Abstract
Objectives
Guidelines for treatment of healthcare-associated pneumonia (HCAP) recommend empirical therapy with broad-spectrum antimicrobials. Our objective was to examine the association between guideline-based therapy (GBT) and outcomes for patients with HCAP.
Patients and methods
We conducted a pharmacoepidemiological cohort study at 346 US hospitals. We included adults hospitalized between July 2007 and June 2010 for HCAP, defined as patients admitted from a nursing home, with end-stage renal disease or immunosuppression, or discharged from a hospital in the previous 90 days. Outcome measures included in-hospital mortality, length of stay and costs.
Results
Of 85 097 patients at 346 hospitals, 31 949 (37.5%) received GBT (one agent against MRSA and at least one against Pseudomonas). Compared with patients who received non-GBT, those who received GBT had a heavier burden of chronic disease and more severe pneumonia. GBT was associated with higher mortality (17.1% versus 7.7%, P < 0.001). Adjustment for demographics, comorbidities, propensity for treatment with GBT and initial severity of disease decreased, but did not eliminate, the association (OR 1.39, 95% CI 1.32–1.47). Using an adaptation of an instrumental variable analysis, GBT was not associated with higher mortality (OR 0.93, 95% CI 0.75–1.16). Adjusted length of stay and costs were also higher with GBT.
Conclusions
Among patients who met HCAP criteria, GBT was not associated with lower adjusted mortality, length of stay or costs in any analyses. Better criteria are needed to identify patients at risk for MDR infections who might benefit from broad-spectrum antimicrobial coverage.
Keywords: cohort studies, healthcare-associated infections, mortality
Introduction
Pneumonia is a common reason for hospital admission in the USA and a leading cause of death.1 Patients who have frequent contact with the healthcare system have pneumonia outcomes that are worse than patients without such exposures,2,3 a finding thought to be associated with their increased risk for harbouring resistant organisms. Inadequate antibiotic therapy has been associated with higher mortality,4,5 and small observational studies in healthcare-associated pneumonia (HCAP) have demonstrated improved outcomes with administration of broad-spectrum antimicrobials.6,7
Consequently, the American Thoracic Society (ATS) and IDSA guidelines recommend that initial treatment of HCAP include combination therapy consisting of one agent with activity against MRSA and two agents with activity against Pseudomonas aeruginosa.8 However, these guidelines are not followed routinely.9 Moreover, several observational studies have noted increased mortality among patients who received guideline-concordant therapy.10
In a large population of HCAP patients, we aimed to evaluate the effectiveness of guideline-based therapy (GBT) compared with other antimicrobial regimens and to identify subgroups of patients with HCAP who received the greatest benefit from GBT.
Patients and methods
Setting and patients
We identified patients discharged between July 2007 and June 2010 from 346 US hospitals that participated in the Premier Alliance.11 Member hospitals represent all geographical regions and generally reflect US hospitals overall. The Premier database contains sociodemographic information, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, and hospital and physician information. It also includes a date-stamped log of billed items and services, including diagnostic tests, medications and other treatments. Because the data do not contain identifiable information, the Institutional Review Board at Baystate Medical Center determined that this study did not constitute human subjects research.
We included all patients aged ≥18 years with a principal ICD-9-CM diagnosis of pneumonia or a secondary diagnosis of pneumonia paired with a principal diagnosis of respiratory failure, acute respiratory distress syndrome, respiratory arrest, sepsis or influenza (Figure S1, available as Supplementary data at JAC Online). We excluded: transfer patients, because we could not assess initial severity or outcomes; those with length of stay of ≤1 day; patients with cystic fibrosis; those whose attending physician was in a specialty not expected to treat pneumonia (e.g. psychiatry); those with a diagnosis related group inconsistent with pneumonia or a code indicating that the pneumonia was not present on admission; and any patient who did not have a chest radiograph and begin antimicrobials within 48 h of admission. For patients with multiple admissions, one admission was randomly selected.
Patients were designated as having HCAP if they had a diagnosis of end-stage renal disease or haemodialysis in the first 2 hospital days, if they were admitted from a skilled nursing facility or had been discharged from hospital in the past 90 days. We also included patients taking immunosuppressant drugs.
Data elements
For each patient, we extracted age, sex, race, marital and insurance status, comorbidities, tests, medications and treatments associated with severity of illness, as well as the specialty of the attending physician. Comorbidities were identified using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser et al.,12 and by use of medications in the first 2 days of hospitalization. Hospitals were categorized by region (north-east, south, mid-west or west), bed size, setting (urban versus rural) and teaching status.
Antimicrobial treatment and outcome variables
GBT, our main predictor variable, was defined as treatment with an antimicrobial regimen similar to those recommended by the ATS-IDSA guidelines (Table 1) begun on hospital day 1 or 2. The guidelines stress the importance of adequate coverage for MRSA and Pseudomonas, but the necessity of routinely using two agents with activity against Pseudomonas is debated.13 Therefore, patients receiving one antimicrobial active against MRSA and any agent against Pseudomonas were classified as receiving GBT. All others were classified as receiving non-GBT.
Table 1.
Characteristics of patients receiving GBT and non-GBT for HCAP
| Description | Total, n = 85 097 (100%) | GBT, n = 31 949 (37.5%) | Non-GBT, n = 53 148 (62.5%) | P (two-sided) |
|---|---|---|---|---|
| Age (years), median (IQR) | 73 (61–83) | 72 (60–81) | 75 (62–83) | <0.0001 |
| Gender, n (%) | <0.0001 | |||
| female | 44 086 (51.8) | 15 699 (49.1) | 28 387 (53.4) | |
| male | 41 011 (48.2) | 16 250 (50.9) | 24 761 (46.6) | |
| Race/ethnicity, n (%) | <0.0001 | |||
| white | 57 571 (67.7) | 20 895 (65.4) | 36 676 (69.0) | |
| black | 10 925 (12.8) | 4769 (14.9) | 6156 (11.6) | |
| Hispanic | 3900 (4.6) | 1565 (4.9) | 2335 (4.4) | |
| other | 12 701 (14.9) | 4720 (14.8) | 7981 (15.0) | |
| Insurance payer, n (%) | <0.0001 | |||
| Medicare | 64 307 (75.6) | 23 614 (73.9) | 40 693 (76.6) | |
| Medicaid | 6807 (8) | 2896 (9.1) | 3911 (7.4) | |
| managed care | 8465 (10.0) | 3434 (10.7) | 5031 (9.5) | |
| commercial indemnity | 2455 (2.9) | 834 (2.6) | 1621 (3.0) | |
| other | 3063 (3.6) | 1171 (3.7) | 1892 (3.6) | |
| HCAP individual components, n (%) | <0.0001 | |||
| admission source is SNF/ICF | 8299 (9.8) | 3119 (9.8) | 5180 (9.7) | |
| haemodialysis | 5296 (6.2) | 2119 (6.6) | 3177 (6.0) | |
| admission in previous 90 days | 34 677 (40.8) | 11 002 (34.4) | 23 675 (44.5) | |
| immunosuppressed | 15 456 (18.2) | 5092 (15.9) | 10 364 (19.5) | |
| two or more components | 21 369 (25.1) | 10 617 (33.2) | 10 752 (20.2) | |
| Comorbidities, n (%) | ||||
| congestive heart failure | 20 851 (24.5) | 7349 (23) | 13 502 (25.4) | <0.0001 |
| valvular disease | 7504 (8.8) | 2188 (6.8) | 5316 (10.0) | <0.0001 |
| hypertension | 38 314 (45.0) | 12 593 (39.4) | 25 721 (48.4) | <0.0001 |
| paralysis | 2814 (3.3) | 1305 (4.1) | 1509 (2.8) | <0.0001 |
| other neurological disorders | 9698 (11.4) | 3667 (11.5) | 6031 (11.3) | 0.563 |
| chronic pulmonary disease | 40 719 (47.85) | 14 526 (45.5) | 26 193 (49.3) | <0.0001 |
| diabetes | 21 146 (24.9) | 7562 (23.7) | 13 584 (25.6) | <0.0001 |
| hypothyroidism | 9883 (11.6) | 3179 (10.0) | 6704 (12.6) | <0.0001 |
| metastatic cancer | 3186 (3.7) | 1370 (4.3) | 1816 (3.4) | <0.0001 |
| weight loss | 6972 (8.2) | 3258 (10.2) | 3714 (7.0) | <0.0001 |
| depression | 8709 (10.2) | 2835 (8.9) | 5874 (11.1) | <0.0001 |
| chronic kidney disease | 15 069 (17.7) | 6048 (18.9) | 9021 (17.0) | <0.0001 |
| Principal diagnosis, n (%) | <0.0001 | |||
| pneumonia/influenza | 53 284 (62.6) | 14 287 (44.7) | 38 997 (73.4) | |
| sepsis | 23 374 (27.5) | 14 189 (44.4) | 9185 (17.3) | |
| respiratory failure/arrest | 8439 (9.9) | 3473 (10.9) | 4966 (9.3) | |
| Admission to ICU, n (%) | ||||
| ICU | 20 891 (24.6) | 12 897 (40.4) | 7994 (15.0) | <0.0001 |
| cardiac ICU | 4348 (5.1) | 2394 (7.5) | 1954 (3.7) | <0.0001 |
| intermediate care (step down) | 1668 (2.0) | 721 (2.3) | 947 (1.8) | <0.0001 |
| Measures of severity of illness,a n (%) | ||||
| any oral medications | 74 753 (87.8) | 26 503 (83.0) | 48 250 (90.8) | <0.0001 |
| calcium (intravenous) | 4018 (4.7) | 2691 (8.4) | 1327 (2.5) | <0.0001 |
| Foley catheter | 12 662 (14.9) | 6215 (19.5) | 6447 (12.1) | <0.0001 |
| bicarbonate | 4613 (5.4) | 3023 (9.5) | 1590 (3.0) | <0.0001 |
| vasopressors | 11 496 (13.5) | 8044 (25.2) | 3452 (6.5) | <0.0001 |
| benzodiazepines | 12 379 (14.6) | 6584 (20.6) | 5795 (10.9) | <0.0001 |
| insulin | 27 699 (32.6) | 12 418 (38.9) | 15 281 (28.8) | <0.0001 |
| arterial and venous blood gas | 36 817 (43.3) | 18 304 (57.3) | 18 513 (34.8) | <0.0001 |
| blood cultures | 77 282 (90.8) | 30 492 (95.4) | 46 790 (88.0) | <0.0001 |
| blood lactate | 19 982 (23.5) | 12 172 (38.1) | 7810 (14.7) | <0.0001 |
| non-invasive ventilation | 8604 (10.1) | 4235 (13.3) | 4369 (8.2) | <0.0001 |
| invasive mechanical ventilation | 11 683 (13.7) | 7637 (23.9) | 4046 (7.6) | <0.0001 |
| central line | 3294 (3.9) | 2262 (7.1) | 1032 (1.9) | <0.0001 |
| Hospital characteristics | ||||
| bed size, n (%) | <0.0001 | |||
| ≤200 beds | 13 953 (16.4) | 4026 (12.6) | 9927 (18.7) | |
| 201–400 beds | 32 986 (38.8) | 11 124 (34.8) | 21 862 (41.1) | |
| >400 beds | 38 158 (44.8) | 16 799 (52.6) | 21 359 (40.2) | |
| rural/urban status, n (%) | <0.0001 | |||
| urban | 74 498 (87.5) | 29 194 (91.4) | 45 304 (85.2) | |
| rural | 10 599 (12.5) | 2755 (8.6) | 7844 (14.8) | |
| teaching status, n (%) | <0.0001 | |||
| non-teaching | 52 801 (62.0) | 17 654 (55.3) | 35 147 (66.1) | |
| teaching | 32 296 (38.0) | 14 295 (44.7) | 18 001 (33.9) | |
| region, n (%) | <0.0001 | |||
| north-east | 15 192 (17.9) | 5717 (17.9) | 9475 (17.8) | |
| mid-west | 20 459 (24.0) | 7304 (22.9) | 13 155 (24.8) | |
| west | 13 233 (15.6) | 4453 (13.9) | 8780 (16.5) | |
| south | 36 213 (42.6) | 14 475 (45.3) | 21 738 (40.9) | |
| Outcomes | ||||
| inpatient mortality, n (%) | 9575 (11.3) | 5470 (17.1) | 4105 (7.7) | <0.0001 |
| 7 day mortality,b n (%) | 4966 (5.8) | 2888 (9.0) | 2078 (3.9) | <0.0001 |
| length of stay (days), median (IQR) | 6 (4–10) | 7 (5–12) | 5 (3–8) | <0.0001 |
| cost ($), median (IQR) | 9846 (5993–17 979) | 13 978 (8321–25 263) | 8123 (5224–13 919) | <0.0001 |
aIn first 2 hospital days.
bPatients discharged alive within 7 days were assumed to survive.
Our primary outcome was in-hospital mortality. Secondary outcomes included 7 day mortality, initiation of mechanical ventilation or admission to the ICU after hospital day 2, readmission to hospital within 30 days, cost, length of stay and Clostridium difficile infection (CDI). The latter was defined by ICD-9 code, treatment with metronidazole or oral vancomycin begun after hospital day 3 or readmission within 30 days for C. difficile.
Statistical analysis
Summary statistics are presented as frequencies and proportions for categorical variables and medians with IQRs for continuous variables. Associations between GBT and patient characteristics were assessed using χ2 tests for categorical variables and using Kruskal–Wallis tests for continuous variables.
We developed a hierarchical logistic regression model for treatment to produce propensity scores for GBT versus non-GBT. The model produced a predicted probability, or propensity for treatment, for each patient. It included all patient and hospital characteristics as well as the individual's predicted mortality based on a previously published mortality model.14
Multivariable hierarchical regression models15 were developed to assess the effect of GBT on mortality, all-cause readmission within 30 days, admission to ICU on or after hospital day 3, length of stay, costs and adverse events adjusted for patient characteristics, other early treatments, propensity score and hospital characteristics.
Models with length of stay and costs were trimmed at three standard deviations above mean and natural log-transformed values were modelled to account for extreme skew. SAS software procedure GLIMMIX was used to account for the hierarchical nature of the data (patients clustered within hospital with hospital as a random effect). Logit link models were used for binary outcomes and identity link models were used for continuous outcomes. In addition, we evaluated log-binomial models, Poisson and negative binomial models due to concern that the OR would overestimate the risk ratio for frequent outcomes. Risk ratios were close to ORs; hence we present ORs only.
In additional analyses, we matched each GBT patient based on propensity for GBT with a non-GBT patient. Using conditional logistic regression, we assessed the association between treatment and outcomes, adjusting for factors unbalanced between groups (P < 0.05). Stratified analyses assessed outcomes among quintiles of predicted mortality and for each subcategory of HCAP patient.
Finally, we developed grouped treatment models, an adaptation of instrumental variable analysis,16 to address the threat of residual confounding by indication. Each patient was assigned a group rate of GBT at the hospital where they were treated. We then applied hierarchical models as described above.
A sensitivity analysis was conducted to explore the effect of a hypothetical unmeasured confounder associated with both GBT and mortality. We varied the prevalence of the unmeasured confounder and hypothesized a range of ORs for mortality for the unmeasured confounder between 1.5 and 3.0, within the range of factors found in our data. Using a method proposed by Lin et al.,17 we explored the combination of prevalence and effect sizes that would be necessary to find that GBT reduced mortality relative to non-GBT.
All tests were two-sided with a significance level of 0.05. All analyses were performed using the Statistical Analysis System (version 9.3, SAS Institute Inc., Cary, NC, USA) and STATA (StataCorp. 2011, Stata Statistical Software: Release 12, College Station, TX, USA: StataCorp LP).
Results
Our final sample included 85 097 patients from 346 hospitals; 31 949 (37.5%) patients received GBT. Of those not receiving GBT, 82% received standard therapy for community-acquired pneumonia. Patient characteristics appear in Table 1 and Table S1. Compared with patients who received non-GBT, those who received GBT were younger and more likely to be male. They had a heavier burden of chronic diseases such as weight loss (10.2% versus 7.0%) or metastatic cancer (4.3% versus 3.4%) and presented with more severe pneumonia as evidenced by more frequent treatment with mechanical ventilation (23.9% versus 7.6%) or vasopressors (25.2% versus 6.5%) in the first 2 hospital days.
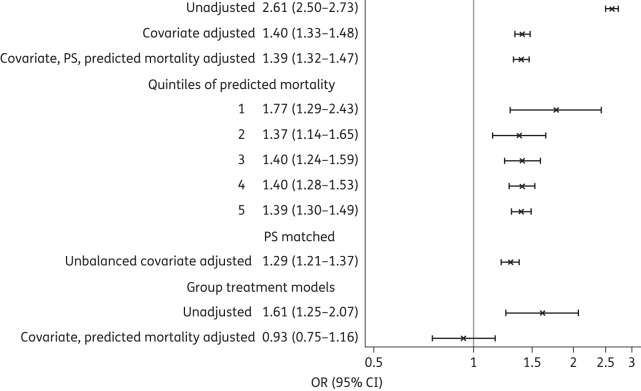
Those who received GBT had higher mortality than those who received non-GBT (17.1% versus 7.7%, P < 0.001). Adjustment for patient characteristics (including demographics, comorbidities, propensity for treatment and treatments in the first 2 hospital days that reflect initial severity of disease) decreased, but did not eliminate, this difference in mortality, with the OR falling from 2.61 to 1.40 (Figure 1). We successfully matched 65.7% of GBT patients with non-GBT patients based on propensity score. Matching balanced all covariates except for minimal differences in peripheral vascular disease and early use of dobutamine and antipsychotics (Table S2). In the matched analysis, adjusted for unbalanced covariates, GBT was still associated with higher mortality. Odds of mortality were increased across all quintiles of predicted mortality, but the higher the predicted mortality, the smaller the increased odds of mortality.
Figure 1.
In-hospital mortality with GBT versus non-GBT. PS, propensity score.
Hospital rates of GBT ranged from 0% to 87%. In the grouped treatment model, in which the hospital's rate of GBT was used in place of the actual therapy received, the odds of mortality with GBT versus non-GBT therapy fell to 0.93 (0.75–1.16). Results for 7 day mortality were almost identical (Table 2). Readmissions followed a pattern similar to mortality for all models, but the magnitude of the effect was smaller. Late ICU admission was slightly higher among the GBT group, even in the grouped treatment model. Adjusted costs and length of stay were also higher with GBT.
Table 2.
Outcomes of patients receiving GBT and non-GBT for HCAP, OR (95% CI)
| Outcome | Unadjusted | Covariate adjusted | Propensity matcheda | Group treatmenta |
|---|---|---|---|---|
| 7 day mortalityb | 2.53 (2.39–2.69) | 1.49 (1.39–1.60) | 1.41 (1.30–1.53) | 0.90 (0.70–1.16) |
| All-cause readmission | 1.28 (1.22–1.33) | 1.17 (1.11–1.23) | 1.14 (1.06–1.21) | 1.01 (0.98–1.03) |
| Late ICU admissionc | 1.61 (1.49–1.75) | 1.18 (1.08–1.28) | 1.16 (1.03–1.30) | 1.05 (1.00–1.11) |
| CDI | 1.87 (1.63–2.15) | 1.24 (1.07–1.44) | 1.09 (0.91–1.30) | 1.01 (1.00–1.02) |
| Ln length of stay | 1.31 (1.30–1.33) | 1.10 (1.09–1.11) | 1.10 (1.08–1.11) | |
| Ln cost | 1.62 (1.60–1.64) | 1.17 (1.16–1.18) | 1.15 (1.14–1.17) |
aAdjusted for unbalanced covariates.
bPatients discharged alive within 7 days were assumed to survive.
cPatients admitted to ICU on hospital day 1 or 2 were excluded from this analysis.
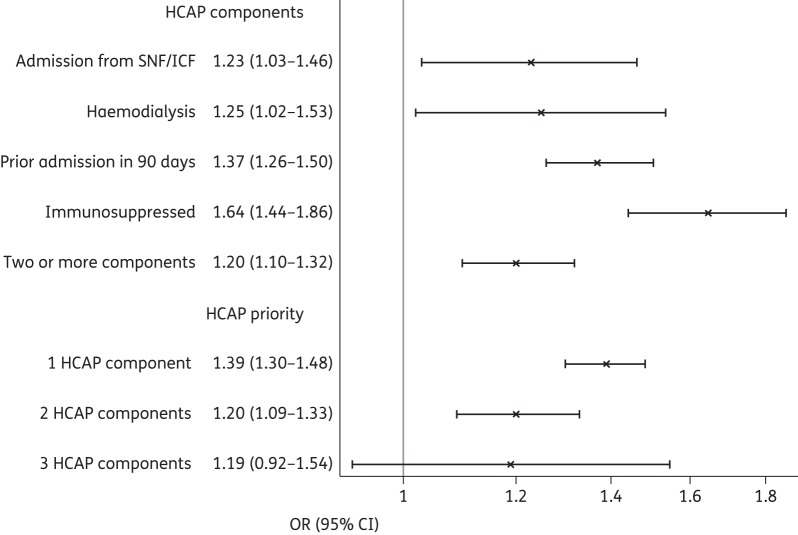
Figure 2 shows the association between mortality and GBT across HCAP subgroups. The association was strongest for immunosuppressed patients and weakest for patients from skilled nursing facilities or on haemodialysis. The magnitude of the association between mortality and GBT declined with each additional HCAP risk factor.
Figure 2.
In-hospital mortality for GBT versus non-GBT among HCAP groups (all models adjusted for propensity score). SNF/ICF, skilled nursing facility/intermediate care facility.
Patients who received GBT were slightly more likely than those receiving non-GBT to experience CDI, but this result was not statistically significant, OR (95% CI) 1.15 (0.98–1.35).
Lastly, we assessed how strong a confounder would be necessary to produce the results we observed if concordant therapy actually reduced mortality. A single potential confounder would have to be present in 30% of the GBT patients (and none of the non-GBT patients) and have an OR of 3.0 in order to find a statistically significant benefit to GBT.
Discussion
In this large observational study of patients with HCAP, we found that patients receiving initial therapy based on HCAP guidelines had higher unadjusted mortality than those receiving other antimicrobial combinations. After adjustment for patient factors, propensity adjustment and propensity matching, the difference between GBT and non-GBT was diminished, but remained. In contrast, a grouped treatment analysis yielded a non-significant 7% mortality reduction associated with GBT. The same pattern occurred for each subgroup of HCAP patients, regardless of HCAP risk factor.
To date, there have been no randomized trials of GBT for HCAP. Several retrospective studies have examined the association between GBT and mortality. The largest examined 15 071 non-critically ill HCAP patients from 150 Veterans' Administration hospitals.18 In a propensity-adjusted analysis, GBT was associated with increased mortality. Of five smaller studies,19–23 only one found that GBT was associated with reduced mortality.20 One additional study observed increased mortality associated with GBT, but for patients with a probability of a resistant organism >60%, mortality was reduced.7 Finally, a quality improvement collaborative aimed at increasing GBT in ICUs also found that GBT was associated with increased mortality, although overall mortality did not change significantly after implementation of the collaborative (from 24% to 27%, P = 0.46) despite an increase in GBT from 33% to 47% of cases.24
We observed that mortality was more than double among patients receiving GBT. One explanation is selection bias due to unmeasured confounders, with physicians reserving GBT for the sickest patients. Indeed, physicians were much more likely to prescribe GBT for critically ill patients. Our grouped treatment model, in which the hospital rate of GBT serves as an instrumental variable, supports this hypothesis, as higher hospital levels of GBT were associated with lower mortality, albeit not statistically so. An alternative explanation is that GBT might harm some patients. Others have postulated that the use of broad-spectrum antimicrobials among patients without MDR pathogens could lead to unnecessary complications, including adverse drug events, development of antimicrobial resistance, superinfections, CDIs and complications of intravenous therapy or prolonged hospital stays.10,24 However, it seems unlikely that these factors could explain the 30% higher adjusted mortality that we observed among patients receiving GBT.
Our findings call into question the necessity of treating all HCAP patients with GBT. The guidelines do not differentiate among HCAP patients, but physicians in our study apparently did, reserving broad-spectrum antimicrobials for those at greatest risk. Most patients received non-GBT and had low mortality nonetheless. While inadequate antimicrobial therapy is a risk factor for mortality among severely ill patients,4,5,25,26 in Europe MDR Gram-negative infections are rare,13 and additional antimicrobials may not affect outcomes.27,28 Rather than identifying exposure to the healthcare system as a risk for MDR organisms, more precise models for identifying at-risk patients could reduce prescribing and improve patient outcomes.29
Our study has several strengths. First, our sample was more than five times as large as all HCAP studies combined, allowing us to assess the impact of GBT in different subpopulations. We found the same pattern regardless of whether patients had been hospitalized, resided in a nursing home or received haemodialysis.
Our study also has limitations. First, its observational nature precludes demonstration of cause and effect. Second, we worked solely with administrative data and could have missed important confounders. Nevertheless, our results were robust. An unmeasured confounder with a strong association with mortality (i.e. similar to admission to ICU) would have had to be present 30% more often, in absolute terms, among patients treated with GBT to produce the observed differences. ICU admission was present 25% more often, so an unobserved confounder with the properties of ICU admission could not account for the observed differences. Third, we lacked microbiology data and therefore cannot comment on the causative organisms or local resistance patterns. Fourth, we did not strictly speaking evaluate the ATS-IDSA guidelines because patients who received a single agent against Pseudomonas were considered to have received GBT. However, patient characteristics and outcomes were similar between those receiving one or two agents. Additionally, we studied in-hospital mortality; results may have been different with 30 day mortality. Finally, in order to increase specificity, we identified patients based on discharge diagnosis rather than admitting diagnosis. Additional patients may have mistakenly received GBT before a correct diagnosis was made.
Eight years after the appearance of published guidelines for treatment of HCAP, physician compliance remains low. As late as 2010, only 40% of patients with HCAP received GBT. Physicians appeared to reserve GBT for patients at highest risk of mortality and most patients who received non-GBT did well. Overall, we found no evidence that GBT reduced mortality. These findings argue for a more nuanced approach to HCAP, with better tools to identify those patients most likely to benefit from broad-spectrum antimicrobial coverage.
Funding
This study was supported by grant # R01HS018723 from the Agency for Healthcare Research and Quality. The funder had no role in the design and conduct of the study, no role in the collection, management, analysis and interpretation of the data, no role in the preparation, review or approval of the manuscript and no role in the decision to submit the manuscript for publication.
T. L. is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL114745.
Transparency declarations
M. D. Z. has received research funding from and has served as a consultant for Pfizer, Astellas, Cubist, Forest, Theravance and Tetraphase. All other authors: none to declare.
Supplementary data
References
- 1.CDC. FastStats: Pneumonia. http://www.cdc.gov/nchs/fastats/pneumonia.htm. [Google Scholar]
- 2.Venditti M, Falcone M, Corrao S, et al. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med 2009; 150: 19–26. [DOI] [PubMed] [Google Scholar]
- 3.Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 2005; 128: 3854–62. [DOI] [PubMed] [Google Scholar]
- 4.Harbarth S, Garbino J, Pugin J, et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med 2003; 115: 529–35. [DOI] [PubMed] [Google Scholar]
- 5.Micek ST, Welch EC, Khan J, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother 2010; 54: 1742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zilberberg MD, Shorr AF, Micek ST, et al. Antimicrobial therapy escalation and hospital mortality among patients with health-care-associated pneumonia: a single-center experience. Chest 2008; 134: 963–8. [DOI] [PubMed] [Google Scholar]
- 7.Madaras-Kelly KJ, Remington RE, Sloan KL, et al. Guideline-based antibiotics and mortality in healthcare-associated pneumonia. J Gen Intern Med 2012; 27: 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171: 388–416. [DOI] [PubMed] [Google Scholar]
- 9.Seymann GB, Di Francesco L, Sharpe B, et al. The HCAP gap: differences between self-reported practice patterns and published guidelines for health care-associated pneumonia. Clin Infect Dis 2009; 49: 1868–74. [DOI] [PubMed] [Google Scholar]
- 10.Troitino AX, Porhomayon J, El-Solh AA. Guideline-concordant antimicrobial therapy for healthcare-associated pneumonia: a systematic review and meta-analysis. Lung 2013; 191: 229–37. [DOI] [PubMed] [Google Scholar]
- 11.Lindenauer PK, Pekow P, Gao S, et al. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2006; 144: 894–903. [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998; 36: 8–27. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers JD, Rother C, Salih W, et al. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014; 58: 330–9. [DOI] [PubMed] [Google Scholar]
- 14.Rothberg MB, Pekow PS, Priya A, et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One 2014; 9: e87382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelman A, Hill J. Data Analysis using Regression and Mulitlevel/Hierarchical Models. New York: Cambridge University Press, 2007. [Google Scholar]
- 16.Stukel TA, Fisher ES, Wennberg DE, et al. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 2007; 297: 278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998; 54: 948–63. [PubMed] [Google Scholar]
- 18.Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J 2011; 38: 878–87. [DOI] [PubMed] [Google Scholar]
- 19.El Solh AA, Akinnusi ME, Alfarah Z, et al. Effect of antibiotic guidelines on outcomes of hospitalized patients with nursing home-acquired pneumonia. J Am Geriatr Soc 2009; 57: 1030–5. [DOI] [PubMed] [Google Scholar]
- 20.Falcone M, Corrao S, Licata G, et al. Clinical impact of broad-spectrum empirical antibiotic therapy in patients with healthcare-associated pneumonia: a multicenter interventional study. Intern Emerg Med 2012; 7: 523–31. [DOI] [PubMed] [Google Scholar]
- 21.Grenier C, Pepin J, Nault V, et al. Impact of guideline-consistent therapy on outcome of patients with healthcare-associated and community-acquired pneumonia. J Antimicrob Chemother 2011; 66: 1617–24. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SP, Taylor BT. Health care-associated pneumonia in haemodialysis patients: clinical outcomes in patients treated with narrow versus broad spectrum antibiotic therapy. Respirology 2013; 18: 364–8. [DOI] [PubMed] [Google Scholar]
- 23.Webb BJ, Dangerfield BS, Pasha JS, et al. Guideline-concordant antibiotic therapy and clinical outcomes in healthcare-associated pneumonia. Respir Med 2012; 106: 1606–12. [DOI] [PubMed] [Google Scholar]
- 24.Kett DH, Cano E, Quartin AA, et al. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis 2011; 11: 181–9. [DOI] [PubMed] [Google Scholar]
- 25.Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999; 115: 462–74. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–96. [DOI] [PubMed] [Google Scholar]
- 27.Heyland DK, Dodek P, Muscedere J, et al. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med 2008; 36: 737–44. [DOI] [PubMed] [Google Scholar]
- 28.Cometta A, Baumgartner JD, Lew D, et al. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrob Agents Chemother 1994; 38: 1309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Restrepo MI, Aliberti S. Healthcare-associated pneumonia: where do we go next? Clin Infect Dis 2014; 58: 340–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.