Abstract
Aim
To measure the prevalence of abnormal rest perfusion in a population of consecutive patients with known hypertrophic cardiomyopathy (HCM) referred for cardiovascular MRI (CMR), and to assess any associations between abnormal rest perfusion and the presence, pattern, and severity of myocardial scar and the presence of risk factors for sudden death.
Materials and methods
Eighty consecutive patients with known HCM referred for CMR underwent functional imaging, rest first-pass perfusion, and late gadolinium enhancement (LGE).
Results
Thirty percent of the patients had abnormal rest perfusion, all of them corresponding to areas of mid-myocardial LGE and to a higher degree of segmental hypertrophy. Rest perfusion abnormalities correlated with more extensive and confluent LGE. The subgroup of patients with myocardial fibrosis and rest perfusion abnormalities (fibrosis+/perfusion+) had more than twice the incidence of episodes of non-sustained ventricular tachycardia on Holter monitoring in comparison to patients with myocardial fibrosis and normal rest perfusion (fibrosis+/perfusion–) and patients with no fibrosis and normal rest perfusion (fibrosis–/perfusion–).
Conclusions
First-pass perfusion CMR identifies abnormal rest perfusion in a significant proportion of patients with HCM. These abnormalities are associated with the presence and distribution of myocardial scar and the degree of hypertrophy. Rest perfusion abnormalities identify patients with increased incidence of episodes of non-sustained ventricular tachycardia on Holter monitoring, independently from the presence of myocardial fibrosis.
Highlights
-
•
30% of patients with HCM have perfusion abnormalities related to scar.
-
•
No rest perfusion abnormalities were observed in areas of viable myocardium.
-
•
Scar-related perfusion abnormalities were associated with the incidence of NSVT.
Introduction
Sudden cardiac death (SCD) and progressive left ventricular (LV) impairment are complications associated with hypertrophic cardiomyopathy (HCM).1 Interstitial fibrosis and scarring are linked with progressive LV dysfunction and are associated with SCD. Also abnormalities of myocardial perfusion are of importance in the pathophysiology of the disease. A few studies have used cardiovascular MRI (CMR) to describe LV perfusion abnormalities in patients with HCM, during adenosine stress2,3 or at rest.4–6 Abnormal stress perfusion has been related to the presence of microvascular disease. Conversely, abnormal rest perfusion has been connected to the degree of hypertrophy and the extent of fibrosis detected with late gadolinium enhancement (LGE).
The aims of the present study were to measure the prevalence of abnormal rest perfusion in a population of patients with HCM referred for CMR, and to define the relationship between rest perfusion abnormalities and the extent and pattern of LGE and the presence of risk factors for sudden death.
Materials and methods
This multicentre study involved 80 consecutive patients with HCM referred for CMR from dedicated HCM clinics at the Ospedale di Rivoli (Rivoli, Torino, Italy; n = 56) and at the Ospedale Cardinal Massaia (Asti, Italy; n = 24) on the date of their yearly routine clinical follow-up. Patients with contraindications to CMR or history of anaphylactic reactions and patients with atrial fibrillation were excluded.
Risk stratification
Four risk factors were used to stratify patients' risk for SCD: family history of HCM-related premature SCD, unexplained syncope, presence of severe LV hypertrophy (≥30 mm) and episodes of non-sustained ventricular tachycardia (NSVT) evaluated on an ECG-Holter performed on the enrolment visit (1 month before the CMR examination). Echocardiographic parameters were considered from the most recent available examination.
Image acquisition
CMR was performed according to standard protocols7,8 using a 1.5 T system (Siemens Avanto, Erlangen, Germany) with fast gradients (45 mT/m; 200 T/m/s slew-rate), using a 12-element cardiac phased-array coil and cardiac package (Siemens Syngo-VB13, Erlangen, Germany). Steady-state free precession loops [repetition time (TR) 3.6 ms, echo time (TE) 1.8 ms, flip angle 65°, 30 acquired cardiac phases, typical voxel size after adapting the field of view to the anatomy of the chest of the patient 1.7 × 1.4 × 8 mm] were acquired in LV long-axis and short-axis views for LV mass and function measurements.
First-pass perfusion imaging was performed during injection of a gadolinium contrast agent (gadobutrol; Gadovist, Schering AG, Berlin, Germany) at a dose of 0.1 mmol/kg of body weight of gadolinium administered with a power injector (Medrad Spectris Solaris, Medrad Europe BV, Maastricht, The Netherlands) at a rate of 5 ml/s, followed by 20 ml saline. Perfusion images were acquired using a saturation-recovery sequence (prepulse delay 100 ms; turbo fast low-angle shot) with an acquisition time of 172 ms per section, TR 1.5 ms, TE 0.99 ms, flip angle 12°, typical voxel size after adapting the field of view 1.7 × 2.6 × 10 mm. Perfusion series were acquired in three short-axis views covering the basal, mid-ventricular and apical segments and additionally in a four-chamber long-axis view when heart rate allowed it. The minimum duration of acquisition of the perfusion sequence was 1 min, corresponding to 45–90 dynamics depending on rest heart rate. Free breathing was permitted after first passage of the contrast agent and the remaining dose was administered after the completion of the perfusion sequence (up to a total of 0.2 mmol/kg of body weight). LGE was performed approximately 20 min after the second injection using an inversion-recovery spoiled gradient-echo sequence (TR 2.4 ms, TE 0.96 ms, flip angle 15°, typical voxel size after adapting the field of view 1.8 × 1.3 × 8 mm).
Image analysis
All images were analysed using a workstation (Argus; Siemens, Germany) by three of the authors whose joint opinion was reached in consensus. Segmentation into 16 myocardial segments9 was used to describe the findings. Wall thickness was measured in end-diastole at the point of maximum thickness of each segment. Basal and mid-ventricular segments were measured on short-axis views, apical segments were measured in four- and two-chamber long axis views, as appropriate. The endocardial and epicardial border of the LV were traced in each short-axis view in the end-diastolic and end-systolic frames, to measure LV global systolic function and LV mass. The area of the atria was measured in end-systole in the four-chamber views. Perfusion images were evaluated qualitatively by visual inspection. Abnormal rest perfusion was defined as a reduced wash-in of the contrast agent in relation to other parts of the myocardium, persisting for at least five heartbeats.2 The distribution of the perfusion defects was compared with the LV myocardial thickness and the distribution of LGE. LGE was evaluated visually (presence/absence; number of LGE positive segments). The pattern of LGE was scored according to Moon et al.10 Moreover, semi-automated quantification of LGE was performed according to published methods.11–13 LGE volume was computed based on a 5 SD threshold above the mean remote myocardial signal13 using dedicated software (CVI 42 version 4, Circle Cardiovascular Imaging, Calgary, Alberta, Canada).
Statistical analysis and ethics
Patients with abnormal rest perfusion were compared with patients with normal rest perfusion for the presence of risk factors, echocardiographic parameters, and CMR findings. Continuous data are presented as mean ± SD; dichotomous data are presented as numbers and percentages. The Kolmogorov–Smirnov test was used for distribution checks of continuous variables. Differences in baseline characteristics between groups were evaluated with unpaired Student's t-tests, Mann–Whitney U-tests or Fisher's exact tests as appropriate. All tests were two sided, and a value of p < 0.05 was considered statistically significant. The association between perfusion abnormalities and hypertrophy was assessed with logistic regression. The association between prevalence of abnormal rest perfusion and LGE, hypertrophy, and the association between LGE and hypertrophy were performed with mixed effects models. All statistical analyses were carried out using the statistical software packages SAS 9.2 (SAS Institute, NC, USA) and SPSS v.19 (SPSS, Chicago, IL, USA). This study complies with the Declaration of Helsinki and was conducted according to the standards set by the institutional ethics committee. No changes to the clinical protocol in use for the evaluation of patients with HCM were needed to carry out the study; therefore, no formal approval from the institutional ethics committee was sought. Informed consent was obtained from all patients.
Results
Abnormal rest perfusion was present in 24 patients (30%), involving 62 segments (2.6 segments per-patient among positives; range 1–10). Patients with normal and abnormal rest perfusion were identical for most demographics and echocardiographic characteristics after indexing for body surface area (BSA; Table 1). Patients with abnormal perfusion showed a higher indexed end-systolic volume, although the difference of LV ejection fraction was not significant (Table 2). All areas with abnormal rest perfusion showed LGE (Fig 1) with mid-myocardial localization. None of the patients showed any perfusion defects or LGE with patterns and distribution compatible with coronary artery disease.
Table 1.
Characteristics, demographics, and risk factors for sudden cardiac death of the study group and comparison between patients with abnormal and normal rest perfusion.
| All patients (n = 80) | Group 1 (abnormal rest perfusion, n = 24) | Group 2 (normal rest perfusion, n = 56) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 49.8 ± 18 | 45.9 ± 16 | 51.3 ± 18 | 0.250 |
| Male sex | 53 (70%) | 18 (82%) | 35 (65%) | 0.176 |
| Height, cm | 169.4 ± 10 | 174.1 ± 10 | 167.5 ± 9 | 0.010 |
| Weight, kg | 73.1 ± 15 | 77.7 ± 11 | 71.1 ± 16 | 0.082 |
| Body mass index, kg/m2 | 25.6 ± 4 | 25.6 ± 3 | 25.6 ± 5 | 0.926 |
| Body surface area, m2 | 1.9 ± 0 | 1.9 ± 0 | 1.8 ± 0 | 0.018 |
| New York Heart Association functional class | 1.4 ± 1 | 1.4 ± 1 | 1.3 ± 1 | 0.834 |
| Echocardiographic parameters | ||||
| Mitral regurgitation | 36 (47%) | 11 (50%) | 25 (46%) | 0.443 |
| LV outflow tract obstruction | 27 (35%) | 11 (50%) | 16 (30%) | 0.102 |
| Systolic anterior movement mitral valve | 25 (33%) | 9 (40%) | 16 (30%) | 0.601 |
| Echocardiographic ejection fraction | 57.3 ± 19 | 56.3 ± 20 | 57.7 ± 18 | 0.782 |
| Risk factors for sudden cardiac death | ||||
| Family history of HCM-related sudden cardiac death, % | 22.4 | 13.6 | 25.9 | 0.364 |
| Syncope, % | 11.8 | 9.1 | 13 | 1.000 |
| Non-sustained ventricular tachycardia (Holter monitoring), % | 22.4 | 40.9 | 14.8 | 0.030 |
| Severe hypertrophy (>30 mm) | 17.1% | 22.7% | 14.8% | 0.504 |
| Number of risk factors for sudden cardiac death | 0.8 ± 1 | 0.9 ± 1 | 0.7 ± 1 | 0.470 |
LV, left ventricular, HCM, hypertrophic cardiomyopathy.
Table 2.
Cardiovascular MRI (CMR) measurements of the study group and comparison between patients with abnormal and normal rest perfusion.
| All patients (n = 80) | Group 1 (abnormal rest perfusion, n = 24) | Group 2 (normal rest perfusion, n = 56) | p-Value | |
|---|---|---|---|---|
| Left ventricular function and mass | ||||
| Indexed left atrium (cm2/m2) | 16 ± 4 | 17 ± 5 | 15 ± 4 | 0.107 |
| Indexed right atrium (cm2/m2) | 12 ± 3 | 12 ± 2 | 11 ± 3 | 0.218 |
| Ejection fraction: left ventricle | 58.6 ± 9 | 55.4 ± 10 | 59.9 ± 9 | 0.069 |
| Indexed stroke volume (ml/beat/m2) | 45.4 ± 8 | 45.4 ± 8 | 45.3 ± 8 | 0.920 |
| Indexed end-diastolic volume: left ventricle (ml/m2) | 79.1 ± 15 | 84.6 ± 16 | 77.0 ± 15 | 0.070 |
| Indexed end-systolic volume: left ventricle (ml/m2) | 34.0 ± 13 | 39.2 ± 14 | 32.0 ± 12 | 0.038 |
| Indexed left ventricular wall mass (g/m2) | 102.3 ± 40 | 116.5 ± 46 | 96.6 ± 36 | 0.066 |
| Maximum left ventricular myocardial thickness | 23.0 ± 7 | 24.6 ± 7 | 22.4 ± 7 | 0.194 |
| Late gadolinium enhancement | ||||
| Presence of fibrosis | 64 (80%) | 24 (100%) | 40 (71%) | 0.003 |
| Number of segments with fibrosis | 3.4 ± 3 | 5.4 ± 3 | 2.6 ± 2 | 0.001 |
| Fibrosis pattern | 0.007 | |||
| Confluent | 39 (49%) | 18 (75%) | 21 (38%) | 0.002 |
| Diffuse | 14 (18%) | 3 (13%) | 11 (14%) | 0.441 |
| Mixed | 11 (14%) | 3 (13%) | 8 (14%) | 0.832 |
| Fibrosis % volume (5 SD above mean of normal myocardium) | 18 ± 15 | 28 ± 17 | 14 ± 12 | 0.001 |
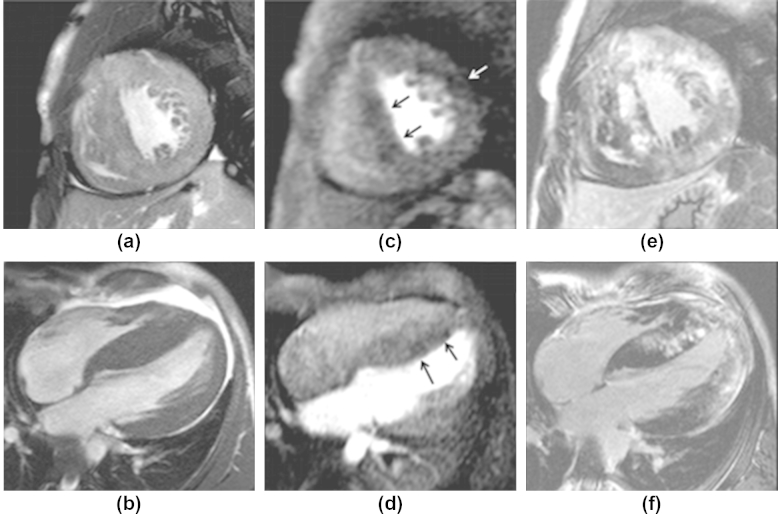
Figure 1.
Findings in a 35-year-old patient with severe hypertrophy. (a) Short-axis and (b) long-axis images showing the segments with maximum hypertrophy (the short axis cine sequence shown (a) was acquired soon after gadolinium injection, showing raised signal in correspondence with the areas of enhancement seen in (e). Perfusion images at peak enhancement of normal myocardium, showing rest perfusion abnormalities in short-axis (c) and long-axis (d), corresponding to areas of LGE (e–f).
Comparison between perfusion and LGE
Sixty-four patients (80%) showed areas of LGE, with a total of 270 positive segments (21.1%; 3.4 ± 2.8 segments/patient; range 0–12). LGE was present in all patients with rest perfusion abnormalities and in 71% of those with normal rest perfusion (p = 0.003; Table 2). The prevalence of LGE was associated with the distribution of hypertrophy (p < 0.001) and with the prevalence of perfusion abnormalities (p < 0.001) in mixed-effects models.
Patients with abnormal rest perfusion presented with a more extensive LGE in comparison with patients with normal rest perfusion on both visual assessment (5.4 ± 2.9 LGE positive segments, range 1–12 segments versus 2.6 ± 2.3 segments, range 0–9 segments, respectively; p = 0.001; Table 2) and quantitative assessment (28% ± 17% versus 14%±12%, respectively; p = 0.001; Table 2). Finally, patients with abnormal rest perfusion showed a significantly higher prevalence of a confluent pattern of LGE (75% versus 38%; p = 0.002; Table 2).
Comparison between perfusion and hypertrophy
The LV maximum thickness was 23 ± 7 mm (15–46 mm; patients with abnormal rest perfusion 16–40 mm; patients with normal rest perfusion 15–46 mm; p = 0.19), with a total of 442 hypertrophic segments of 1280 analysed (34.5%; 5.7 ± 3 per patient). The basal antero-septal segment showed the highest average maximal thickness (20.1 ± 7 mm), followed by the mid-ventricular infero-septal segment (18.5 ± 7 mm) and the anterior septum at mid-ventricular level (17.3 ± 8 mm). The localization of perfusion abnormalities was associated with the degree of hypertrophy in a mixed-effects model (p < 0.001).
Prevalence of risk factors for SCD
Seventeen patients (21%) had two or more risk factors for SCD, 23 patients (29%) had one risk factor, and 40 patients (50%) had no risk factors. The average number of risk factors per patient in the groups with abnormal and normal rest perfusion was not different from the average of the population (Table 1). An association was found between the presence of rest perfusion abnormalities and the prevalence of NSVT (p = 0.03). No association was found between the presence of rest perfusion abnormalities and any of the other considered risk factors (Table 1).
A higher prevalence of NSVT was also associated with the presence of significant hypertrophy (50% amongst patients with maximum LV thickness >30 mm and 16% amongst the others; p = 0.002) and with the volume of LGE both for 2 SD and 5 SD (p = 0.03 and p = 0.022, respectively).
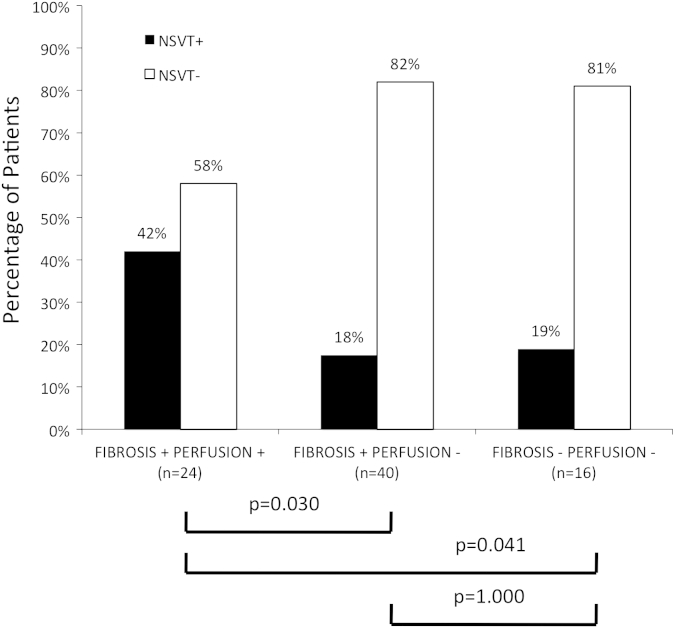
Fig 2 shows the percentage of patients with NSVT for three different patient categories based on the presence of scar tissue (fibrosis+/−) in combination with abnormal rest perfusion (perfusion+/−). Patients with fibrosis and rest perfusion abnormalities (fibrosis+/perfusion+) showed a prevalence of NSVT that was more than twice as high as in patients showing fibrosis with normal rest perfusion (fibrosis+/perfusion–; p = 0.03) and in patients without fibrosis and without rest perfusion abnormalities (fibrosis−/perfusion−; p = 0.04). Interestingly, no difference was found between patients with fibrosis and normal rest perfusion and patients without fibrosis and without rest perfusion abnormalities (p = 1.000).
Figure 2.
Percentage of patients with NSVT for three different groups based on the presence of absence of fibrosis (fibrosis+/fibrosis–) and rest perfusion abnormalities (perfusion+/perfusion–).
Discussion
The main findings of the present study are that (1) rest perfusion abnormalities are observed in 30% of patients with HCM; (2) rest perfusion abnormalities correlate with the severity of LGE and with the degree of hypertrophy; and (3) abnormal rest perfusion identifies a subgroup of patients with more advanced and clinically significant fibrosis.
The high prevalence of LGE in patients with HCM has been reported in many studies.11,12,14 LGE pattern and distribution also contribute to the differential diagnosis of HCM from other cardiomyopathies.15,16 LGE is associated with the degree of hypertrophy17,18 and has been associated with the occurrence of ventricular arrhythmias,19,20 progressive ventricular dilatation, markers of SCD,10 with all-cause and cardiac mortality,11 as well as with major cardiovascular events, hospitalization, heart failure, and arrhythmic events.12
In recent years, several authors have proposed the use of either rest or stress perfusion CMR for the assessment of patients with HCM. Matsunaka et al.4 was the first to describe a very high prevalence (75%) of rest perfusion abnormalities in a small selected population of 12 patients with HCM and related its presence to the distribution of LGE and the reduction of regional contractile function. The authors hypothesized a relationship between abnormal rest perfusion and the mechanical and anatomical effects of the increase in extracellular water fraction and the reduction in capillary density, respectively, observed in areas of LGE. In a different study, Soler et al.6 related abnormal rest perfusion (seen with a prevalence of 30%) to an impairment of systolic regional contraction. More recently, Melacini et al.5 demonstrated LGE and rest perfusion abnormalities in 46% of patients with HCM.
In the present study, abnormal rest perfusion was found in 30% of patients, in accordance with previous studies. To the authors' knowledge, the present study enrolled the largest population of patients so far. All perfusion abnormalities in the present cohort were spatially associated with regions of LGE. Perfusion defects were mostly associated with areas of confluent LGE. This likely represents an extreme degree of microvascular remodelling in areas of more dense scar,21,22 resulting in a slower inflow of contrast agent during first-pass.23 Accordingly, more severe fibrosis relates to more pronounced microvascular remodelling and to a higher probability of rest perfusion abnormalities. Quantitative LGE analysis for 5 SD threshold confirmed the significant correlation between rest perfusion abnormalities and volume of LGE. Therefore, based on the present results, rest perfusion abnormalities should not be considered an expression of myocardial ischaemia but rather a marker of severe myocardial fibrosis.
Although the present study focused on rest perfusion abnormalities, adenosine-induced perfusion abnormalities have also been reported in the literature.2,3 These are considered instead a marker of microvascular dysfunction and have also been associated in previous studies with the degree of hypertrophy. However, adenosine-induced perfusion abnormalities have a subendocardial localization that does not resemble the distribution of LGE,2 probably indicating a different underlying pathophysiological mechanism.
The possibility that severe myocardial fibrosis can cause perfusion abnormalities should, however, be considered when adenosine stress perfusion imaging is prescribed in patients with HCM to assess the LV ischaemic burden. Scar-related perfusion abnormalities and adenosine-induced perfusion abnormalities are, in fact, likely to coexist in a significant proportion of patients. However, the true ischaemic burden is likely to be better represented by adenosine-induced perfusion defects, without considering areas of abnormal perfusion correlated with severe myocardial fibrosis. Combined high-resolution quantification of stress perfusion and LV fibrosis could in future improve the assessment of patients with HCM and perhaps even enable the use of perfusion as a novel independent predictor of events.
Currently, risk stratification schemes identify only a limited proportion of patients at risk of SCD,1,24–28 which is the most threatening expression of the disease. LGE has been proposed as a method to improve risk stratification and CMR is being used in an increasing number of patients.29,30 However, the reported high prevalence of LGE among patients with HCM and the relatively low incidence of arrhythmic events at follow-up31 clearly delineates an important overlap between groups of patients with different degrees of myocardial damage and risk,32 underscoring the need to improve risk stratification beyond the mere presence or absence of LGE.33
The correlation between imaging findings and risk factors for SCD was examined in the present cohort population. Patients with abnormal rest perfusion and LGE had more than double the prevalence of NSVT when compared with the group of patients showing LGE and normal rest perfusion. The latter did not differ from the group of patients without LGE (Fig 2). As such, abnormalities of rest perfusion could be a useful marker to identify those patients with more severe microvascular remodelling, and severe and dense scarring. As rest perfusion examination is an easily applicable, rapid, and reproducible technique, it is feasible to obtain important information from its regular application at the expense of a trivial time delay.
Study limitations
The present findings are based on a population of patients with a clinical profile of low–intermediate risk. The validity of these findings in a population of high-risk patients with HCM should be further evaluated. Quantitative analysis of rest perfusion abnormalities was not performed but visual assessment was performed; the perfusion acquisition protocol was optimized for this purpose. A stress perfusion study was also not performed.
In conclusion, first-pass perfusion CMR can identify abnormal rest perfusion in a significant proportion of patients with HCM. These abnormalities are associated with areas of severe fibrosis and are most likely due to a reduction in the number of capillaries in areas of scar tissue. Adequately powered prospective studies relating the CMR findings, and in particular, the presence of rest perfusion abnormalities, to risk stratification would be needed in future.
Acknowledgements
The Centre of Excellence in Medical Engineering is funded by the Wellcome Trust and EPSRC under grant number WT 088641/Z/09/Z. The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London. K.B. received grant support from the Hellenic Society of Cardiology. A.C. received grant support from Philips Healthcare. E.N. received research grants from Phillips Healthcare and Bayer Schering Pharma. The other authors report no conflicts of interest. No further comments on Acknowledgments.
References
- 1.Maron B.J., McKenna W.J., Danielson G.K. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–1713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- 2.Petersen S.E., Jerosch-Herold M., Hudsmith L.E. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115:2418–2425. doi: 10.1161/CIRCULATIONAHA.106.657023. [DOI] [PubMed] [Google Scholar]
- 3.Sipola P., Lauerma K., Husso-Saastamoinen M. First-pass MR imaging in the assessment of perfusion impairment in patients with hypertrophic cardiomyopathy and the Asp175Asn mutation of the alpha-tropomyosin gene. Radiology. 2003;226:129–137. doi: 10.1148/radiol.2261011874. [DOI] [PubMed] [Google Scholar]
- 4.Matsunaka T., Hamada M., Matsumoto Y. First-pass myocardial perfusion defect and delayed contrast enhancement in hypertrophic cardiomyopathy assessed with MRI. Magn Reson Med Sci. 2003;2:61–69. doi: 10.2463/mrms.2.61. [DOI] [PubMed] [Google Scholar]
- 5.Melacini P., Corbetti F., Calore C. Cardiovascular magnetic resonance signs of ischemia in hypertrophic cardiomyopathy. Int J Cardiol. 2008;128:364–373. doi: 10.1016/j.ijcard.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Soler R., Rodríguez E., Monserrat L. Magnetic resonance imaging of delayed enhancement in hypertrophic cardiomyopathy: relationship with left ventricular perfusion and contractile function. J Comput Assist Tomogr. 2006;30:412–420. doi: 10.1097/00004728-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kramer C.M., Barkhausen J.R., Flamm S.D. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer C., Barkhausen J., Flamm S. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008;10:35. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerqueira M.D., Weissman N.J., Dilsizian V. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 10.Moon J.C.C., McKenna W.J., McCrohon J.A. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561–1567. doi: 10.1016/s0735-1097(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 11.Bruder O., Wagner A., Jensen C.J. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–887. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 12.O'hanlon R., Grasso A., Roughton M. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Flett A.S., Hasleton J., Cook C. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4:150–156. doi: 10.1016/j.jcmg.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury L., Mahrholdt H., Wagner A. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–2164. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 15.Hunold P., Schlosser T., Vogt F.M. Myocardial late enhancement in contrast-enhanced cardiac MRI: distinction between infarction scar and non-infarction-related disease. AJR Am J Roentgenol. 2005;184:1420–1426. doi: 10.2214/ajr.184.5.01841420. [DOI] [PubMed] [Google Scholar]
- 16.Matoh F., Satoh H., Shiraki K. Usefulness of delayed enhancement magnetic resonance imaging to differentiate dilated phase of hypertrophic cardiomyopathy and dilated cardiomyopathy. J Card Fail. 2007;13:372–379. doi: 10.1016/j.cardfail.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Amano Y., Takayama M., Takahama K. Delayed hyper-enhancement of myocardium in hypertrophic cardiomyopathy with asymmetrical septal hypertrophy: comparison with global and regional cardiac MR imaging appearances. J Magn Reson Imaging. 2004;20:595–600. doi: 10.1002/jmri.20172. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.J., Choi B.W., Hur J. Delayed enhancement in hypertrophic cardiomyopathy: comparison with myocardial tagging MRI. J Magn Reson Imaging. 2008;27:1054–1060. doi: 10.1002/jmri.21366. [DOI] [PubMed] [Google Scholar]
- 19.Kwon D.H., Smedira N.G., Rodriguez E.R. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol. 2009;54:242–249. doi: 10.1016/j.jacc.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Suk T., Edwards C., Hart H. Myocardial scar detected by contrast-enhanced cardiac magnetic resonance imaging is associated with ventricular tachycardia in hypertrophic cardiomyopathy patients. Heart, Lung Circ. 2008;17:370–374. doi: 10.1016/j.hlc.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 21.Camici P.G., Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 22.Moon J.C.C., Reed E., Sheppard M.N. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43:2260–2264. doi: 10.1016/j.jacc.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Conte M.R., Bongioanni S., Chiribiri A. Late gadolinium enhancement on cardiac magnetic resonance and phenotypic expression in hypertrophic cardiomyopathy. Am Heart J. 2011;161:1073–1077. doi: 10.1016/j.ahj.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Elliott P.M., Gimeno Blanes J.R., Mahon N.G. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–424. doi: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 25.Elliott P.M., Poloniecki J., Dickie S. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. doi: 10.1016/s0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 26.Maron B.J., Shen W.K., Link M.S. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365–373. doi: 10.1056/NEJM200002103420601. [DOI] [PubMed] [Google Scholar]
- 27.Olivotto I., Gistri R., Petrone P. Maximum left ventricular thickness and risk of sudden death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41:315–321. doi: 10.1016/s0735-1097(02)02713-4. [DOI] [PubMed] [Google Scholar]
- 28.Spirito P., Bellone P., Harris K.M. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 29.Maron M.S., Maron B.J., Harrigan C. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54:220–228. doi: 10.1016/j.jacc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Bongioanni S., Spirito P., Masi A.S. Extensive myocardial fibrosis in a patient with hypertrophic cardiomyopathy and ventricular tachycardia without traditional high-risk features. Circ Cardiovasc Imaging. 2009;2:349–350. doi: 10.1161/CIRCIMAGING.108.824839. [DOI] [PubMed] [Google Scholar]
- 31.Maron B.J., Olivotto I., Spirito P. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102:858–864. doi: 10.1161/01.cir.102.8.858. [DOI] [PubMed] [Google Scholar]
- 32.Kim R.J., Judd R.M. Gadolinium-enhanced magnetic resonance imaging in hypertrophic cardiomyopathy: in vivo imaging of the pathologic substrate for premature cardiac death? J Am Coll Cardiol. 2003;41:1568–1572. doi: 10.1016/s0735-1097(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 33.Chiribiri A., Conte M.R., Bonamini R. Late gadolinium enhancement and sudden cardiac death in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2011;57:1402. doi: 10.1016/j.jacc.2010.09.076. author reply1402–3. [DOI] [PubMed] [Google Scholar]