrations of celecoxib and sildenafil have the potential to be a new therapeutic approach for cancer.
Cyclooxygenase 2 (COX2) is one of the three prostaglandin endoperoxide synthase enzymes which convert arachidonic acid to prostaglandins, leading to an inflammatory response (Chandrasekharan et al., 2002; Nandakishore et al., 2014; Vosooghi and Amini, 2014). Inhibition of COX1–3 will thus tend to suppress inflammation and a variety of well-established non-steroidal anti-inflammatory drugs such as aspirin and ibuprofen act to block these enzymes (Flower, 2003). More recently developed NSAID drugs have a greater degree of specificity for COX2 over COX1, potentially reducing systemic toxicity due to a lack of COX1 inhibition; drugs such as celecoxib (trade mark, Celebrex) (Swiergiel and Dunn, 2002; Hsieh et al., 2008).
COX2 is over-expressed in several tumor types and drugs that inhibit COX2, that is, celecoxib, can cause in a dose-dependent fashion, tumor cell killing (Hsu et al., 2000; Williams et al., 2000; Johnson et al., 2001). However, in many studies the in vitro doses of celecoxib used to kill tumor cells are above those achievable in human tissues after a 200–800 mg drug dose, that is, ~2.5–7.5 μM (Werner et al., 2002; see http://dailymed.nlm.nih.gov/). There is also solid evidence that COX2 inhibitors have cancer chemo-preventative actions in patients, for example, colorectal polyps using 400 mg BID (Kim and Giardiello, 2011; Mao et al., 2011; Saba et al., 2013). However, although drugs, such as celecoxib have anti-cancer effects, it has been observed that tumor cells exhibiting low levels of COX2 can exhibit sensitivity to these agents, arguing that COX2 inhibitors are likely to have multiple COX2-independent cellular targets in terms of their effects on cancer biology (Grösch et al., 2001; Chuang et al., 2008; Schiffmann et al., 2008; Bastos-Pereira et al., 2010). The reported mechanisms by which COX2 inhibitors regulate tumor cell viability are diverse and include altered levels of autophagy and endoplasmic reticulum stress signaling; increased death receptor expression and reduced levels of c-FLIP-s; inhibition of the AKT protein kinase; modulation of PPARγ function; increased mitochondrial injury and down-regulation of protective BCL-2 family proteins; ceramide generation; and activation of protein kinase G (PKG) (Liu et al., 2004; Pyrko et al., 2007; Soh et al., 2008; Schiffmann et al., 2010; Chen et al., 2011; Huang et al., 2013; Piplani et al., 2013; Ramer et al., 2013; Song et al., 2013).
Phosphodiesterase 5 (PDE5) inhibitors were originally developed as agents to manipulate cardio-vascular biology that were in parallel noted to treat erectile dysfunction (Watanabe et al., 2002; Bruzziches et al., 2013). Inhibition of PDE5 suppresses the degradation of cyclic GMP resulting in the activation of PKG (Francis et al., 2010). cGMP/PKG, through its stimulatory actions upon the ERK, p38 MAPK, JNK and NFκB pathways can increase the expression of inducible nitric oxide synthase (iNOS), resulting in the production of nitric oxide (NO) (Komalavilas et al., 1999; Das et al., 2008; Choi et al., 2009). NO and cGMP/PKG have multiple cellular targets including (to name but a few) ion channels, receptors, phospholipases, Rho A, altered protein nitrosylation, ceramide generation and death receptor signaling (Hayden et al., 2001; Florio et al., 2003; Francis et al., 2010; Kots et al., 2011; Russwurm et al., 2013).
Prior studies from our laboratories have demonstrated that PDE5 inhibitors enhance the toxicities of multiple well established cytotoxic chemotherapies (Das et al., 2010; Booth et al., 2014; Roberts et al., 2014). In these studies, PDE5 inhibitors, in an NOS-dependent fashion, were shown to enhance chemotherapy killing through activation of the CD95 death receptor pathway, the generation of reactive oxygen species and mitochondrial dysfunction. The mechanism(s) by which PDE5 inhibitors and chemotherapies interacted to activate CD95 were not further explored.
The present studies grew out of those described in refs. (Das et al., 2010; Booth et al., 2014; Roberts et al., 2014), and were designed to determine whether PDE5 inhibitors enhanced celecoxib toxicity in tumor cells and if so, the molecular mechanisms by which the drugs interact. PDE5 inhibitors and celecoxib, in an NOS-dependent and COX2-independent fashion, killed multiple tumor cell types, including tumor stem cells and anoikis resistant tumor cells. Cell killing required expression of the death receptor CD95, though tyrosine phosphorylation of the receptor was not required for the death signal. The drug combination induced ER stress signaling and autophagy that were both causal in tumor cell killing.
Materials and Methods
Materials
Phospho-/total-antibodies were purchased from Cell Signaling Technologies (Danvers, MA) and Santa Cruz Biotech. (Santa Cruz, CA). All drugs were purchased from Selleckchem (Houston, TX). Commercially available validated short hairpin RNA molecules to knock down RNA/protein levels were from Qiagen (Valencia, CA). Antibody reagents, other kinase inhibitors, caspase inhibitors cell culture reagents, and non-commercial recombinant adenoviruses have been previously described (Park et al., 2010; Tang et al., 2012; Cruickshanks et al., 2013). Previously characterized semi-established GBM5/GBM6/GBM12/GBM14, glioblastoma cells were supplied by Dr. C.D. James (University of California, San Francisco) and Dr. J.N. Sarkaria (Mayo Clinic, Rochester, MN) and were not further characterized by ourselves (Giannini et al., 2005). The primary human GBM isolates (patient 1; patient 2; patient 3) and primary human medulloblastoma isolates (HOSS1, VC312, CON1) were obtained/isolated from discarded tumor tissue after standard of care surgery. Patients had previously given informed consent under an IRB protocol to the use of tumor tissue. Tumor samples were made anonymous of all patient identifiers by the VCU TDAAC prior to hand-over to the Dent laboratory.
Methods
Cell culture and in vitro exposure of cells to drugs
All fully established cancer lines were cultured at 37°C (5% (v/v CO2) in vitro using RPMI supplemented with 10% (v/v) fetal calf serum and 10% (v/v) non-essential amino acids. All primary human GBM cells were cultured at 37°C (5% (v/v CO2) in vitro using RPMI supplemented with 2% (v/v) fetal calf serum and 10% (v/v) non- essential amino acids at 37°C (5% (v/v CO2). GBM5/6/12/14 stem cells were cultured in StemCell Technologies NeuroCult NS-A Basal Medium supplemented with 20 μg/ml bFGF, 20 μg/ml EGF and 2 mm heparin. For short-term cell killing assays and immunoblotting, cells were plated at a density of 3 × 103/cm2 and 24 h after plating were treated with various drugs, as indicated. In vitro small molecule inhibitor treatments were from a 100 mM stock solution of each drug and the maximal concentration of Vehicle (DMSO) in media was 0.02% (v/v). Cells were not cultured in reduced serum media during any study.
Cell treatments, SDS-PAGE and Western blot analysis
Cells were treated with various drug concentrations, as indicated in the Figure legends. Samples were isolated at the indicated times and SDS PAGE and immunoblotting was performed as described in refs (Park et al., 2010; Tang et al., 2012; Cruickshanks et al., 2013). Blots were observed by using an Odyssey IR imaging system (LI-COR Biosciences, Lincoln, NE).
Recombinant adenoviral vectors; infection in vitro
We generated and purchased previously noted recombinant adenoviruses as per refs. (Park et al., 2010; Tang et al., 2012; Cruickshanks et al., 2013). Cells were infected with these adenoviruses at an approximate m.o.i. as indicated in the Figure Legend (usually an moi of 50). Cells were incubated for 24 h to ensure adequate expression of transduced gene products prior to drug exposures.
Detection of cell death by Trypan Blue assay
Cells were harvested by trypsinization with Trypsin/EDTA for ~10 min at 37°C. Harvested cells were combined with the culture media containing unattached cells and the mixture centrifuged (800 rpm, 5 min). Cell pellets were resuspended in PBS and mixed with trypan blue agent. Viability was determined microscopically using a hemocytometer (Park et al., 2010; Tang et al., 2012; Cruickshanks et al., 2013). Five hundred cells from randomly chosen fields were counted and the number of dead cells was counted and expressed as a percentage of the total number of cells counted. Cell killing was confirmed using the Sceptor instrument (Millipore, Billerica, MA) with a 60 μm tip, which measured tumor cell size/sub G1 DNA as an indication of tumor cell viability.
Soft agar colony formation assay
Tumor cells were triturated to form single cells and were plated into soft agar in sextuplicate using established procedures (500–1000 cells per 60 mm dish) (Cruickshanks et al., 2013). Cells were treated with vehicle (DMSO), Celecoxib (5.0 μM), sildenafil (as indicated, μM) or the drugs combined. Twenty four hours after drug treatment, the plates were washed with drug free media and the cover media was replaced with drug free media. Colonies were permitted to form over the following 20 days, after which they were stained and colonies counted.
Assessment of autophagy
Cells were transfected with a plasmid to express a green fluorescent protein (GFP) and red fluorescent protein (RFP) tagged form of LC3 (ATG8). For analysis of cells transfected with the GFP-RFP-LC3 construct, the GFP/RFP-positive vesicularized cells were examined under the X40 objective of a Zeiss Axiovert fluorescent microscope.
Plasmid transfection
Plasmids
Cells were plated as described above and 24 h after plating, transfected. Plasmids (0.5 μg) expressing a specific mRNA or appropriate vector control plasmid DNA was diluted in 50 μl serum-free and antibiotic-free medium (1 portion for each sample). Concurrently, 2 μl Lipofectamine 2000 (Invitrogen), was diluted into 50 μl of serum-free and antibiotic-free medium. Diluted DNA was added to the diluted Lipofectamine 2000 for each sample and incubated at room temperature for 30 min. This mixture was added to each well/dish of cells containing 200 μl serum-free and antibiotic-free medium for a total volume of 300 μl and the cells were incubated for 4 h at 37°C. An equal volume of 2X medium was then added to each well. Cells were incubated for 48 h, then treated with drugs. To assess transfection efficiency of plasmids we used a plasmid to express GFP and defined the percentage of cells being infected as the percentage of GFP+ cells. For all cell lines the infection efficiency was >70%.
siRNA
Cells were plated in 60 mm dishes from a fresh culture growing in log phase as described above, and 24 h after plating transfected. Prior to transfection, the medium was aspirated and 1 ml serum-free medium was added to each plate. For transfection, 10 nM of the annealed siRNA, the positive sense control doubled stranded siRNA targeting GAPDH or the negative control (a “scrambled” sequence with no significant homology to any known gene sequences from mouse, rat or human cell lines) were used (predominantly Qiagen, Valencia, CA; occasional alternate siRNA molecules were purchased from Ambion, Inc., Austin, TX). Ten nanometer siRNA (scrambled or experimental) was diluted in serum-free media. Four microliter Hiperfect (Qiagen) was added to this mixture and the solution was mixed by pipetting up and down several times. This solution was incubated at room temp for 10 min, then added drop-wise to each dish. The medium in each dish was swirled gently to mix, then incubated at 37°C for 2 h. One mililiter of 10% (v/v) serum-containing medium was added to each plate, and cells were incubated at 37°C for 24–48 h before re-plating (50 × 103 cells each) onto 12-well plates. Cells were allowed to attach overnight, then treated with drugs (0–48 h). Trypan blue exclusion assays and SDS PAGE/immunoblotting analyzes were then performed at the indicated time points.
Immunohistochemistry and live-dead assays
Immunohistochemistry and live-dead assays were performed in 96 well plates using a Hermes Wiscan instrument (IDEA Bio-medical, Rehovot, Israel). Cells were plated and treated with drugs for 12–24 h as indicated in the Figure Legend. After treatment plates were centrifuged to deposit floating cells onto the plate (800 rpm, 5 min). Cells were then either fixed in place (4% paraformaldehyde in PBS) or were subjected to live-dead assay using a commercially available kit and performed according to the manufacturer’s method (Life Technologies, Grand Island, NY). Cell viability was measured using Wisoft software. Fixed cells were subjected to Ki67/IL-6/TNFα staining using standard procedures (Park et al., 2010).
Isolation of activated microglia
Following acquisition of fresh GBM tumor tissue, we isolated activated microglia using CD11b microglia microbeads (MACS, Miltenyi Biotec., San Diego, CA). Tumor tissue was macerated and single cell suspensions obtained using a Miltenyi neural tissue dissociation kit.
Animal studies
For studies with human mammary carcinoma cells, athymic Nu/Nu mice (8 week old, female) were injected, into the 4th mammary fat pad, with 1 × 106/1 × 107 BT474 cells or 3 × 106/1 × 107 BT549 cells. Tumors of ~150 mm3 grew over the following month. Animals were segregated into tumor volumes of approximate equivalent mean tumor size and standard error. The animals were administered vehicle diluent (cremophore), sildenafil (2.5/5.0 mg/kg), celecoxib (2.5, 10 or 25 mg/kg) or the drug combination by oral gavage once every day for 5 days. Other tumor studies used FTY720 (0.05 mg/kg). We performed the experiment twice (n =2) with a total of 8 animals per treatment group. Tumor volumes are measured every 2–3 days as indicated.
Mass spectrometry measurements
Equal numbers of cells (5.98 +/− 0.02 × 106) cells were treated for 6 h and lipids were extracted. Bioactive lipid levels were quantified by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) with a Shimadzu LC-20AD binary pump HPLC system (Shimadzu, Kyoto, Japan) and an Applied Biosystems 4000 QTRAP operating in a triple quadrupole mode, as described previously (Hait NC et al., 2009).
Data analysis
Comparison of the effects of various treatments was performed using one way analysis of variance and a two tailed Student’s t-test. Statistical examination of in vivo animal survival data utilized log rank statistical analyzes between the different treatment groups. Differences with a P-value of <0.05 were considered statistically significant. Experiments shown are the means of multiple individual points from multiple experiments (±SEM).
Results
Initial studies examined whether there was a toxic interaction between the PDE5 inhibitor sildenafil and the non-steroidal anti-inflammatory drug celecoxib. In mammary carcinoma cell lines growing in 10% fetal calf serum, sildenafil and celecoxib interacted in a greater than additive fashion to kill parental wild type tumor cells as well as anoikis resistant variants of these cells at doses as low as 0.5 μM sildenafil and 1 μm celecoxib (Figs. 1A–C). A similar toxic interaction between sildenafil and celecoxib was observed in hepatoma, colorectal cancer, glioblastoma and medulloblastoma cells (Figs. 1D and E; Figs. S1A–S1F). Stem-like GBM12 cells and other stem-like GBM cell derivatives expressed higher levels of CD133 and other stem cell markers than parental GBM cells (Fig. S1 G). Not only did sildenafil and celecoxib treatment kill cells; it also profoundly reduced tumor cell proliferation as judged by reduced Ki67 staining (Fig. 1F, upper IHC). In median dose effect colony formation assays, sildenafil and celecoxib interacted in a greater than additive fashion to kill breast cancer cells with combination index values lower than 0.70 (Fig. 1F, lower graphs). As judged by increased phosphorylation of the protein VASP-1 at S239 (a PKG site), following sildenafil treatment, we were increasing cGMP levels (Fig. 1G). As judged by increased phosphorylation of the endoplasmic reticulum stress marker eIF2α, following celecoxib treatment, we were inducing an ER stress response (Fig. 1H). As judged by decreased expression of the sphingosine-1-phosphate receptor 1 (EDG-1), a target of the multiple sclerosis medicine FTY720 (Fingolimod, Gilenya), FTY720 alone and [sildenafil +celecoxib] treatments reduced EDG-1 expression (Fig. 1I).
Fig. 1.
The PDE5 inhibitor sildenafil interacts with celecoxib to kill cancer cell lines. (A) SKBR3 and BT549 cells were treated with celecoxib (5 μM) and increasing concentrations of sildenafil (0–2 μM). SKBR3 and BT549 cells were treated with sildenafil (2 μM) and increasing concentrations of celecoxib (0–5 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (B) Breast cancer cells, wild type (WT) or anoikis resistant (AR) variants, were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. Upper inset pictures: BT474 cells in 96 well plates were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined using a live-dead assay (red cells =dead; green cells =alive) (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (C) Breast cancer cells, wild type (WT) or anoikis resistant (AR) variants, were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. Upper inset pictures: HCC38 cells in 96 well plates were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined using a live-dead assay (red cells =dead; green cells =alive) (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (D) GBM12 cells and selected GBM12 stem cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. Upper inset pictures GBM12 cells in 96 well plates were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined using a live-dead assay (red cells =dead; green cells =alive) (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (E) Medulloblastoma cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. Upper inset pictures: DAOY medulloblastoma cells in 96 well plates were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined using a live-dead assay (red cells =dead; green cells =alive) (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (F) Upper IHC: BT549 cells in 96 well plates were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and fixed. Immunohistochemistry was performed to determine the levels of Ki67 staining in cells under each treatment condition. Lower graphs: BT549 triple negative breast and SUM149 triple negative inflammatory breast cancer cells were plated as single cells in sextuplicate (250–1500 cells per plate) and treated with vehicle (DMSO), sildenafil (1–3 μM), celecoxib (5 μM) or the drugs in combination as indicated for 24 h. The media was removed and drug free media added to the cultures. Colonies were permitted to form for 10 days after which colonies were stained and counted (n =3 +/− SEM). (G)–(I) Tumor cells (as indicated) were treated with sildenafil (2 μM); celecoxib (5 μM); FTY720 (50 nM) or ATRA (150 nM). One, two and six hours after treatment cells, as indicated, were fixed in place and permeabilized. Immunohistochemistry was performed to determine the levels of Phospho-VASP-1 (S239); Phospho-eIF2α; total EDG-1, and counter staining was with DAPI.
Studies were then performed to define on- and off-target actions of our drugs and their effects on cell viability. The chemically dissimilar PDE5 inhibitor tadalafil interacted with celecoxib to kill tumor cells (Figs. 2A and B). We noted that the expression of PDE5 was elevated in breast, lung and liver tumor tissue compared to matched non-tumor tissues from the same organs arguing for a relatively more specific on-target effect in tumor compared to normal tissues (Figs. 2C–E). The celecoxib derivative 2,5-dimethyl celecoxib, that does not inhibit COX2, was as efficacious as celecoxib at enhancing sildenafil toxicity (Fig. 2F). Knock down of COX2 did not alter the lethality of the sildenafil and celecoxib drug combination in breast cancer cells (Fig. 2G). Knock down of PDE5 expression enhanced celecoxib lethality (Fig. 2H). The nitric oxide synthase (NOS) inhibitor L-NG-Nitroarginine Methyl Ester (L-NAME) inhibited cell killing by sildenafil and celecoxib, consistent with the ability of PDE5 inhibitors to increase iNOS and NO production (Fig. 2I and J). Sildenafil and celecoxib treatment radiosensitized tumor cells (Fig. 2K). Collectively these findings argue that celecoxib, in a COX2–independent fashion, promotes tumor cell killing in cells lacking PDE5 expression and does so in an NOS-dependent manner.
Fig. 2.
PDE5 inhibitors enhance celecoxib toxicity. (A) DAOY and HOSS1 medulloblastoma cells were treated with celecoxib (CEL 5.0 μM) and/or tadalafil (TAD, 0.5 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (B) SKBR3 and BT474 breast cancer cells were treated with celecoxib (CEL 5.0 μM) and/or tadalafil (TAD, 0.5 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (C)–(E) We purchased tissue arrays of mammary/lung/liver carcinoma and matching normal tissues. Tissue was de-parafinized, blocked and stained for expression of PDE5. The data shows representative images of matched normal and tumor tissue. (F) Tumor cells were treated with 2,5 dimethyl celecoxib (25DMC 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (G) SUM149 cells were transfected with a scrambled siRNA (siSCR) or an siRNA to knock down COX2. Thirty-six hours after transfection cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in vehicle control. (H) BT549 cells were transfected with a scrambled siRNA (siSCR) or an siRNA to knock down PDE5. Thirty-six hours after transfection cells were treated with increasing concentrations of celecoxib (0–7.5 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than corresponding value in siSCR control. (I) and (J) BT549, BT474 breast and HOSS1 medulloblastoma cells were pre-treated with vehicle or the NOS inhibitor L-NAME (1 μM). After 30 min, cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 h and viability determined by trypan blue exclusion (n =3, +/− SEM) *P <0.05 greater than vehicle control; #P <0.05 less than corresponding value in vehicle control. (K) BT474 and BT549 cells were plated as single cells in sextuplicate. Twelve hours after plating cells were treated with vehicle (DMSO), celecoxib (2 μM), sildenafil (1 μM) or the drugs in combination as indicated. Thirty minutes after drug treatment cells were irradiated (2 Gy) or mock exposed (time out of incubator ~20 min). The media was replaced after 12 h with drug free media and colonies permitted to form over the following 10 days. Colonies were fixed, stained and counted (n =3 +/− SEM). #P <0.05 less than corresponding value in unirradiated cells.
In Figure 1, we noted that celecoxib and sildenafil interacted to kill GBM/GBM stem-like cells as well as GBM cells freshly isolated from patients. The growth of all tumors is influenced by stromal cell signaling; in the brain activated, microglia play a pivotal role in the proliferation and invasive potential of malignant glial tumors. Hence, we next examined the impact of sildenafil and celecoxib treatment on activated microglia freshly isolated from GBM tumors ex vivo and on the synthesis of paracrine ligands by the activated microglia. Celecoxib and sildenafil interacted in a greater than additive fashion to kill activated microglia (Fig. S2A). Treatment of activated microglia with celecoxib and sildenafil rapidly reduced expression of interleukin 6 and of tumor necrosis factor α (Fig. S2B). Thus, sildenafil and celecoxib treatment has the potential to kill activated stromal cells in the brain and to suppress their production of growth promoting and inflammatory cytokines.
We next examined the roles signal transduction pathways play in regulating the response of tumor cells to sildenafil and celecoxib treatment. Drug combination exposure almost abolished ERK1/2 activity and reduced phosphorylation of AKT, mTOR, p70 S6K and p65 NFκB at regulatory sites (Fig. 3A). Initially, we made use of HCT116 colon cancer cells expressing in wild-type cells a mutant active K-RAS D13 or mutant forms of H-RAS V12 that specifically activated RAF-1 or PI3K (Carón et al., 2005). Expression of H-RAS V12 protected cells from celecoxib and sildenafil toxicity to a greater extent than did expression of K-RAS D13. The protective effect of H-RAS V12 was lost in cells expressing a mutant H-RAS V12 protein that specifically activated only RAF-1 or a H-RAS V12 protein that specifically activated only PI3K (Fig. 3B). In BT549 breast cancer cells which are null for phosphatase and tensin homolog (PTEN), expression of activated AKT or MEK1 significantly reduced drug combination toxicity and expression of dominant negative AKT or MEK1 enhanced drug combination lethality (Figs. 3C and D). Expression of activated forms of p70 S6K or mTOR also suppressed drug combination killing, as did inhibition of JNK signaling (Figs. 3E and F). Surprisingly, expression of dominant negative p38 MAPK enhanced drug toxicity. Incubation of cells with the NOS inhibitor L-NAME suppressed both sildenafil-induced and (sildenafil +celecoxib)-induced activation of JNK (Fig. 3G).
Fig. 3.
The toxic interaction between PDE5 inhibitors and celecoxib is regulated by ERK, AKT, p70 S6K, p38 MAPK and mTOR. (A) BT549 cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 12 h and lysates prepared for SDS PAGE and immunoblotting against the indicated phospho-proteins and proteins. (B) HCT116 colon cancer cells (Wild-type cells expressing K-RAS D13; null −/− cells with K-RAS D13 deleted; null cells expressing H-RAS V12; null cells expressing H-RAS V12–35 that activates RAF-1; null cells expressing H-RAS V12–40 that activates PI3K) were treated with vehicle (DMSO), celecoxib (5.0 μM), sildenafil (2 μM) or the drugs combined. Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). *P <0.05 greater than vehicle control; **P <0.05 greater than corresponding value in HCT116 −/− H-RAS V12 cells. (C) BT549 cells were infected with recombinant adenoviruses to express empty vector (CMV); constitutively active AKT; dominant negative AKT (50 moi). Twenty-four hours after infection cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). *P <0.05 greater than corresponding value in CMV infected cells; #P <0.05 less than corresponding value in CMV infected cells. (D) BT549 cells were infected with recombinant adenoviruses to express empty vector (CMV); constitutively active MEK1; dominant negative MEK1 (50 moi). Twenty-four hours after infection cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). *P <0.05 greater than corresponding value in CMV infected cells; #P <0.05 less than corresponding value in CMV-infected cells. (E) BT549 cells were transfected with plasmids to express empty vector (CMV); constitutively active p70 S6K; constitutively active mTOR; dominant negative p38. Twenty-four hours after transfection cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). *P <0.05 greater than corresponding value in CMV transfected cells; #P <0.05 less than corresponding value in CMV transfected cells. (F) BT549 and HOSS1 cells were pre-treated with vehicle (DMSO) or the JNK inhibitory peptide (JNK-IP, 10 μM). Cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). *P <0.05 greater than celecoxib treatment alone; #P <0.05 less than corresponding value in vehicle treated cells. (G) GBM5/6/12/14 cells as indicated pre-treated with vehicle or the NOS inhibitor L-NAME (1 μM). After 30 min cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 12 h and the phosphorylation of JNK and total JNK expression determined.
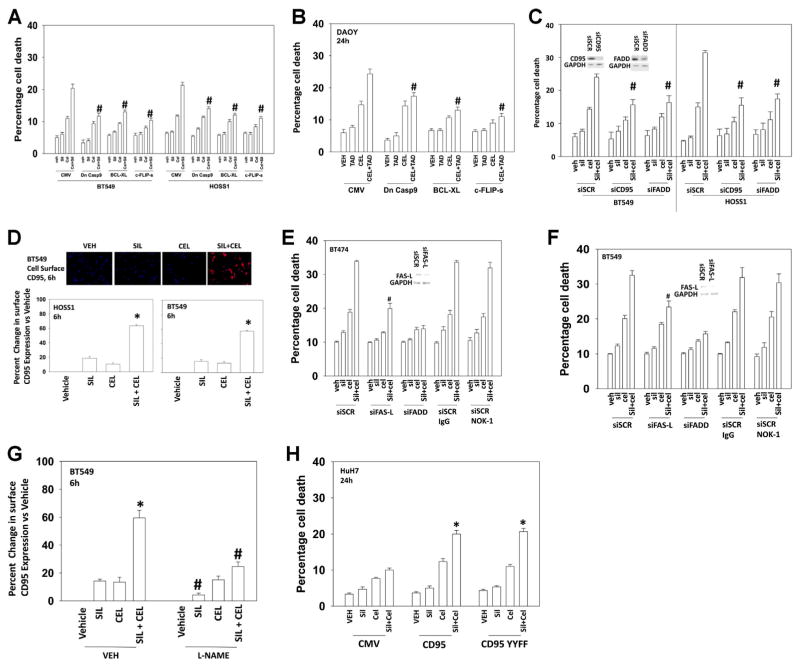
We next defined the cell survival regulatory pathways by which celecoxib and sildenafil interacted to kill tumor cells. Inhibition of the intrinsic apoptosis pathway by over-expression of BCL-XL or expression of dominant negative caspase 9 reduced cell killing (Figs. 4A and B). Of note, however was that, over-expression of the caspase 8 inhibitor c-FLIP-s also protected cells, suggestive that the extrinsic apoptosis pathway was being activated. In general, agreement with the hypothesis that the extrinsic pathway was being activated, knock down of the docking protein FAS associated death domain (FADD) or of the death receptor CD95 attenuated the toxic interaction between sildenafil and celecoxib (Fig. 4C). In further agreement with a role for CD95 in the killing process, drug combination exposure caused a rapid plasma membrane localization and clustering of CD95, indicative of receptor activation (Fig. 4D). Of particular note, whilst knock down of FAS-Ligand reduced killing by ~50% use of a neutralizing anti-FAS-L antibody had no impact on cell killing, despite the antibody being able to block exogenous FAS-L killing (Figs. 4E and F, data not shown). Thus, the mechanisms by which knock down of total FAS ligand expression can alter CD95 signaling remain to be resolved. Incubation of cells with the NOS inhibitor L-NAME prevented CD95 plasma membrane localization (Fig. 4G). There are multiple mechanisms by which CD95 can become activated including the actions of ceramides for receptor clustering and tyrosine phosphorylation for receptor oligomerization (Eberle et al., 2007; Park et al., 2010). To test whether tyrosine phosphorylation of CD95 played a role in sildenafil/celecoxib biology, we utilized HuH7 hepatoma cells that are CD95 null together with constructs to express either wild-type CD95 or a CD95 protein that lacks the two sites of regulatory tyrosine phosphorylation (CD95 Y232F Y291F) (Hamed et al., 2013). Regardless of tyrosine phosphorylation status, expression of CD95 facilitated the toxic interaction between sildenafil and celecoxib (Fig. 4H).
Fig. 4.
Celecoxib and sildenafil-induced lethality is suppressed by inhibition of the intrinsic and extrinsic apoptosis pathways. (A) and (B) BT549, HOSS1 and DAOY cells were infected with recombinant adenoviruses to express empty vector (CMV); dominant negative caspase 9; BCL-XL; or c-FLIP-s. Twenty-four hours after infection cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). #P <0.05 less than corresponding value in CMV infected cells. (C) BT549 and HOSS1 cells were transfected with a scrambled control siRNA (siSCR) or siRNA molecules to knock down expression of CD95 (siCD95) or FADD (siFADD). Thirty-six hours after transfection cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). #P <0.05 less than corresponding value in siSCR transfected cells. (D) BT549 and HOSS1 cells in 96 well plates were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Six hours after treatment cells were fixed to the plate and immunohistochemistry performed to determine the plasma membrane levels of CD95. The upper images obtained using the Hermes Wiscan imaging system is representative of the data. The intensity of CD95 immunostaining was determined using the Wisoft data analysis package (n =3 +/− SEM). *P <0.05 value greater than celecoxib treatment alone. (E) and (F) BT474 and BT549 cells were transfected with scrambled siRNA (siSCR) or siRNA molecules to knock down expression of FAS-L or FADD, as indicated. Thirty-six hours after transfection cells were treated with a control IgG antibody (10 mg) or with a neutralizing anti-FAS-L antibody (NOK-1). Cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). #P <0.05 less than corresponding value in siSCR transfected cells. (G) BT549 in 96 well plates were pre-treated with vehicle or L-NAME (1 μM). Cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Six hours after treatment cells were fixed to the plate and immunohistochemistry performed to determine the plasma membrane levels of CD95. The intensity of CD95 immunostaining was determined using the Wisoft data analysis package (n =3 +/− SEM). *P <0.05 value greater than celecoxib treatment alone; #P <0.05 less than corresponding value in vehicle-treated cells. (H) HuH7 cells were transfected with either an empty vector plasmid (CMV); a plasmid to express CD95-GFP or a plasmid to express CD95-GFP-Y232F Y291F (46,47). Twenty-four hours after transfection cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). *P <0.05 greater than corresponding value in CMV transfected cells.
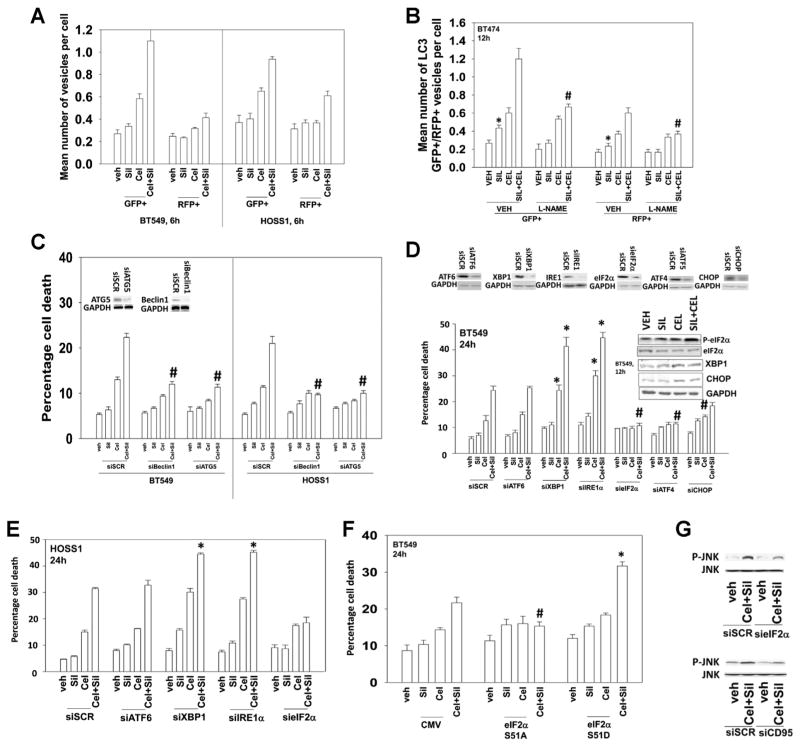
Studies from our laboratory using the non-coxib derivative of celecoxib, OSU-03012, have shown that this compound kills tumor cells through the induction of endoplasmic reticulum (ER) stress and autophagy (Yacoub et al., 2006; Park et al., 2008; Booth et al., 2012). Initial studies to examine autophagy in the present studies made use of cells transfected to express an LC3-GFP-RFP construct. Treatment of cells with sildenafil and celecoxib increased the numbers of early autophagosome GFP+ and late RFP+ autolysosome vesicles in cells suggestive that autophagic flux was being induced (Fig. 5A). Incubation of cells with the nitric oxide synthase inhibitor L-NAME suppressed the drug-induced induction of GFP+ and RFP+ vesicles (Fig. 5B). Knock down of the autophagy regulatory proteins Beclin1 or ATG5 abolished the induction of autophagy and significantly reduced the toxicity of sildenafil and celecoxib treatment (Fig. 5C, data not shown). We next examined the role of ER stress signaling in the cellular response to sildenafil and celecoxib treatment. Inhibition of the IRE1/XBP1 arm of the ER stress response increased the toxicity of sildenafil and celecoxib treatment (Figs. 5D and E). Inhibition of the eIF2α/ATF4 arm of the ER stress response almost abolished the lethality of sildenafil and celecoxib treatment; knock down of CHOP had a more modest effect at reducing cell killing. The data with eIF2α knock down correlated with the drug combination causing increasing phosphorylation of eIF2α (Fig. 5D, inset panel; see also data in Fig. 1). Expression of a dominant negative form of eIF2α (S51A) protected cells from sildenafil and celecoxib treatment toxicity whereas expression of an activated mutant of eIF2α (S51D) enhanced drug-induced killing (Fig. 5F). Inhibition of eIF2α or CD95 function (knock down) suppressed the activation of JNK caused by sildenafil and celecoxib treatment (Fig. 5G [cf data in Fig. 3F]).
Fig. 5.
Sildenafil and celecoxib increase autophagy and promote ER stress which regulate the cell killing process. (A). BT549 and HOSS1 cells were transfected with a plasmid to express LC3-GFP-RFP. Twenty-four hours after transfection cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Six hours after drug treatment cells were analyzed for the numbers of GFP+ and RFP+ intense staining vesicles per cell (n =3 +/− SEM). (B) BT474 cells were transfected with a plasmid to express LC3-GFP-RFP. Twenty-four hours after transfection cells were pre-treated with vehicle or the NOS inhibitor L-NAME (1 μM). After 30 min cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twelve hours after drug treatment cells were analyzed for the numbers of GFP+ and RFP+ intense staining vesicles per cell (n =3 +/− SEM). #P <0.05 less than corresponding value in vehicle treated cells; *P <0.05 greater than vehicle treatment. (C) BT549 and HOSS1 cells were transfected with a scrambled control siRNA (siSCR) or siRNA molecules to knock down expression of Beclin1 (siBeclin1) or ATG5 (siATG5). Thirty-six hours after transfection cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). #P <0.05 less than corresponding value in siSCR transfected cells. (D) and (E) BT549 and HOSS1 cells were transfected as indicated to with a scrambled siRNA (siSCR) or siRNA molecules to knock down expression of ATF6 (siATF6), XBP1 (siXBP1), IRE1α (siIRE1α), eIF2α (sieIF2α), ATF4 (ATF6), or CHOP (siCHOP). Thirty-six hours after transfection cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). #P <0.05 less than corresponding value in siSCR transfected cells *P <0.05 greater than corresponding value in siSCR transfected cells. Inset Panel: BT549 cells were treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM) and isolated after 12 h to determine the phosphorylation of eIF2α. (F) BT549 cells were transfected with an empty vector plasmid (CMV); a plasmid to express eIF2α S51A (dominant negative); a plasmid to express eIF2α S51D (activated). Twenty-four hours after transfection cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Twenty-four hours after drug treatment cells were isolated and viability determined by trypan blue exclusion assay (n =3 +/− SEM). #P <0.05 less than corresponding value in CMV transfected cells *P <0.05 greater than corresponding value in CMV transfected cells. (G) BT549 cells were transfected as indicated with a scrambled siRNA (siSCR) or siRNA molecules to knock down expression of CD95 (siCD95) or eIF2α (sieIF2α). Thirty-six hours after transfection cells were then treated with celecoxib (CEL 5.0 μM) and/or sildenafil (SIL, 2.0 μM). Six hours after drug treatment cells were isolated and subjected to SDS PAGE and immunoblotting to determine the phosphorylation of JNK.
The generation of ceramide species is known to play an important role in the activation of CD95 and JNK following exposure to a variety of stressful stimuli. We and others have previously shown that ceramide generation can mediate CD95 activation (Park et al., 2010). Indeed, inhibition of de-novo ceramide synthesis using fumonisin B1 reduced drug-induced activation of both CD95 and JNK, and suppressed killing by the drug combination (Figs. S3A and B). Intriguingly, 6 h after celecoxib exposure, dihydro-ceramide levels significantly increased (indicative of ceramide synthase 2/3/6 activation) (Fig. S3C) and dihydro-sphingosine-1-phosphate levels were decreased (Fig. S3D). Both of these changes in dihydro sphingolipids were at least partially prevented by pre-treatment with the nitric oxide synthase inhibitor L-NAME (Figs. S3C–H). However, of particular concern was that as a single agent, celecoxib increased the levels of sphingosine-1-phosphate (Fig. S3H). Knock down of ceramide synthase 6, but not ceramide synthase 2, reduced cell killing by the drug combination (Fig. S3I).
Based on our data in Fig. S3A–I, we determined whether treatment of [celecoxib + sildenafil] cells with clinically relevant FDA approved agents which also increase ceramide levels and reduce sphingosine-1-phosphate levels could further enhance the killing effect caused by [celecoxib + sildenafil]. FTY720 (Fingolimod, Gilenya) is an FDA approved drug for the treatment of multiple sclerosis that acts to suppress sphingosine-1-phosphate signaling processes as well as sphingosine-1-phosphate production and also causes an increase in ceramide levels (see data in Fig. 1). FTY720 can also act as a histone deacetylase inhibitor against HDAC1 and HDAC2 and prior studies from our laboratory have shown that HDAC inhibitors increase expression of CD95 (Park et al., 2010; Cruickshanks et al., 2013). Fenretinide (4HPR) is a much trialed synthetic chemical related to vitamin A that inhibits the desaturase enzyme which metabolizes dihydro-ceramides, and which has been shown to both concentrate in the breast and to reduce breast cancer relapse in pre-menopausal women (Formelli et al., 1993; Sabichi et al., 2003; Veronesi et al., 2006; Wang et al., 2008; Shin et al., 2012; Holliday et al., 2013). ATRA (all-trans retinoic acid) is also a vitamin A analogue that is a highly effective therapeutic agent in acute promyelocitic leukemia patients.
Treatment of mammary tumor cells with clinically relevant concentrations of FTY720 (~50 nM) rapidly increased the levels of multiple dihydro-ceramide species (Fig. S3J). At clinically relevant single agent concentrations that lacked individual toxicity, FTY720 or 4HPR significantly enhanced the lethality of [celecoxib + sildenafil] treatment in multiple tumor cell types (Figs. S3K–M). Similar data to that with FTY720 or 4HPR were also observed using ATRA (Fig. S3N).
FTY720 is known to act as an HDAC inhibitor, and prior studies from our group have demonstrated that multiple HDAC inhibitors can increase expression of CD95. In agreement with FTY720 being an HDAC inhibitor, the acetylation of histones in treated tumor cells was increased by the drug which was also associated with increased expression of CD95 (Fig. S3O). Knock down of HDAC1 and HDAC2, the HDACI targets of FTY720, also increased CD95 expression, and knock down of CD95 or incubation of cells with fumonisin B1 suppressed killing by FTY720 + celecoxib + sildenafil treatment (Fig. S3P). Similar data were obtained using 4HPR and ATRA (Figs. S3Q and R).
As noted for [celecoxib + sildenafil] treatment previously, treatment of cells with [celecoxib + sildenafil + FTY720] had a radiosensitizing effect (Fig. S3S). Finally, based on prior and present knowledge that FTY720 increases ceramide levels and that 4HPR inhibits ceramide degradation, we compared the ability of FTY720 and 4HPR treatment to enhance [celecoxib + sildenafil] toxicity. Combined treatment of tumor cells with FTY720 and 4HPR and with [celecoxib + sildenafil] resulted in profound levels of cell killing at times 9 h after exposure (Fig. S3T). Of note, both fumonisin B1 and myriocin prevented killing by [CEL/SIL/FTY] but myriocin only partially protected cells from [CEL/SIL/FTY/4HPR] (Fig. S3T).
We next determined whether our in vitro data with sildenafil and celecoxib and with [sildenafil + celecoxib] + FTY720 translated into animal model systems (Fig. 6). In our first series of studies, we injected 1 ×106 cells and pre-formed large mammary tumors in the fat pads of female athymic mice (~150 mm3). Animals were treated for 6 days with either vehicle, sildenafil, celecoxib or both drugs simultaneously. Treatment of animals with celecoxib and sildenafil significantly reduced the growth of tumors below that of celecoxib alone (Fig. 6A). In the second series of studies, we injected 3 × 106 cells and 4 days after injection animals were treated for 7 days with either vehicle, sildenafil, celecoxib or both drugs simultaneously. Treatment of animals with celecoxib and sildenafil significantly reduced the formation and growth of tumors below that of celecoxib alone (Fig. 6B). Similar data to that in Fig. 6A were obtained using triple negative BC cells treated with drugs for 5 days (Fig. 6C). We next injected 1 × 107 BT549 cells into the fat pad and 4 days after injection tumors of ~150 mm3 had formed, and animals were treated with either vehicle, [sildenafil + celecoxib], FTY720 or all three drugs simultaneously. Treatment of animals with celecoxib and sildenafil and FTY720 significantly reduced the growth of tumors below that of celecoxib and sildenafil alone (Fig. 6D). This was associated with increased animal survival (Fig. 6E). Similar data to that in breast cancer was observed in GBM (Fig. S3U). Of note, treatment of animals with higher doses of sildenafil and celecoxib and FTY720 above those used in tumor studies and for the same amount of time did not cause noticeable normal tissue toxicity as judged using H&E staining (Fig. S4). Thus sildenafil and celecoxib treatment safely suppresses BC and GBM tumor growth in vivo which is enhanced by the FDA approved multiple sclerosis drug FTY720 (Fingolimod, Gilenya).
Fig. 6.
Sildenafil and celecoxib interact to suppress mammary tumor growth in vivo. Panel A. BT474 cells (1 × 106) were injected into the 4th mammary fat pad of athymic mice and tumors permitted to form for 21 days until the mean tumor volume was ~150 mm3. Animals were segregated into groups with very similar mean tumor volumes. Animals were treated with vehicle (cremophore), sildenafil (5 mg/kg), celecoxib (10 mg/kg) or both drugs simultaneously for 5 days QD. Tumor volumes were measured every third day and the –Fold increase in tumor volume calculated (with Day 0 =1.00) (2 studies, 8 animals total per group +/− SEM) *P <0.05 lower than celecoxib alone value. Panel B. BT474 cells (3 × 106) were injected into the 4th mammary fat pad of athymic mice and 4 days after injection animals were randomly segregated into four groups. Animals were treated with vehicle (cremophore), sildenafil (5 mg/kg), celecoxib (10 mg/kg) or both drugs simultaneously for 7 days QD. Tumor volumes were measured beginning at Day 7 and then every second day and the actual increase in tumor volume calculated (with Day 0 =0 mm3) (2 studies, 8 animals total per group +/− SEM) *P <0.05 lower than celecoxib alone value. Panel C. BT549 cells (1 × 106) were injected into the 4th mammary fat pad of athymic mice and tumors permitted to form for 21 days until the mean tumor volume was ~150 mm3. Animals were segregated into groups with very similar mean tumor volumes. Animals were treated with vehicle (cremophore), sildenafil (5 mg/kg), celecoxib (25 mg/kg) or both drugs simultaneously for 5 days QD. Tumor volumes were measured every third day and the –Fold increase in tumor volume calculated (with Day 0 =1.00) (2 studies, 8 animals total per group +/− SEM) *P <0.05 lower than celecoxib alone value. Panel D. BT549 cells (1 × 107) were injected into the 4th mammary fat pad of athymic mice and tumors permitted to form until the mean tumor volume was ~150 mm3. Animals were segregated into groups with very similar mean tumor volumes. Animals were treated with vehicle (cremophore), (sildenafil (2.5 mg/kg) and celecoxib (2.5 mg/kg)) and/or FTY720 (0.05 mg/kg) simultaneously for 7 days QD. Tumor volumes were measured approximately every third day and the tumor volume calculated (with Day 0 =1.00) (2 studies, 8 animals total per group +/− SEM) *P <0.05 lower than celecoxib + sildenafil value. Panel E. Kaplan–Meir survival curves based on data presented in Panel D. #greater survival that vehicle-treated animals; ##greater survival than [celecoxib + sildenafil] treatment alone.
Discussion
The present studies were performed to determine whether clinically relevant PDE5 inhibitors interacted with the COX2 inhibitor celecoxib to kill cancer cells. In multiple tumor cell types, including anoikis resistant and stem cell-like cells, sildenafil (and tadalafil) interacted with celecoxib to promote killing in short-term assays and long-term colony formation assays. Knock down of PDE5 recapitulated the combinatorial effect of a PDE5 inhibitor when combined with celecoxib. PDE5 inhibitors are known to enhance cGMP and NO levels; inhibition of NOS enzymes by L-NAME prevented the killing interaction between sildenafil and celecoxib. Our data also argued that celecoxib was acting in a COX2-independent manner when combined with sildenafil to promote cell death. Thus celecoxib, in a COX2–independent fashion, promotes tumor cell killing in cells lacking PDE5 expression and does so in an NOS-dependent manner.
The molecular mechanisms by which sildenafil and celecoxib interact to kill were then investigated. Drug combination treatment tended to inactivate signaling pathways normally associated with cell survival (ERK, AKT, p70 S6K, mTOR, NFκB) and to activate pathways normally associated with cell killing (JNK, p38 MAPK). Based on the use of site specific RAS mutants and molecular tools, we noted that combined signaling by ERK and AKT, or activation of mTOR, was required to prevent the synergy of drug interaction. Of note, others have argued that NO-induced DNA damage through ATM can suppress mTOR function (Tripathi et al., 2013). Inhibition of p38 MAPK and to a greater extent JNK blocked cell killing by the combination. As noted above, L-NAME prevented the killing interaction between sildenafil and celecoxib, and incubation of cells with the NOS inhibitor L-NAME blocked JNK activation.
The cell death pathway(s) by which sildenafil and celecoxib interacted to kill were also determined. Inhibition of both the intrinsic and extrinsic apoptosis pathways reduced killing. The drug combination activated the death receptor CD95 in a tyrosine phosphorylation independent fashion and knock down of CD95 or FADD suppressed the toxic drug interaction. JNK activation, already shown to be a toxic signal, was CD95 dependent. And, activation of CD95 was suppressed by inhibition of NOS enzymes using L-NAME. Thus, celecoxib and sildenafil in an NOS-dependent fashion activate CD95 which plays a key role in the activation of JNK which signals towards cell death. Studies by others have shown that nitrosylation of CD95 at its regulatory tyrosine residues inhibits CD95 signaling (Reinehr et al., 2004). Others, however, have argued that NO signaling and CD95 activation can cooperate to induce cell death (Gandelman et al., 2013; González et al., 2013). The precise mechanism(s) by which NO signaling regulates CD95 function in our system in combination with celecoxib will require studies beyond the scope of the present manuscript.
In addition to activating the extrinsic apoptosis pathway, the drug combination also increased the numbers of autophagosomes and autolysosomes, that is, “autophagy,” in a time-dependent fashion that was inhibited by incubation of the cells with L-NAME. Inhibition of autophagy suppressed cell killing. NO signaling has been linked by others to the regulation of autophagy; in some primary non-transformed cells, NO can inhibit autophagy by inhibiting JNK signaling (Benavides et al., 2013; Shen et al., 2014). In some transformed cells, NO signaling can promote autophagy in an mTOR-dependent fashion which seems to play a role in tumor cell killing (Yu et al., 2012; Tripathi et al., 2013). We discovered that the drug combination inactivated mTOR, and expression of an activated form of mTOR suppressed killing, as well as autophagy as judged by GFP+ and RFP+ cells (unpublished data). The inactivation of mTOR was also blocked by incubation with L-NAME (unpublished data). Thus nitric oxide signaling plays a key role in both de-repressing a brake on toxic autophagy (mTOR) as well as promoting activation of the CD95/JNK apoptosis pathway.
The regulation of CD95 activation has been proposed to require tyrosine phosphorylation of the receptor but also the generation of ceramide species (e.g., Cremesti et al., 2001). In prior studies by our group using different therapeutic drug combinations, we have found that ceramide synthase 6-mediated generation of ceramide played an important role in CD95 activation and tumor cell killing (e.g., Park et al., 2010). Celecoxib and sildenafil treatment increased the levels of [C16:0, C24:1, C24:0] dihydro-ceramide species; indicative that ceramide synthases 2, 3 and 6 were activated. Pan inhibition of ceramide synthase activity using fumonisin B1 suppressed the activation of CD95 and JNK, and enhanced levels of autophagy. Knock down of ceramide synthase 6 protected cells from drug combination toxicity. In smooth and cardiac muscle cells, inhibition of ceramide synthase enzymes blocks nitric oxide toxicity (Rabkin, 2002; Pilane and LaBelle, 2004).
Recently, several studies have demonstrated that inhibitors of ceramide desaturase, the last enzyme in the de novo synthesis of ceramide, also elevate levels of dihydro-ceramide species and consequently induce cell killing and autophagy of diverse cancer cell lines, that is, the vitamin A analogue fenretinide (4HPR). And in addition, FTY720 is known to decrease sphingosine-1-phosphate signaling and to increase dihydro-ceramide levels. Our data demonstrated that either 4HPR, ATRA or FTY720 at clinically achievable concentrations could enhance the lethality of [celecoxib + sildenafil] treatment in vitro and in vivo. These findings suggest that the planned for clinical translation of the celecoxib and sildenafil concept should also possibly include a lagging arm of the trial in which the toxicity of a three drug combination with FTY720 (or ATRA) is determined. Unfortunately Novartis, the makers of Fingolimod, do not wish to support the oncologic translation of this drug due to its financial relevance to the company and the possible discovery of additional contraindications in cancer patients. As Fingolimod/Gilenya (standard of care, 0.5 mg/day per patient) costs ~$7,500/month, it is highly unlikely that our three drug combination approach will reach the clinical arena in the foreseeable future. Additional studies will also be required to determine whether ceramide synthase and desaturase enzyme activities are regulated by protein nitration or by celecoxib-induced ER stress signaling.
Celecoxib levels in human plasma and tissues after a 200–800 mg single dose can reach up to ~2.5–7.5 μM and it is evident that the drug at these concentrations has chemopreventative effects in patients. Although, the majority of celecoxib is albumin bound in plasma after a 200–800 mg single dose, the partitioning of celecoxib into multiple organs occurs rapidly and after 5 days of dosing the levels of celecoxib in the plasma and in tissues reach equilibrium at ~5–10 μM. This delivery and partitioning process is similar in concept to the delivery of paclitaxel using albumin as a carrier (i.e., Abraxane). As celecoxib has been safely dosed on a daily basis at up to 2,600 mg QD for 2 weeks our in vitro studies using 5 μM celecoxib will be well within the physiologically achievable range of free drug in a human tumor. Sildenafil has been safely administered to patients at doses of up to 200 mg, with peak plasma levels in the ~3 μm range and a bioavailability of ~40% (Nichols et al., 2002). Our data in Figure 1 would argue that at sildenafil concentrations as low as 0.5 μM, we can observe a significant interaction with celecoxib, again suggesting that we are within the physiologically achievable range of free drug. Similarly, using clinically achievable concentrations of 4HPR (200 nM), ATRA (150 nM) and FTY720 (50 nM) we were able to demonstrate drug interactions in vitro and in vivo. As FTY720 is known to concentrate in the brain (and lung and adipose tissue) our data strongly argue that the clinical translation of [celecoxib + sildenafil + FTY720] in mammary tissue in situ, NSCLC and GBM should be explored.
Celecoxib at much higher concentrations than used in the present studies has been reported to cause ER stress signaling (Tsutsumi et al., 2004; Kardosh et al., 2008; Chen et al., 2010). We noted that whilst celecoxib as a single agent at 5 μm did not strongly increase eIF2α phosphorylation the drug combination did increase the phosphorylation of eIF2α, and we then interrogated the three arms of the ER stress response as to whether they controlled the level of cell killing. Inhibition of the IRE1α/XBP1 arm of the stress response enhanced both celecoxib and (celecoxib + sildenafil) lethality whereas inhibition of the eIF2α/ATF4/CHOP arm of the response protected cells. Celecoxib at 10-fold higher levels, through an eIF2α/ATF4/CHOP pathway, has been proposed to increase expression of the death receptor DR5 (Oh et al., 2010). Others have shown celecoxib to increase CD95 levels (Kern et al., 2006). Thus, it is probable that our observed activation of death receptor signaling may be augmented in the long term by signaling by newly synthesized death receptors. Additional studies beyond the scope of this manuscript will be required to identify the proteins downstream of the IRE1α/XBP1 arm of the ER stress response that control the apoptotic threshold.
In conclusion, we have discovered that the approved NSAID celecoxib can interact with approved PDE5 inhibitors to kill a wide range of tumor cell types at physiologically relevant concentrations. In some Westerner European nations, it has frequently been difficult for cancer patients to obtain the latest most expensive standard of care therapeutics drugs due to Governmental committee decisions, for example, as previously reported for Velcade, Sorafenib, Abiraterone. The studies in this manuscript, particularly those combining the generic drugs Celebrex, Viagra and ATRA could thus become a cost-effective approach to treat cancer patients with a wide variety of solid malignancies in countries with limited financial resources. Based on the studies presented in this manuscript, it is hoped that clinical translation of the two drug [celecoxib + sildenafil] combination will occur in the near future.
Supplementary Material
Acknowledgments
Support for the present study was funded from PHS grants from the National Institutes of Health [R01-CA141704, R01-CA150214, R01-DK52825, R01-CA61774]. Thanks to Mrs. Grizzard for her support to the Dent lab and to the Betts family fund for support in the purchase of the Hermes Wiscan instrument. Thanks to the VCU TDAAC, Dr. G. Tye and Dr. W.C. Broaddus in the procurement and rapid delivery of fresh tumor tissue from the OR. We gratefully acknowledge the essential assistance of the VCU lipidomics/Metabolomics core in performing our ceramide and S1P analyzes, which is supported in part by funding from the NIH-NCI Massey Cancer Center support grant CA016059. We acknowledge that the basic histology of normal tissue sections was performed at the VCU Massey Cancer Center Macromolecule Core Facility sponsored in part with funding from NCI Cancer Core Grant P30 CA016059. PD is the holder of the Universal Inc. Chair in Signal Transduction Research. The authors have no conflicts of interest to report. This manuscript is black and white but red all over and is dedicated to the memory of Crazy Horse and Wor Jackie.
Abbreviations
- ERK
extracellular regulated kinase
- MEK
mitogen activated extracellular regulated kinase
- PI3K
phosphatidyl inositol 3 kinase
- MAPK
mitogen activated protein kinase
- ca
constitutively active
- dn
dominant negative
- CMV
empty vector plasmid or virus
- si
small interfering
- SCR
scrambled
- Ad
adenovirus
- TUNEL
Terminal deoxynucleotidyl transferase UTP nick end labeling
- VEH
vehicle
- SIL
sildenafil
- VAR
vardenafil
- TAD
tadenafil
- CEL
celecoxib
- PDE
phosphodiesterase
- NECRO
necrostatin 1
- FADD
Fas-associated death domain protein
- CD95
cluster of determinants 95
- AIF
apoptosis inducing factor
Footnotes
Conflict of interest: None.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
Literature Cited
- Bastos-Pereira AL, Lugarini D, Oliveira-Christoff Ad, Ávila TV, Teixeira S, do Pires A, Muscará MN, Cadena SM, Donatti L, Cristina da Silva, de Assis H, Acco A. Celecoxib prevents tumor growth in an animal model by a COX-2 independent mechanism. Cancer Chemother Pharmacol. 2010;65:267–276. doi: 10.1007/s00280-009-1031-8. [DOI] [PubMed] [Google Scholar]
- Benavides GA, Liang Q, Dodson M, Darley-Usmar V, Zhang J. Inhibition of autophagy and glycolysis by nitric oxide during hypoxia-reoxygenation impairs cellular bioenergetics and promotes cell death in primary neurons. Free Radic Biol Med. 2013;65:1215–1228. doi: 10.1016/j.freeradbiomed.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzziches R, Francomano D, Gareri P, Lenzi A, Aversa A. An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin Pharmacother. 2013;14:1333–1344. doi: 10.1517/14656566.2013.799665. [DOI] [PubMed] [Google Scholar]
- Booth L, Cazanave SC, Hamed HA, Yacoub A, Ogretmen B, Chen CS, Grant S, Dent P. OSU-03012 suppresses GRP78/BiP expression that causes PERK-dependent increases in tumor cell killing. Cancer Biol Ther. 2012;13:224–236. doi: 10.4161/cbt.13.4.18877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth L, Roberts JL, Cruickshanks N, Conley A, Durrant DE, Das A, Fisher PB, Kukreja RC, Grant S, Poklepovic A, Dent P. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol Pharmacol. 2014;85:408–419. doi: 10.1124/mol.113.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carón RW, Yacoub A, Li M, Zhu X, Mitchell C, Hong Y, Hawkins W, Sasazuki T, Shirasawa S, Kozikowski AP, Dennis PA, Hagan MP, Grant S, Dent P. Activated forms of H-RAS and K-RAS differentially regulate membrane association of PI3K, PDK-1, and AKT and the effect of therapeutic kinase inhibitors on cell survival. Mol Cancer Ther. 2005;4:257–270. [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Cao W, Yue P, Hao C, Khuri FR, Sun SY. Celecoxib promotes c-FLIP degradation through Akt-independent inhibition of GSK3. Cancer Res. 2011;71:6270–6281. doi: 10.1158/0008-5472.CAN-11-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ST, Thomas S, Gaffney KJ, Louie SG, Petasis NA, Schönthal AH. Cytotoxic effects of celecoxib on Raji lymphoma cells correlate with aggravated endoplasmic reticulum stress but not with inhibition of cyclooxygenase-2. Leuk Res. 2010;34:250–253. doi: 10.1016/j.leukres.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Choi DE, Jeong JY, Lim BJ, Chung S, Chang YK, Lee SJ, Na KR, Kim SY, Shin YT, Lee KW. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009;297:F362–F370. doi: 10.1152/ajprenal.90609.2008. [DOI] [PubMed] [Google Scholar]
- Chuang HC, Kardosh A, Gaffney KJ, Petasis NA, Schönthal AH. COX-2 inhibition is neither necessary nor sufficient for celecoxib to suppress tumor cell proliferation and focus formation in vitro. Mol Cancer. 2008;7:38. doi: 10.1186/1476-4598-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremesti A, Paris F, Grassmé H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- Cruickshanks N, Hamed HA, Booth L, Tavallai S, Syed J, Sajithlal GB, Grant S, Poklepovic A, Dent P. Histone deacetylase inhibitors restore toxic BH3 domain protein expression in anoikis-resistant mammary and brain cancer stem cells, thereby enhancing the response to anti-ERBB1/ERBB2 therapy. Cancer Biol Ther. 2013;14:982–996. doi: 10.4161/cbt.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, Park MA, Qureshi I, Lee R, Dent P, Kukreja RC. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci USA. 2010;107:18202–18207. doi: 10.1073/pnas.1006965107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle A, Reinehr R, Becker S, Keitel V, Häussinger D. CD95 tyrosine phosphorylation is required for CD95 oligomerization. Apoptosis. 2007;12:719–729. doi: 10.1007/s10495-006-0003-2. [DOI] [PubMed] [Google Scholar]
- Florio T, Arena S, Pattarozzi A, Thellung S, Corsaro A, Villa V, Massa A, Diana F, Spoto G, Forcella S, Damonte G, Filocamo M, Benatti U, Schettini G. Basic fibroblast growth factor activates endothelial nitric-oxide synthase in CHO-K1 cells via the activation of ceramide synthesis. Mol Pharmacol. 2003;63:297–310. doi: 10.1124/mol.63.2.297. [DOI] [PubMed] [Google Scholar]
- Flower RJ. The development of COX2 inhibitors. Nat Rev Drug Discov. 2003;2:179–191. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- Formelli F, Clerici M, Campa T, Di Mauro M, Magni A, Mascotti G, Moglia D, De Palo G, Costa A, Veronesi U. Five-year administration of fenretinide: Pharmacokinetics and effects on plasma retinol concentrations. J Clin Oncol. 1993;11:2036–2042. doi: 10.1200/JCO.1993.11.10.2036. [DOI] [PubMed] [Google Scholar]
- Francis SH, Busch JL, Corbin JD, Sibley D. CGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman M, Levy M, Cassina P, Barbeito L, Beckman JS. P2×7 receptor-induced death of motor neurons by a peroxynitrite/FAS-dependent pathway. J Neurochem. 2013;126:382–388. doi: 10.1111/jnc.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, Schroeder MA, James CD. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González R, Ferrín G, Aguilar-Melero P, Ranchal I, Linares CI, Bello RI, De la Mata M, Gogvadze V, Bárcena JA, Alamo JM, Orrenius S, Padillo FJ, Zhivotovsky B, Muntané J. Targeting hepatoma using nitric oxide donor strategies. Antioxid Redox Signal. 2013;18:491–506. doi: 10.1089/ars.2011.4476. [DOI] [PubMed] [Google Scholar]
- Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- Hamed HA, Yamaguchi Y, Fisher PB, Grant S, Dent P. Sorafenib and HDAC inhibitors synergize with TRAIL to kill tumor cells. J Cell Physiol. 2013;228:1996–2005. doi: 10.1002/jcp.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MA, Lange PA, Nakayama DK. Nitric oxide and cyclic guanosine monophosphate stimulate apoptosis via activation of the Fas-FasL pathway. J Surg Res. 2001;101:183–189. doi: 10.1006/jsre.2001.6257. [DOI] [PubMed] [Google Scholar]
- Holliday MW, Jr, Cox SB, Kang MH, Maurer BJ. C22:0- and C24:0-dihydroceramides confer mixed cytotoxicity in T-cell acute lymphoblastic leukemia cell lines. PLoS ONE. 2013;8:e74768. doi: 10.1371/journal.pone.0074768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh PS, Tsai HC, Kuo CH, Chan JY, Shyu JF, Cheng WT, Liu TT. Selective COX2 inhibition improves whole body and muscular insulin resistance in fructose-fed rats. Eur J Clin Invest. 2008;38:812–819. doi: 10.1111/j.1365-2362.2008.02026.x. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- Huang KH, Kuo KL, Ho IL, Chang HC, Chuang YT, Lin WC, Lee PY, Chang SC, Chiang CK, Pu YS, Chou CT, Hsu CH, Liu SH. Celecoxib-induced cytotoxic effect is potentiated by inhibition of autophagy in human urothelial carcinoma cells. PLoS ONE. 2013;8:e82034. doi: 10.1371/journal.pone.0082034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Song X, Hsu A, Chen C. Apoptosis signaling pathways mediated by cyclooxygenase-2 inhibitors in prostate cancer cells. Adv Enzyme Regul. 2001;41:221–235. doi: 10.1016/s0065-2571(00)00015-7. [DOI] [PubMed] [Google Scholar]
- Kardosh A, Golden EB, Pyrko P, Uddin J, Hofman FM, Chen TC, Louie SG, Petasis NA, Schönthal AH. Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma cell killing by bortezomib in combination with celecoxib or its non-coxib analogue, 2,5-dimethyl-celecoxib. Cancer Res. 2008;68:843–851. doi: 10.1158/0008-5472.CAN-07-5555. [DOI] [PubMed] [Google Scholar]
- Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H, Fleischer B, Trautwein C, Michalski C, Schulze-Bergkamen H, Friess H, Stremmel W, Krammer PH, Schirmacher P, Müller M. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006;66:7059–7066. doi: 10.1158/0008-5472.CAN-06-0325. [DOI] [PubMed] [Google Scholar]
- Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607–622. doi: 10.1016/j.bpg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komalavilas P, Shah PK, Jo H, Lincoln TM. Activation of mitogen-activated protein kinase pathways by cyclic GMP and cyclic GMP-dependent protein kinase in contractile vascular smooth muscle cells. J Biol Chem. 1999;274:34301–34309. doi: 10.1074/jbc.274.48.34301. [DOI] [PubMed] [Google Scholar]
- Kots AY, Bian K, Murad F. Nitric oxide and cyclic GMP signaling pathway as a focus for drug development. Curr Med Chem. 2011;18:3299–3305. doi: 10.2174/092986711796504646. [DOI] [PubMed] [Google Scholar]
- Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–1780. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- Mao JT, Roth MD, Fishbein MC, Aberle DR, Zhang ZF, Rao JY, Tashkin DP, Goodglick L, Holmes EC, Cameron RB, Dubinett SM, Elashoff R, Szabo E, Elashoff D. Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev Res (Phila) 2011;4:984–993. doi: 10.1158/1940-6207.CAPR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakishore R, Ravikiran Y, Rajapranathi M. Selective Cyclooxygenase Inhibitors: Current Status. Curr Drug Discov Technol. 2014;11:127–132. doi: 10.2174/1570163811666140127123717. [DOI] [PubMed] [Google Scholar]
- Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53:5S–12S. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YT, Liu X, Yue P, Kang S, Chen J, Taunton J, Khuri FR, Sun SY. ERK/ribosomal S6 kinase (RSK) signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J Biol Chem. 2010;285:41310–41319. doi: 10.1074/jbc.M110.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Häussinger D, Reinehr R, Larner A, Spiegel S, Fisher PB, Voelkel-Johnson C, Ogretmen B, Grant S, Dent P. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res. 2010;70:6313–6324. doi: 10.1158/0008-5472.CAN-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Yacoub A, Rahmani M, Zhang G, Hart L, Hagan MP, Calderwood SK, Sherman MY, Koumenis C, Spiegel S, Chen CS, Graf M, Curiel DT, Fisher PB, Grant S, Dent P. OSU-03012 stimulates PKR-like endoplasmic reticulum-dependent increases in 70-kDa heat shock protein expression, attenuating its lethal actions in transformed cells. Mol Pharmacol. 2008;73:1168–1184. doi: 10.1124/mol.107.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilane CM, LaBelle EF. NO induced apoptosis of vascular smooth muscle cells accompanied by ceramide increase. J Cell Physiol. 2004;199:310–315. doi: 10.1002/jcp.10464. [DOI] [PubMed] [Google Scholar]
- Piplani H, Rana C, Vaish V, Vaiphei K, Sanyal SN. Dolastatin, along with Celecoxib, stimulates apoptosis by a mechanism involving oxidative stress, membrane potential change and PI3-K/AKT pathway down regulation. Biochim Biophys Acta. 2013;1830:5142–5156. doi: 10.1016/j.bbagen.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Pyrko P, Kardosh A, Liu YT, Soriano N, Xiong W, Chow RH, Uddin J, Petasis NA, Mircheff AK, Farley RA, Louie SG, Chen TC, Schönthal AH. Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther. 2007;6:1262–1275. doi: 10.1158/1535-7163.MCT-06-0629. [DOI] [PubMed] [Google Scholar]
- Rabkin SW. Fumonisin blunts nitric oxide-induced and nitroprusside-induced cardiomyocyte death. Nitric Oxide. 2002;7:229–235. doi: 10.1016/s1089-8603(02)00115-5. [DOI] [PubMed] [Google Scholar]
- Ramer R, Walther U, Borchert P, Laufer S, Linnebacher M, Hinz B. Induction but not inhibition of COX-2 confers human lung cancer cell apoptosis by celecoxib. J Lipid Res. 2013;54:3116–3129. doi: 10.1194/jlr.M042283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr R, Görg B, Höngen A, Häussinger D. CD95-tyrosine nitration inhibits hyperosmotic and CD95 ligand-induced CD95 activation in rat hepatocytes. J Biol Chem. 2004;279:10364–10373. doi: 10.1074/jbc.M311997200. [DOI] [PubMed] [Google Scholar]
- Roberts JL, Conley A, Booth L, Cruickshanks N, Malkin M, Kukreja RC, Grant S, Poklepovic A, Dent P. IN PRESS PDE5 inhibitors enhance the lethality of standard of care chemotherapy in pediatric CNS tumor cells. Cancer Biol Ther. 2014 doi: 10.4161/cbt.28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russwurm M, Russwurm C, Koesling D, Mergia E. NO/cGMP: The past, the present, and the future. Methods Mol Biol. 2013;1020:1–16. doi: 10.1007/978-1-62703-459-3_1. [DOI] [PubMed] [Google Scholar]
- Saba N, Hurwitz SJ, Kono S, Yang CS, Zhao Y, Chen Z, Sica GL, Muller S, Moreno-Williams R, Lewis M, Grist W, Chen AY, Moore CE, Owonikoko TK, Ramalingam SS, Beitler JJ, Nannapaneni S, Shin HJ, Grandis JR, Khuri FR, Chen ZG, Shin DM. Chemoprevention of head and neck cancer with celecoxib and erlotinib: Results of a phase 1b and pharmacokinetic study. Cancer Prev Res (Phila) 2013 Oct 3; doi: 10.1158/1940-6207.CAPR-13-0215. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabichi A, Modiano M, Lee JJ, Peng YM, Xu MJ, Vallar H, Dalton W, Lippman S. Breast Tissue Accumulation of Retinamides in a Randomized Short-term Study of Fenretinide. Clin Cancer Res. 2003;9:2400–2405. [PubMed] [Google Scholar]
- Schiffmann S, Ziebell S, Sandner J, Birod K, Deckmann K, Hartmann D, Rode S, Schmidt H, Angioni C, Geisslinger G, Grösch S. Activation of ceramide synthase 6 by celecoxib leads to a selective induction of C16:0-ceramide. Biochem Pharmacol. 2010;80:1632–1640. doi: 10.1016/j.bcp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Schiffmann S, Maier TJ, Wobst I, Janssen A, Corban-Wilhelm H, Angioni C, Geisslinger G, Grösch S. The anti-proliferative potency of celecoxib is not a class effect of coxibs. Biochem Pharmacol. 2008;76:179–187. doi: 10.1016/j.bcp.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Shen C, Yan J, Erkocak OF, Zheng XF, Chen XD. Nitric oxide inhibits autophagy via suppression of JNK in meniscal cells. Rheumatology (Oxford) 2014;53:1022–1033. doi: 10.1093/rheumatology/ket471. [DOI] [PubMed] [Google Scholar]
- Shin KOI, Park NY, Seo CH, Hong SP, Oh KW, Hong JT, Han SK, Lee YM. Inhibition of sphingolipid metabolism enhances resveratrol chemotherapy in human gastric cancer cells. Biomol Ther (Seoul) 2012;20:470–476. doi: 10.4062/biomolther.2012.20.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh JW, Kazi JU, Li H, Thompson WJ, Weinstein IB. Celecoxib-induced growth inhibition in SW480 colon cancer cells is associated with activation of protein kinase G. Mol Carcinog. 2008;47:519–525. doi: 10.1002/mc.20409. [DOI] [PubMed] [Google Scholar]
- Song J, Chen Q, Xing D. Enhanced apoptotic effects by downregulating Mcl-1: Evidence for the improvement of photodynamic therapy with Celecoxib. Exp Cell Res. 2013;319:1491–1504. doi: 10.1016/j.yexcr.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Distinct roles for cyclooxygenases 1 and 2 in interleukin-1-induced behavioral changes. J Pharmacol Exp Ther. 2002;302:1031–1036. doi: 10.1124/jpet.102.036640. [DOI] [PubMed] [Google Scholar]
- Tang Y, Hamed HA, Cruickshanks N, Fisher PB, Grant S, Dent P. Obatoclax and lapatinib interact to induce toxic autophagy through NOXA. Mol Pharmacol. 2012;81:527–540. doi: 10.1124/mol.111.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi DN, Chowdhury R, Trudel LJ, Tee AR, Slack RS, Walker CL, Wogan GN. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc Natl Acad Sci USA. 2013;110:E2950–E2957. doi: 10.1073/pnas.1307736110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, Mizushima T. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 2004;11:1009–1016. doi: 10.1038/sj.cdd.4401436. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Mariani L, Decensi A, Formelli F, Camerini T, Miceli R, Di Mauro MG, Costa A, Marubini E, Sporn MB, De Palo G. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17:1065–1071. doi: 10.1093/annonc/mdl047. [DOI] [PubMed] [Google Scholar]
- Vosooghi M, Amini M. The discovery and development of cyclooxygenase-2 inhibitors as potential anticancer therapies. Expert Opin Drug Discov. 2014;9:255–267. doi: 10.1517/17460441.2014.883377. [DOI] [PubMed] [Google Scholar]
- Wang H, Maurer BJ, Liu YY, Wang E, Allegood JC, Kelly S, Symolon H, Liu Y, Merrill AH, Jr, Gouazé-Andersson V, Yu JY, Giuliano AE, Cabot MC. N-(4-Hydroxyphenyl) retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol Cancer Ther. 2008;7:2967–2976. doi: 10.1158/1535-7163.MCT-08-0549. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Ohashi K, Takeuchi K, Yamashita K, Yokoyama T, Tran QK, Satoh H, Terada H, Ohashi H, Hayashi H. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther. 2002;71:398–402. doi: 10.1067/mcp.2002.123554. [DOI] [PubMed] [Google Scholar]
- Werner U, Werner D, Pahl A, Mundkowski R, Gillich M, Brune K. Investigation of the pharmacokinetics of celecoxib by liquid chromatography-mass spectrometry. Biomed Chromatogr. 2002;16:56–60. doi: 10.1002/bmc.115. [DOI] [PubMed] [Google Scholar]
- Williams CS, Watson AJ, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–6051. [PubMed] [Google Scholar]
- Yacoub A, Park MA, Hanna D, Hong Y, Mitchell C, Pandya AP, Harada H, Powis G, Chen CS, Koumenis C, Grant S, Dent P. OSU-03012 promotes caspase-independent but PERK-, cathepsin B-, BID-, and AIF-dependent killing of transformed cells. Mol Pharmacol. 2006;70:589–603. doi: 10.1124/mol.106.025007. [DOI] [PubMed] [Google Scholar]
- Yu Y, Fan SM, Yuan SJ, Tashiro S, Onodera S, Ikejima T. Nitric oxide (•NO) generation but not ROS plays a major role in silibinin-induced autophagic and apoptotic death in human epidermoid carcinoma A431 cells. Free Radic Res. 2012;46:1346–1360. doi: 10.3109/10715762.2012.715369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.