Abstract
Objective
Up to 90% of adults with rheumatoid arthritis (RA) in clinical remission have persistent synovitis and/or bone marrow lesions (BMLs) on magnetic resonance imaging (MRI). MRI findings in patients with juvenile idiopathic arthritis (JIA) in clinical remission have not been described. We utilized 3T MRI with contrast enhancement to examine JIA patients with hand and/or wrist involvement who were in clinical remission and compared them with a cohort of adult RA patients.
Methods
In total, 11 JIA patients and 10 RA patients with arthritis involving the hands and/or wrists were identified by their primary rheumatologist as being in physician-defined clinical remission, having no signs or symptoms of active arthritis and no medication changes for at least 6 months. A study rheumatologist performed a joint evaluation for tenderness, swelling, and limitation of motion, and study participants self-reported tender joint counts. The participants underwent MRI with intravenous contrast enhancement of 1 hand and wrist with a history of prior symptoms. A pediatric musculoskeletal radiologist blinded to the clinical data scored the MRIs for synovitis, tenosynovitis, and/or BMLs.
Results
Sixty-three percent of the JIA cohort and 70% of the RA cohort had MRI findings of synovitis, BMLs, and/or tenosynovitis. All pediatric patients with MRI abnormalities had normal physician tender and swollen joint counts. The patients’ self-report of painful joint counts did not predict MRI abnormalities.
Conclusion
Over one-half of the patients in clinical remission had MRI evidence of persistent inflammation, defined as the presence of synovitis, tenosynovitis, or BMLs. A substantial portion of patients with JIA may have subclinical disease despite clinical remission.
INTRODUCTION
The goal of therapy for juvenile idiopathic arthritis (JIA) and rheumatoid arthritis (RA) is an absence of disease activity, or remission (1). Studies of newer drugs and more aggressive combination therapy have shown that remission is obtainable (2–4). Several studies of RA and JIA patients have shown that achieving remission early in the disease course leads to a longer duration of remission and improved outcomes for arthritis patients (5–7).
A uniform definition of remission in certain subtypes of JIA for use in clinical trials has been developed and preliminarily validated (8,9). Retrospective cohort studies of JIA using these criteria have shown that remission is achieved in 26–40% of patients (10–14). These studies also showed that remission rates differed among JIA subtypes. Recently, preliminary criteria for defining clinical inactive disease in JIA have also been developed (9). The criteria for remission in RA were first developed in 1981 and have recently been revised (15).
There has been an ongoing discussion of the significance of clinical versus imaging remission in RA. Brown et al showed that over 90% of RA patients in clinical remission have evidence of subclinical synovitis and bone marrow lesions (BMLs) on magnetic resonance imaging (MRI) and/or sonography (16). A followup study showed that evidence of subclinical disease activity on MRI resulted in subsequent joint damage and disease progression (17). More recently, Saleem et al showed that patients classified using a more strict definition of remission versus DAS28 remission still had evidence of subclinical disease activity by power Doppler (18). The authors suggested that imaging results should be incorporated into the definition of remission. Wakefield et al used sonography with Doppler evaluation to demonstrate continuing improvement in sonography scores even after the patient had achieved clinical remission with treatment, suggesting there should be a more prolonged course of treatment before considering whether to discontinue therapy (19). The authors also showed that although clinical remission had been achieved, imaging remission was not achieved for any of the 10 patients during a 12-month followup period. These studies of RA patients suggest that there is significant clinical disease activity detectable by MRI or sonography in the absence of other clinical signs and symptoms of disease activity.
The results of the imaging and remission studies in RA should not be extrapolated to JIA. JIA is a heterogeneous group of diseases that are clinically and pathophysiologically different from RA. Rheumatoid factor (RF) positivity and cutaneous nodules that are not common in JIA are common in RA (20). Some JIA subtypes have a monophasic course, and often JIA patients are able to maintain remission when not taking medication (14). RA often has a prolonged and progressive clinical course, and patients do not maintain remission when stopping medications (21). Therefore, it is important to evaluate the potential differences in the relationship between imaging results and the remission disease state in both RA and JIA.
In the present study, we evaluated JIA patients that met the new criteria for clinical remission or for clinical remission while taking medication using intravenous contrast-enhanced MRI at 3T and compared the rates of MRI-detectable inflammation of this JIA remission group with an adult RA remission group. Our study is the first to look for evidence of imaging-detected subclinical synovitis in patients with JIA who are in remission, and it is the first to compare pediatric and adult arthritis patients who are in remission.
PATIENTS AND METHODS
Patients
In this cross-sectional cohort pilot study, 11 JIA and 10 RA patients with hand or wrist involvement who met the inclusion criteria and had a physician-determined impression of remission from the University of Pittsburgh Medical Center Rheumatology Clinics were studied. The study was approved by the University of Pittsburgh Institutional Review Board. Written informed consent was obtained from all subjects or their guardians, and consent was obtained from all pediatric patients when age appropriate prior to inclusion in the study.
The JIA patients met the International League of Associations for Rheumatology criteria for JIA and had at least 12 months of disease duration (22). The adult RA patients met the 1987 American College of Rheumatology (ACR) classification criteria for an RA diagnosis (23). The JIA patients were identified by their pediatric rheumatologist as being in a physician-determined impression of remission and were screened for the absence of joints with active arthritis (tenderness and swelling) as well as the absence of fever, rash, serositis, splenomegaly, or generalized lymphadenopathy for at least the past 6 months. The adult patients were identified by their rheumatologist as being in a physician-determined impression of remission, and subjects were screened to confirm that they did not have joint swelling or tender joints for at least the past 6 months. Both JIA and RA patients still taking medication could not have had any history of medication changes or clinical indication to change their medication in the previous 6 months. Those with any history of steroid joint injections within the previous 6 months were excluded.
To ensure patient safety for MRI, all patients were screened for any previous reaction to gadolinium-containing intravenous contrast and the ability to keep their hand motionless during imaging. Patients were also excluded if they had a history of comorbidities that would increase patient safety risk in receiving the intravenous contrast, such as abnormal renal function, diabetes mellitus, hypertension, renal disease, or liver disease. Subjects older than 60 years of age had to have documentation of normal levels of blood urea nitrogen and creatinine in the prior 30 days.
Definition of remission
From a clinical perspective, both the JIA and RA patients were identified as being in remission by their primary rheumatologist (which qualified the patients for enrollment into the study); that is, a patient’s physician determined that the patient was asymptomatic with no objective findings of arthritis by physical examination, such as painful, tender, or swollen joints. We also applied formal RA and JIA remission criteria. As outlined by the 2011 ACR/European League Against Rheumatism (EULAR) definition of remission in RA for clinical trials, the patients must have a tender joint count ≤1, swollen joint count ≤1, C-reactive protein (CRP) level ≤1 mg/dl, and patient’s global assessment score ≤1 (range 0–10), or must fulfill the index-based definition of remission, a Simplified Disease Activity Index (SDAI) score of ≤3.3 (24). To fulfill the 2011 RA remission criteria, there is no specified length of time that the patient must be in this disease state. The preliminary criteria for remission in JIA were used for the JIA patients (9). In these criteria, inactive disease is defined as no joints with active arthritis (defined as a joint with swelling or, if no swelling is present, a joint with limitation of motion accompanied by pain on motion and/or tenderness); no fever, rash, serositis, splenomegaly, or generalized lymphadenopathy secondary to JIA; no active uveitis; a normal erythrocyte sedimentation rate (ESR) or CRP level (if both are tested, both must be normal); and a physician’s global assessment of disease activity indicating no disease activity (the best score on the scale used). There were 2 types of clinical remission proposed: clinical remission on medication (if the criteria for inactive disease were met for a minimum of 6 continuous months while the patient was still taking medication) or clinical remission off medication (if the criteria for inactive disease were met for a minimum of 12 continuous months while the patient had stopped taking all antiarthritis and antiuveitis medications).
Assessments of demographic and clinical characteristics
Demographic data (age, sex, and ethnicity) and clinical data, such as current medications, the date of diagnosis, the duration of remission, and other medical history, were recorded. When available, RF status was documented for RA and JIA patients, as well as antinuclear antibody (ANA) status for JIA patients. The clinical data collected for all patients included 28-joint self-report of tender joints, patient report of joint pain by a numerical rating scale (NRS), and patient report for disease activity using an NRS. A study rheumatologist (AB) trained in the Disease Activity Score in 28 joints (DAS28) joint count performed the physician’s 28-joint count for tenderness and swelling and the NRS for the physician’s assessment of disease activity. The JIA patients were also evaluated for loss of motion for the 28-joint count. The Health Assessment Questionnaire (HAQ) or the Childhood HAQ (C-HAQ) score was obtained for the RA and JIA patients, respectively (25,26). Disease activity and remission scores were calculated using the DAS28-ESR or DAS-CRP, the Clinical Disease Activity Index (CDAI), and the SDAI (27–29). While these scores have not been validated in the pediatric population, there is a significant correlation with these measures and the ACR pediatric criteria for improvement in polyarticular JIA (30).
Laboratory assessments
All adult patients had their ESR drawn at the study visit or recorded from the medical record if it was performed within 90 days of the study visit. Pediatric patients had their ESR drawn on a voluntary basis or recorded from the medical record if it was performed within 90 days of the study visit. CRP levels were recorded when available.
MRI
Each patient underwent MRI of the asymptomatic hand and wrist (a single hand and wrist was chosen if the patient had a history of bilateral arthritis). MRI was performed using a 3T clinical scanner (Magnetom Trio, Siemens Medical Solutions) with a receive-only quadrature extremity or wrist transmit–receive coil. Prior to the beginning of the scan, an intravenous catheter was placed in the upper extremity opposite to the side to be imaged. Each patient was scanned while supine with the hand of interest in a neutral position down by the side or prone with the hand of interest placed overhead. The position was determined by the patient’s size. The MRI protocol included the following sequences: coronal T1 weighted turbo spin-echo (TSE) without fat saturation, coronal and axial TSE T2 weighted with fat saturation, sagittal TSE intermediate weighted with fat saturation, and, following the administration of an intravenous contrast (0.1 mmoles/kg body weight [0.2 ml/kg] up to 20 ml, gadobenate dimeglumine [MultiHance, Bracco Diagnostics]) by hand bolus injection, coronal and axial fast spin-echo T1 weighted with fat saturation. All sequences performed following intravenous contrast administration were completed within 10 minutes of the bolus injection. The imaging field of view was 10–16 cm based on patient size and extended from the distal radioulnar joint to the proximal inrerpha-langeal joint.
MRI examination scoring
Each MRI examination was reviewed by a pediatric musculoskeletal radiologist with 20 years of experience who had been blinded to clinical information (TL). The hand and wrist of each patient were evaluated for synovitis, tenosynovitis, and bone marrow edema using a modified Rheumatoid Arthritis Magnetic Resonance Imaging Scoring (RAMRIS) system (31,32).
Statistical analysis
The scores for the HAQ, C-HAQ, DAS28, SDAI, and CDAI were calculated. The Wilcoxon-Mann-Whitney test was used to compare the pediatric and adult disease activity scores. Fisher’s exact test, was performed to compare the MRI scores between the KA and JIA cohorts. All analyses were done using SAS, version 9.2.
RESULTS
Demographic and clinical features of the patients
The demographic and clinical features of the patients are shown in Table 1. For the JIA patients, the mean age was 17 years, and the sample was predominantly female and white. Of the JIA patients tested, 71% (5 of 7) were ANA positive and 17% (1 of 6) were RF positive. The mean duration of disease was 6.5 years (range 2–17 years). The mean age at diagnosis was 11 years (range 2–15 years). The mean length of symptoms prior to diagnosis was 4 months. Nine JIA patients had the polyarticular subtype of JIA, 1 had systemic JIA, and 1 had extended oligoarthritis. The current therapy for JIA included methotrexate (55%), biologic agent therapy (36%), and both disease-modifying antirheumatic drug (DMARD) and biologic agent therapy (1 patient). Three JIA patients were in remission off medication. Two of these patients had stopped therapy for just over 3 years, and 1 had stopped therapy for 10 months. All 3 had the polyarticular subtype of JIA. While nobody was currently taking steroids, 4 JIA patients had a history of steroid therapy; 1 had been taking steroids during the course of disease and the other had a history of arthrocentesis with steroid injection. Ninety percent of the JIA patients had been taking DMARDs at some point in their treatment course.
Table 1.
Demographic and clinical characteristics of the JIA and RA patients*
| JIA | RA | |
|---|---|---|
| Age, mean (range) years | 17 (12–22) | 60 (20–70) |
| Female | 64 | 80 |
| Non-Hispanic white | 100 | 90 |
| African American | 0 | 10 |
| ANA positive† | 71 | 0 |
| RF positive‡ | 17 | 88 |
| Anti-CCP antibody positive§ | – | 100 |
| Taking DMARDs | 82 | 100 |
| Taking biologic agents | 36 | 40 |
| Previously taken DMARDs or biologic agents, no. | ||
| 1 | 2 | 1 |
| 2 | 2 | 1 |
| 3 | 1 | 0 |
| Currently taking NSAIDs | 50 | 40 |
| Previously taken NSAIDs | 60 | 40 |
| Currently taking steroids | 0 | 20 |
| Previously taken steroids | 36 | 30 |
| Age at diagnosis, mean (range) years | 11 (2–15) | 46 (19–63) |
| Length of symptoms prior to diagnosis, mean (range) months | 4 (0–12) | 1 (0–2) |
| Duration of disease, mean (range) years | 7 (2–17) | 7 (1–10) |
| Duration of remission, mean(range) months | 17 (6–48) | 41 (11–127) |
Values are the percentage unless otherwise indicated. JIA = juvenile idiopathic arthritis; RA = rheumatoid arthritis; ANA = antinuclear antibody; RF = rheumatoid factor; anti-CCP = anti–cyclic citrullinated peptide; DMARDs = disease-modifying antirheumatic drugs; NSAIDs = nonsteroidal antiinflammatory drugs.
JIA n = 7, RA n = 6.
JIA n = 6, RA n = 8.
JIA n = 0, RA n = 7.
For the adult RA patients, the mean age was 60 years (range 20–70 years) and 80% were women. The mean duration of RA was 7 years and the mean duration of disease remission was 40 months. The mean age at diagnosis was 46 years with a mean of 1 month of experiencing symptoms prior to diagnosis. All of the RA patients were receiving DMARD therapy at the study entry, and 40% were taking a biologic medication as well. Also at study entry, 40% of the RA patients were receiving nonsteroidal antiinflammatory drug therapy, and 20% were receiving steroid therapy. Thirty percent of the RA patients had a history of steroid therapy by medical record review.
Clinical, laboratory, and function assessments
For the pediatric patients, all available laboratory studies were normal (n = 8). The median for all joint counts was 0 (Table 2). Ninety percent of the self-reported and physician-reported joint counts in the JIA patients were 0. Overall, the group had good functional status, with a median C-HAQ score of 0. Median scores for the DAS28, CDA1, and SDA1 were consistent with being in remission, with scores of 1.6, 0.5, and 1.1, respectively.
Table 2.
Clinical, laboratory, function, and quality of life data for the JIA and RA patients*
| JIA (n = 11) | RA (n = 10) | |
|---|---|---|
| Patient-reported NRS joint pain | 0.5 (0–5) | 0 (0–8.5) |
| Patient-reported tender joint count | 0 (0–4) | 0.5 (0–17) |
| Patient’s global assessment NRS | 1 (0–3) | 0.8 (0–7) |
| Physician-reported tender joint count | 0(0) | 0 (0–9) |
| Physician-reported swollen joint count | 0 (0–4) | 0 (0–7) |
| Physician-reported LOM joint count | 0(0) | N/A |
| Physician’s global assessment NRS | 0 (0–0.5) | 0.3 (0–3) |
| Laboratory assessments | ||
| ESR, mm/hour† | 9.5 (3–13) | 42 (2–42) |
| CRP level, mg/dl‡ | 0.06 (0.02–0.46) | 0.3 (0.2–0.5) |
| C-HAQ/HAQ score | 0 (0–1.38) | 0.8 (0–2.1) |
| DAS28 score§ | 1.6 (0.98–1.96) | 2.5 (0.5–5) |
| CDAI score | 0.5 (0–7.0) | 3 (0–20) |
| SDAI score¶ | 1.1 (0.1–5.8) | 2.5 (0.4–18.9) |
| In ACR/EULAR remission, % | 82 | 56 |
Values are the median (range) unless otherwise indicated. JIA = juvenile idiopathic arthritis; RA = rheumatoid arthritis; NRS = numerical rating scale; LOM = loss of motion; N/A = not applicable; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; C-HAQ = Childhood Health Assessment Questionnaire; HAQ = Health Assessment Questionnaire; DAS28 = Disease Activity Score in 28 joints; CDAI = Clinical Disease Activity Index; SDAI = Simplified Disease Activity Index; ACR = American College of Rheumatology; EULAR = European League Against Rheumatism.
JIA n = 8, RA n = 9.
JIA n = 7, RA n = 4.
JIA n = 8, RA n = 9.
JIA n = 8, RA n = 4.
For the adult patients, 2 had an elevated ESR, and 1 did not undergo a blood draw. All of the other RA patients had a normal ESR. The median for self-report of tender joints was 0.5 (range 0–17), A single RA patient reported 17 tender joints, while most reported 0–2. The median physician-reported tender and swollen joint counts performed by the study rheumatologist were 0 (range 0–9 and range 0–9, respectively). A single RA patient had a physician-reported tender joint count of 9, while most patients had counts of 1–3. The median HAQ score was 0.8 (range 0–2.1). The medians for the DAS28, CDA1, and SDA1 scores were 2.5, 3, and 2.5, respectively.
Comparison of pediatric and adult patients
The median DAS28 score was significantly higher in the RA cohort compared to the JIA cohort (2.5 versus 1.6; P = 0.034), but the differences between the JIA and RA cohorts in the median CDAI score (0.5 versus 3,0; P = 0.114) and the median SDAI score (1.1 versus 2.5; P = 0.233) did not reach statistical significance.
Fulfillment of formal criteria for remission
Of the JIA patients who were classified by their rheumatologist as being in remission, 82% met the preliminary criteria for remission. The 2 JIA patients that did not meet the remission criteria because of a joint count >0 had no findings on MRI.
Of the RA patients who were classified by their rheumatologist as being in remission, 40% met the ACR/EULAR criteria for remission based on swollen and tender joint counts, patient’s global assessment, and CRP level. Fifty-six percent of the RA patients met the revised ACR criteria for remission based on an SDAI score ≤3.3 and 78% met the DAS28 criteria for remission or low disease activity.
MRI findings
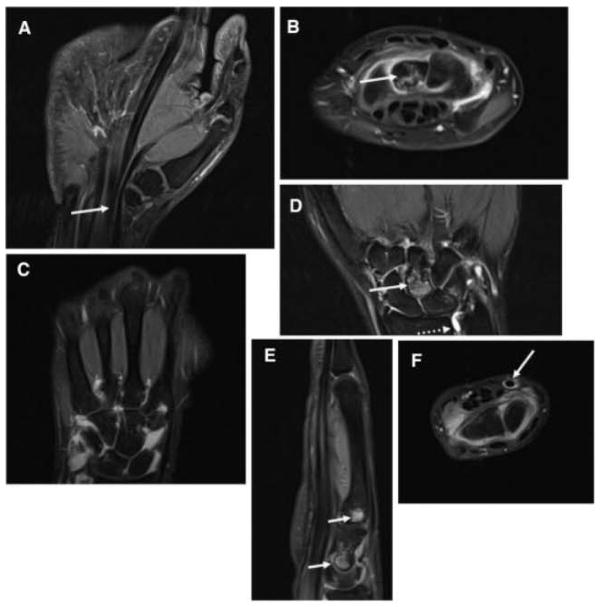
Sixty-three percent of the JIA patients had an abnormality on MRI (Table 3); 5 had synovitis, 3 had BMLs, and 6 had tenosynovitis. Of these JIA patients, 4 had >1 type of abnormality on MRI. One JIA patient had synovitis and tenosynovitis, and 3 had a combination of synovitis, HMLs, and tenosynovitis. Two JIA patients did not have the distal radioulnar joint included on the images. Examples of the pediatric MRI findings are shown in Figure 1.
Table 3.
MRI assessments*
| Pediatric MRI (n = 11) | Adult MRI (n = 10) | |
|---|---|---|
| Imaging-detected abnormalities | ||
| Synovitis | 45 | 70 |
| BMLs | 27 | 60 |
| Tenosynovitis | 55 | 40 |
| Synovitis and BMLs | 0 | 30 |
| Synovitis and tenosynovitis | 9 | 10 |
| BMLs and tenosynovitis | 0 | 0 |
| Synovitis and BMLs and tenosynovitis | 27 | 30 |
| Synovitis or BMLs or tenosynovitis | 64 | 70 |
| Frequency of MRI-detected pathology | ||
| Synovitis | ||
| Radiocarpal joint | 30 | 60 |
| Distal radioulnar joint† | 36 | 60 |
| Proximal intercarpal joint‡ | 36 | 60 |
| Distal intercarpal joint‡ | 27 | 50 |
| Carpometacarpal joints‡ | 27 | 50 |
| Metacarpophalangeal joints§ | 27 | 50 |
| Proximal interphalangeal joints§ | 9 | 40 |
| Flexor tendons | 55 | 40 |
| Extensor tendons | 9 | 20 |
| BMLs | ||
| Radius‡ | 0 | 20 |
| Ulna‡ | 0 | 10 |
| Carpal joints‡ | 27 | 50 |
| Metacarpal joints | 27 | 40 |
| Proximal phalangeal joints§ | 0 | 100 |
Values are the percentage. MRI = magnetic resonance imaging; BMLs = bone marrow lesions.
2 pediatric patients without this joint included on images; 1 adult patient with wrist not included on images.
1 adult patient with wrist not included on images.
1 adult patient with images obscured by artifact.
Figure 1.
Pediatric findings of magnetic resonance imaging (all panels are contrast enhanced and T1 weighted unless otherwise specified). A, Mild flexor tendon tenosynovitis (arrow). B, Intercarpal joint synovitis and bone marrow edema pattern of the capitate (arrow). C, Severe intercarpal joint synovitis, moderate carpometacarpal joint synovitis. D, Capitate bone marrow edema pattern (arrow), radioulnar synovitis (dotted arrow; noncontrast image). E, Capitate and base of the third metacarpal joint focal bone marrow edema pattern (arrows). F, Flexor tendon tenosynovitis (arrow).
Seventy percent of the RA patients demonstrated abnormalities on MRI (Table 3); 7 had synovitis, 6 had BMLs, and 4 had tenosynovitis. Of these RA patients, 3 had both synovitis and BMLs, 1 had synovitis and tenosynovitis, and 3 had synovitis, tenosynovitis, and BMLs. MRI for 1 RA patient did not show adequate imaging of the wrist to allow scoring. One RA patient had an artifact that did not allow reading of the second, third, and fourth proximal phalangeal joints.
JIA and RA comparison
In the RA cohort, there was a trend toward more evidence of persistent inflammation (i.e., the presence of synovitis or BMLs) when compared to the JIA cohort, but this did not reach statistical significance (P = 0.09).
DISCUSSION
This study demonstrates evidence of subclinical disease activity in JIA patients in clinical remission by MRI. One study that used ultrasound to longitudinally evaluate JIA patients with inactive disease showed that 100% of JIA patients that had a subsequent disease flare had evidence of previous subclinical synovial hypertrophy (33). Although this study was published only in abstract form, the results suggest that subclinical disease is clinically significant in JIA. In another study that compared 2 definitions of remission in the same RA cohort (a more strict definition of remission versus DAS28 remission), there was no change in the proportion of joints that had subclinical disease detected by sonography (18). In our study, 82% of the JIA patients met the preliminary criteria for clinical remission or clinical remission on medication. The 2 patients that did not meet the remission criteria due to the observed physician joint count of >0 did not have any abnormalities on MRI. This discrepancy likely reflects the lack of reliability of physician-reported joint assessment (34) rather than actual failure to meet criteria for remission. While the preliminary criteria for remission in JIA were not designed to identify imaging remission or biologic inactivity, our study results highlight the difficulty in defining remission solely by clinical variables. Saleem et al have suggested that evidence of the absence of synovitis on imaging, such as sonography, should be a component of remission criteria in RA (18). In a study of newly diagnosed RA patients, Wakefield et al demonstrated a lag between fulfilling clinical remission of disease activity and imaging remission (19). Although the synovitis scores on imaging of these early RA patients in clinical remission continued to improve over time, no patients achieved an imaging remission within the 1-year followup period. This suggests that in RA, subclinical disease activity can persist for at least a year after clinical remission is reached. There is an absence of data in the literature to indicate whether long-term followup of our JIA cohort would show an eventual resolution of subclinical inflammation over time or would lead to disease flare or progression.
In RA patients, a longitudinal study demonstrated radiographic progression of disease correlated with areas of subclinical disease activity as seen on MRI and/or sonography (17). Prior to this study, it was thought that radiographic disease progression that did not correlate with clinical signs and symptoms of active arthritis indicated a disassociation between BMLs and synovitis (35). In JIA, evidence of subclinical disease activity has been reported using sonography or MRI. Haslam et al demonstrated subclinical synovitis in one-third of early JIA patients with oligoarthritis (36). These sonographic findings led to 1 of 17 patients being reclassified as having polyarthritis. Magni-Manzoni et al reported that subclinical synovitis as detected by sonography is common in JIA, with 5 of 32 patients being reclassified as having polyarthritis based on sonography findings (37). Cannizzaro et al and Weiss et al described reclassification of JIA patients initially thought to have oligoarticular arthritis; however, over 80% of these patients were reclassified to the polyarticular subtype of JIA when temporomandibular joint imaging was completed (38,39). Gardner-Medwin et al demonstrated the presence of subclinical disease in the unaffected knee of children recently diagnosed with monarthritis of the knee. All 4 of the patients that had progression of disease to other joints also had abnormal MRI findings in the unaffected knee (40). These studies have important clinical implications, especially with the release of new treatment guidelines that recommend treatment based on the number of joints affected by JIA (41). Studies of subclinical disease in early JIA suggest that we could be misclassifying patients by relying on the clinical examination alone, and therefore potentially undertreating JIA.
Tenosynovitis was found in 55% of pediatric participants in our study. Tenosynovitis is not part of the preliminary criteria for remission in JIA. The majority of pediatric participants in this study were classified as polyarticular JIA. It is known that polyarticular JIA, systemic JIA, and enthesitis-related arthritis subtypes are less likely to achieve remission or an inactive disease state with treatment (42,43). It is important to note that this study population may represent a subset of patients with disease that is less responsive to treatment and may not be representative of all JIA subtypes.
This study is unique for its comparison of JIA and RA cohorts. While the disease durations of both cohorts were similar, the JIA cohort had been in clinical remission for a shorter length of time. Both groups had a similar rate of DMARD and biologic agent use; however, the JIA cohort had 3 patients in remission off medication, while the RA cohort had none. Despite equivalent disease durations, the JIA cohort trended toward lower scores on the C-HAQ than comparable HAQ scores in the RA cohort, but this difference was not statistically significant. The RA cohort trended toward greater frequency of BMLs. Generally, the JIA cohort had better quality of life and fewer findings on MRI compared to the RA cohort. Only the JIA cohort included patients that had achieved clinical remission off medication, but 2 of the 3 patients still had evidence of inflammation on MRI (i.e., 1 had mild tenosynovitis, and 1 had synovitis, tenosynovitis, and BMLs). While rheuma-tologists for both the adult and pediatric patients referred those that they determined to be in clinical remission, the RA patients were less likely to meet formal criteria for remission compared to the JIA patients. The differences in the findings between our JIA and RA cohorts highlight the difficulties that may arise when trying to extrapolate results from RA studies to the JIA population. The existing literature lacks longitudinal imaging studies in JIA that have the potential to increase our knowledge of the natural history the disease and improve our treatment approaches. There is also a lack of a formal scoring method of MRI for JIA. It has been demonstrated, however, that RAMRIS may be useful for scoring MRI in JIA (31). There is also a gap in the knowledge of what normal MRI findings are in skele-tally immature children. A recent study by Muller et al found a significant amount of bone marrow edema in children without arthritis (44). JIA patients in clinical remission were more likely to have an absence of MRI abnormalities than RA patients. While this did not reach statistical significance (P = 0.09), likely due to a lack of power with our small sample size, it is consistent with our clinical impression that disease is more likely to truly remit in patients with JIA than RA. This is also reflected in the stricter criteria used for remission in JIA than RA. The ACR/EULAR criteria for remission in RA allow 1 swollen joint, while the preliminary criteria for remission in JIA require that there are no swollen joints.
The limitations of this study include a small sample size and a bias toward selection of patients with polyarticular arthritis patients due to the decision to image the hand and wrist. It is known that clinical remission is achieved less often in polyarticular disease, and the selection of this patient group may show a bias toward more disease activity than other JIA subtypes (45). This is a cross-sectional study, and long-term outcomes for patients in remission with subclinical disease activity remain unknown.
To improve outcomes and reduce the morbidity associated with JIA, the goal of treatment should be disease remission. In our study, we found evidence of subclinical disease in patients that met the definition of clinical remission. Other JIA studies have demonstrated that disease flares and disease progression to other joints are associated with subclinical disease activity. A significant portion of the JIA population is potentially being undertreated due to a lack of clinical symptoms. We suggest that imaging to detect subclinical inflammation should be a part of the evaluation of disease status in JIA.
Significance & Innovations.
Our results demonstrate evidence of subclinical inflammation in juvenile idiopathic arthritis (JIA) patients in remission for at least 6 months by magnetic resonance imaging (MRI).
A total of 64% of JIA patients and 70% of rheumatoid arthritis patients in physician-determined clinical remission had signs of disease activity by 3T MRI with contrast.
Overall, 82% of the JIA patients met the 2004 criteria for clinical remission or clinical remission on medication.
In JIA, tenosynovitis of the flexor tendons was the most common abnormality; bone marrow lesions were limited to the carpal and metacarpal joints.
Acknowledgments
Supported in part by the Children’s Hospital of Pittsburgh. Dr. Hirsch’s work was supported by the NIH (grants R21-AR-056690 and T32-AR-052282). Dr. Kwoh’s work was supported by the NIH (grant P60-AR-054731).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Kwoh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Brown, Hirsch, Laor, Kwoh.
Acquisition of data. Brown, Hirsch, Laor, Levesque, Starz, Francis.
Analysis and interpretation of data. Brown, Hirsch, Hannon, Kwoh.
References
- 1.Wallace CA. Current management of juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2006;20:279–300. doi: 10.1016/j.berh.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Bartoli M, Taro M, Magni-Manzoni S, Pistorio A, Traverso F, Viola S, et al. The magnitude of early response to methotrexate therapy predicts long-term outcome of patients with juvenile idiopathic arthritis. Ann Rheum Dis. 2008;67:370–4. doi: 10.1136/ard.2007.073445. [DOI] [PubMed] [Google Scholar]
- 3.Prince FH, Twilt M, ten Cate R, van Rossum MA, Armbrust W, Hoppenreijs EP, et al. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis. 2009;68:635–41. doi: 10.1136/ard.2007.087411. [DOI] [PubMed] [Google Scholar]
- 4.Woo P. Theoretical and practical basis for early aggressive therapy in paediatric autoimmune disorders. Curr Opin Rheumatol. 2009;21:552–7. doi: 10.1097/BOR.0b013e32832f142e. [DOI] [PubMed] [Google Scholar]
- 5.Breedveld FC, Emery P, Keystone E, Patel K, Furst DE, Kalden JR, et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004;63:149–55. doi: 10.1136/ard.2003.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landewe RB, Boers M, Verhoeven AC, Westhovens R, van de Laar MA, Markusse HM, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347–56. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- 7.Magnani A, Pistorio A, Magni-Manzoni S, Falcone A, Lombardini G, Bandeira M, et al. Achievement of a state of inactive disease at least once in the first 5 years predicts better outcome of patients with polyarticular juvenile idiopathic arthritis. J Rheumatol. 2009;36:628–34. doi: 10.3899/jrheum.080560. [DOI] [PubMed] [Google Scholar]
- 8.Wallace CA, Ravelli A, Huang B, Giannini EH. Preliminary validation of clinical remission criteria using the OMERACT filter for select categories of juvenile idiopathic arthritis. J Rheumatol. 2006;33:789–95. [PubMed] [Google Scholar]
- 9.Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–4. [PubMed] [Google Scholar]
- 10.Fantini F, Gerloni V, Gattinara M, Cimaz R, Arnoldi C, Lupi E. Remission in juvenile chronic arthritis: a cohort study of 683 consecutive cases with a mean 10-year followup. J Rheumatol. 2003;30:579–84. [PubMed] [Google Scholar]
- 11.Fernandes TA, Corrente JE, Magalhaes CS. Remission status follow-up in children with juvenile idiopathic arthritis. J Pediatr (Rio J) 2007;83:141–8. doi: 10.2223/JPED.1601. [DOI] [PubMed] [Google Scholar]
- 12.Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schontube M, et al. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2392–401. doi: 10.1002/art.10444. [DOI] [PubMed] [Google Scholar]
- 13.Oen K, Malleson PN, Cabral DA, Rosenberg AM, Petty RE, Cheang M. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29:1989–99. [PubMed] [Google Scholar]
- 14.Wallace CA, Huang B, Bandeira M, Ravelli A, Giannini EH. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:3554–62. doi: 10.1002/art.21389. [DOI] [PubMed] [Google Scholar]
- 15.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 16.Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug–induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–73. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- 17.Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 18.Saleem B, Brown AK, Keen H, Nizam S, Freeston J, Wakefield R, et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis. 2011;70:792–8. doi: 10.1136/ard.2010.134445. [DOI] [PubMed] [Google Scholar]
- 19.Wakefield RJ, Freeston JE, Hensor EM, Bryer D, Quinn MA, Emery P. Delay in imaging versus clinical response: a rationale for prolonged treatment with anti–tumor necrosis factor medication in early rheumatoid arthritis. Arthritis Rheum. 2007;57:1564–7. doi: 10.1002/art.23097. [DOI] [PubMed] [Google Scholar]
- 20.Prieur AM, Chedeville G. Prognostic factors in juvenile idiopathic arthritis. Curr Rheumatol Rep. 2001;3:371–8. doi: 10.1007/s11926-996-0006-6. [DOI] [PubMed] [Google Scholar]
- 21.Harris E, Jr, Firestein G. Clinical features of rheumatoid arthritis. In: Firestein G, editor. Kelley’s textbook of rheumatology. 8. Philadelphia: W. B. Saunders Company; 2008. pp. 1087–118. [Google Scholar]
- 22.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63:573–86. doi: 10.1002/art.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761–9. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 27.Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796–806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 29.Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- 30.Ringold S, Chon Y, Singer NG. Associations between the American College of Rheumatology pediatric response measures and the continuous measures of disease activity used in adult rheumatoid arthritis: a secondary analysis of clinical trial data from children with polyarticular-course juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:3776–83. doi: 10.1002/art.24983. [DOI] [PubMed] [Google Scholar]
- 31.Malattia C, Damasio MB, Pistorio A, Ioseliani M, Vilca I, Valle M, et al. Development and preliminary validation of a paediatric-targeted MRI scoring system for the assessment of disease activity and damage in juvenile idiopathic arthritis. Ann Rheum Dis. 2011;70:440–6. doi: 10.1136/ard.2009.126862. [DOI] [PubMed] [Google Scholar]
- 32.Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies: core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6. [PubMed] [Google Scholar]
- 33.Magni-Manzoni S, Scire CA, Ravelli A, Klersy C, Rossi S, Muratore V. High frequency of subclinical ultrasound-detected synovitis in juvenile idiopathic arthritis patients with clinically-defined inactive disease [abstract] Arthritis Rheum. 2010;62 (Suppl):S933. [Google Scholar]
- 34.Pincus T. Limitations of a quantitative swollen and tender joint count to assess and monitor patients with rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2008;66:216–23. [PubMed] [Google Scholar]
- 35.Mulherin D, Fitzgerald O, Bresnihan B. Clinical improvement and radiological deterioration in rheumatoid arthritis: evidence that the pathogenesis of synovial inflammation and articular erosion may differ. Br J Rheumatol. 1996;35:1263–8. doi: 10.1093/rheumatology/35.12.1263. [DOI] [PubMed] [Google Scholar]
- 36.Haslam KE, McCann LJ, Wyatt S, Wakefield RJ. The detection of subclinical synovitis by ultrasound in oligoarticular juvenile idiopathic arthritis: a pilot study. Rheumatology (Oxford) 2010;49:123–7. doi: 10.1093/rheumatology/kep339. [DOI] [PubMed] [Google Scholar]
- 37.Magni-Manzoni S, Epis O, Ravelli A, Klersy C, Veisconti C, Lanni S, et al. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:1497–504. doi: 10.1002/art.24823. [DOI] [PubMed] [Google Scholar]
- 38.Cannizzaro E, Schroeder S, Muller LM, Kellenberger CJ, Saurenmann RK. Temporomandibular joint involvement in children with juvenile idiopathic arthritis. J Rheumatol. 2011;38:510–5. doi: 10.3899/jrheum.100325. [DOI] [PubMed] [Google Scholar]
- 39.Weiss PF, Arabshahi B, Johnson A, Bilaniuk LT, Zarnow D, Cahill AM, et al. High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis, as detected by magnetic resonance imaging but not by ultrasound. Arthritis Rheum. 2008;58:1189–96. doi: 10.1002/art.23401. [DOI] [PubMed] [Google Scholar]
- 40.Gardner-Medwin JM, Killeen OG, Ryder CA, Bradshaw K, Johnson K. Magnetic resonance imaging identifies features in clinically unaffected knees predicting extension of arthritis in children with monoarthritis. J Rheumatol. 2006;33:2337–43. [PubMed] [Google Scholar]
- 41.Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63:465–82. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donnithorne KJ, Cron RQ, Beukelman T. Attainment of inactive disease status following initiation of TNF-α inhibitor therapy for juvenile idiopathic arthritis: enthesitis-related arthritis predicts persistent active disease. J Rheumatol. 2011;38:2675–81. doi: 10.3899/jrheum.110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otten MH, Prince FH, Armbrust W, ten Cate R, Hoppenreijs EP, Twilt M, et al. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA. 2011;306:2340–7. doi: 10.1001/jama.2011.1671. [DOI] [PubMed] [Google Scholar]
- 44.Muller LS, Avenarius D, Damasio B, Eldevik OP, Malattia C, Lambot-Juhan K, et al. The paediatric wrist revisited: redefining MR findings in healthy children. Ann Rheum Dis. 2011;70:605–10. doi: 10.1136/ard.2010.135244. [DOI] [PubMed] [Google Scholar]
- 45.Shenoi S, Wallace CA. Remission in juvenile idiopathic arthritis: current facts. Curr Rheumatol Rep. 2010;12:80–6. doi: 10.1007/s11926-010-0085-2. [DOI] [PubMed] [Google Scholar]