Abstract
Background
In the setting of a statewide quality collaborative approach to the review of cardiac surgical mortalities in intensive care units (ICUs), variations in complication-related outcomes became apparent. Utilizing “failure to rescue” methodology, (FTR; the probability of death after a complication), we compared FTR rates after adult cardiac surgery in low, medium, and high mortality centers from a voluntary, 33-center quality collaborative.
Methods
We identified 45,904 patients with a Society of Thoracic Surgeons predicted risk of mortality who underwent cardiac surgery between 2006 and 2010. The 33 centers were ranked according to observed-to-expected (O/E) ratios for mortality and were categorized into 3 equal groups. We then compared rates of complications and FTR.
Results
Overall unadjusted mortality was 2.6%, ranging from 1.5% in the low-mortality group to 3.6% in the high group. The rate of 17 complications ranged from 19.1% in the low group to 22.9% in the high group while FTR rates were 6.6% in the low group, 10.4% in the medium group, and 13.5% in the high group (p<0.001). The FTR rate was significantly better in the low mortality group for the majority of complications (11 of 17) with the most significant findings for cardiac arrest, dialysis, prolonged ventilation, and pneumonia.
Conclusion
Low mortality hospitals have superior ability to rescue patients from complications after cardiac surgery procedures. Outcomes review incorporating a collaborative multi-hospital approach can provide an ideal opportunity to review processes that anticipate and manage complications in the ICU and help recognize and share “differentiators” in care.
Keywords: Outcomes, Database, Patient safety, Statistics, Surgery, Complications
Introduction
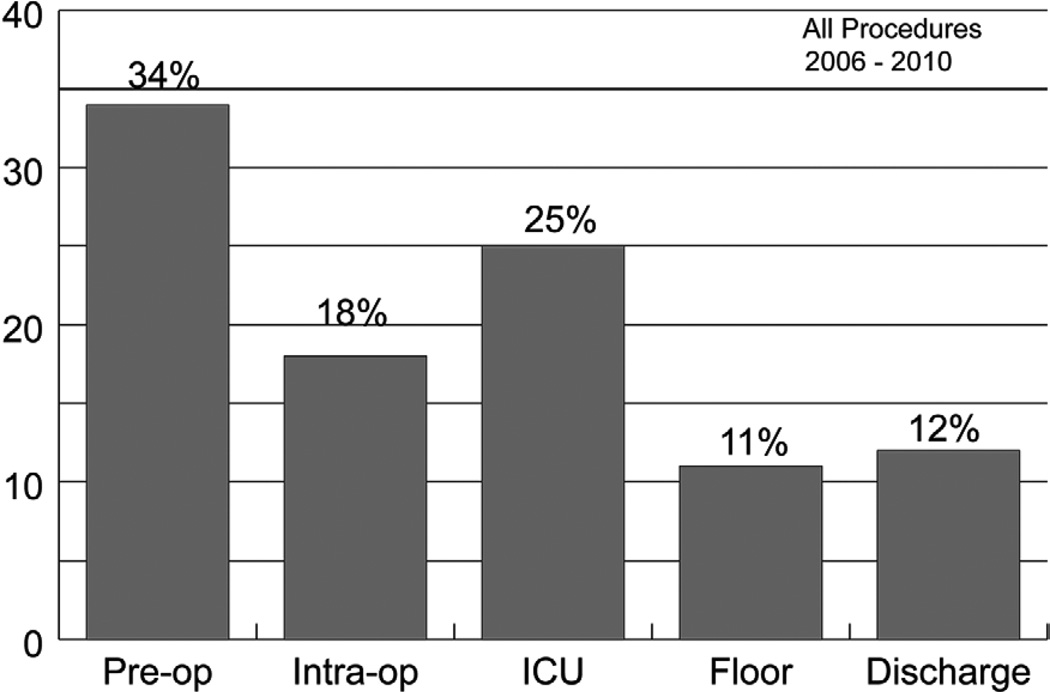
The Michigan Society of Thoracic and Cardiovascular Surgeons (MSTCVS) serves as the platform for a statewide, surgeon directed, voluntary, quality collaborative program. With participation of all 33 cardiac surgical programs in the state, Society of Thoracic Surgeons (STS) adult cardiac surgery data have been utilized in an unblinded fashion to promote discussion and share approaches to improve outcomes and care processes in cardiac surgery [1]. Quarterly review of all mortalities published in previous work identified post-operative intensive care unit (ICU) care as a significant source of potentially avoidable mortality (Figure 1) [2]. Furthermore, despite improved overall outcomes in cardiac surgery over the past several decades, there remains significant inter-hospital variation in mortality [3].
Figure 1.
Phase of Care Mortality Analysis (POCMA) Profile 2006 – 2010
ICU = Intensive Care Unit Phase; Intra-op = Intra-operative Phase; Pre-op = Preoperative Phase
In attempts to reduce this variation in outcomes, multiple efforts have been proposed to reduce post-operative complication rates, which no doubt contribute to post-operative mortality. However, there is a growing body of evidence that “failure to rescue” (FTR) – mortality among patients with a major complication – is an important mechanism underlying post-operative mortality [4]. This has been demonstrated to be an important source of inter-hospital variation in general surgery, vascular surgery, and other surgical disciplines [3, 5–7]. Failure to rescue is now used by Agency of Health Care Research and Quality as one of 20 patient safety indicators [8]. The degree to which FTR is an important contributor to variation in adult cardiac surgery is less established.
In this context, we sought to determine whether variations in surgical mortality among patients undergoing cardiac surgery in Michigan are due to differences in the incidence of complications or differences in the success of managing complications once they occur (i.e. FTR). Using audited MSTCVS data, we compared the rates of complications and FTR in cardiac surgery across hospitals, with a particular focus on 17 major complications.
Patients and Methods
Data Source
The MSTCVS Quality Collaborative is a multidisciplinary group consisting of all 33 hospitals that perform adult cardiac surgery in the state of Michigan. All programs use the Society of Thoracic Surgeons (STS) data collection form and submit data on a quarterly basis to both the STS database and the MSTCVS collaborative warehouse with state-specific data fields including phase of care mortality analysis (POCMA). Data collected include perioperative, operative, and outcomes data on all patients undergoing cardiac surgery at all 33 participating hospitals. Data managers meet quarterly for ongoing education and training in data abstraction and outcomes reporting. In addition, there are scheduled conference calls and web-based seminars that focus specifically on issues related to institutional quality initiatives or data definitions. Yearly data audits are performed to enhance reliability.
Data Definitions
We defined operative mortality as (1) all deaths occurring during the hospital period in which the operation was performed; and (2) those deaths occurring after hospital discharge, but within 30 days of the procedure. Failure to rescue (FTR), as previously described, was defined as operative mortality after suffering a complication [5]. Seventeen complications were examined: multi-system organ failure (MSOF), coma, cardiac arrest, renal dialysis, sepsis, anticoagulation event, gastrointestinal event, intensive care unit (ICU) readmission, prolonged ventilation, reoperation for bleeding, pneumonia, stroke, cardiac tamponade, pulmonary embolism, deep sternal wound infection, heart block, and aortic dissection.
Study Population
We analyzed a total of 45,904 patients who underwent any cardiac surgery procedure from 2006 to 2010 at the 33 participating MSTCVS hospitals that had a STS predicted risk of mortality (76% of all patients). The STS risk model for predicted mortality has been described previously and was most recently updated in 2008 [9–11]. Procedures included isolated coronary artery bypass grafting (CABG) surgery, isolated aortic valve replacement (AVR), isolated mitral valve replacement (MVR), AVR plus CABG, MVR plus CABG, mitral valve repair, and mitral valve repair plus CABG.
Statistical Analysis
Study variables were described using standard summary statistics. Outcomes were calculated at the hospital level, including operative mortality rate (number of total operative deaths divided by number of total patients), complication rate (number of patients with any of the 17 post-operative complications described divided by number of total patients), and FTR rate (number of deaths in those with any of the 17 post-operative complications divided by number of total patients with any of the 17 post-operative complications). Predicted mortality probabilities were summed at each hospital to estimate the expected number of deaths. We then calculated the ratio of observed-to-expected (O/E) deaths for each hospital. Hospitals were then ranked according to these O/E ratios and divided into three equal-sized groups (tertiles). The low-mortality group included those sites whose O/E ratios were 0.23 – 0.77, the medium-mortality group was 0.77 – 0.96, and the high-mortality group was 0.96 – 1.56. We next compared the complication rates and FTR rates across these tertiles of mortality. To determine whether specific complications had different rates of FTR, we calculated a FTR rate for each specific complication. Statistical analyses were carried out using SAS 9.3 (SAS Institute, Cary, NC).
Results
Descriptive statistics are shown in Table 1. The low mortality hospitals treated a higher proportion of males and Caucasians. Less patients in the low mortality hospitals had diabetes, renal failure, hypertension, chronic lung disease, peripheral vascular disease, prior stroke, and were on immunosuppression. More patients in the low mortality hospitals had no previous history of myocardial infarction. Interestingly, there were more patients in the low mortality hospitals that underwent isolated valve and CABG plus valve surgery and less isolated CABG patients than the medium mortality and high mortality groups. All these factors were accounted for in the STS risk prediction model, and hospitals were ranked according to their observed-to-expected mortality ratios.
Table 1.
Demographics and Clinical Characteristics of Patients, Stratified by Hospital Tertile of Mortality
| Variable | Low Mortality (N=15842) |
Medium Mortality (N=14181) |
High Mortality (N=15881) |
p value |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr), median | 66.00 | 66.00 | 66.00 | 1.000 |
| Male sex (%) | 70.5% | 69.1% | 68.2% | < 0.001 |
| Non-white race (%) | 3.5% | 4.3% | 5.8% | < 0.001 |
| Risk Factors | ||||
| Diabetes mellitus (%) | 36.2% | 39.3% | 38.0% | < 0.001 |
| Dialysis (%) | 2.2% | 2.1% | 3.2% | < 0.001 |
| Hypertension (%) | 81.7% | 84.6% | 86.0% | < 0.001 |
| Chronic lung disease | ||||
| None | 81.3% | 76.8% | 77.7% | |
| Mild | 11.3% | 14.4% | 13.9% | < 0.001 |
| Moderate | 4.8% | 4.7% | 5.1% | |
| Severe | 2.5% | 4.2% | 3.3% | |
| Immunosuppressive therapy (%) | 2.3% | 2.6% | 4.6% | < 0.001 |
| Peripheral vascular disease (%) | 14.5% | 15.4% | 16.9% | < 0.001 |
| Prior cerebrovascular accident (%) | 13.3% | 15.3% | 16.1% | < 0.001 |
|
Previous Cardiovascular Interventions |
||||
| Previous CABG (%) | 5.0% | 4.5% | 5.0% | 0.080 |
| Previous valve surgery (%) | 2.1% | 0.9% | 1.7% | < 0.001 |
| Previous PCI (%) | 22.3% | 24.5% | 25.0% | < 0.001 |
| Preoperative Cardiac Status | ||||
| Previous myocardial infarction (%) | 39.4% | 45.1% | 43.2% | < 0.001 |
| Congestive heart failure (%) | ||||
| NYHA Class I-III | 12.0% | 11.4% | 13.0% | < 0.001 |
| NYHA Class IV | 6.8% | 4.3% | 7.4% | <0.001 |
|
Hemodynamics and Catheterization data |
||||
| Three vessel coronary disease (%) | 61.9% | 64.9% | 64.5% | < 0.001 |
| Ejection fraction (%), mean | 51.8 | 51.0 | 51.4 | < 0.001 |
| Operative Characteristics | ||||
| First cardiovascular surgery (%) | 93.3% | 94.7% | 93.6% | < 0.001 |
| Elective status (%) | 45.4% | 38.7% | 44.6% | < 0.001 |
| Procedure | ||||
| Isolated CABG (%) | 70.7% | 79.9% | 77.7% | < 0.001 |
| Isolated valve surgery (%) | 16.6% | 10.5% | 12.6% | < 0.001 |
| CABG plus valve surgery (%) | 12.8% | 9.7% | 9.7% | < 0.001 |
| Mortality | ||||
| Expected mortality (%) | 2.82 | 2.88 | 3.11 | < 0.001 |
| Observed-to-expected ratio | 0.23–0.77 | 0.78–0.96 | 0.97–1.56 | - |
CABG=coronary artery bypass graft, PCI=previous cardiovascular intervention, MI=myocardial infarction, NYHA=New York Heart Association, CPR=cardiopulmonary resuscitation
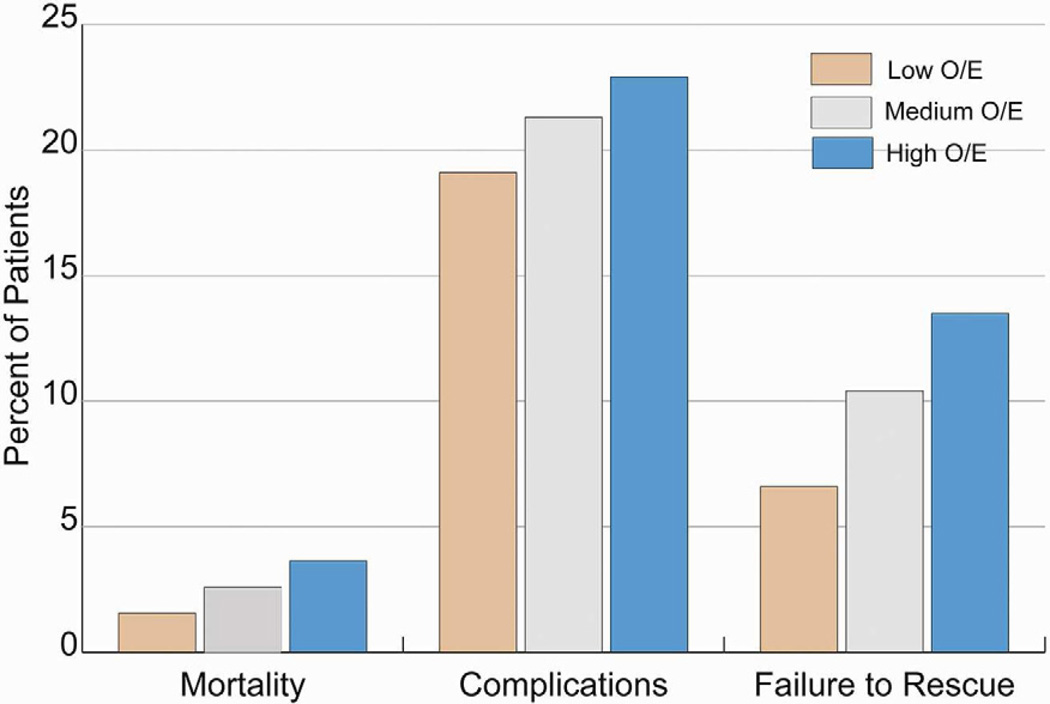
The overall unadjusted mortality was 2.6% and varied by a factor of 2.4 across the hospital tertiles, from 1.5% in the low-mortality group to 3.6% in the high-mortality group (p<0.001) (Figure 2). The overall complication rates between the three groups were significantly different (19.1 vs. 21.3% vs. 22.9%, p<0.001). While the complication rates between the three groups had a significant p value, the percentage differences were small supporting the fact that reaching statistical significance does not equal a clinically significant difference. However, the failure-to-rescue rate was markedly elevated in hospitals with higher overall mortality. Patients treated at high-mortality hospitals had greater than two times the likelihood of death after developing a complication when compared to patients treated at low-mortality hospitals (13.5% vs. 6.6%, p<0.001).
Figure 2.
Rates of Mortality, Complications and Failure to Rescue (2006–2010)
O/E = Observed Over Expected Mortality Group
The FTR rate was significantly better in the low-mortality group for the majority (11 of 17) of complications (Table 2). The largest differences in FTR between high-mortality and low-mortality hospitals were observed in patients with cardiac arrest (62.2% vs 38.2%, p<0.001), renal dialysis (40.7% vs. 24.6%, p<0.001), prolonged ventilation (15.6% vs. 8.6%, p<0.001), and pneumonia (20.1% vs 7.6%, p<0.001). There were no statistically significant differences in FTR for six complications: stroke, tamponade, pulmonary embolism, deep space wound infection, heart block, and aortic dissection.
Table 2.
Incidence of Mortality, Complications, and Failure-to-Rescue, Stratified by Hospital Tertile of Mortality
| Mortality | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Odds Ratio High vs. Low Mortality (95% CI) |
Low | Medium | High | Odds Ratio High vs. Low Mortality (95% CI) |
|
| Variable | ||||||||
| Incidence of Complication | Incidence of Failure to Rescue | |||||||
| Percent of patients | Percent of patients | |||||||
| Overall (any of 17 listed) | 19.1 | 21.3 | 22.9 | 1.26 (1.19, 1.33) | 6.6 | 10.4 | 13.5 | 2.21 (1.86, 2.62) |
| Multi-system organ failure | 0.2 | 0.5 | 0.8 | 3.44 (2.39, 4.97) | 67.6 | 83.1 | 85.8 | 2.91 (1.24, 6.80) |
| Coma | 0.3 | 0.3 | 0.5 | 1.38 (0.97, 1.98) | 44.2 | 70.7 | 72.2 | 3.28 (1.55, 6.95) |
| Cardiac arrest | 1.5 | 1.9 | 2.4 | 1.60 (1.36, 1.88) | 38.2 | 52.6 | 62.2 | 2.65 (1.89, 3.71) |
| Renal dialysis | 1.5 | 1.6 | 1.7 | 1.13 (0.95, 1.35) | 24.6 | 30.6 | 40.7 | 2.11 (1.43, 3.10) |
| Sepsis | 0.8 | 1.1 | 1.6 | 1.93 (1.56, 2.38) | 20.3 | 26.9 | 33.7 | 2.00 (1.22, 3.28) |
| Anticoagulation Event | 0.6 | 0.7 | 0.9 | 1.50 (1.15, 1.95) | 15.4 | 23.5 | 30.9 | 2.46 (1.25, 4.83) |
| Gastrointestinal Event | 2.5 | 2.8 | 2.8 | 1.13 (0.98, 1.30) | 11.0 | 14.7 | 19.3 | 1.93 (1.30, 2.87) |
| ICU readmission | 2.8 | 3.4 | 3.4 | 1.20 (1.06, 1.37) | 8.3 | 15.4 | 15.1 | 1.97 (1.31, 2.98) |
| Prolonged ventilation | 11.8 | 13.0 | 14.9 | 1.31 (1.22, 1.39) | 8.6 | 13.3 | 15.6 | 1.95 (1.61, 2.28) |
| Reoperation for bleeding | 2.6 | 2.7 | 2.8 | 1.09 (0.95, 1.25) | 7.7 | 11.7 | 14.8 | 2.09 (1.33, 3.28) |
| Pneumonia | 3.1 | 4.7 | 4.5 | 1.46 (1.30, 1.63) | 7.6 | 10.9 | 20.1 | 3.05 (2.09, 4.45) |
| Stroke | 1.2 | 1.3 | 1.6 | 1.34 (1.11, 1.62) | 18.0 | 21.0 | 24.5 | 1.49 (0.94, 2.36) |
| Tamponade | 0.5 | 0.4 | 0.3 | 0.60 (0.35, 1.04) | 21.2 | 36.0 | 38.1 | 2.29 (0.68, 7.69) |
| Pulmonary embolism | 0.2 | 0.1 | 0.2 | 1.26 (0.78, 204) | 6.7 | 10.5 | 15.8 | 2.63 (0.49, 14.07) |
| Deep sternal wound infection | 0.6 | 0.4 | 0.7 | 1.20 (0.92, 1.57) | 10.2 | 8.0 | 10.2 | 1.00 (0.41, 2.42) |
| Heart block | 1.5 | 1.6 | 1.4 | 0.96 (0.80, 1.15) | 3.5 | 4.9 | 5.8 | 1.73 (0.70, 4.27) |
| Aortic dissection | 0.03 | 0.02 | 0.04 | 1.75 (0.51, 5.97) | 25.0 | 0.0 | 42.9 | 2.25 (0.15, 33.93) |
Comment
The results of this study help explain the variation in mortality rates between hospitals. There was a 2.4-fold difference in mortality between the low-mortality hospitals and high-mortality hospitals. The incidence of complications, though different between these hospitals, varied to a much smaller extent. However, the rates of failure to rescue varied markedly between high-performing and low-performing hospitals. These data suggest that while patients at low-mortality hospitals suffer fewer complications than high-mortality hospitals, what truly distinguishes these high-performing hospitals is their superior ability to recognize and rescue patients from complications that arise after cardiac surgery procedures.
Our results are consistent with a growing body of evidence that supports “failure to rescue” as a major mechanism explaining variation in hospital mortality rates among hospitals. Failure to rescue was first popularized by Silber and colleagues [4] and validated in surgical patient populations by multiple subsequent analyses. Ghaferi and colleagues [5] studied 84,370 patients who had undergone general or vascular surgery from 2005 to 2007 using data from the American College of Surgeons National Surgical Quality Improvement Program and found similar rates of postoperative complications between high mortality and low mortality centers, but a drastically different rate of failure to rescue in high-performing versus low-performing hospitals (6.8% vs. 16.7%). An analysis of Medicare beneficiaries undergoing 6 major operations that included CABG, AVR, and MVR yielded similar results; complication rates were similar at worst (bottom 20%) and best (top 20%) hospitals, but FTR rates were much higher at worst compared with best hospitals (16.7% vs. 6.8%) [3]. Breakdown into individual operations revealed slight differences in complication rates between the best and worst hospitals and markedly different rates in FTR. Analyses in pediatric heart surgery patients with the STS Congenital Heart Surgery Database [7] and trauma patients using the National Trauma Databank [6] also reveal similar complication rates and drastically different FTR rates among high-performing and low-performing hospitals. Our study is the first study to our knowledge that examines the adult cardiac surgery patient population using a prospectively-collected clinical database, ensuring both adequate risk adjustment using a well-validated model and accurate ascertainment of postoperative complications.
Our study has several limitations. First, our analysis was limited to MSTCVS hospitals, which may not be representative of all hospitals in the United States. However, the MSTCVS consists of all hospitals in Michigan that perform cardiac surgery, and rates of mortality and complications are similar to like hospitals in STS national data [12] and failure to rescue analysis is similar to previous analysis of the national Medicare population [3]. Second, the database does not capture every possible postoperative complication. Therefore, we may have underestimated the degree to which other uncaptured complications varied across hospitals. However, greater than 85% of deaths were preceded by at least one of the complications captured in the database. In addition, in this analysis, we examined the correlation between hospital mortality and FTR; however, surgical mortality rankings alone are limited in their ability to evaluate overall hospital quality due to small sample sizes for certain operations. It may be that a composite measure of various factors may be more reliable in predicting surgical mortality [13–15].
The factors underlying failure to rescue have yet to be fully elucidated; however, Silber and colleagues demonstrated that while complication rates were associated primarily with patient characteristics, failure to rescue was associated more with hospital characteristics [4]. Hospital characteristics that have been associated with low FTR rates in pancreatectomy patients include teaching status, hospital size greater than 200 beds, average daily census greater than 50% capacity, increased nurse-to-patient ratios, and high hospital technology [16]. Aiken and colleagues demonstrated that each additional patient per nurse was associated with a 7% increase in the odds of failure to rescue [17]. Nurse education, communication, job satisfaction, and burnout have all been implicated as factors contributing to failure to rescue [18]. Ghaferi and colleagues categorized contributors to FTR into two broad classes: timely recognition of a complication and effective management [5]. To address the latter, rapid response teams and increasing ICU physician staffing ratios have been trialed, however retrospective data exist to support the observation that there is still a lack of early recognition of complications [19]. Still more work needs to be done to better understand the mechanisms underlying failure to rescue. Pronovost and colleagues showed an association between physician staffing levels in the ICU and patient mortality [20]. To that effect, in follow-up of this analysis of our data, we have sent a detailed questionnaire to each of the 33 participating MSTCVS hospitals to better understand the hospital structures and processes in place in the operating rooms and ICUs at each institution.
Conclusions
This study suggests that the variation in mortality rates among hospitals is largely attributable to the marked differences in mortality after complications among hospitals. Low mortality hospitals are better able to recognize and treat life-threatening complications. Further characterization of hospital structures and processes is needed to better understand the variation in failure to rescue rates between hospitals.
Acknowledgments
Acknowledgments and Disclosures
Terry Shih is supported by grant from the National Institutes of Health (5T32HL07612309).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Southern Thoracic Surgical Association 59th annual meeting in Naples, Florida on November 8, 2012
References
- 1.Prager RL, Armenti FR, Bassett JS, et al. Cardiac surgeons and the quality movement: the Michigan experience. Semin Thorac Cardiovasc Surg. 2009;21:20–27. doi: 10.1053/j.semtcvs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Shannon FL, Fazzalari FL, Theurer PF, et al. A method to evaluate cardiac surgery mortality: phase of care mortality analysis. Ann Thorac Surg. 2012;93:36–43. doi: 10.1016/j.athoracsur.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 3.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in Medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 4.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–629. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 6.Glance LG, Dick AW, Meredith JW, Mukamel DB. Variation in hospital complication rates and failure-to-rescue for trauma patients. Ann Surg. 2011;253:811–816. doi: 10.1097/SLA.0b013e318211d872. [DOI] [PubMed] [Google Scholar]
- 7.Pasquali SK, He X, Jacobs JP, Jacobs ML, O'Brien SM, Gaynor JW. Evaluation of failure to rescue as a quality metric in pediatric heart surgery: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94:573–580. doi: 10.1016/j.athoracsur.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agency for Healthcare Research and Quality (AHRQ) Quality Indicators. [Accessed October 15, 2012];Patient Safety Indicators: Technical Specifications. Available at: http://www.qualityindicators.ahrq.gov/downloads/modules/psi/v30/psi_technical_specs_v30.pdf.
- 9.Shahian DM, O'Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 11.Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3--valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S43–S62. doi: 10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed October 22, 2012];Society of Thoracic Surgeons Adult Cardiac Surgery Database Executive Summary 10 years. Available at: http://www.sts.org/sites/default/files/documents/2012%20-%20AC%20%202ndHarvestExecutiveSummary.pdf.
- 13.Dimick JB, Staiger DO, Baser O, Birkmeyer JD. Composite measures for predicting surgical mortality in the hospital. Health Aff (Millwood) 2009;28:1189–1198. doi: 10.1377/hlthaff.28.4.1189. [DOI] [PubMed] [Google Scholar]
- 14.Shahian DM, Edwards FH, Ferraris VA, et al. Quality measurement in adult cardiac surgery: part 1--Conceptual framework and measure selection. Ann Thorac Surg. 2007;83:S3–S12. doi: 10.1016/j.athoracsur.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien SM, Shahian DM, Delong ER, et al. Quality measurement in adult cardiac surgery: part 2--Statistical considerations in composite measure scoring and provider rating. Ann Thorac Surg. 2007;83:S13–S26. doi: 10.1016/j.athoracsur.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 16.Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg. 2010;211:325–330. doi: 10.1016/j.jamcollsurg.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288:1987–1993. doi: 10.1001/jama.288.16.1987. [DOI] [PubMed] [Google Scholar]
- 18.Hravnak M, Schmid A, Ott L, Pinsky MR. Causes of Failure to Rescue. In: DeVita MA, Hillman K, Bellomo R, editors. Textbook of Rapid Response Systems. 1st ed. New York, NY: Springer; 2011. pp. 141–150. [Google Scholar]
- 19.Taenzer AH, Pyke JB, McGrath SP. A review of current and emerging approaches to address failure-to-rescue. Anesthesiology. 2011;115:421–431. doi: 10.1097/ALN.0b013e318219d633. [DOI] [PubMed] [Google Scholar]
- 20.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]