Abstract
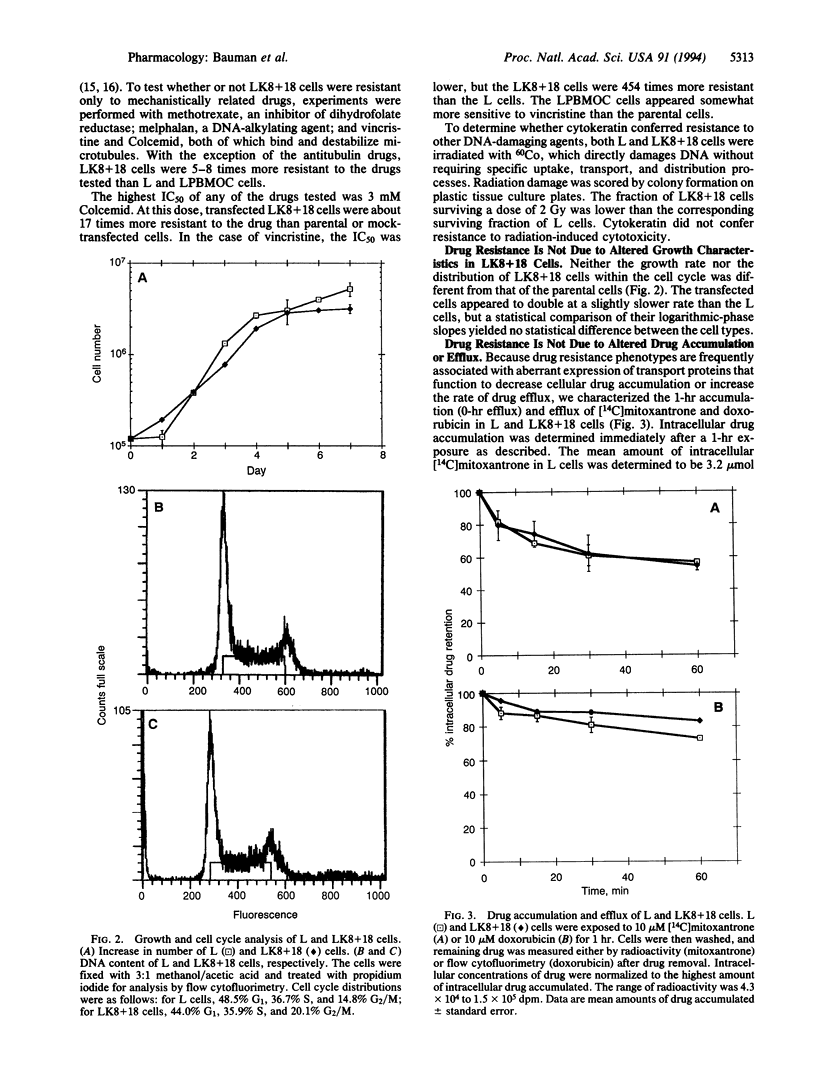
The cytokeratin network is an extensive filamentous structure in the cytoplasm whose biological function(s) is unknown. Based upon previous data showing the modification of cytokeratin by mitoxantrone, we investigated the ability of cytokeratin networks to influence the survival response of cells to chemotherapeutic agents. We have compared the survival of mouse L fibroblasts lacking cytokeratins with that of L cells transfected with cytokeratins 8 and 18 in the presence of chemotherapeutic drugs. The expression of cytokeratins 8 and 18 conferred a multiple drug resistance phenotype on cells exposed to mitoxantrone, doxorubicin, methotrexate, melphalan, Colcemid, and vincristine. The degree of drug resistance was 5-454 times that of parental cells, depending upon the agent used. Drug resistance could not be attributed to altered growth characteristics, altered drug accumulation, or an altered drug efflux in the transfected cells. Cytokeratin does not confer resistance to ionizing radiation, which damages DNA independently of intracellular transport mechanisms. These data suggest a role for cytokeratin networks in conferring a drug resistance phenotype.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin V., Haseltine W. A. Reduction of adriamycin to a semiquinone-free radical by NADPH cytochrome P-450 reductase produces DNA cleavage in a reaction mediated by molecular oxygen. J Biol Chem. 1981 May 25;256(10):4747–4756. [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Chan R., Rossitto P. V., Edwards B. F., Cardiff R. D. Presence of proteolytically processed keratins in the culture medium of MCF-7 cells. Cancer Res. 1986 Dec;46(12 Pt 1):6353–6359. [PubMed] [Google Scholar]
- Cress A. E., Roberts R. A., Bowden G. T., Dalton W. S. Modification of keratin by the chemotherapeutic drug mitoxantrone. Biochem Pharmacol. 1988 Aug 1;37(15):3043–3046. doi: 10.1016/0006-2952(88)90296-1. [DOI] [PubMed] [Google Scholar]
- Denny W. A. DNA-intercalating ligands as anti-cancer drugs: prospects for future design. Anticancer Drug Des. 1989 Dec;4(4):241–263. [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins. Cell. 1983 Jul;33(3):915–924. doi: 10.1016/0092-8674(83)90034-x. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Bachant J. B., Domingo A. Functions of intermediate filaments. Cell Motil Cytoskeleton. 1989;14(3):309–331. doi: 10.1002/cm.970140302. [DOI] [PubMed] [Google Scholar]
- Knapp L. W., O'Guin W. M., Sawyer R. H. Drug-induced alterations of cytokeratin organization in cultured epithelial cells. Science. 1983 Feb 4;219(4584):501–503. doi: 10.1126/science.6186022. [DOI] [PubMed] [Google Scholar]
- Kolodziejczyk P., Reszka K., Lown J. W. Enzymatic oxidative activation and transformation of the antitumor agent mitoxantrone. Free Radic Biol Med. 1988;5(1):13–25. doi: 10.1016/0891-5849(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Kulesh D. A., Ceceña G., Darmon Y. M., Vasseur M., Oshima R. G. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989 Apr;9(4):1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Schiller D. L., Hatzfeld M., Achtstätter T., Moll R., Jorcano J. L., Magin T. M., Franke W. W. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Roberts R. A., Cress A. E., Dalton W. S. Persistent intracellular binding of mitoxantrone in a human colon carcinoma cell line. Biochem Pharmacol. 1989 Dec 1;38(23):4283–4290. doi: 10.1016/0006-2952(89)90527-3. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]




