Abstract
This study aimed to investigate the protective effects of dietary glutamate and aspartate supplementations on diquat-induced oxidative stress in piglets. Diquat injection significantly reduced growth performance, including body weight, average daily weight gain, and feed intake (P<0.05). Meanwhile, diquat administration induced oxidative stress evidenced by the decreased serum nitric oxide (NO) and elevated malondialdeyhde (MDA) concentration (P<0.05). Furthermore, diquat-induced oxidative stress disrupted intestinal absorption system and decreased serum threonine, serine, and glycine levels. Dietary supplementation with glutamate improved final body weight, antioxidant system, and expressions of amino acids transporters and enhanced serum glutamate concentration compared with diquat group (P<0.05). While aspartate failed to alleviate diquat-induced oxidative stress, growth depression, and dysfunction of nutrients absorption except for liver relative weight. In conclusion, dietary supplementation with glutamate confers beneficial effects on diquat-induced oxidative stress in piglets, while aspartate exhibits little effects.
Introduction
Oxidative stress can be induced by various factors during the animal growth and development, including physical (weaning, housing, transport, and novel handling), social (relocation with unfamiliar penmates), and pathological environments [1–3]. We found that pathological factors such as mold-contaminated feed, porcine circovirus type 2 infection, and dextran sulfate sodium-induced colitis exhibit an inhibitory effect on activities of antioxidant enzymes and induce oxidative stress in pigs and mice [3–5]. Our latest studies also revealed that birth and weaning processes disrupt oxidative balance and cause oxidative injury in piglets [2,6]. Oxidative stress correlates with the modification of protein, lipid oxidation, and nucleic acid breaks, and compelling evidences have demonstrated that oxidative stress involves in the development of many diseases [1].
Glutamate and aspartate are functional amino acids and have been shown to exert various functions in nutrients metabolisms, energy requirements, immune responses, oxidative stress, regulation of signaling pathways, and synaptic transmitting [7–9]. Dietary supplementation with glutamate exhibits a beneficial role in deoxynivalenol and mycotoxins challenged pigs [10,11]. Furthermore, Pi et al. reported that dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge [12]. However, little is known about effects of glutamate and aspartate on oxidative stress. Diquat has been widely used to induce oxidative stress in vivo and injection of diquat exerts inhibitory effects on growth performance [13] and nutrients metabolism [14]. Thus, the current study was to investigate the protective roles of dietary glutamate and aspartate in diquat-induced oxidative stress in piglets.
Materials and Methods
This study was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving animal subjects were approved by the animal welfare committee of the Institute of Subtropical Agriculture, University of Chinese Academy of Sciences [13].
Twenty-four healthy piglets of similar body weight (9.92 ± 0.30 kg) (Landrace× Large White) (ZhengHong Co., China) were randomly divided into four groups (n = 6): one control group (control), one diquat group (diquat), one glutamate group in which piglets were fed 2% glutamate (glutamate group), and one aspartate group in which piglets received 2% aspartate (aspartate group). All piglets were fed basal diet for 5 days, then injected intraperitoneally (i.p.) with either 10 mL saline or 10 mg/kg body weight diquat in 10 ml saline to induce oxidative stress according to previous report [14]. After injection of diquat, feed in glutamate and aspartate groups was added 2% glutamate and 2% aspartate, respectively. Feed intake was recorded daily to calculate average daily feed intake (ADFI). After 7 days of experimental period, body weight was weighed and blood was sampled from a jugular vein before slaughter [15]. All piglets were anesthetized with sodium pentobarbital and killed by jugular puncture [16]. The basal diet was prepared from corn, soybean meal, wheat bran, limestone, CaHPO4, NaCl, and additive premix to meet or exceed the nutritional requirements of piglets according to our previous report [17]. Glutamate and aspartate were added to the feed and mixed uniformly.
Calculation of relative organ weights
The heart, liver, spleen, and kidney were separated and weighed. The relative organ weights were calculated basing the ratio of organ weigh to body weight [18].
Measurements of oxidative stress index
Blood samples were centrifuged at 3000 × g for 10 min and 4°C, and supernatant were collected for serum analysis [19]. Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), nitric oxide (NO), and malondialdeyhde (MDA) in serum were measured using spectrophotometric kits in accordance with the manufacturer’s instructions (Nanjing Jiangcheng Biotechnology Institute, China) [20].
Histomorphometry determination
Samples from jejunal and ileal middle section (3cm) were kept in 4% neutral buffered 10% formalin for H&E staining. Villus height and crypt depth were measured using an image-analysis system [4].
Determination of serum amino acids
Amino acids in serum were determined by LC–MS/MS (HPLC Ultimate3000 and 3200 QTRAP LC–MS/MS) according to our previous report [4].
Real time PCR (RT-PCR)
Extraction of total RNA and its reverse transcription were performed according to our previous reports [4,6]. Primers were designed with Primer 5.0 according to the gene sequence of pig (http://www.ncbi.nlm.nih.gov/pubmed/) to produce an amplification product (Table 1). β-actin was used as a housekeeping gene to normalize target gene transcript levels. Real-time PCR was performed according to our previous studies [4,6]. Relative expression was normalized and expressed as a ratio to the expression in control group.
Table 1. Primers used in this study.
| Gene | Accession No. | Primer squence (5’-3’) | Size (bp) |
|---|---|---|---|
| SLC1A1 | NM_001164649.1 | F:GGCACCGCACTCTACGAAGCA | 177 |
| R:GCCCACGGCACTTAGCACGA | |||
| SLC7A1 | NM_001012613.1 | F: TGCCCATACTTCCCGTCC | 192 |
| R:GGTCCAGGTTACCGTCAG | |||
| NAAT | XM_003355984.2 | F:GATTGTGGAGATGGAGGATGTG | 128 |
| R:TGCGAGTGAAGAGGAAGTAGAT | |||
| β-actin | XM_003124280.3 | F:CTGCGGCATCCACGAAACT | 147 |
| R:AGGGCCGTGATCTCCTTCTG |
F: forward primer; R: reverse primer; SLC1A1: solute carrier family 1, member 1; SLC7A1: solute carrier family 7, member 1; NAAT: neutral amino acid transporter.
Statistical Analysis
All data were analyzed using Grubbs’ test and then performed by using the one-way analysis of variance (ANOVA) to test homogeneity of variances via Levene’s test and followed with Ducan’s multiple comparison test (SPSS 17.0 software). Data are expressed as the mean ± standard error of the mean. Values in the same row with different superscripts are significant (P < 0.05), while values with same superscripts are not significant different (P > 0.05) [21].
Results
Effects of glutamate and aspartate on growth performance in diquat-challenged piglets
The results of growth performance were summarized in Table 2 and S1 Dataset. Injection of diquat significantly decreased final body weight, average daily weight gain, and average daily intake (P < 0.05). Meanwhile, supplementation with glutamate restored the inhibitory effect of body weight caused by diquat (P < 0.05).
Table 2. Growth performance and relative organ weights in four groups.
| Item | Control | Diquat | Glutamate | Aspartate | SE | p-value |
|---|---|---|---|---|---|---|
| Initial body weight (kg) | 9.88±0.70 | 9.90±0.61 | 9.98±0.65 | 9.91±0.59 | 0.30 | 0.999 |
| Final body weight (kg) | 11.36±0.62 a | 8.87±0.75 b | 10.31±0.50 a | 8.82±0.47 b | 0.44 | 0.009 |
| Average daily weight gain (kg) | 0.32±0.05 a | -0.08±0.04 b | -0.06±0.01 b | -0.12±0.04 b | 0.05 | <0.001 |
| Average daily intake (kg) | 0.61±0.07 a | 0.26±0.07 b | 0.32±0.03 b | 0.26±0.05 b | 0.04 | 0.001 |
| Heart (%) | 4.68±0.09 | 6.17±0.66 | 5.57±0.27 | 6.37±0.78 | 0.30 | 0.196 |
| Liver (%) | 26.31±0.29 b | 30.80±0.84 a | 31.72±1.93 a | 26.27±1.66 b | 0.84 | 0.017 |
| Spleen (%) | 2.02±0.05 | 1.90±0.12 | 2.05±0.09 | 2.03±0.07 | 0.04 | 0.599 |
| Kidney (%) | 5.20±0.22 | 6.44±0.58 | 6.28±0.51 | 6.10±0.37 | 0.23 | 0.225 |
a,bWithin a row, means with different superscripts differ (P<0.05). The same as below.
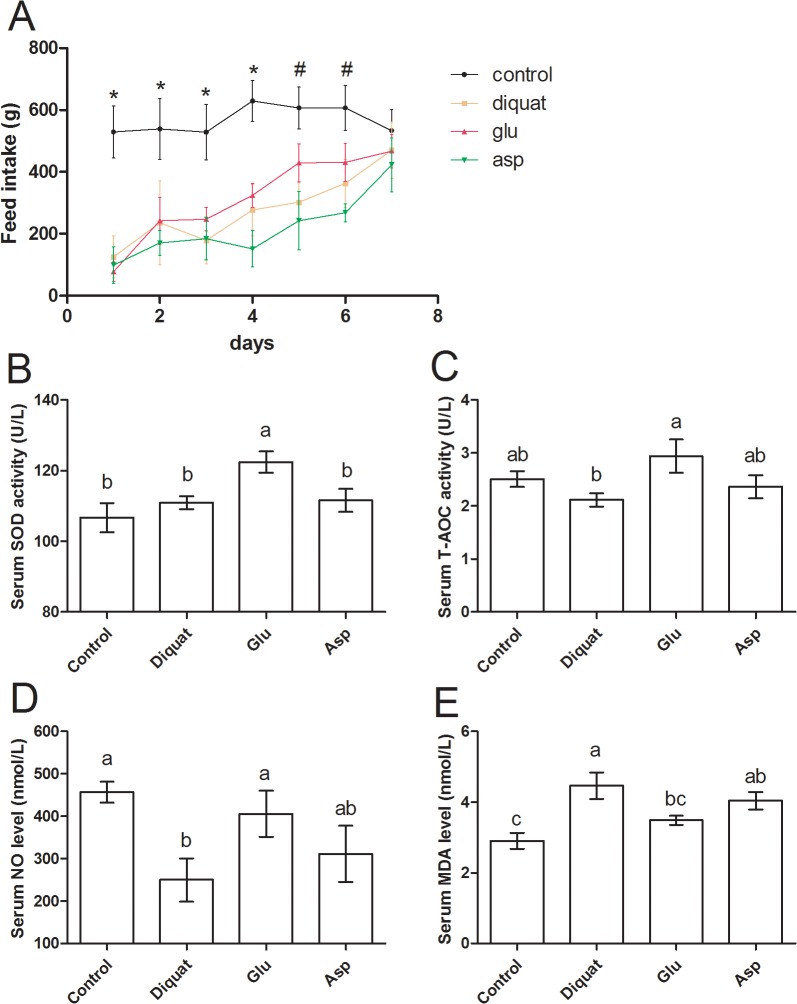
In addition, we monitored the feed intake in all piglets after challenging diquat (Fig 1 A and S1 Dataset). Feed intake was markedly lower after diquat injection compared with control group at day 1 to day 6 (P < 0.05). While at day 5 and 6, feed intake in glutamate group was much higher than that in diquat group (P > 0.05). At day 7, all piglets challenged diquat recovered to normal intake (P > 0.05).
Fig 1. Feed intake (A) and serum SOD, T-AOC, NO, and MDA levels (B, C, D, and E) in four groups after exposure to diquat.
* means feed intake in control is significantly higher than that in other three groups and # means feed intake in control is markedly higher than that in diquat and aspartate groups (P<0.05), but has no difference compared with glutamate group (P>0.05).
Effects of glutamate and aspartate on relative organ weights in diquat-challenged piglets
The effects of glutamate and aspartate on relative organ weights in diquat-challenged piglets were shown in Table 2 and S1 Dataset. There was no difference in heart, spleen, and kidney relative weights between four groups (P > 0.05), while diquat injection significantly increased liver relative weight (P < 0.05). Although supplementation with glutamate failed to exhibit a protective effect on alteration in liver relative weight caused by diquat (P > 0.05), aspartate significantly reduced the liver relative weight compared with diquat group (P < 0.05).
Effects of glutamate and aspartate on oxidative stress in diquat-challenged piglets
Diquat has been reported to induce oxidative stress in mice [22] and pigs [14], so we next determined serum oxidative stress-related indexes, including SOD, T-AOC, NO, and MDA (Fig 1 and S2 Dataset). The results showed that serum SOD activity was not changed after exposure to diquat, while supplementation with glutamate significantly increased SOD activity compared with other groups (P < 0.05). Diquat administration tended to block serum T-AOC (P > 0.05) and glutamate markedly restored (P < 0.05) the inhibitory function compared with diquat qroup. NO, a gas signal related to oxidative stress [23], markedly decreased after exposure to diquat (P < 0.05), dietary supplementation with glutamate increased serum NO level compared with diquat group (P < 0.05). MDA is a metabolite of lipid oxidation [24] and was significant higher in diquat group than that in control piglets. Similarly, glutamate supplementation significantly reduced the MDA level compared with diquat qroup (P < 0.05). However, in the present study, we failed to notice any significant difference in serum SOD, T-AOC, NO, and MDA levels after dietary supplementation with aspartate (P < 0.05).
Effects of glutamate and aspartate on intestinal morphological structure in diquat-challenged piglets
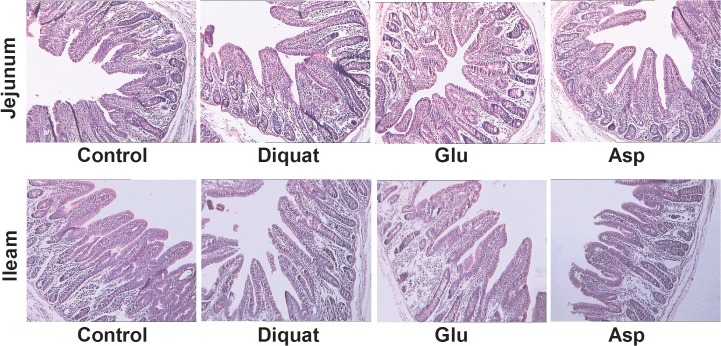
The HE staining results shown at Fig 2, we found that diquat injection failed to cause intestinal morphological injury. We further calculated the intestinal villus height and crypt depth in four groups (Table 3 and S3 Dataset) and there was no difference (P > 0.05).
Fig 2. Histological evaluation of intestinal tissues (HE×250) after exposure to diquat.
Table 3. The intestinal villus height and crypt depth in four groups.
| Item(um) | Control | Diquat | Glutamate | Aspartate | SE | p-value |
|---|---|---|---|---|---|---|
| Jejunam | ||||||
| villus height | 385.82±21.19 | 402.55±27.18 | 357.94±10.32 | 375.56±15.60 | 10.23 | 0.507 |
| Crypt depth | 179.00±13.75 | 175.18±10.55 | 184.03±15.76 | 173.20±8.08 | 5.92 | 0.930 |
| V/C | 2.19±0.13 | 2.16±0.13 | 2.13±0.18 | 2.044±0.12 | 0.07 | 0.899 |
| Ileam | ||||||
| Villus height | 389.33±27.63 | 391.63±23.79 | 370.95±31.49 | 337.28±12.06 | 12.43 | 0.404 |
| Crypt depth | 181.20±22.18 | 185.75±21.56 | 142.56±20.57 | 147.53±10.57 | 9.84 | 0.294 |
| V/C | 2.24±0.18 | 2.03±0.18 | 2.24±0.20 | 2.21±0.08 | 0.08 | 0.806 |
Effects of glutamate and aspartate on serum free amino acids in diquat-challenged piglets
Serum free amino acids analysis after diquat exposure was shown at Table 4 and S3 Dataset. Compared with control group, threonine, serine, and glycine concentrations were significant lower in diquat-challenged piglets (P < 0.05). Although supplementation with glutamate failed to restore the threonine, serine, and glycine concentrations, it significantly enhanced serum glutamate level (P < 0.05). However, aspartate failed to affect serum amino acids concentration (P > 0.05).
Table 4. Serum free amino acids in four groups.
| Item | Control | Diquat | Glutamate | Aspartate | SE | p-valve |
|---|---|---|---|---|---|---|
| Aspartate | 134.14±12.03 | 111.65±16.01 | 149.42±15.10 | 115.59±13.33 | 7.34 | 0.776 |
| Threonine | 842.33±31.23a | 490.00±95.47b | 658.88±58.44ab | 473.99±90.93b | 46.39 | 0.096 |
| Serine | 265.65±13.90a | 188.56±17.94bc | 227.00±20.31ab | 174.59±13.29c | 10.71 | 0.863 |
| Glutamate | 721.79±45.89ab | 681.63±43.59b | 872.23±74.57a | 707.58±38.32b | 28.99 | 0.118 |
| Glycine | 1548.84±74.86a | 1143.86±148.21b | 1286.86±162.39ab | 960.69±85.48b | 73.05 | 0.410 |
| Alanine | 912.77±30.54 | 937.18±92.15 | 1159.52±93.53 | 874.15±144.37 | 51.55 | 0.115 |
| Cysteine | 35.35±2.80b | 47.34±3.87ab | 57.55±4.18a | 49.26±6.41ab | 2.68 | 0.062 |
| Valine | 187.19±23.30 | 214.78±16.33 | 225.52±28.11 | 236.80±29.15 | 12.16 | 0.590 |
| Methionine | 75.57±9.37 | 51.19±7.89 | 75.89±11.61 | 50.78±2.29 | 4.74 | 0.389 |
| Isoleucine | 100.83±8.79 | 113.14±8.76 | 112.55±7.74 | 113.66±11.97 | 4.54 | 0.659 |
| Leucine | 214.20±13.01 | 244.83±10.19 | 261.60±24.51 | 231.67±15.07 | 8.54 | 0.140 |
| Tyrosine | 64.49±9.90 | 47.92±5.18 | 58.77±5.91 | 44.25±4.58 | 3.56 | 0.591 |
| Phenylalanine | 137.52±6.14 | 133.79±9.75 | 138.19±6.09 | 131.80±7.79 | 3.58 | 0.666 |
| Lysine | 472.97±56.37 | 372.02±49.98 | 399.15±55.32 | 372.37±28.24 | 24.33 | 0.499 |
| NH3 | 780.68±25.52 | 804.27±29.82 | 861.79±35.81 | 785.66±35.18 | 16.30 | 0.793 |
| Histidine | 92.60±12.51 | 83.18±6.54 | 109.84±11.89 | 101.12±8.86 | 5.20 | 0.357 |
| Arginine | 189.58±17.31 | 195.50±15.41 | 203.23±19.81 | 188.10±27.67 | 11.37 | 0.402 |
| Proline | 359.98±19.23 | 332.53±40.63 | 409.30±37.12 | 306.43±33.24 | 17.54 | 0.721 |
Effects of glutamate and aspartate on intestinal relative gene expressions in diquat-challenged piglets
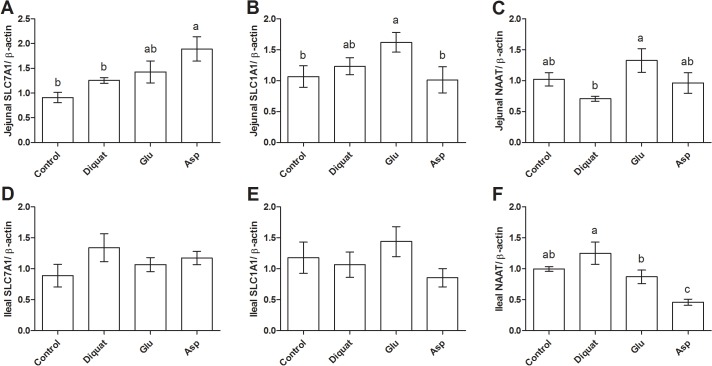
We next preformed RT-PCR to test expressions of solute carrier family 7, member 1 (SLC7A1), solute carrier family 1, member 1 (SLC1A1), and neutral amino acid transporter (NAAT) in the intestine in all piglets (Fig 3 and S4 Dataset). The results exhibited that diquat injection had no effects on these transporters expression in the intestine (P > 0.05). While supplementation with glutamate markedly up-regulated jejunal SLC1A1 expression compared with control group and enhanced jejunal NAAT mRNA abundance compared with diquat group (P < 0.05). Glutamate addition significantly down-regulated ileal NAAT expression compared with diquat treatment (P < 0.05). Furthermore, dietary supplementation with aspartate significantly enhanced jejunal SLC7A1 mRNA abundance compared with the control and diquat group (P < 0.05), while inhibited ileal NAAT expression compared with other groups (P < 0.05).
Fig 3. Intestinal relative mRNA abundances in four groups after exposure to diquat.
Discussion
Diquat, a bipyridyl herbicide, can convert molecular oxygen into superoxide anion radical and stimulate cellular production of free radical species via undergoing cyclic reduction–oxidation processes. Thus, diquat has been widely used to induce animal oxidative stress in vivo and diquat-induced oxidative stress has been reported to affect growth performance and nutritional metabolism [14,25,26]. For example, Lv et al. found that 10 mg/kg diquat significantly enhances serum MDA concentration and inhibited activity of antioxidant enzymes, including SOD and GSH-Px [14]. In the present study, administration of diquat markedly decreased serum NO level and increased serum MDA concentration, indicating a significant disruption in the oxidative balance after exposure to diquat injection.
Glutamate has been demonstrated to improve growth performance and health in pigs [27–32], and we found that supplementation with glutamate significantly increased the final body weight compared with diquat group, suggesting a potentially important role for glutamate in mitigating adverse effects of oxidative stress on piglets. Furthermore, Pi et al. reported that addition of aspartate alleviates growth suppression of weaned pigs after the LPS challenge [12], while the present study failed to exhibit significant difference in growth performance after dietary aspartate. The reason might be different model and different dosage of aspartate.
Our previous reports have shown that supplementation with glutamate can restore deoxynivalenol and mycotoxins induced oxidative stress in pigs [10,11]. The present data indicated that glutamate alleviated diquat-induced oxidative stress via enhancing SOD, T-AOC, and NO levels and inhibiting lipid oxidation subsequent with MDA generation. It is widely recognized that glutamate exhibits an antioxidant function as glutamate is a precursor for glutathione (GSH) along with cysteine and glycine [33]. In our lab, we have found that glutamate is the main limiting substrate compared with cysteine and glycine for liver GSH synthesis in mice (unpublished data). GSH homeostasis in body is an important cellular defense against oxidative stress and involves in the cellular redox state and in the detoxification process [1]. Thus, we speculated glutamate alleviates diquat-induced oxidative stress in piglets through increasing GSH synthesis mechanism. But further data about the serum and hepatic GSH synthesis function are needed to validate this explanation.
Diquat-induced oxidative stress decreased serum threonine, serine, and glycine concentrations in the present study. It is likely that degradation and metabolism of dietary threonine, serine, and glycine in the intestine are increased in response to the oxidative stress, resulting in their deficiencies in piglets. Various reports have shown that the serine/threonine kinase and these amino acids metabolisms are essential for oxidative stress response [34–35] and glycine can reduce the oxidative stress and elevate the enzymic and non-enzymic antioxidants in animals [36]. The current study also showed that supplementation with glutamate increased serum glutamate concentration after diquat challenge. An increase in the influx of glutamate from the lumen of the small intestine into the enterocyte can enhance tissue protein synthesis and improve antioxidant system via enhancing GSH synthesis.
The amino acids transporters mainly contribute to the absorption of luminal amino acids into serum [37]. Thus, we next measured several transporters expressions in the intestine after exposure to diquat-induced oxidative stress. Although there were no differences in mRNA abundances for SLC7A1, SLC1A1, and NAAT between control and diquat groups, supplementation with glutamate and aspartate affected these transporters expression in the intestine, which may mediate glutamate absorption in response to diquat-induced oxidative stress. Indeed, our previous reports have indicated that dietary supplementation with amino acids modulate intestinal activities of nutrients transporters in pathological conditions [4,10, 38].
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present work was supported by grants from the National Natural Science Foundation of China (Nos. 31330075, 31110103909, 31101729, 31402088, 31201813, and 31272463) and the Hunan Provincial Natural Science Foundation of China (Nos. 12JJ2014, 2013RS4065, and 12JJ2020). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yin J, Ren WK, Wu XS, Yang G, Wang J, Li T, et al. (2013) Oxidative stress-mediated signaling pathways: A review. Journal of Food Agriculture & Environment 11: 132–139. [Google Scholar]
- 2. Yin J, Wu MM, Xiao H, Ren WK, Duan JL, Yang G, et al. (2014) Development of an antioxidant system after early weaning in piglets. J Anim Sci 92: 612–619. 10.2527/jas.2013-6986 [DOI] [PubMed] [Google Scholar]
- 3. Ren W, Yin Y, Liu G, Yu X, Li Y, Yang G, et al. (2012) Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42: 2089–2094. 10.1007/s00726-011-0942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin J, Ren W, Duan J, Wu L, Chen S, Li T, et al. (2014) Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids 46: 883–892. 10.1007/s00726-013-1643-5 [DOI] [PubMed] [Google Scholar]
- 5. Ren W, Yin J, Wu M, Liu G, Yang G, Xiong Y, et al. (2014) Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS One 9: e88335 10.1371/journal.pone.0088335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin J, Ren W, Liu G, Duan J, Yang G, Wu L, et al. (2013) Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic Res 47: 1027–1035. 10.3109/10715762.2013.848277 [DOI] [PubMed] [Google Scholar]
- 7. Johnson JL (1972) Glutamic acid as a synaptic transmitter in the nervous system. A review. Brain Res 37: 1–19. [DOI] [PubMed] [Google Scholar]
- 8. Watford M (2008) Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr 138: 2003S–2007S. [DOI] [PubMed] [Google Scholar]
- 9. Burrin DG, Stoll B (2009) Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr 90: 850S–856S. 10.3945/ajcn.2009.27462Y [DOI] [PubMed] [Google Scholar]
- 10. Duan J, Yin J, Wu M, Liao P, Deng D, Gasng L, et al. (2014) Dietary glutamate supplementation ameliorates mycotoxin-induced abnormalities in the intestinal structure and expression of amino Acid transporters in young pigs. PLoS One 9: e112357 10.1371/journal.pone.0112357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang Y, Ma S, Zhang Y, Wang Y, Cheng Q, Wu Y, et al. (2014) IL-1beta and TLR4 signaling are involved in the aggravated murine acute graft-versus-host disease caused by delayed bortezomib administration. J Immunol 192: 1277–1285. 10.4049/jimmunol.1203428 [DOI] [PubMed] [Google Scholar]
- 12. Pi D, Liu Y, Shi H, Li S, Odle J, Lin X, et al. (2014) Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J Nutr Biochem 25: 456–462. 10.1016/j.jnutbio.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 13. Yin FG, Zhang ZZ, Huang J, Yin YL (2010) Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. British Journal of Nutrition 103: 1404–1412. 10.1017/S0007114509993321 [DOI] [PubMed] [Google Scholar]
- 14. Lv M, Yu B, Mao XB, Zheng P, He J, Chen D (2012) Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 6: 928–934. 10.1017/S1751731111002382 [DOI] [PubMed] [Google Scholar]
- 15. Tan BE, Li XG, Wu GY, Yin Y (2012) Dynamic changes in blood flow and oxygen consumption in the portal-drained viscera of growing pigs receiving acute administration of L-arginine. Amino Acids, 43: 2481–2489. 10.1007/s00726-012-1328-5 [DOI] [PubMed] [Google Scholar]
- 16. Wu X, Xie C, Yin Y, Li F, Li T, Huang R, et al. (2013) Effect of L-arginine on HSP70 expression in liver in weanling piglets. BMC Veterinary Research 9:63 10.1186/1746-6148-9-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu M, Xiao H, Ren W, Yin J, Tan B, Liu G, et al. (2014) Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol. PLoS One 9: e100591 10.1371/journal.pone.0100591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu L, Wang W, Yao K, Zhou T, Yin J, Li T, et al. (2013) Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS One 8: e69502 10.1371/journal.pone.0069502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yin Y, Huang R, Li T, Ruan Z, Xie M, Deng Z, et al. (2010) Amino acid metabolism in the portal-drained viscera of young pigs: effects of dietary supplementation with chitosan and pea hull. Amino Acids 39: 1581–1587. 10.1007/s00726-010-0577-4 [DOI] [PubMed] [Google Scholar]
- 20. Li L, Yin Y, Liu Y, Hou D, Hou Z, Yang C, et al. (2007). Intramuscular administration of zinc metallothionein to preslaughter stressed pigs improves anti-oxidative status and porl quality. Asian- Australasian Journal of Animal Science 20:761–767. [Google Scholar]
- 21. Libao-Mercado AJ, Yin Y, Van Eys J, ang De Lange CFM, (2006) True ileal amino acid digestibility and endogenous ileal amino acid losses in growing pigs fed wheat shorts- or casein-based diets. Journal of Animal Science 84: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 22. Rogers LK, Bates CM, Welty SE, Smith CV (2006) Diquat induces renal proximal tubule injury in glutathione reductase-deficient mice. Toxicol Appl Pharmacol 217: 289–298. [DOI] [PubMed] [Google Scholar]
- 23. Zoerner F, Wiklund L, Miclescu A, ang Martijn C (2013) Therapeutic hypothermia activates the endothelin and nitric oxide systems after cardiac arrest in a pig model of cardiopulmonary resuscitation. PLoS One 8: e64792 10.1371/journal.pone.0064792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiang M, Xu YJ, Lu Y, He YG, Han CS, Liu Y, et al. (2014) Autofluorescence of MDA-modified proteins as an in vitro and in vivo probe in oxidative stress analysis. Protein & Cell 5: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu T, Piao XL, Zhang Q, Wang D, Piao XS, Kim S (2010) Protective effects of Forsythia suspensa extract against oxidative stress induced by diquat in rats. Food Chem Toxicol 48: 764–770. 10.1016/j.fct.2009.12.018 [DOI] [PubMed] [Google Scholar]
- 26. Wu KC, Zhang Y, Klaassen CD (2012) Nrf2 protects against diquat-induced liver and lung injury. Free Radic Res 46: 1220–1229. 10.3109/10715762.2012.700709 [DOI] [PubMed] [Google Scholar]
- 27. le Floc'h N, Seve B, Henry Y (1994) The addition of glutamic acid or protein to a threonine-deficient diet differentially affects growth performance and threonine dehydrogenase activity in fattening pigs. J Nutr 124: 1987–1995. [DOI] [PubMed] [Google Scholar]
- 28. Wu X, Zhang Y, Liu Z, Li T, Yin Y, (2012) Effects of oral supplementation with glutamate or combination of glutamate and N-carbamylglutamate on intestinal mucosa in piglets. Journal of Animal Science, 90: 337–339 10.2527/jas.53752 [DOI] [PubMed] [Google Scholar]
- 29. Wu X, Shu X, Xie C, Li J, Hu J, Deng Z, et al. (2013) The Acute and Chronic Effects of Monosodium L-Glutamate on Serum Iron and Total Iron-Binding Capacity in the Jugular Artery and Vein of Pigs. Biol Trace Elem Res. 153:191–195 10.1007/s12011-013-9668-x [DOI] [PubMed] [Google Scholar]
- 30. Chen G, Zhang J, Zhang Y, Liao P, Li T, Chen L, et al. (2014) Oral MSG administration alters hepatic expression of genesfor lipid and nitrogen metabolism in suckling piglets Amino Acids. 46:245–250 10.1007/s00726-013-1615-9 [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Yin Y, Shu X, Li T, Li F, Tan b, et al. (2013) Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 45:1169–1177 10.1007/s00726-013-1573-2 [DOI] [PubMed] [Google Scholar]
- 32. Feng Z, Zhou X, Wu F, Yao K, Kong X, Li T, et al. (2014). Both dietary supplementation with monosodium L-glutamate and fat modify circulating and tissue amino acid pools in growing pigs, but with little interactive effect. PLOS ONE 9: e84533 10.1371/journal.pone.0084533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng Y, Xu ZF, Liu W, Xu B, Yang HB, Wei Y, et al. (2012) Riluzole-triggered GSH synthesis via activation of glutamate transporters to antagonize methylmercury-induced oxidative stress in rat cerebral cortex. Oxid Med Cell Longev 2012: 534705 10.1155/2012/534705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanchez-Piris M, Posas F, Alemany V, Winge I, Hidalgo E, Bachs O, et al. (2002) The serine/threonine kinase Cmk2 is required for oxidative stress response in fission yeast. J Biol Chem 277: 17722–17727. [DOI] [PubMed] [Google Scholar]
- 35. Enomoto A, Kido N, Ito M, Takamatsu N, Miyagawa K (2012) Serine-threonine kinase 38 is regulated by glycogen synthase kinase-3 and modulates oxidative stress-induced cell death. Free Radic Biol Med 52: 507–515. 10.1016/j.freeradbiomed.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 36. Senthilkumar R, Viswanathan P, Nalini N (2004) Effect of glycine on oxidative stress in rats with alcohol induced liver injury. Pharmazie 59: 55–60. [PubMed] [Google Scholar]
- 37. Wu G (2013) Functional amino acids in nutrition and health. Amino Acids 45: 407–411. 10.1007/s00726-013-1500-6 [DOI] [PubMed] [Google Scholar]
- 38. Yin J, Duan J, Cui Z, Ren W, Li T, Yin Y (2015) Hydrogen peroxide-induced oxidative stress activates NF-[small kappa]B and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Advances 20: 15479–15486 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.