Abstract
5-hydroxymethylcytosine (5-hmC) is a novel environmentally sensitive DNA modification that is highly enriched in post-mitotic neurons and is associated with active transcription of neuronal genes. Recently, 5-hmC was functionally linked to learning and cognition and these studies revealed an accumulation of 5-hmC in the prefrontal cortex of mice undergoing fear extinction. These studies led us to hypothesize a role for 5-hmC in response to stress. To test this hypothesis, we combined immunohistochemistry, tandem mass spectrometry, and tet-assisted sodium bisulfite sequencing (TAB-seq) analyses on tissue and DNA from the hippocampus of 7-week old male mice exposed to a single thirty-minute restraint stress. After first identifying that the broad neuronal distribution of 5-hmC is not disrupted by acute stress, we used TAB-seq to find a stress-induced increase of 5-hmC in the 3’UTR of the glucocorticoid receptor gene (Nr3c1). Nr3c1 has a well-defined role in the stress pathway and these data suggest that 5-hmC contributes to these processes. Together, these data indicate that a deeper investigation of stress-related 5-hmC levels may reveal an environmental impact on this newly discovered epigenetic mark in the brain.
Keywords: acute stress, epigenetics, DNA methylation, 5-hmC
Environmentally sensitive molecular mechanisms (e.g. epigenetic modifications) in the brain have become a significant focus of neuroscience research because of growing evidence that suggests they are critical to the development of psychiatric disorders, including depression, anxiety, post-traumatic stress disorder, and schizophrenia (Abdolmaleky et al. 2006; Grayson et al. 2005; Hunter et al. 2009; Kuratomi et al. 2008; Poulter et al. 2008). DNA methylation was the first epigenetic modification discovered and is characterized by the addition of a methyl group on cytosine (5-mC) in a CpG dinucleotide context. Recent advances in technology have enabled unprecedented resolution of this mark and have identified subtle features in DNA methylation that include distinctive fluctuations that are highly conserved among eukaryotes and function in both gene silencing and gene activation via a variety of mechanisms such as the blocking or recruiting of transcription factors. The recent identification of another modified form of cytosine, 5-hydroxymethylcytosine (5-hmC) raises new questions as to the role of this base in mediating epigenetic effects. The oxidation of 5-mC to 5-hmC is mediated by the three members of the ten-eleven translocation family of proteins (Tet1-3), which are influenced by environmental cues such as oxidative stress (Chia et al. 2011). Recently, we and others have developed genome-wide methods capable of providing a distinction between 5-mC and 5-hmC. These studies found that 5-hmC is highly enriched in the brain, associated with active transcription of neuronal genes, and can be stable, accumulating throughout the lifespan of an organism (Chopra et al. 2014; Szulwach et al. 2011). Functionally, this epigenetic mark is critically involved in neuronal differentiation and in the reprogramming of pluripotent stem cells. Although a role for 5-hmC in the brain is only starting to emerge, with recent links to learning and cognition (Li et al. 2014; Rudenko et al. 2013), much work remains to fully unravel the relationship between this epigenetic modification and environmental stimuli.
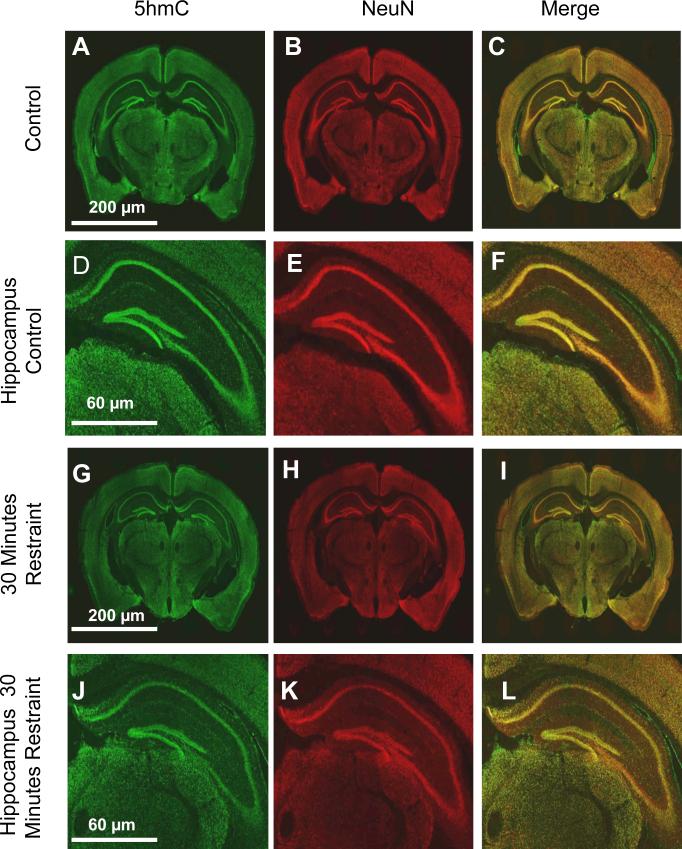
The hippocampus was the first brain region to show an epigenetic response to an exogenous stimulus when genome-wide alterations in histone modifications and gene expression were found following a single acute restraint stress (Crosio et al. 2003; Gray et al. 2014; Hunter et al. 2009). The recent identification of 5-hmC being highly enriched in the brain and associated with active transcription of neuronal genes led us to hypothesize a role for 5hmC in response to acute stress. To test this hypothesis, seven-week-old male C57BL/6J mice were randomly divided into experimental or control groups (N = 5 for each group). The experimental mice were restrained for thirty minutes using a 50 ml conical tube followed by a one-hour recovery period in an individually housed cage prior to anesthetization with isoflurane. Whole brains of experimental and control mice were perfused with 4% paraformaldehyde, submerged in a 30% sucrose solution for a minimum of 48 hours, and sectioned using a microtome (−1.07 to −2.79 mm posterior to bregma). To characterize the abundance of 5-hmC throughout the mouse brain sections were stained with two primary antibodies, 5-hmC (1:500; Active Motif) and a neuronal marker (NeuN; 1:500; Millipore) followed by two secondary antibodies (Alexa Fluor 488 goat anti-rabbit, 1:200, and Alexa Fluor 546 donkey anti-mouse IgG, 1:200; Invitrogen). The staining protocol followed a standard procedure (Ferrazzano et al. 2013), with the exception that sections were incubated for antigen retrieval (2N HCL for 30 minutes at 37° C). All stained sections were visualized with a confocal microscope using the same settings (Nikon A1R-Si; 512*512 format, 400 speed, 1.5 zoom factor, 2 frame average, 1 pinnacle, 0.5 optic zoom). Particle analysis was performed to examine the total intensity density of the cells in a given region (e.g., hippocampus, cortex, or thalamus), which was then normalized to the total area of that region. The intensity densities were ultimately normalized to the average intensity density of the same region in a control mouse and this difference was compared to the NeuN expression level. Consistent with previous studies there was a substantial overlap between 5-hmC+/NeuN+ neuronal cells (Figure 1); this is particularly evident in the dentate gyrus of the hippocampus (Figure 1d-f, j-l) (Szulwach et al. 2011). Notably, this finding was not a result of poor antibody specificity, as we also found 5-hmC+/NeuN− cells (Supplementary Figure 1). Statistical analysis using a t-test showed no significant difference between the 5-hmC intensity density measured in several brain regions of experimental and control groups, suggesting that acute stress does not alter the gross distribution of 5-hmC in the brain (Figure 1).
Fig. 1.
Distribution of 5-hmC throughout the mouse whole brain and hippocampus. (A-L) Representative sections of control mouse whole brain (A-C) and hippocampus (D-F) and restrained mouse whole brain (G-I) and hippocampus (J-L). Tissues were stained with specific antibodies against 5-hmC (A, D, G, J) and NeuN (B, E, H, K); the merge (C, F, I, L) of 5-hmC and NeuN is also shown. The scale of each whole brain (200μm) and hippocampus (60μm) image is shown.
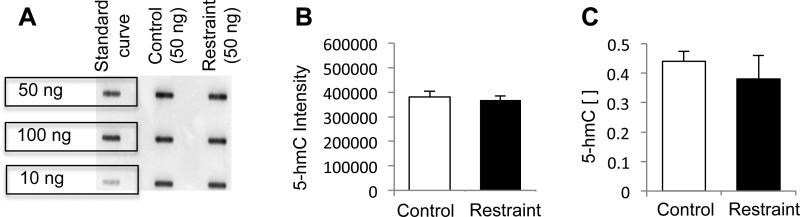
Immunohistochemtry approaches may not be sensitive enough to detect subtle stress-related changes in the gross distribution of 5-hmC; thus, we next employed immunoblotting methodologies with a 5-hmC specific antibody to quantitatively investigate 5-hmC in the hippocampus following acute stress. Hippocampal tissue was excised by micropunch (bregma −0.95 mm to −3.79 mm) from experimental and control mice and approximately thirty milligrams of tissue was homogenized with glass beads (Sigma). DNA was extracted (Qiagen) from the tissue and duplicates of fifty nanograms of DNA from each mouse was blotted onto a positively charged membrane using a slot blot apparatus as previously described (Hoefer Scientific Instruments) (Szulwach et al. 2011), except that the primary antibody was applied at a dilution of 1:8000. The average intensity for the experimental and control groups were 3.7 ×105 ± 1.6 ×104 and 3.8 ×105 ± 1.6 ×104, respectively, which were not significantly different from each other (Figure 2a,b; duplicates of N = 4/group; p > 0.05, t-test). These data further support that acute stress does not alter gross changes in the genome-wide distribution of 5-hmC in the hippocampus.
Fig. 2.
Quantitative investigation of 5-hmC in the hippocampus following acute stress. (A) Representative imunnoblot with a standard curve and 50 ng of hippocampal DNA from control and restrained mice; quantification (B) using the normalized intensity density (y-axis) is also shown. (C) Liquid chromatography (LC) and tandem mass spectrometry (MS/MS) quantification of 5-hmC in the hippocampus of control and restrained mice. The concentration (y-axis) is normalized to the total DNA input.
Since this finding was in contrast to previous reports of acute stress-related changes in histone modifications in the hippocampus (Hunter et al. 2009), we further examined the distribution of 5-hmC using a highly sensitive liquid chromatography (LC) and tandem mass spectrometry (MS/MS) methodology. This analysis was carried out on the LC/MS/MS Linear Ion Trap Quadrupole system consisting of a high pressure liquid chromatograph (HPLC; Agilent 1100) placed on the front end of a Q-Trap Mass Spectrometer (Applied Biosystems 3200) equipped with a spray source (Turbo V™) running in the Multiple Reaction Monitoring (MRM) mode. Chromatography separations were accomplished using an endcapped reversed-phase column (Phenomenex Synergi Polar-RP 2.0×150mm; 4 μm particles, 80 Å pores) onto which 10μl of each diluted sample (1:1; 0.1% formic acid) was automatically loaded. The solvents that were delivered by the HPLC throughout this assay included 0.02% (v/v) acetic acid in water (buffer A) and 100% methanol at 0.2 ml/min (buffer B). After three minutes of loading and equilibration at 98% of buffer A, the analytes were eluted from column directly into the electrospray orifice over a 13 minutes primary gradient (2% to 15% buffer B), followed by a 9 minutes secondary gradient (15% to 30% buffer B) with 1 minute hold at 30% buffer B and 8 minute post gradient equilibration at 98% buffer A. As the analytes were eluted from the HPLC-column into the electrospray source the MRM mode selectively measured spectra for 5-mC and 5-hmC based on their precursor and specific fragment mass. The LC/MS/MS method revealed that equal amounts of genomic DNA from the experimental and control groups had a normalized 5-hmC concentration of 0.40 ± 0.03 and 0.38 ± 0.08, respectively, which was not significantly different from each other (Figure 2c; N = 3/group; p > 0.05, t-test). Notably, the average concentration of 5mC was much higher in both the experimental and control groups (3.78 ± 0.2 and 3.40 ± 0.6, respectively) and these levels also were not significantly different between the groups (data not shown; N = 3/group; p > 0.05, t-test). Together, the immunohistochemistry, immunoblotting, and the LC/MS/MS data suggest that acute stress does not disrupt the gross distribution of 5-hmC throughout the brain.
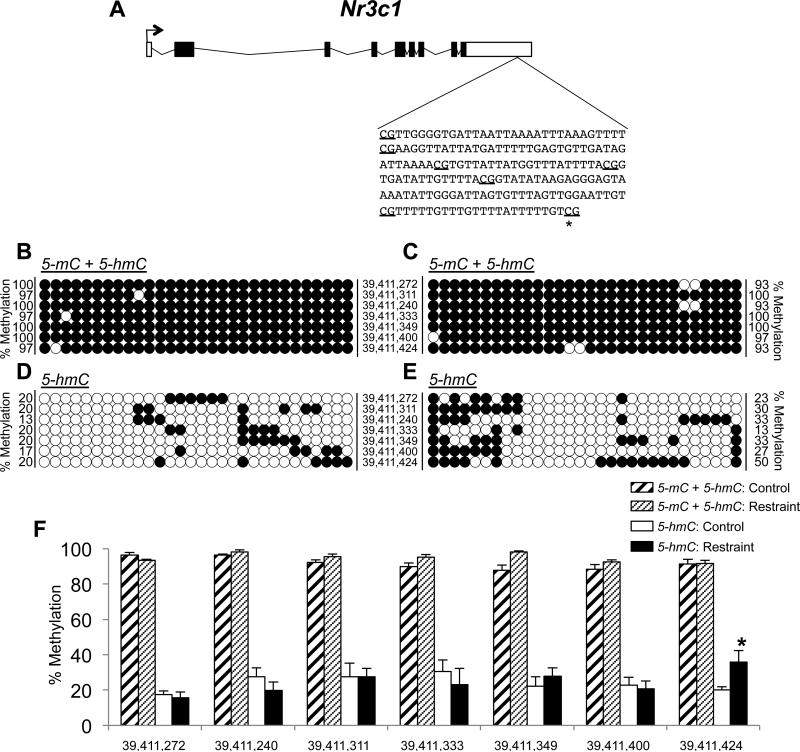
Stress response is highly mediated by the glucocorticoid receptor (Nr3c1) in the hippocampus (Zhang et al. 2013). Thus, despite finding that acute stress does not disrupt 5-hmC distributions in the hippocampus at the genome level, we hypothesize that it may alter stress-related changes in 5-hmC at individual loci in the hippocampus (e.g., Nr3c1). To test this hypothesis, we next assessed the abundance of 5-hmC on Nr3c1 following acute stress. Examination of publically available data from a recent study that characterized 5-hmC levels in hippocampus tissue from six-week old naïve mice revealed that the well-characterized Nr3c1 promoter, which harbors the NGF1-A binding site in exon 1-F that becomes hypermethylated (5-mC) in response to stress (McGowan et al. 2009), lacks 5-hmC in naïve mice (Szulwach et al. 2011). However, Nr3c1 does have a significant abundance of 5-hmC (p-value < 7.6 × 10−5) further downstream, in its 3’ untranslated region (UTR). This region is four hundred base pairs long and contains seven CpG dinucleotides (Figure 3a). While the biological relevance of 5-hmC in the 3’UTR of a stress-related gene is unknown, we sought to determine if exposure to an acute stress could alter the levels of 5-hmC in this region of Nr3c1 using tet-assisted sodium bisulfite sequencing (TAB-seq) of hippocampal DNA from experimental and control mice (N = 3 per group). Hippocampal genomic DNA was extracted as described above and was split into two aliquots, a sodium bisulfite-only aliquot and a Tet1/sodium bisulfite treated aliquot (TAB). One microgram of sonicated genomic DNA from the TAB aliquot was glucosylated and oxidized (Wisegene) (Yu et al. 2012). Then both aliquots were treated with sodium bisulfite (Zymo Research) and the bisulfite-converted DNAs were amplified using standard amplification parameters (48°C annealing temperature) and primer sets for the Nr3c1 gene: forward: ATTGAAATTAGGTATATAAGAA and reverse: CCCACCAACAAAACAAAC. The resultant PCR products were size-fractionated on a 1% agarose gel, purified (Qiagen), and cloned into a sequencing vector (Invitrogen). Thirty clones from each treatment per animal were sequenced to ensure that we would have the power to calculate statistical significance of any findings. These analyses identified a significant stress-related increase in 5-hmC at one of the seven CpG dinucleotides examined (Figure 3d-f; effect of treatment F(1,8) = 243.7, p < 0.001, 2-way ANOVA). This CpG had a 1.8-fold increase in 5-hmC levels following exposure to acute stress (20% and 36% 5-hmC in control and experimental mice, respectively; p < 0.02, tukey post-hoc test). Since the presence of 5-hmC in the brain is associated with active transcription of neuronal genes (Szulwach et al. 2011), these data suggest that this stress-related increase in 5-hmC is associated with an increase in Nr3c1 expression. This conclusion is consistent with previous work that found an increase in Nr3c1 expression approaches significance in a small sample size of male 7-week-old mice (N = 3) exposed to a single acute stress (Gray et al. 2014). Notably, standard sodium bisulfite sequencing, which can not distinguish between 5-mC and 5-hmC, did not detect a stress-related change in methylation (i.e., 5-mC + 5-hmC) at any of these seven CpGs (Figure 3b,c,f; 2-way ANOVA). Together, these data suggest that 5-hmC plays a role in response to acute stress and that the total DNA methylation content (i.e., 5mC + 5hmC) remains static (Figure 3b,c,f), meaning that the stress-related epigenetic information may be primarily contained in the 5-hmC profile (Figure 3d-f). This finding represents the first example of acute stress altering this newly discovered epigenetic mark and supports further investigation into both the extent that 5-hmC is disrupted at individual loci and its molecular function in response to stress.
Fig. 3.
Altered abundance of 5-hmC in the 3’UTR of Nr3c1. (A) A schematic of the glucocorticoid receptor gene (Nr3c1), highlighting the region in the 3’UTR that was examined for stress-related levels of 5-hmC. The seven CpG dinucleotides interrogated are underlined and an asterisk marks the dinucleotide with a significant stress-related increase in 5-hmC. (B-E) Circle plots showing the representative 5-mC + 5hmC (B-C) and 5-hmC (D-E) status of the seven individual CpG loci. Each circle represents the methylation status of an independent clone (open circle = unmethylated; closed circle = methylated). The numbers on the left (B,D) and right (C,E) of each panel of circle plots indicates the percent methylation. The numbers between each panel of circle plots indicates the genomic position on mouse chromosome 18, as referenced from UCSC build 38/mm10. F) Bar plot showing the quantitative methylation levels (5-mC + 5-hmC and 5-hmC) of the seven CpG loci (x-axis; Chr18 genomic position) for control and restrained mice, a significant difference is indicated by an asterisk (p < 0.05).
Previous studies showed that a single acute stress had a large magnitude effect on histone modifications throughout the genome, which was the result of their association with retrotransposons, a highly abundant genomic element (Hunter et al. 2009). In contrast to what was shown for histone modification, a single acute stress does not appear to have a large magnitude effect on 5-hmC, as indicated here by the immunoblot and LC/MS/MS data, which did not reveal genomic differences in the hippocampus of stressed and control mice. Although this finding warrants deeper investigation using more definitive genome-wide 5-hmC detection methods (e.g. ChIP-seq), these data suggest that 5-hmc has a different and/or more limited role than histone modifications in response to stress.
While we find stress-related 5-hmC in the 3’UTR of Nr3c1, it is notable that standard sodium bisulfite sequencing of this same region did not detect stress-related methylation differences (5mC + 5hmC; Figure 2b,c,f). These data suggest that previous interpretations regarding the role of DNA methylation in health and disease should be reconsidered, due to the inability of earlier DNA methylation detection methods to distinguish 5-mC and 5-hmC. It will be imperative for future studies to incorporate analyses of both the 5-mC and 5-hmC into their study designs.
As we begin to unravel the links between environmental stimuli and different DNA methylation modifications, studies will need to expand their surveys to the entire genome and to behavioral phenotypes. The previously described relationship between 5-mC and psychiatric disorders may only represent the tip of the iceberg for DNA methylation contributions to complex behavioral phenotypes. The finding here that stress-related changes in 5-hmC occur on a gene best known for its role in the stress pathway supports a role for this epigenetic mark in psychopathology. Further elucidation of behavior-related methylation may provide potentially modifiable molecular substrates that could be treatment targets for preventing the onset of these disorders.
Supplementary Material
Fig. 1. Specificity of the 5-hmC antibody in mouse brain. Representative sections of mouse brain tissue stained with specific antibodies against 5-hmC, NeuN, and a fluorescent stain that binds strongly to A-T rich regions in DNA (4',6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI)); the merge of 5-hmC, NeuN, and DAPI is also shown. Arrows denote 5-hmC+/NeuN−/DAPI+ cells, which represent non-neuronal cells with 5-hmC staining and show that 5-hmC staining is not only specific to neuronal cells.
HIGHLIGHTS.
The characterization of the distribution of 5-hmC in the whole mouse brain following acute stress exposure.
Identification of stress-related changes to 5-hmC in the glucocorticoid receptor gene.
These findings, for the first time, link 5-hmC and the stress pathway.
Acknowledgements
The authors would like to thank Kim Sorens, the WISPIC animal facility, Dr. Michael R. Sussman, and the UW biotechnology center. This work was supported in part by the University of Wisconsin-Madison department of Psychiatry (RSA), the University of Wisconsin Neuroscience training grant T32-GM007507 (SL), the Clinical and Translational Science Award program of NCATS UL1 TR0000427 and KL2 TR000428 (PC), UL1TR000427 to the UW ICTR from NIH/NCATS and funds from the Waisman Center (PC), and NIH P30 HD03352 (Waisman Center). All experiments in this study were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin (UW IACUC protocol #M02529).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Abdolmaleky HM, et al. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006;15(21):3132–45. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia N, et al. Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics. 2011;6(7):853–6. doi: 10.4161/epi.6.7.16461. [DOI] [PubMed] [Google Scholar]
- Chopra P, et al. Array-based assay detects genome-wide 5-mC and 5-hmC in the brains of humans, non-human primates, and mice. BMC Genomics. 2014;15:131. doi: 10.1186/1471-2164-15-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosio C, et al. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J Cell Sci. 2003;116(Pt 24):4905–14. doi: 10.1242/jcs.00804. [DOI] [PubMed] [Google Scholar]
- Ferrazzano P, et al. Age-dependent microglial activation in immature brains after hypoxia- ischemia. CNS Neurol Disord Drug Targets. 2013;12(3):338–49. doi: 10.2174/1871527311312030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, et al. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry. 2014;19(11):1171–8. doi: 10.1038/mp.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102(26):9341–6. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, et al. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci U S A. 2009;106(49):20912–7. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratomi G, et al. Aberrant DNA methylation associated with bipolar disorder identified from discordant monozygotic twins. Mol Psychiatry. 2008;13(4):429–41. doi: 10.1038/sj.mp.4002001. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc Natl Acad Sci U S A. 2014;111(19):7120–5. doi: 10.1073/pnas.1318906111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter MO, et al. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. 2008;64(8):645–52. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Rudenko A, et al. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron. 2013;79(6):1109–22. doi: 10.1016/j.neuron.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach KE, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci. 2011;14(12):1607–16. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, et al. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149(6):1368–80. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, et al. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38(1):111–23. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1. Specificity of the 5-hmC antibody in mouse brain. Representative sections of mouse brain tissue stained with specific antibodies against 5-hmC, NeuN, and a fluorescent stain that binds strongly to A-T rich regions in DNA (4',6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI)); the merge of 5-hmC, NeuN, and DAPI is also shown. Arrows denote 5-hmC+/NeuN−/DAPI+ cells, which represent non-neuronal cells with 5-hmC staining and show that 5-hmC staining is not only specific to neuronal cells.