Abstract
Objectives
Despite a strong link between antibiotic use and resistance, and highly variable antibiotic consumption rates across the USA, drivers of differences in consumption rates are not fully understood. The objective of this study was to examine how provider density affects antibiotic prescribing rates across socioeconomic groups in the USA.
Methods
We aggregated data on all outpatient antibiotic prescriptions filled in retail pharmacies in the USA in 2000 and 2010 from IMS Health into 3436 geographically distinct hospital service areas and combined this with socioeconomic and structural factors that affect antibiotic prescribing from the US Census. We then used fixed-effect models to estimate the interaction between poverty and the number of physician offices per capita (i.e. physician density) and the presence of urgent care and retail clinics on antibiotic prescribing rates.
Results
We found large geographical variation in prescribing, driven in part by the number of physician offices per capita. For an increase of one standard deviation in the number of physician offices per capita there was a 25.9% increase in prescriptions per capita. However, the determinants of the prescription rate were dependent on socioeconomic conditions. In poorer areas, clinics substitute for traditional physician offices, reducing the impact of physician density. In wealthier areas, clinics increase the effect of physician density on the prescribing rate.
Conclusions
In areas with higher poverty rates, access to providers drives the prescribing rate. However, in wealthier areas, where access is less of a problem, a higher density of providers and clinics increases the prescribing rate, potentially due to competition.
Keywords: antibiotic resistance, public health, pharmacoepidemiology
Introduction
Antibiotic resistance is a significant public health challenge and contributes to poor inpatient outcomes.1–3 Large differences in the frequency of resistant infections have been noted, both among regions of the USA4,5 and across European countries.6 Variations in antibiotic consumption rates among countries6 and regions of countries7–11 offer one possible explanation for variation in resistance.
The main drivers of geographical variations in antibiotic consumption have been attributed to: (i) socioeconomic differences (e.g. education level,12 financial well-being,9,12,13 access to health insurance,11,14 use of childcare centres15); (ii) structural differences (e.g. physician density,9,13 physician remuneration,13 antibiotic costs and competition9,16); and (iii) cultural differences (e.g. prescribing norms,12 patient demand12). Healthcare providers, as the only ones that can prescribe antibiotics in the USA, play an important role in antibiotic consumption. However, despite the importance of understanding the role that providers play in driving antibiotic use, and the greater cost-related barriers and lower efficiency of care in the USA vis-à-vis other countries,17,18 there is only limited research on how provider density, or the number of providers per capita, affects antibiotic prescribing in the USA, particularly across socioeconomic groups.
We hypothesized that provider density may affect prescribing behaviour through competition to retain patients, as has been seen in some limited studies in other countries.19,20 One potential source of competition for providers is non-traditional prescribing outlets, such as urgent care or retail clinics (areas incorporated into a retail store), where patients can receive medical services; these have been shown to have high rates of antibiotic prescribing.21 These types of establishment, which primarily exist in the USA, are viewed as a competitive threat by physicians' organizations.22 Since increased provider density and urgent and retail clinics are associated with more advantaged areas,23,24 we hypothesized that the presence of clinics would have differential effects on provider prescribing across socioeconomic strata.
Methods
Study data
Data on the annual number of dispensed drug prescriptions collected from retail pharmacies in the USA were obtained from IMS Health's Xponent database for the years 2000 and 2010. IMS data provide the total number of prescriptions dispensed by age at the zip-code level, and have been extensively used in prior studies.14,25–27 We aggregated zip-code level data up to 3436 geographically distinct hospital service areas (HSAs), which are collections of zip codes in which residents receive most of their hospitalizations from the hospitals in that area,28 and thus are likely to have providers with similar prescribing norms. HSAs also allow us to compare areas across time.28 We then calculated the number of prescriptions written per capita in each HSA using population data from the US Census (www.census.gov).
Socioeconomic and structural indicators were obtained from the Census Bureau and were selected based on existing literature of the determinants of antibiotic prescribing.7,9,12,16 Structural variables of interest at the HSA level were population density, number of childcare centres per capita (attendance correlates with antibiotic use15), the number of hospitals per capita (which included hospitals with emergency departments), the number of physician offices per capita and the number of clinics per capita.
Socioeconomic variables included the proportion of the population living in poverty in a given year (census estimate based on income and family size), the population age distribution (percentages of the population under 5 and over 65), race/ethnicity (white, African–American and other), education (percentage of the population that graduated from college) and unemployment. For the general population level of health, we included the number of dialysis centres per capita.
Infection level in the population, though not a major driver, has been associated with increased prescriptions as well.12 Because detailed data on infection level for the entire population are not available, we controlled for infection level using the elderly population (who account for ∼16% of all antibiotic prescriptions) by including the number of Medicare discharges per 1000 Medicare enrollees.28 Geographical differences in climate may affect the prescription rate and thus we included the average temperature difference between January and July (www.noaa.gov).
Finally, because the Census Bureau includes additional healthcare establishments in its clinic classifications (for full definition of the clinics variable see the Supplementary data available at JAC Online), we obtained data from Urgent Care Locations, LLC (www.urgentcarelocations.com) on the locations of urgent care and retail clinics in the USA to use as a robustness check. Table 1 lists the variables included in the analysis.
Table 1.
Socioeconomic indicators across HSAs
| Mean (SD) | 25–75 percentiles | Source | |
|---|---|---|---|
| Offices of physicians per 10 000 people (NAICS 621111) | 5.46 (4.07) | 3.03–7.19 | US Census Business Survey |
| Clinics (NAICS 621493/621498) | 0.84 (1.94) | 0.00–5.66 | US Census Business Survey |
| Kidney dialysis centres per million people (NAICS 621492) | 12.2 (23.1) | 0.0–16.6 | US Census Business Survey |
| General medical and surgical hospitals per 100 000 people (NAICS 6221) | 6.87 (10.96) | 1.38–7.36 | US Census Business Survey |
| Childcare centres per 10 000 children under five (NAICS 62441) | 42.29 (29.63) | 24.39–53.12 | US Census Business Survey |
| Difference between mean January and July temperatures (°F) | 42.96 (11.2) | 38.00–49.70 | NOAA Local Climatological Data |
| Percentage of population under 5 | 6.31 (1.19) | 5.57–6.93 | 2000, 2010 Census (SF1) |
| Percentage of population over 65 | 15.29 (4.6) | 12.41–17.69 | 2000, 2010 Census (SF1) |
| Percentage of population non-white or African–American | 9.24 (12.29) | 2.47–10.94 | 2000, 2010 Census (SF1) |
| Percentage of population African–American alone | 8.07 (13.41) | 0.47–8.74 | 2000, 2010 Census (SF1) |
| Percentage of civilian population in the labour force that are unemployed | 7.08 (3.37) | 4.70–8.90 | 2000, 2010 Census (DP3) |
| People per km2 | 245.62 (897.27) | 10.09–87.53 | 2000, 2010 Census (SF1), Dartmouth Atlas of Health |
| Medical discharges per 1000 Medicare enrollees | 256.86 (77.53) | 206.39–292.21 | Dartmouth Atlas of Health |
| Percentage of population 25+ with a BA or greater | 19.86 (10.25) | 13.00–23.58 | 2000 Census (SF3), 2010 American Community Survey (S1501) |
| Percentage of population with income below poverty line in past 12 months | 14.07 (6.62) | 9.40–17.45 | 2000, 2010 Census (DP3) |
| Population total | 85 862 (189 504) | 12 760–85 608 | 2000, 2010 Census (SF1) |
| Urgent care and retail clinics per 100 000 people (2010 only) | 1.67 (2.77) | 0.00–2.72 | Urgent Care Locations, LLC (www.urgentcarelocations.com) |
| Retail clinics per 100 000 people (excluding HSAs without retail clinic) (2010 only) | 1.35 (1.02) | 0.68–1.67 | Urgent Care Locations, LLC (www.urgentcarelocations.com) |
NAICS, North American Industry Classification System.
Statistical analysis
Because the US Census only occurs every 10 years, we included data from both 2000 and 2010 in our analyses. We used a two-way fixed-effect ordinary least squares (OLS) regression model that accounted for inherent differences in state regulations regarding prescribing as well as differences between years. Our base model centred on both the number of physician offices and clinics per capita in an HSA, and included the previously described socioeconomic, structural and infection level control variables. To further examine the socioeconomic determinants of differences in prescribing, we augmented the model to include a set of interaction terms, which allowed the measurement of the synergistic effect of physician density, poverty and clinics. The interaction terms help identify the two mechanisms simultaneously motivating differences in prescription rates across socioeconomic strata: differences in the accessibility of physicians to the local population and differences in the competitive landscape facing physicians. For model specification see the Supplementary data available at JAC Online.
For each regression model, a finer-grained analysis was done on the effect of clinics using only urgent and retail clinic data. However, these additional analyses only included data from 2010 because data for finer-grained analyses were not available for 2000.
To further distinguish between ‘access’ and ‘competition’ effects, we also used quantile regression to estimate effects of the independent variables across the entire distribution of prescriptions (for prescribing rate distribution see Figure S1) using the same model equations. Lastly, we examined the robustness of our results using the density of dialysis centres, which are comparable to clinics in that they are health provision centres that occupy similar physical spaces, are similarly located and hire similar employees, but would lack competition with physicians.
Results
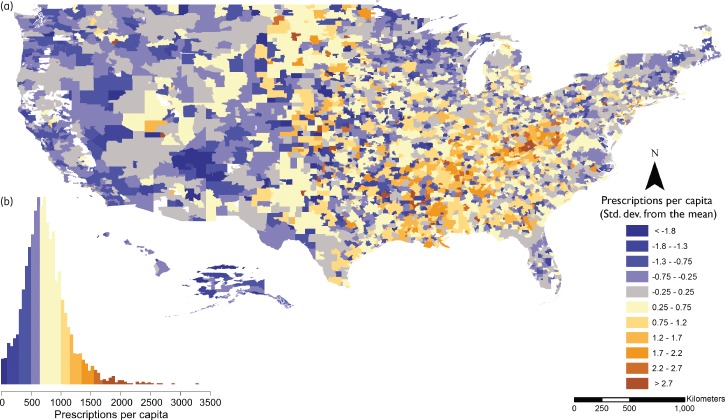
There were large differences in the rates of antibiotic prescribing by HSA (Figure 1 and Figure S2). The average rate of prescribing across HSAs was 793 [standard deviation (SD) 382] prescriptions per 1000 people. While there were fewer prescriptions written in 2010 compared with 2000, the patterns of prescribing by HSAs were similar across years (Figure S3).
Figure 1.
Antibiotic prescribing rates by HSA, 2010. Rates are defined as the number of prescriptions per capita. (a) Geographical variation in prescribing rates defined as SDs from the mean prescribing rate across all HSAs. There was a clear geographical variation, with more prescriptions on average in the south-east and to some extent the upper mid-west, while in the west the rates were significantly lower on average. (b) Number of prescriptions per capita colour-coded to match the SD breaks of the map. Source: IMS Xponent®, 2010, IMS Health Incorporated. All Rights Reserved.
We found a strong positive correlation between physician offices per capita and the number of prescriptions per capita (Table 2, column 1). For an increase of 1 SD in the number of physician offices per capita there was a 25.9% increase in prescriptions per capita. We also found that clinics were strongly correlated with increases in the prescription rate (10.5% increase per SD). Other variables that were strongly correlated with changes in the prescribing rate included: the proportion of the population over 65 (7.4% increase per SD); the number of childcare centres per capita (1.8% increase per SD); the percentage of the population with a bachelor's degree (12.5% increase per SD); the number of dialysis centres (4.6% increase per SD); the percentage of the population neither white nor African–American (7.9% decrease per SD); unemployment (0.9% decrease per 1 percentage point increase); and rural residence (12% decrease). The percentage of the population under age 5 was also strongly correlated, but exhibited a non-linear relationship with respect to prescriptions (Figure S4). While the results from our finer-grained analysis were less precise (larger standard errors) because clinic identification data were only available for 2010, the results were nearly identical to the prior results (Table 2, columns 2 and 3).
Table 2.
OLS regression results on dependent variable prescriptions per capita
| Prescriptions per inhabitanta |
|||
|---|---|---|---|
| clinics from census | urgent care and retail clinics | retail clinics | |
| Offices of physiciansa | 0.35 (0.03)*** | 0.33 (0.04)*** | 0.34 (0.04)*** |
| Clinics (NAICS 621493/621498)a,b | 0.07 (0.01)*** | ||
| Urgent care and retail clinicsa,b | 0.09 (0.02)*** | ||
| Retail clinicsa,b | 0.01 (0.03) | ||
| Number of kidney dialysis centresa | 0.02 (0.01)*** | 0.02 (0.01)*** | 0.02 (0.01)*** |
| Number of general medical and surgical hospitalsa | −0.01 (0.02) | 0.02 (0.03) | 0.003 (0.027) |
| Number of childcare centresa | 0.03 (0.01)* | 0.03 (0.01)* | 0.03 (0.01)** |
| Difference between mean January and July temperatures | 0.004 (0.003) | 0.002 (0.003) | 0.002 (0.003) |
| Percentage of population under 5 | 40.76 (8.75)*** | 40.48 (9.61)*** | 41.42 (9.51)*** |
| Percentage of population under 5 squared | −278.35 (66.89)*** | −269.43 (70.55)*** | −275.63 (70.22)*** |
| Percentage of population over 65a | 0.24 (0.09)** | 0.25 (0.10)** | 0.24 (0.10)** |
| Percentage of population non-white or African–Americana | −0.06 (0.03)** | −0.06 (0.03)** | −0.06 (0.03)** |
| Percentage of population African–American alonea | 0.003 (0.017) | 0.018 (0.018) | 0.015 (0.018) |
| Medical discharges per 1000 Medicare enrollees | 0.0004 (0.0002)** | 0.0006 (0.0003)* | 0.0005 (0.0003)* |
| Percentage of population 25+ with a BA or greatera | 0.24 (0.06)*** | 0.25 (0.06)*** | 0.28 (0.07)*** |
| Percentage unemployed | −2.11 (0.80)** | −1.61 (0.71)** | −1.56 (0.70)** |
| Rural | −0.12 (0.04)*** | −0.14 (0.05)*** | −0.14 (0.05)*** |
| Percentage living in poverty | 0.43 (0.27) | 0.94 (0.32) | 0.84 (0.32)** |
| Year (2010)b | −0.12 (0.04)*** | ||
| Constant | −3.95 (0.55)*** | −4.19 (0.64)*** | −4.23 (0.64)*** |
| N | 6862 | 3433 | 3433 |
| R2 | 0.31 | 0.31 | 0.30 |
Standard errors in parentheses.
***Significant at 1% level.
**Significant at 5% level.
*Significant at 10% level.
aLogged variable.
bEach column refers to a regression with a different definition for clinics. The first column uses data from the census, which includes additional healthcare establishments but has data for both 2000 and 2010. The second and third columns only include data for 2010 but use data from Urgent Care Locations, LLC (www.urgentcarelocations.com), which has more precise data on clinics. Column 2 includes urgent care and retail clinics, while column 3 only includes retail clinics.
Prescribing differences across socioeconomic strata
Our interaction terms help to elucidate how socioeconomic conditions affected the prescribing rate. While the marginal effect of the interaction of variables is identified by the coefficient on the relevant interaction term, the net effect on the baseline prescription rate in any context requires the addition of the relevant coefficients. For example, to identify the cumulative effect of physician density in a high-poverty area with a clinic requires summing the physician coefficient, each of the pairwise interactions involving physicians, and the triple interaction term. We found that the coefficients for the poverty–physician and poverty–clinic interaction terms were positive and significant, indicating that increases in prescriptions per capita were more strongly correlated to both the number of physician offices per capita and the presence of a clinic in areas with high poverty rates (Table 3, column 1). However, our triple interaction term, which measured the effect of physician offices on poorer areas with clinics, was negatively correlated with prescribing, indicating the per capita prescribing rate was not as strongly correlated with an increasing number of physician offices per capita in poor areas with clinics as in poor areas without clinics. Conversely, our positive clinic–physician interaction term indicates that in non-poor areas the opposite was true: the presence of clinics in a non-poor area increased the correlation between the number of physician offices per capita and the prescribing rate when compared with areas without clinics. Results from the finer-grained analysis were generally the same (i.e. same signs and similar magnitude, but less precision due to fewer data). While the precision of the interaction with poverty was lessened, the interaction with physician offices was strengthened.
Table 3.
OLS regression results on dependent variable prescriptions per capita with interactions
| Prescriptions per inhabitanta |
|||
|---|---|---|---|
| clinics from census | urgent care and retail clinics | retail clinics | |
| Offices of physiciansa | 0.25 (0.03)*** | 0.25 (0.04)*** | 0.30 (0.04)*** |
| Poverty indicator (1/0)b | −0.45 (0.15)*** | −0.11 (0.14) | −0.08 (0.11) |
| Clinic present indicator (1/0)c | −0.00 (0.08) | −0.16 (0.10) | −0.22 (0.10)** |
| Clinic–physician interactionc | 0.08 (0.04)* | 0.17 (0.05)*** | 0.12 (0.05)** |
| Poverty–physician interaction | 0.34 (0.09)*** | 0.13 (0.08) | 0.11 (0.06)* |
| Poverty–clinic interactionc | 0.54 (0.16)*** | 0.25 (0.22) | 0.29 (0.35) |
| Poverty–clinic–physician interactionc | −0.32 (0.08) *** | −0.13 (0.12) | −0.15 (0.18) |
| Number of kidney dialysis centresa | 0.02 (0.01)*** | 0.02 (0.01)** | 0.02 (0.01)*** |
| Number of general medical and surgical hospitalsa | −0.01 (0.02) | 0.03 (0.03) | 0.002 (0.027) |
| Number of childcare centresa | 0.03 (0.01)** | 0.03 (0.01)** | 0.03 (0.01)** |
| Difference between mean January and July temperatures | 0.004 (0.003) | 0.002 (0.003) | 0.002 (0.003) |
| Percentage of population under 5 | 37.60 (8.00)*** | 36.27 (9.26)*** | 38.03 (9.19)*** |
| Percentage of population under 5 squared | −257.51 (60.65)*** | −240.48 (68.14)*** | −249.90 (67.91)*** |
| Percentage of population over 65a | 0.25 (0.09)*** | 0.21 (0.10)** | 0.23 (0.10)** |
| Percentage of population non-white or African–Americana | −0.05 (0.02)* | −0.06 (0.03)** | −0.05 (0.03)* |
| Percentage of population African–American alonea | −0.002 (0.015) | 0.01 (0.02) | 0.01 (0.02) |
| Medical discharges per 1000 Medicare enrollees | 0.0003 (0.0002) ** | 0.0006 (0.0003)** | 0.0005 (0.0002)* |
| Percentage of population 25+ with a BA or greatera | 0.24 (0.05) *** | 0.21 (0.06)*** | 0.27 (0.06)*** |
| Percentage unemployed | −1.91 (0.77)** | −1.12 (0.65)* | −1.19 (0.65)* |
| Rural (1/0) | −0.12 (0.04)*** | −0.13 (0.05)*** | −0.14 (0.05)*** |
| Year (2010)c | −0.13 (0.04)*** | ||
| Constant | −3.71 (0.52)*** | −3.64 (0.63)*** | −3.93 (0.63)*** |
| N | 6862 | 3433 | 3433 |
| R2 | 0.32 | 0.32 | 0.30 |
Standard errors in parentheses.
***Significant at 1% level.
**Significant at 5% level.
*Significant at 10% level.
aLogged variable.
b25% of HSAs with the highest percentage of individuals living in poverty.
cEach column refers to a regression with a different definition for clinics. The first column uses data from the census, which includes additional healthcare establishments but has data for both 2000 and 2010. The second and third columns use data from Urgent Care Locations, LLC (www.urgentcarelocations.com), and include urgent care and retail clinics in column 2 and only retail clinics in column 3, but only include data for 2010.
Robustness
If unobservable factors associated with increasing the number of centres that deliver healthcare outside the hospital were driving our results, they would likely show up as correlations with dialysis centres. Therefore, we examined the robustness of our results to this assumption by replacing clinics with dialysis centres in our interaction analysis. We observed a positive association between dialysis centres and the physician prescribing rate, as would be expected in a sicker population. However, there was no other statistical effect on prescribing. In addition, if we included both clinics and dialysis centres in the regression, the clinic effect dominated the effect of dialysis centres (Table S1).
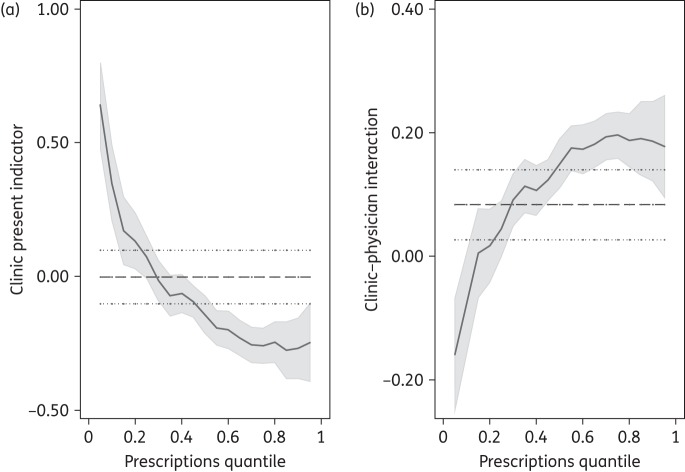
Our quantile regression analysis augmented the described findings, showing that the effect of clinics was different across the prescribing rate distribution. Where the prescribing rate was low, a clinic was correlated with a strong and significant positive effect on prescribing; however, as the rate of prescribing increased, the effect of a clinic alone was less strongly associated with prescribing (Figure 2a). However, the effect of the number of physician offices per capita was magnified by the presence of a clinic across the prescribing rate distribution, and this effect was more pronounced in areas with higher prescription rates (Figure 2b).
Figure 2.
Quantile regression plot. (a) Clinic present indicator variable, dummy variable indicating the presence of clinic(s) in an HSA. The presence of a clinic had a positive effect on the lower quantiles, in line with the ‘access’ hypothesis, and a slight negative effect on the upper quantiles, reflecting the better health of these populations and thus lower prescribing rate. (b) Clinic–physician interaction shows the effects of competition; the presence of a clinic augmented the rate at which prescriptions increased with the number of physicians; this was most pronounced at the higher end of the distribution, suggesting that it may be the result of competition for patients rather than access to alternative sources of care.
Discussion
In this study we found that both the number of physicians per capita and clinics were significant drivers of the per capita antibiotic prescription rate. These results are consistent with the literature showing that increasing physician density drives consumption of healthcare services.29 However, we also found a suggestion that clinics altered the prescribing rate differently in poor and non-poor areas. This is not surprising since clinics provide an experience that is generally less expensive and more convenient (more hours, no appointments, less expensive) than physician offices or emergency departments.21,30 Evidence also suggests that a large proportion of their clientele are underserved by the rest of the medical system,31 particularly individuals without insurance.21 Thus, we examined how poverty and clinics interacted to elucidate the different mechanisms driving prescribing rates in different socioeconomic areas.
Our results suggest that, in poor areas, while prescription rates increased with both physician office density and the presence of a clinic, the effect of physician office density was mitigated by the presence of a clinic. In other words, these results suggest that adding a clinic or a physician office in a poor area increases prescribing, but once a clinic already exists in an area introducing more physician offices has a limited impact. These are the expected results if access is the fundamental issue.
In higher-income areas, on the other hand, we found that the presence of a clinic augmented physician prescribing. This correlation was particularly strong when we only used retail clinics, which are largely concentrated in higher-income areas.24 This suggests that in wealthier areas, which are generally already well served by physicians,23 clinic presence increases the prescribing rate for providers. The significant lack of an interaction with poverty also supports the notion that retail clinics were largely affecting prescribing habits in wealthier areas. Our quantile regression approach also strongly supported this result, showing that this effect was strongest in areas that received the most prescriptions.
There are two potential reasons for the per capita increase in prescribing in non-poor areas: (i) the probability of prescribing an antibiotic at a visit increases when clinics are introduced; and (ii) prescribing rates are constant per visit, but the presence of a clinic augments the office visit rate. Data from The National Ambulatory Medical Care Survey (NAMCS) suggest that while the prescribing rates remain constant across poverty levels, the visit rate to outpatient ambulatory care physician offices is 3.5-fold higher in wealthy areas compared with poor areas (Figure S5). While the greater office visit rate suggests that the primary mechanism driving higher prescribing rates is more office/clinic visits, prior studies have found that increasing physician density increases prescribing rates through efforts to retain patients19 and to maintain good patient relationships.20 In addition, when retail clinics open nearby, physicians may change their operations, including providing increased access to same-day care and extended hours.32 These responses, which are a form of non-price competition, can drive up the office visit rate and by extension the antibiotic prescribing rate. Further study in this area is warranted.
We have rigorously controlled for model specification and possible omissions of contributing factors by using OLS and quantile regression and a robustness check using dialysis centre density, controlling for state and year differences and using carefully selected control variables. However, our analysis is subject to some limitations. First, our results are correlative and not causative and we cannot capture every factor that may affect prescribing behaviour. Second, the variables included may not adequately identify all the factors leading to differences in prescribing. This is particularly true for cultural factors, which have been shown to vary broadly at the country level,12 but additional analysis is needed to understand whether cultural differences drive variation in prescribing rates in the USA. Lastly, we only had data on the number of physician offices and not the number of providers. However, due to the scale of the analysis (>3400 geographical regions), this is unlikely to vary in any systematic way that would bias the results.
Conclusions
Our results suggest that in the USA, as in other countries,9,13 an increase in provider density is associated with an increase in per capita antibiotic prescribing. However, we find evidence that, rather than this association being driven by supply-induced demand for prescriptions,9,29 it is due to competition between providers. Therefore, new intervention campaigns must be broad-based, acknowledging the changing healthcare delivery landscape and emphasizing more local coordination among diverse groups of practitioners.
Funding
This work was supported by The Models of Infectious Disease Agent Study (MIDAS) awarded by the National Institutes of General Medical Sciences at the National Institutes of Health (U54 GM088491) and The Extending the Cure Project funded by a Pioneer Portfolio grant of the Robert Wood Johnson Foundation. The funders had no role in the design and conduct of the study, the collection, management, analysis and interpretation of the data, or the preparation, review and approval of the manuscript.
Transparency declarations
None to declare.
Disclaimer
The statements, findings, conclusions, views and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): Xponent™ (2000–2010), IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
Supplementary data
References
- 1.Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol 2010; 8: 260–71. [DOI] [PubMed] [Google Scholar]
- 2.Niederman MS. Impact of antibiotic resistance on clinical outcomes and the cost of care. Crit Care Med 2001; 29: N114–20. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove SE, Kaye KS, Eliopoulous GM, et al. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med 2002; 162: 185–90. [DOI] [PubMed] [Google Scholar]
- 4.Braykov N, Eber MR, Klein EY, et al. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999–2010. Infect Control Hosp Epidemiol 2013; 34: 259–68. [DOI] [PubMed] [Google Scholar]
- 5.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 2007; 13: 1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365: 579–87. [DOI] [PubMed] [Google Scholar]
- 7.Steinman MA, Landefeld C, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA 2003; 289: 719–25. [DOI] [PubMed] [Google Scholar]
- 8.Nitzan O, Low M, Lavi I, et al. Variability in outpatient antimicrobial consumption in Israel. Infection 2010; 38: 12–8. [DOI] [PubMed] [Google Scholar]
- 9.Filippini M, Masiero G, Moschetti K. Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy 2006; 78: 77–92. [DOI] [PubMed] [Google Scholar]
- 10.Glass SK, Pearl DL, McEwen SA, et al. Canadian province-level risk factor analysis of macrolide consumption patterns (2000–2006). J Antimicrob Chemother 2010; 65: 148–55. [DOI] [PubMed] [Google Scholar]
- 11.Matuz M, Benko R, Doro P, et al. Regional variations in community consumption of antibiotics in Hungary, 1996–2003. Br J Clin Pharmacol 2006; 61: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbarth S, Monnet D. Cultural and socioeconomic determinants of antibiotic use. In: Gould I, Meer J, eds. Antibiotic Policies: Fighting Resistance. New York: Springer US, 2008; 29–40. [Google Scholar]
- 13.Masiero G, Filippini M, Ferech M, et al. Socioeconomic determinants of outpatient antibiotic use in Europe. Int J Public Health 2010; 55: 469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Laxminarayan R. Are physicians' prescribing decisions sensitive to drug prices? Evidence from a free-antibiotics program. Health Econ 2013; doi:10.1002/hec.3008. [DOI] [PubMed] [Google Scholar]
- 15.Harbarth S, Albrich W, Brun-Buisson C. Outpatient antibiotic use and prevalence of antibiotic-resistant pneumococci in France and Germany: a sociocultural perspective. Emerg Infect Dis 2002; 8: 1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monnet DL, Ferech M, Frimodt-Møller N, et al. The more antibacterial trade names, the more consumption of antibacterials: a European study. Clin Infect Dis 2005; 41: 114–7. [DOI] [PubMed] [Google Scholar]
- 17.Anderson GF, Reinhardt UE, Hussey PS, et al. It's the prices, stupid: why the United States is so different from other countries. Health Aff (Millwood) 2003; 22: 89–105. [DOI] [PubMed] [Google Scholar]
- 18.Schoen C, Osborn R, Doty MM, et al. Toward higher-performance health systems: adults' health care experiences in seven countries, 2007. Health Aff (Millwood) 2007; 26: w717–34. [DOI] [PubMed] [Google Scholar]
- 19.Bennett D, Hung C-L, Lauderdale T-L. Health care competition and antibiotic use in Taiwan. University of Chicago Press, Chicago, 2014. http://home.uchicago.edu/~dmbennett/abx.pdf. [Google Scholar]
- 20.Butler CC, Rollnick S, Pill R, et al. Understanding the culture of prescribing: qualitative study of general practitioners' and patients' perceptions of antibiotics for sore throats. BMJ 1998; 317: 637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinick RM, Burns RM, Mehrotra A. Many emergency department visits could be managed at urgent care centers and retail clinics. Health Aff (Millwood) 2010; 29: 1630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudavsky R, Mehrotra A. Sociodemographic characteristics of communities served by retail clinics. J Am Board Fam Med 2010; 23: 42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto M, Inoue K, Bowman R, et al. Geographical distributions of physicians in Japan and us: impact of healthcare system on physician dispersal pattern. Health Policy 2010; 96: 255–61. [DOI] [PubMed] [Google Scholar]
- 24.Pollack C, Armstrong K. The geographic accessibility of retail clinics for underserved populations. Arch Intern Med 2009; 169: 945–9. [DOI] [PubMed] [Google Scholar]
- 25.Polgreen P, Yang M, Laxminarayan R, et al. Respiratory fluoroquinolone use and influenza. Infect Control Hosp Epidemiol 2011; 32: 706–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Klein EY, Laxminarayan R. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin Infect Dis 2012; 55: 687–94. [DOI] [PubMed] [Google Scholar]
- 27.Polgreen PM, Yang M, Kuntz JL, et al. Using oral vancomycin prescriptions as a proxy measure for Clostridium difficile infections: a spatial and time series analysis. Infect Control Hosp Epidemiol 2011; 32: 723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wennberg JE, Cooper MM. The Dartmouth Atlas of Health Care. Chicago: American Hospital Publishing, 1996. [PubMed] [Google Scholar]
- 29.Léonard C, Stordeur S, Roberfroid D. Association between physician density and health care consumption: a systematic review of the evidence. Health Policy 2009; 91: 121–34. [DOI] [PubMed] [Google Scholar]
- 30.Mehrotra A, Liu H, Adams JL, et al. Comparing costs and quality of care at retail clinics with that of other medical settings for 3 common illnesses. Ann Intern Med 2009; 151: 321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehrotra A, Wang MC, Lave JR, et al. Retail clinics, primary care physicians, and emergency departments: a comparison of patients' visits. Health Aff (Millwood) 2008; 27: 1272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachman J. What do retail clinics mean for family medicine? Fam Pract Manag 2006; 13: 19–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.