Abstract
Conventional allograft therapy for corneal scarring is widespread and successful, but donor tissue is not universally available, and some grafts fail owing to rejection and complications such as endothelial failure. We investigated direct treatment of corneal scarring using autologous stem cells, a therapy that, if successful, could reduce the need for corneal grafts. Mesenchymal cells were expanded from small superficial, clinically replicable limbal biopsies of human cadaveric corneo-scleral rims. Limbal biopsy–derived stromal cells (LBSCs) expanded rapidly in media containing human serum, were highly clonogenic, and could generate spheres expressing stem cell genes (ABCG2, Nestin, NGFR, Oct4, PAX6, and Sox2). Human LBSCs differentiated into keratocytes expressing characteristic marker genes (ALDH3A1, AQP1, KERA, and PTGDS) and organized a thick lamellar stroma-like tissue containing aligned collagen and keratan sulfate proteoglycans when cultured on aligned nanofiber substrata. When engrafted into mouse corneal wounds, LBSCs prevented formation of light-scattering scar tissue containing fibrotic matrix components. The presence of LBSCs induced regeneration of ablated stroma with tissue exhibiting lamellar structure and collagen organization indistinguishable from that of native tissue. Because the limbus can be easily biopsied from either eye of an affected individual and LBSCs capable of corneal stromal remodeling can be expanded under xeno-free autologous conditions, these cells present a potential for autologous stem cell–based treatment of corneal stromal blindness.
INTRODUCTION
The cornea is like a watch glass on the external surface of the eye, and its optical clarity is essential for vision. When the cornea is injured or inflamed, normally quiescent cells of the corneal stroma, the keratocytes, transform into fibroblasts to repair the damage by secreting scar tissue (1, 2). The presence of fibrotic extracellular matrix (ECM) components in stromal scars results in scatter of incident light, causing a deterioration of visual function (3, 4). Visual impairment and blindness owing to corneal scarring affect millions worldwide (5, 6) and is the commonest indication for corneal transplantation (penetrating keratoplasty) in the developing world (7). Penetrating keratoplasty is the most widespread organ grafting procedure and usually highly effective in improving vision (8); however, global demand for donor corneal tissue vastly exceeds its availability. Moreover, postoperative complications, particularly immune rejection, reduce the functional survival time for corneal allografts (9), especially in developing countries (10). For these reasons, there is increasing interest in the development of therapeutic alternatives to corneal transplantation, including stem cell therapy, cell-free collagen scaffolds, bioengineered constructs, and corneal prostheses (11–14).
The discovery of multipotent stem cells in the corneal stroma (15, 16) has opened up the possibility of developing a cell-based approach to treating blinding corneal stromal disorders. In a mouse model of corneal opacity, human stromal stem cells were effective in regenerating normal corneal ECM and repairing collagen fibril defects (17). The ability of mesenchymal stem cells to restore corneal transparency in lumican knockout mice was subsequently confirmed with umbilical cord stem cells (18). The presence of adult stem cells of mesenchymal lineage in human corneal stroma has been the subject of several reports (13, 19–21). Mesenchymal stem cells are immune-suppressant and therefore may have a role not only in remodeling but also in preventing inflammation, scarring, and immune rejection of transplanted tissue (22, 23).
The transitional region between optically clear cornea and opaque sclera, known as limbus, is the location of a population of epithelial stem cells that can be obtained by biopsy and expanded in vitro. Limbal epithelial cells, obtained from surface biopsy, have been used successfully for autologous treatment of limbal stem cell deficiency in clinical trials (24–28). Cultures from limbal biopsy procedures also contain a population of mesenchymal cells with properties similar to mesenchymal stem cells from bone marrow (21). Other studies also have identified mesenchymal stem cells in the limbal region of the corneal stroma (19–22). We have proposed that these reports all describe a single population of cells, the corneal stromal stem cells we have shown to differentiate to keratocytes and to regenerate stromal tissue in vivo (13). The implication that corneal stromal stem cells may be present in biopsy samples suggests their availability for autologous use.
Our current study addresses the hypothesis that mesenchymal cells obtained from limbal biopsy tissue from human corneas can be expanded in a culture medium containing autologous serum and can differentiate to keratocytes with the potential for use in cell-based therapy. To demonstrate this property, we used a therapeutic model in which human limbal biopsy–derived stromal cells (LBSCs) embedded in fibrin gel were applied to the surface of a healing murine debridement wound. We report that human stromal cells derived from limbal biopsies can not only differentiate to functional keratocytes in vitro but also induce regeneration of damaged stromal tissue in vivo, resulting in a matrix indistinguishable from that of native cornea.
RESULTS
Stromal cells can be obtained from limbal biopsies
Cells were obtained from donated human corneas from which a central button had been taken for penetrating keratoplasty. Tissue samples were excised in a manner simulating limbal biopsies used to obtain epithelial cells from living human patients (fig. S1) (24, 25, 29, 30). In initial trials, stromal cells were released by collagenase digestion with or without prior removal of epithelial cells, using the neutral protease dispase. Previously, we found that an initial dispase treatment yielded mesenchymal cell cultures free of epithelial cells (15). Isolation of cells using only collagenase (omitting dispase) produced primary cultures (P0) cells with both epithelial and mesenchymal morphologies (Fig. 1A). After expansion (P3), cells with epithelial morphology were lost, and cultures consisted of homogeneous small mesenchymal cells (Fig. 1A). Collagenase-only isolation of cells resulted in significantly faster proliferation of LBSC than dispase/collagenase isolation (Fig. 1B and table S1).
Fig. 1. Ex vivo expansion and clonogenicity of limbal biopsy stromal cells.
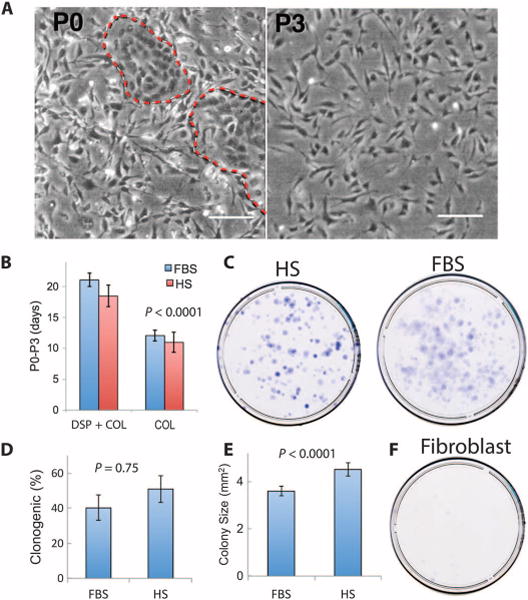
(A) Phase-contrast images of primary cells cultured from limbal biopsy tissue prepared by digestion with collagenase only. In initial plating (P0), red dashed lines mark islands of epithelial cells. Scale bars, 50 μm. (B) The length of time (in days) required to expand cells from P0 to P3 was compared for LBSCs prepared with dispase and collagenase (DSP + COL) or collagenase only (COL), expanding cells in either HS or FBS. Data are means ± SD (n = 4). P value determined by two-way analysis of variance (ANOVA). (C) Clonal growth of LBSCs in HS and FBS. (D) Percentage of clonogenic cells in P3 cultures. Data are means ± SD (n = 4). (E) Colony size was calculated with Fiji image analysis software. Data are means ± SD (n > 400). P values in (D) and (E) were determined by a two-sided t test. (F) Corneal fibroblasts in FBS did not exhibit clonal growth (n = 4).
LBSCs exhibit stem cell–like properties
Mesenchymal cells isolated from limbal biopsies were highly clonogenic irrespective of the culture conditions (Fig. 1C). About 40% of P3 cells grew as colonies from cells expanded in the presence of fetal bovine serum (FBS) or human serum (HS) (Fig. 1D and table S2). Cells cultured in FBS had significantly smaller colonies compared to clones in HS (Fig. 1E and table S3). Stromal fibroblasts, mesenchymal cells from central cornea, did not form colonies under identical conditions (Fig. 1F). When cultured under substratum-free conditions, LBSCs organized into free-floating spheres (fig. S2A). Under these conditions, LBSCs up-regulated expression of genes associated with both adult and pluripotent stem cells (ABCG2, Nestin, OCT4, PAX6, NGFR, and SOX2) (fig. S2B).
LBSCs differentiate into keratocytes in vitro
LBSCs cultured on a collagen gel substratum in ascorbate-containing, serum-free keratocyte differentiation medium (KDM) exhibited a marked decrease in the expression of the stem cell genes ABCG2 and Nestin and an increase in expression of the following genes associated with keratocyte differentiation: ALDH3A1, AQP1, KERA, and PTGDS (Fig. 2) (16, 31, 32). When LBSCs were cultured on a substratum of parallel, aligned nanofibers (14, 33) in KDM, the cells secreted a thick ECM of fibrillar collagen type I and keratan sulfate–containing proteoglycans (Fig. 3). The cells and collagen fibrils showed strong alignment with the nanofiber substratum, but collagen deposited 30 to 40 μm above the substratum exhibited an orientation rotated by about 40° with respect to the lower layers (Fig. 3A and movie S1). This rotation is similar to that of stromal lamellae in vivo, demonstrating a lamellar organization similar to that of corneal stroma. After 30 days in culture, the collagen construct was 35 to 40 μm in thickness (Fig. 3B), a value independent of the culture medium. Conditioned media pooled from cultures contained high molecular weight (>130 kD) keratan sulfate–containing proteoglycans, unique components of corneal ECM (Fig. 3C).
Fig. 2. Gene expression during ex vivo differentiation of LBSCs.
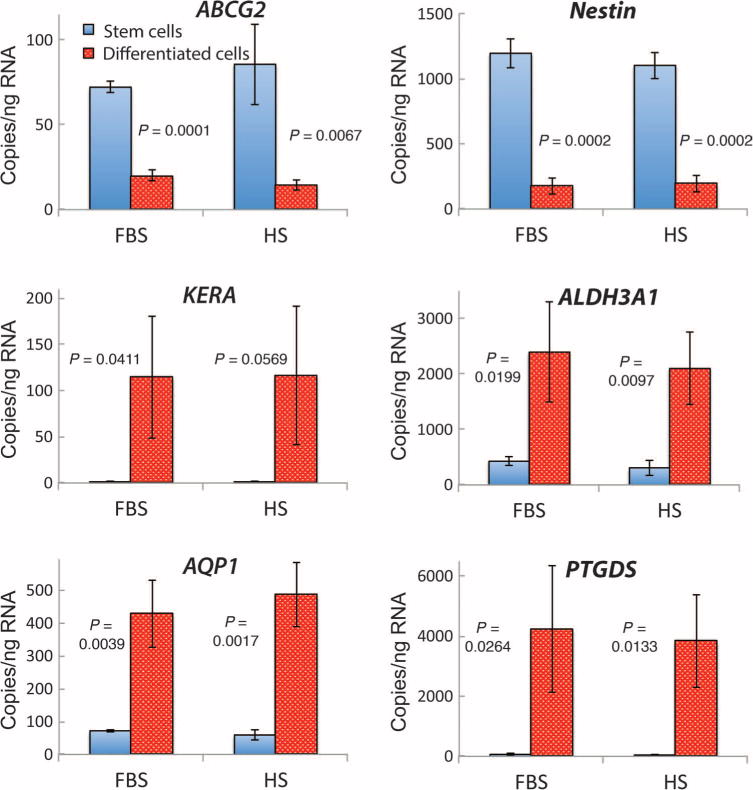
LBSCs expanded to P3 in FBS or HS were cultured in differentiation conditions on collagen gels for 1 week. mRNA was quantified as copies per nanogram of total cellular RNA, determined by quantitative polymerase chain reaction (qPCR). Data are averages ± SD from four different cell lines, each obtained from a different donor cornea. P values were determined by two-sided t test.
Fig. 3. Generation of a stroma-like three-dimensional matrix ex vivo.
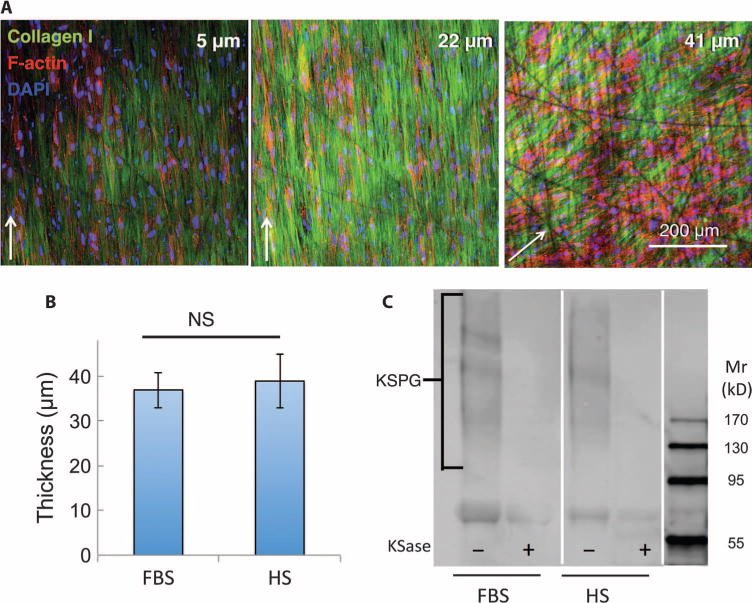
ECM produced by LBSCs cultured on aligned nanofiber substrate for 4 weeks was imaged by confocal microscopy capturing optical sections at different z levels above the substratum. (A) Type I collagen fibrils (green) and keratocytes (nuclei, blue; F-actin, red) are shown at different depths of the construct. (B) Thickness of collagenous matrix at 4 weeks in HS or FBS was determined from confocal analysis. Data in (B) show averages ± SD from cell lines from four different donors. Lack of significance (NS; P > 0.05) was determined by a two-sided t test. (C) Cornea-specific keratan sulfate proteoglycan (KSPG) was detected by immunoblotting. Alternate lanes show sensitivity of the heterogeneous KSPG band (130 to 300 kD) to keratanase (KSase). Mr, relative molecular mass.
Human LBSCs engraft in murine cornea in vivo
The ability of LBSCs to prevent and/or remediate corneal scarring was examined with a mouse corneal debridement model, which induces fibrotic matrix deposition and long-term disruption of the organization of the stromal collagenous ECM structure and produces visible stromal scars (23). At the time of wounding, 50,000 LBSCs were applied to the wound bed in a solution of fibrinogen, which gelled in response to thrombin (fig. S3). At 1 week after wounding, 3,3′-dioctadecyloxacarbocyanine (DiO)–labeled LBSCs remained in the cornea distributed throughout the wounded area (Fig. 4A and fig. S4). LBSCs remained in the anterior stroma for at least 4 weeks, during which time the average number of engrafted cells decreased by about half (fig. S4). During that time, no inflammation or rejection was observed in response to these cells. Four weeks after wounding, anterior stromal tissue subjacent to the corneal epithelium and near engrafted LBSCs contained human keratocan and type I collagen, components of normal transparent stromal ECM (Fig. 4B).
Fig. 4. LBSC engraftment and stromal matrix synthesis in mouse cornea in vivo.
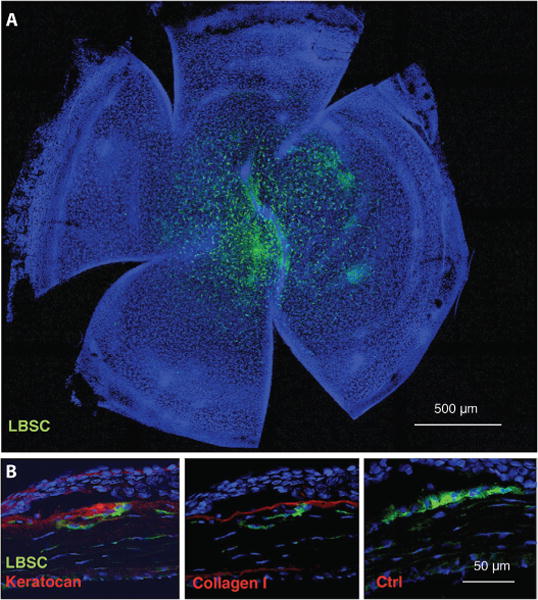
Fluorescent DiO-labeled human LBSCs were transferred to a superficially debrided mouse cornea in a fibrin gel as depicted in fig. S3. (A) One week after wounding, whole-mount staining showed persistence of the human LBSCs (green) in the central corneal region. (B) At 1 month, histological sections immunostained with human-specific antibodies show human keratocan and collagen type I. Omission of primary antibody controlled nonspecific staining. In all images, nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Anterior of the eye is oriented up in each image in (B), and the corneal epithelium is visible as a dense layer of cells near the top of each image. Ctrl, control.
LBSCs promote regeneration of native stromal tissue during wound repair
Wound repair in the corneal stroma typically results in the accumulation of a number of ECM components associated with light-scattering scar tissue, absent in normal stroma, including fibronectin, tenascin C, biglycan, hyaluronan, type III collagen, and SPARC (secreted protein acidic and rich in cysteine) (1, 34–38). In wounds allowed to heal without addition of LBSC (Fig. 5A, left panels), fibrotic markers hyaluronan, fibronectin, tenascin C, biglycan, and decorin were abundant in anterior stroma, indicating scar formation. In wounded corneas treated with LBSCs, only the proteoglycan decorin, a component of normal stromal matrix, was detected (Fig. 5A). Similarly, mRNAs for mouse type III collagen and SPARC were up-regulated 2 weeks after wounding in debrided corneas; however, the presence of LBSCs significantly reduced the up-regulation to levels similar to unwounded controls (Fig. 5B).
Fig. 5. LBSCs block deposition of fibrotic matrix in healing murine corneas.
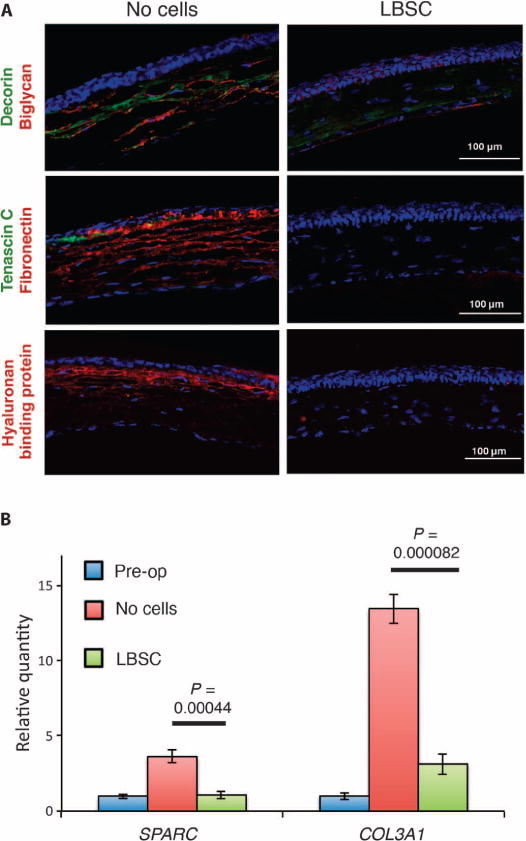
(A) Debridement-wounded mouse corneas were treated with fibrin gel only (no cells) or with 50,000 LBSCs in fibrin gel. After 4 weeks of healing, histological sections (epithelium oriented up) were stained for fibrotic markers decorin, biglycan, tenascin C, fibronectin, and hyaluronan. Images are representative of sections from three corneas for each condition. (B) Quantification of SPARC and type III collagen (COL3A1) mRNA pre-operative (Pre-op) and 2 weeks after treatment with LBSCs or no cells. Data are averages ± SD (n = 3). P values were determined by a two-sided t test.
Low-magnification photos of wounded corneas with diffuse lighting revealed the presence of visible scarring in all eyes that healed without LBSCs, whereas visible scars were absent in all eyes receiving LBSCs (Fig. 6A). Light scatter by corneal scars, a cause of reduced visual acuity, was assessed using spectral-domain optical coherence tomography (OCT). As shown in Fig. 6B, scatter in individual cross-sectional OCT scans was revealed as bright stromal regions in untreated corneas 2 and 4 weeks after debridement. Thresholding of these bright pixels in en face projections allowed a qualitative assessment of scar area and volume (Fig. 6C). Quantification of the thresholded images revealed a significant increase in light scatter in the untreated scars at both 2 and 4 weeks after wounding (Fig. 6D). In eyes treated with LBSCs at the time of wounding, however, light scatter was not increased compared to the preoperative normal corneas (Fig. 6D). Statistical analyses of the OCT data are presented in table S4.
Fig. 6. LBSC treatment influences light transmission properties of ECM deposited after debridement.
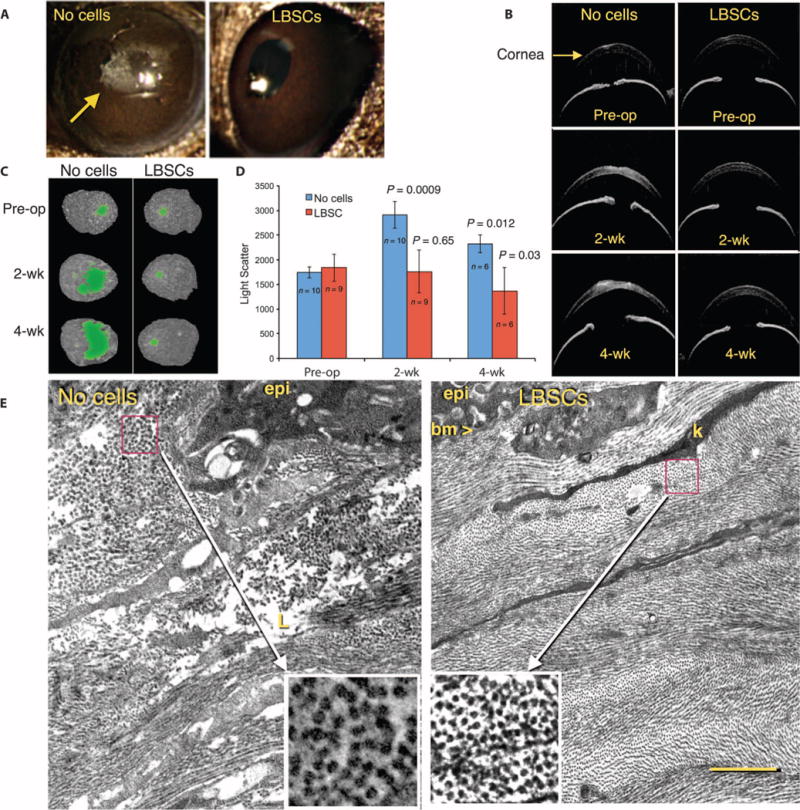
(A) Macroscopic images of mouse eyes in diffuse lighting reveal opaque scars (arrow) in untreated (no cells) eyes but none in LBSC-treated corneas. (B) OCT imaging shows transverse optical sections of preoperative (Pre-op) eyes and those 2 and 4 weeks after debridement, with scarring visible as bright pixels in the corneal stroma. (C) Thresholding of high-intensity pixels in three-dimensional (3D) OCT images of individual corneas defines scarred region (green) at 2 and 4 weeks after debridement. (D) Light scatter in 3D OCT scans at 2 and 4 weeks was compared with the values in preoperative eyes. Data are means ± SD. The number of eyes is indicated in the graph. P values were determined with unpaired t tests at each time point compared to respective Pre-op values (table S4). (E) Transmission electron micrographs 4 weeks after debridement show the ablated region of the anterior stroma. epi, epithelial cells; bm, basement membrane; k, keratocyte processes; L, amorphous matrix deposit (lake). Insets show magnification of a box (1 μm × 1 μm) from the indicated region containing orthogonal views of collagen fibrils. Scale bar, 2 μm. Images are representative of n = 3 animals.
Corneal transparency relies on a high level of ultrastructural organization of the collagenous ECM of the stroma (39, 40). It was therefore important to assess whether LBSC-treated eyes showed differences in collagen organization compared to wounds that did not receive stem cells. Transmission electron micrography of the healed untreated wounds showed the ablated region of the anterior stroma to be reconstituted with ECM typical of that of corneal scars (Fig. 6E, left panel). New ECM lacked characteristic lamellae organization. Collagen fibrils were large (inset) and typically not tightly packed or in parallel orientation, and the tissue contained amorphous deposits and empty regions known as lakes (41). In healed corneas treated with LBSCs, collagen organization in the stroma was essentially indistinguishable from that of native tissue (Fig. 6E). Collagen was organized into distinct layers (lamellae) containing small, uniform, tightly packed fibrils. Keratocyte processes were seen sandwiched between lamellae. Epithelial cells had elaborated a continuous basement membrane. Higher magnification of the fibrils (Fig. 6E, insets) showed fibril diameters in LBSC-treated tissue to be significantly smaller than those of the untreated scars [31.0 ± 2.9 nm versus 50.6 ± 6.6 nm, respectively (n = 28); P = 0.001, single-sample t test].
LBSC treatment reduced corneal vascularization in mice
Adult cornea is a nonvascularized tissue, and neovascularization in response to inflammation or trauma can represent a threat to vision. Immunostaining of healed corneas for CD31, a marker of vascular endothelial cells, revealed blood vessels in the central region of all (five of five) corneas that healed without addition of LBSCs (Fig. 7A). Conversely, three of five corneas that healed with LBSC treatment were free of central blood vessels (Fig. 7B). χ2 analysis of these results demonstrated a significant reduction in the vascularization in response to LBSC treatment (Fig. 7C).
Fig. 7. Vascularization of debridement wounds.
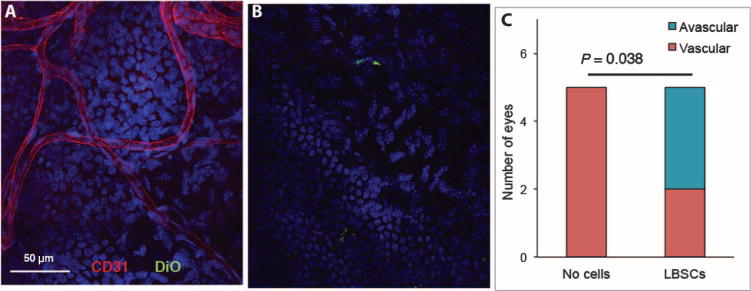
One month after wounding, whole-mount corneas were stained with antibody to CD31 (red) to detect ingrowth of blood vessels. Cell nuclei were imaged with DAPI (blue), and added human LBSCs appear green. (A) Vessels in a healed cornea in which no cells were added. (B) DiO-labeled LBSCs are visible (green), but no vessels were present in the central LBSC-treated wound. (C) A stacked bar graph shows the proportion of vascularized corneas in human LBSC-treated (n = 5) and untreated (n = 5) mouse eyes analyzed by staining as in (A). P value was obtained from a two-tailed χ2 test with a 2 × 2 contingency table.
DISCUSSION
Over the past decade, we have characterized multipotent mesenchymal stem cells in human corneal stroma that differentiate to keratocytes and elaborate a multilamellar collagenous ECM resembling that of cornea stroma (14–17, 31, 33). In vivo, these cells restore transparency to lumican knockout mice, which have hazy stromas and disorganized stromal collagen (17). The current study reveals two new properties of these cells. First, these cells can be obtained in a biopsy procedure that will permit autologous use. Second, we show that in an actively healing wound, these cells suppress accumulation of fibrotic scar tissue and promote regeneration of new native, transparent corneal ECM. Together, these findings present an argument for investigating clinical use of LBSCs to treat corneal scarring.
Our data demonstrate that corneal stromal stem cells can be obtained from a small surface ocular biopsy. In the current study, we emulated a biopsy procedure, but clinical use of such biopsies to obtain limbal epithelial stem cells is well established (24, 25, 29). The presence of mesenchymal cells in limbal biopsy tissue has been described (21). The current study confirms that mesenchymal stem cells obtained from limbal biopsies are functionally equivalent to corneal stromal stem cells we have previously described, on the basis of sphere-forming ability (fig. S2), gene expression patterns (Fig. 2), and the ability to organize stromal ECM in vitro (Fig. 3). The importance of this finding is that we are now able to use a clinically established procedure to obtain autologous stem cells with regenerative potential. These cells also grow rapidly in HS, thus allowing production of a fully autologous, xenobiotic-free cell-based reagent.
Recent reports have described mesenchymal stromal cells proximal to the epithelial basement membrane in limbal regions near epithelial stem cells. These mesenchymal “niche cells” are thought to help maintain the phenotype of limbal epithelial stem cells in vivo (42–44). Niche cells can form spheres in vitro and express stem cell genes, including ABCG2, SOX2, Nanog, OCT4, and Nestin (20, 43, 45, 46). LBSCs isolated in this study from human limbal biopsies closely resembled niche cells. They grew more rapidly when only collagenase was used in their isolation, and they were highly clonogenic, formed spheres, and expressed genes characteristic of both adult and pluripotent cells. By these criteria, LBSCs, corneal stromal stem cells, and limbal niche cells appear indistinguishable and probably represent the same population of neural crest-derived mesenchymal stem cells.
An important advance contributed by this study is observation that LBSCs induce deposition of a native stromal tissue rather than scar tissue in healing wounds. Previous in vivo studies have used lumican-null mice, which develop stromal haze owing to abnormal collagen fibrillogenesis (17, 18). Although the disruption of stromal matrix in these mice resembles that in scars, it is not evident that restoration of transparency to lumican-null corneas is fully analogous to remediation of corneal scars. In the current study, we found that human LBSCs in the healing wound allowed regeneration of a fully transparent native stromal tissue. The new tissue had no expression of fibrotic mRNA or proteins, had no change in light scatter, and had highly organized stromal ECM indistinguishable from that of normal mouse cornea. Few mammalian tissues heal by regenerating native tissue. Scarring provides a strong and rapid structural repair, but tissue functionality can be impaired by scar tissue. This is particularly true for cornea in which collagen fibril diameter, parallel alignment, packing, and lamellar organization are essential for vision. Traumatic damage to stroma typically heals, leaving scar tissue with disorganized collagen that scatters light and produces long-term disruption of vision. The ability of LBSCs to induce the replacement of ablated tissue with transparent ECM containing native components with collagen organization indistinguishable from that of normal stroma is an exciting finding that points to the clear potential for use of these cells in clinical applications to treat human corneal scars.
Here, LBSCs were engrafted only in the most anterior portion of the stroma and were in low abundance in comparison to the mouse stromal cells. Amelioration of fibrotic matrix, light scatter, and disruption of the stromal organization occurred both where LBSCs were located and in more posterior regions of the stoma. The lack of colocalization of LBSCs and scarring suggests that LBSCs exert their effects indirectly. Rather than simply replacing mouse stromal ECM with that produced by the differentiated LBSC, these cells are likely exerting a paracrine influence on the mouse stromal cells repopulating the wounded region. The mechanism of this regenerative effect is unknown. The ability of the stem cells to work at a distance may be an advantage in cell-based therapy of existing scars, in that it may not be necessary to saturate scar tissue with stem cells but rather only to deliver them proximal to the region needing to be regenerated. Whether existing scars can be treated with these cells remains an open question.
Much work over the previous decade has focused on corneal limbal epithelial stem cells for treatment of limbal stem cell deficiency, a potentially blinding but rare condition. Autologous limbal epithelial stem cells have been successfully used to correct this condition in several human trials (24–28, 47). Corneal epithelium and stroma are structurally and functionally distinct tissues, and epithelial stem cells are not suitable for stromal therapy. However, the positive results of pioneering clinical trials with epithelial stem cells and the data presented in our current study strongly support the idea that autologous mesenchymal stem cells (the LBSCs) may be successful in treating stromal scarring, the major cause of corneal blindness in the world. Because these cells can be obtained and cultured in an autologous, xenobiotic-free fashion and because fibrinogen-based adhesives are currently approved for ocular applications, barriers to bringing this treatment to clinical trial may be modest.
In summary, we have found a population of mesenchymal cells expanded from human limbal biopsy tissue with the potential to differentiate into keratocytes, to generate stromal tissue in vitro, to block corneal scarring, and to stimulate regeneration of transparent stromal tissue in murine healing wounds in vivo. The ability to obtain cells from clinically reliable biopsies and to expand them in HS presents the opportunity to use these cells clinically to remediate cornea wounds and scars.
MATERIALS AND METHODS
Study design
The purpose of this study was to determine if mesenchymal cells present in human corneal limbal biopsies (LBSCs) differentiate into corneal keratocytes in vitro and whether they can prevent corneal scarring in a murine model in vivo. In vitro, stem cell potential was assessed using clonogenic potential, sphere formation, and expression of stem cell genes. Keratocyte differentiation was examined by gene and protein expressions and by secretion of typical stromal ECM. In vivo, LBSCs were introduced into mouse corneal debridement wounds, and the effect on corneal transparency, fibrotic ECM expression, and ECM ultrastructural organization was assessed. Statistical analyses used two-sided t tests and two-way ANOVA. In vivo experiments were designed to provide a power of 0.8 on the basis of results from our previous study (23) with animals randomized as to treatment. Collection and analysis of in vivo data were carried out with observers masked as to treatment groups. No adverse events were observed, and no data points were excluded from analyses.
Limbal biopsy
Human corneo-scleral rims from donors younger than 60 years with less than 5 days of preservation were obtained from the Center for Organ Recovery and Education (www.core.org). Rims were rinsed twice in Dulbecco’s modified Eagle’s medium (DMEM/F12) containing gentamicin, penicillin, and streptomycin for 10 min each. As illustrated in fig. S1, under a dissecting microscope, residual conjunctiva was grasped with a toothed forceps, and subconjunctival dissection was carried out with Vannas scissors toward the anatomical limbus extending 0.5 mm into the clear cornea. Conjunctiva was excised, and dissection of anterior limbal tissue was carried out circumferentially in the same plane. An annular ring of tissue consisting of the superficial limbal epithelium and stroma with 0.5 mm of cornea and sclera on either side was excised. This was divided into equal halves, and each half was further divided into four equal segments (total of eight segments) ~4.5 mm each.
Isolation and culture of stromal cells
Four limbal segments were incubated in collagenase (0.5 mg/ml) (Sigma-Aldrich, type L) overnight at 37°C, without epithelial removal. The remaining four segments were first incubated for 40 min in Dispase II neutral protease (Zen-Bio) at 37°C. Epithelial cells were removed by gentle scraping, and the stripped limbal segments were incubated in collagenase similar to the first four segments. The digests were ticturated, incubated for an additional hour, and filtered through a 70-μm nylon screen to obtain a single-cell suspension. Cells obtained from each segment were seeded onto FNC (AthenaES)–coated wells of a 12-well tissue culture plate in stem cell growth medium (17) containing 2% (v/v) FBS or pooled HS (Innovative Research). Culture media were changed every other day, and cells were subcultured by brief digestion with TrypLE Express (Life Technologies) when 90% confluent into six-well plates, 25-cm2 T-flasks, and 175-cm2 T-flasks at P1, P2, and P3, respectively. Corneal fibroblasts were isolated by collagenase digestion of the peripheral 1- to 2-mm corneal rim left behind after excising the limbus and the sclera and expanded in DMEM/F12 with 10% FBS and used at P4 to P6 (15).
Clonogenic assay (fibroblast colony-forming units)
P3 cells were plated at a density of 250 cells in a 10-cm diameter dish (~5 cells/cm2) in the same media that were originally used to culture the cells. After 10 days, media were removed, and cells were fixed with cold methanol for 10 min and then stained with 0.1% crystal violet in 20% ethanol for 5 min. Colonies of >50 cells were evaluated as to size and number with Fiji (ImageJ) software. Colony counts were determined in duplicate for cell lines derived from four donor corneas (n = 8). Diameter of colonies in four dishes was determined for each condition (n > 400).
Sphere formation
P3 LBSCs were placed in six-well plates coated with poly(2-hydroxyethyl methacrylate) (polyHEMA) (48) at a density of 3000 cells per well in sphere medium [Advanced DMEM (containing GlutaMAX, Gibco, 12491), B27 (1:50), basic fibroblast growth factor (10 ng/ml), epidermal growth factor (20 ng/ml), gentamicin (50 μg/ml), streptomycin (100 μg/ml), and penicillin (100 IU/ml)]. The individual spheres were photographed and collected for RNA analysis on day 7.
Differentiation to keratocytes
P3 LBSCs were cultured on collagen gel–coated 12-well plates at a density of 100,000 cells per well. After 24 hours, medium was changed to KDM consisting of Advanced DMEM (containing GlutaMAX, Gibco, 12491), ascorbate-2-phosphate (1 mM), fibroblast growth factor–2 (10 ng/ml), and transforming growth factor – β3 (0.1 ng/ml) (14). One week later, the gel was digested with 0.5 ml of collagenase (2 mg/ml, Sigma-Aldrich, type L). Cells were collected by centrifugation and lysed in RLT buffer (Qiagen) for RNA isolation.
Gene expression before and after differentiation
RNA isolated by Qiagen Miniprep was transcribed into complementary DNA with SuperScript III (Life Technologies) and analyzed for expression of stem cell (ABCG2 and Nestin) and keratocyte (ALDH, AQPR1, CHST6, Keratocan, and PTGDS) gene markers with procedures and primers described previously (16, 17, 31). Assessment of mRNA copy numbers was carried out using standard curves based on linearized plasmids containing the amplified sequences (31).
Production of stromal matrix
P3 cells were plated on 15-mm-diameter poly(ɛ-caprolactone) aligned nanofiber inserts (Nanofiber Solutions, no. 242402) in a 24-well plate at a density of 20,000 cells per well. After 24 hours in stem cell growth medium, cultures were shifted to KDM. The medium was changed every 3 days for 1 month, after which the tissue was fixed in paraformaldehyde and immunostained (as below) for collagen I, for F-actin with phalloidin, and for nuclear DNA with DAPI (14, 33). Stained constructs were imaged with an Olympus FluoView 1000 confocal microscope with a 20× oil immersion objective. Z-stack images captured optical sections throughout the construct and were used to determine construct thickness. Proteoglycans isolated from the culture medium were separated by polyacrylamide gel electrophoresis and detected by immunoblotting with antibody J19 to detect keratan sulfate, as described previously (14).
Immunostaining
Immunostaining of mouse tissue was carried out on 8-μm cryostat sections fixed overnight in 3% paraformaldehyde and blocked with 10% heat-inactivated goat or donkey serum in phosphate-buffered saline (PBS). Primary antibody was incubated at 4°C overnight. Primary antibodies used were as follows: human keratocan [1:150, a gift from C.-Y. Liu (49)], human-specific collagen I (1:100, Sigma, C2456), decorin (1:100, Santa Cruz Biotechnology, sc-22753), biglycan (1:80, R&D Systems, AF2667), fibronectin (1:80, Abcam, ab26245), or tenascin C (10 mg/ml, R&D Systems, MAB 2138). To detect hyaluronan, tissue was fixed in ice-cold 4% paraformaldehyde, 70% ethanol, and 5% glacial acetic acid (v/v) for 10 min. After being washed three times in PBS, slides were blocked with an Endogenous Biotin-Blocking Kit (Life Technologies, E-21390) and stained with biotinylated hyaluronan-binding protein (1:100, Millipore, 385911) overnight at 4°C. Slides were then washed three times in PBS and stained with Alexa Fluor 546-conjugated streptavidin (Life Technologies, S-11225) at 1:1000 for 2 hours at room temperature. Slides were subsequently washed three times in PBS before staining with secondary antibody and DAPI for 2 hours at room temperature. Slides were imaged with an Olympus FluoView 1000 confocal microscope with a 20× or 40× oil objective.
Corneal debridement
All animal procedures were done in accordance with The Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Debridement procedures were done as previously described (23). Briefly, 7-week-old female C57BL/6 mice in randomized groups of 6 to 10 were anesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). Our previous study and power analysis determined that at least six eyes were required for statistical significance in OCT analysis and that 2 and 4 weeks provided appropriate time points for analysis of gene expression and fibrosis (23). One drop of proparacaine hydrochloride (0.5%) was added to each eye before debridement. Corneal epithelial debridement was performed by passing an AlgerBrush II (The Alger Company) over the central 2 mm of the mouse cornea. Once the epithelium was removed, a second application of the AlgerBrush II was used, this time applying more pressure to remove the basement membrane and 10 to 15 mm of anterior stromal tissue. See fig. S3 for a schematic of this procedure. Immediately after the procedure, mice received ketoprofen (3 mg/kg) for analgesia.
Fibrin gel and LBSC application
LBSCs expanded in HS at 1 × 106 cells/ml in DMEM/F-12 were stained with Vybrant DiO (50 μg/ml) (Life Technologies) for 20 min at 37°C. The cells were washed twice with DMEM/F-12 and resuspended in a solution of human fibrinogen, 20 mg/ml in PBS at 2.5 × 107 cells/ml. This concentration was determined to be the maximum number of cells that was retained on the corneal surface during healing. The relationship between cell number and biological efficacy was not investigated. After wounding, 0.5 ml of thrombin (100 U/ml, Sigma) was added to the wound bed, followed immediately by 1 ml of fibrinogen (with or without LBSCs). After 1 to 2 min, the fibrinogen had gelled, and a second round of thrombin and fibrinogen was added. The wound was irrigated with sterile PBS, and a drop of gentamicin ophthalmic solution (0.3%) was added. The corneal epithelium closed the wound in 24 to 36 hours. Immunostaining was performed after 4 weeks, as described above. Eyes were examined daily for signs of rejection and infection for 1 week and weekly thereafter.
Optical coherence tomography
OCT was performed weekly to quantify light scatter, the cause of reduced vision in corneal scars (3, 4). Mice were anesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg), and eyes were scanned with a Bioptigen SDOCT (Bioptigen). Animals were randomized as to order of analysis, and scanning data were collected and analyzed in a masked fashion regarding experimental treatment of the animals. Image processing and analysis were conducted with ImageJ [National Institutes of Health (NIH)] and MetaMorph 7.7.3 (Molecular Devices Inc.). For quantification of corneal light scatter, Imaris (Bitplane USA) was used to isolate the cornea from the lens, iris, and interfering eyelashes. Pixel intensity measurements were taken both of the cornea and of the background with ImageJ and exported to Excel (Microsoft Corp.), where the average background pixel intensity was subtracted from the average corneal pixel intensity. For generating threshold images, corneal epithelium was removed with MetaMorph to isolate only the corneal stroma. Control eyes were used to set the threshold, and scar volume beyond the control threshold was displayed graphically.
Transmission electron microscopy
Three corneas per group were analyzed by transmission electron microscopy. The corneas were processed as described previously (17, 23). Briefly, tissue was fixed immediately postmortem in fresh 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate (pH 7.4), and 8 mM CaCl2, followed by postfixation with 1% osmium tetraoxide. After dehydration in an ethanol series followed by propylene oxide, corneas were infiltrated and embedded in a mixture of EMbed 812, Nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 [2,4,6-tri(dimethylaminomethyl) phenol] (Electron Microscopy Sciences). Thin sections were cut with a Leica Ultracut UCT ultramicrotome equipped with a diamond knife and were stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid (pH 3.2). Anterior and posterior stroma regions of sections from central cornea were captured by investigators masked to the experimental treatment of the eyes. Micrographs were captured at 80 kV with a JEOL-1400 transmission electron microscope (JEOL Ltd.) equipped with a Gatan Orius wide-field side mount charge-coupled digital camera (Gatan Inc.). LBSC-treated eyes were fully sectioned and serially screened to confirm the absence of scarring.
Statistical analyses
LBSCs were isolated from four separate donor eyes, so data in Figs. 1, 2, 3, and 6 and fig. S2 present mean values of four biological replicates. P values for differences were determined by double-sided nonparametric t tests or ANOVA with Tukey’s post hoc analysis, as indicated in figure captions, with GraphPad Prism software. Differences were considered significant when P < 0.05. Error bars show SD of the mean unless otherwise indicated in the figure legend. Determination of differences in vascularization in Fig. 7, used the χ2 test on a 2 × 2 contingency table to determine two-tailed P values.
Supplementary Material
Fig. S1. Ex vivo model of corneal limbal biopsy.
Fig. S2. Sphere formation and stem cell–gene expression by LBSCs.
Fig. S3. Murine corneal debridement model.
Fig. S4. Persistence of engrafted LBSCs in the stroma.
Table S1. Time to reach passage 3 by primary LBSCs under different conditions.
Table S2. Clonogenic potential of LBSCs in different sera.
Table S3. Area of colonies cultured in different sera.
Table S4. OCT analysis of debrided mouse corneas.
Movie S1. Organization of matrix deposited ex vivo by LBSCs.
Acknowledgments
We thank E. K. Steer for assistance in creating schematics used in this publication, M. Geary for assistance with animal work, K. Davoli for assistance with histology, and G. Wollstein for assistance with OCT imaging.
Funding: This work was supported by NIH grants EY016415 (to J.L.F.) and EY05129 (to D.E.B.); NIH Core grant P30-EY08098; and grants from Louis J. Fox Center for Vision Restoration (Pittsburgh), by Hyderabad Eye Research Foundation (Hyderabad, India), and from Research to Prevent Blindness Inc. M.K.B. is a Howard Hughes Medical Research Fellow. A.J.H. was supported by NIH grant T32-EY017271.
Footnotes
Author contributions: S.B. participated in project design, developed the biopsy technique and cell culture methods, analyzed cell phenotype and clonogenicity, carried out surgery, evaluated data, and participated in the writing and proofreading of the manuscript. A.J.H. participated in project design, assisted in surgery, captured OCT images, carried out immunostaining, evaluated data, and participated in the writing and proofreading of the manuscript. M.L.F. maintained records, set up PCR assays, evaluated PCR data, and participated in the writing and proofreading of the manuscript. M.K.B. assisted with surgery, collection, and analysis of OCT data. M.M.M. isolated proteoglycans and carried out immunoblotting experiments. Y.D. consulted on corneal stem cell culture, participated in experimental design and analysis, assisted in animal surgery, and contributed to the writing and proofreading of the manuscript. K.L.L. and F.N.S.-P. developed algorithms for quantitative analysis of OCT images and helped edit the manuscript. S.M.A. and D.E.B. captured and evaluated transmission electron micrograph images and helped with manuscript proofreading. J.L.F. was responsible for project management, provided funding, and participated in experimental design, PCR data analysis, and manuscript writing and proofreading.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data are shown here, and all materials are commercially available.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/6/266/266ra172/DC1
REFERENCES AND NOTES
- 1.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of α-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- 3.Koh S, Maeda N, Nakagawa T, Nishida K. Quality of vision in eyes after selective lamellar keratoplasty. Cornea. 2012;31(Suppl. 1):S45–S49. doi: 10.1097/ICO.0b013e318269c9cd. [DOI] [PubMed] [Google Scholar]
- 4.Patel SV, McLaren JW, Hodge DO, Baratz KH. Scattered light and visual function in a randomized trial of deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2008;145:97–105. doi: 10.1016/j.ajo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya’ale D, Négrel AD, Resnikoff S. 2002 global update of available data on visual impairment: A compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11:67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- 6.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 7.Dandona L, Ragu K, Janarthanan M, Naduvilath TJ, Shenoy R, Rao GN. Indications for penetrating keratoplasty in India. Indian J Ophthalmol. 1997;45:163–168. [PubMed] [Google Scholar]
- 8.Williams KA, Esterman AJ, Bartlett C, Holland H, Hornsby NB, Coster DJ. How effective is penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation. 2006;81:896–901. doi: 10.1097/01.tp.0000185197.37824.35. [DOI] [PubMed] [Google Scholar]
- 9.Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005;140:1112–1122. doi: 10.1016/j.ajo.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Dandona L, Naduvilath TJ, Janarthanan M, Ragu K, Rao GN. Survival analysis and visual outcome in a large series of corneal transplants in India. Br J Ophthalmol. 1997;81:726–731. doi: 10.1136/bjo.81.9.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 12.Polisetti N, Islam MM, Griffith M. The artificial cornea. Methods Mol Biol. 2013;1014:45–52. doi: 10.1007/978-1-62703-432-6_2. [DOI] [PubMed] [Google Scholar]
- 13.Pinnamaneni N, Funderburgh JL. Concise review: Stem cells in the corneal stroma. Stem Cells. 2012;30:1059–1063. doi: 10.1002/stem.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Du Y, Mann MM, Yang E, Funderburgh JL, Wagner WR. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Eng Part A. 2013;19:2063–2075. doi: 10.1089/ten.tea.2012.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Y, Sundarraj N, Funderburgh ML, Harvey SA, Birk DE, Funderburgh JL. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48:5038–5045. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Y, Carlson EC, Funderburgh ML, Birk DE, Pearlman E, Guo N, Kao WW, Funderburgh JL. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–1642. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Zhang J, Liu CY, Wang IJ, Sieber M, Chang J, Jester JV, Kao WW. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: Lumican null mice. PLOS One. 2010;5:e10707. doi: 10.1371/journal.pone.0010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53:5109–5116. doi: 10.1167/iovs.11-8673. [DOI] [PubMed] [Google Scholar]
- 20.Li GG, Zhu YT, Xie HT, Chen SY, Tseng SC. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53:5686–5697. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polisetty N, Fatima A, Madhira SL, Sangwan VS, Vemuganti GK. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 22.Bray LJ, Heazlewood CF, Munster DJ, Hutmacher DW, Atkinson K, Harkin DG. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy. 2014;16:64–73. doi: 10.1016/j.jcyt.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Boote C, Du Y, Morgan S, Harris J, Kamma-Lorger CS, Hayes S, Lathrop KL, Roh DS, Burrow MK, Hiller J, Terrill NJ, Funderburgh JL, Meek KM. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci. 2012;53:2786–2795. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:1504–1509. doi: 10.1136/bjophthalmol-2012-301869. [DOI] [PubMed] [Google Scholar]
- 25.Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, Krishnaiah S, Gaddipati S, Tiwari S, Balasubramanian D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br J Ophthalmol. 2011;95:1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 26.O’Callaghan AR, Daniels JT. Concise review: Limbal epithelial stem cell therapy: Controversies and challenges. Stem Cells. 2011;29:1923–1932. doi: 10.1002/stem.756. [DOI] [PubMed] [Google Scholar]
- 27.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 28.Menzel-Severing J, Kruse FE, Schlötzer-Schrehardt U. Stem cell–based therapy for corneal epithelial reconstruction: Present and future. Can J Ophthalmol. 2013;48:13–21. doi: 10.1016/j.jcjo.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2012;153:643–650.e2. doi: 10.1016/j.ajo.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Lal I, Panchal BU, Basu S, Sangwan VS. In-vivo expansion of autologous limbal stem cell using simple limbal epithelial transplantation for treatment of limbal stem cell deficiency. BMJ Case Rep. 2013;2013:bcr2013009247. doi: 10.1136/bcr-2013-009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karamichos D, Funderburgh ML, Hutcheon AE, Zieske JD, Du Y, Wu J, Funderburgh JL. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLOS One. 2014;9:e86260. doi: 10.1371/journal.pone.0086260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan AA, Hertsenberg AJ, Funderburgh ML, Mann MM, Du Y, Davoli KA, Mich-Basso JD, Yang L, Funderburgh JL. Differentiation of human embryonic stem cells into cells with corneal keratocyte phenotype. PLOS One. 2013;8:e56831. doi: 10.1371/journal.pone.0056831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Du Y, Watkins SC, Funderburgh JL, Wagner WR. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials. 2012;33:1343–1352. doi: 10.1016/j.biomaterials.2011.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzsimmons TD, Fagerholm P, Härfstrand A, Schenholm M. Hyaluronic acid in the rabbit cornea after excimer laser superficial keratectomy. Invest Ophthalmol Vis Sci. 1992;33:3011–3016. [PubMed] [Google Scholar]
- 35.Latvala T, Puolakkainen P, Vesaluoma M, Tervo T. Distribution of SPARC protein (osteonectin) in normal and wounded feline cornea. Exp Eye Res. 1996;63:579–584. doi: 10.1006/exer.1996.0148. [DOI] [PubMed] [Google Scholar]
- 36.Maseruka H, Bonshek RE, Tullo AB. Tenascin-C expression in normal, inflamed, and scarred human corneas. Br J Ophthalmol. 1997;81:677–682. doi: 10.1136/bjo.81.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funderburgh JL, Hevelone ND, Roth MR, Funderburgh ML, Rodrigues MR, Nirankari VS, Conrad GW. Decorin and biglycan of normal and pathologic human corneas. Invest Ophthalmol Vis Sci. 1998;39:1957–1964. [PubMed] [Google Scholar]
- 38.Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor β-induced keratocyte-myofibroblast trans-differentiation. J Biol Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox JL, Farrell RA, Hart RW, Langham ME. The transparency of the mammalian cornea. J Physiol. 1970;210:601–616. doi: 10.1113/jphysiol.1970.sp009230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meek KM, Leonard DW, Connon CJ, Dennis S, Khan S. Transparency, swelling and scarring in the corneal stroma. Eye. 2003;17:927–936. doi: 10.1038/sj.eye.6700574. [DOI] [PubMed] [Google Scholar]
- 42.Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–548. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie HT, Chen SY, Li GG, Tseng SC. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011;29:1874–1885. doi: 10.1002/stem.743. [DOI] [PubMed] [Google Scholar]
- 44.Higa K, Kato N, Yoshida S, Ogawa Y, Shimazaki J, Tsubota K, Shimmura S. Aquaporin 1-positive stromal niche-like cells directly interact with N-cadherin-positive clusters in the basal limbal epithelium. Stem Cell Res. 2013;10:147–155. doi: 10.1016/j.scr.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Xie HT, Chen SY, Li GG, Tseng SC. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:279–286. doi: 10.1167/iovs.11-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida S, Shimmura S, Nagoshi N, Fukuda K, Matsuzaki Y, Okano H, Tsubota K. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 47.Zakaria N, Possemiers T, Dhubhghaill SN, Leysen I, Rozema J, Koppen C, Timmermans JP, Berneman Z, Tassignon MJ. Results of a phase I/II clinical trial: Standardized, non-xenogenic, cultivated limbal stem cell transplantation. J Transl Med. 2014;12:58. doi: 10.1186/1479-5876-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 49.Espana EM, He H, Kawakita T, Di Pascuale MA, Raju VK, Liu CY, Tseng SC. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci. 2003;44:5136–5141. doi: 10.1167/iovs.03-0484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Ex vivo model of corneal limbal biopsy.
Fig. S2. Sphere formation and stem cell–gene expression by LBSCs.
Fig. S3. Murine corneal debridement model.
Fig. S4. Persistence of engrafted LBSCs in the stroma.
Table S1. Time to reach passage 3 by primary LBSCs under different conditions.
Table S2. Clonogenic potential of LBSCs in different sera.
Table S3. Area of colonies cultured in different sera.
Table S4. OCT analysis of debrided mouse corneas.
Movie S1. Organization of matrix deposited ex vivo by LBSCs.


