Abstract
Objectives
This study aims to investigate the impact of newly diagnosed atrial fibrillation (AF) on future major adverse cardiac events (MACE). AF is the most common form of cardiac arrhythmia and is associated with several other cardiovascular (CV) events. Little is known about whether newly diagnosed AF is an independent factor for future MACE, especially in patients without such a history.
Methods and Results
We evaluated data from the National Health Insurance Research Database, which represented a retrospective cohort of 713,288 adults in Taiwan from 2006 to 2010. Individuals with previous MACE were excluded. Newly diagnosed AF patients were identified by assigning International Classification of Diseases codes. Propensity score matching adjusted for gender, age, hypertension, diabetes mellitus and dyslipidemia. Cox proportional hazard models estimated future MACE ratios. We compared a total of 3,737 patients with newly diagnosed AF and 704,225 patients without. After matching, there was no difference in baseline demographic characteristics in patients across newly diagnosed AF and non-AF groups. The result showed that newly diagnosed AF in multivariate analysis were associated with increased incidents of MACE (hazard ratio: 3.11-3.51 in different models) and mortality. Newly diagnosed AF without other CV risk factors had 8.45 times the risk of developing future MACE than healthy adults. The more associated CV risk factors in addition to AF, the increased rate of future CV events.
Conclusions
Newly diagnosed AF is an independent factor that leads to future CV events after gender, age, hypertension, diabetes mellitus and dyslipidemia matching. AF is associated with a higher mortality rate.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and affects more than 1.2% of the general population [1]. AF is a well-documented independent risk factor for stroke [2–5], heart failure (HF) [2, 6], and premature death [2, 6–12]. Meanwhile, AF in patients with cardiac comorbidities, such as HF with or without left ventricular dysfunction [10, 13] and myocardial infarction [8], are also associated with an increased risk of cardiovascular (CV) events and mortality. In previous studies, newly diagnosed AF was specifically analyzed and also disclosed a higher risk of mortality [9, 11]. However, little is known about the influence of newly diagnosed AF on major adverse cardiovascular events (MACE), such as myocardial infarction (MI), percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), HF, stroke, malignant dysrhythmia, thrombolysis and cardiogenic shock, especially in patients without pre-existing events. Furthermore, all of the population-based studies, to our knowledge, were conducted in Western countries and most of the patients were of Caucasian decent. Previously, we have studied the effect of MACE in different populations [14–16]. In this study, we analyzed a large-scale, population-based data in an Asian population from National Health Insurance (NHI) claims records in Taiwan to evaluate the impact of newly diagnosed AF on future CV events in adults without pre-existing MACE.
Methods
Informed consent was waived as the database analysis used de-identified secondary data, and the study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (#98-4060B). All the patient records/information was anonymized and de-identified prior to analysis.
Data Source
The NHI in Taiwan started in March 1995 and provides effective insurance coverage to the entire population. Approximately 96% of the Taiwanese population has registered for the NHI program [17]. Since 1996, the National Health Insurance Research Database (NHIRD) has covered 97% of hospitals and clinics throughout the country [18].
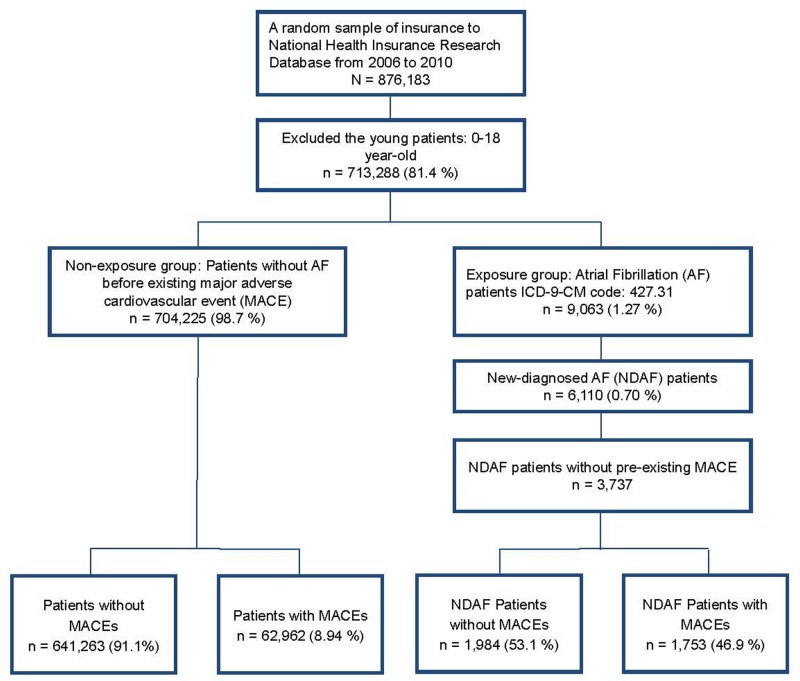
Data on the prevalence and incidence of AF in Taiwanese adults were obtained from the Department of Household Registration Affairs between 2006 and 2010. Randomized NHIRD data from the same period were used for the current study. After excluding individuals younger than 18 years of age, a total of 713,288 participants were analyzed (Fig 1). AF was diagnosed by International Classification of Diseases-9-Clinical Modification (ICD-9-CM) code: 427.31, where gender, age, and medical treatment information were also considered. Patients of newly diagnosed AF without pre-existing MACE were acquired by ICD-9-CM coding during this period (n = 3,737).
Fig 1. Flowchart of the relationship between newly diagnosed AF and MACE.
AF = atrial fibrillation; MACE = major adverse cardiovascular events.
In order to effectively investigate the relationship between newly diagnosed AF and future MACE, pre-existing events were also excluded, including myocardial infarction (MI, ICD-9-CM code: 410–410.9), PCI (operation code: 36.0–36.03, 36.05–36.09), CABG (operation code: 36.1–36.99, V45.81), HF (ICD-9-CM code: 428.0–428.10), stroke (ICD-9-CM code: 430–437), malignant dysrhythmia (IDC-9-CM code: 426.0, 426.12–426.13, 426.51, 426.52, 426.54, 427.1, 427.4, 427.41, 427.42, 427.5), thrombolysis (operation code: 36.0–36.99), cardiogenic shock (ICD-9-CM code: 785.51), pulmonary embolism (ICD-9-CM code: 415.1, 415.11, 415.19, 673), and deep vein thrombosis (ICD-9-CM code: 453.0, 453.2, 453.3, 453.8).
Case matching
In Framingham Heart Study, only age, gender, total cholesterol, high density lipoprotein cholesterol, smoking and systolic blood pressure were used to predict future cardiac events [19]. So we used propensity score matching of SAS macro at a ratio of 4:1 to adjust the influences of other associated CV risk factors, such as hypertension (ICD-9-CM code: 401–405, 437.2, and 362.11), diabetes mellitus (DM, ICD-9-CM code: 250, 357.2, 362.01, 362.02, and 366.41), dyslipidemia (ICD-9-CM code: 272), gender, and age (older than 65 years).
Statistical analysis
Chi-square tests were used to compare categorical variables and Student’s t-test was employed for continuous variables. The Kaplan-Meier method was also used to estimate overall survival, and the log-rank test was used to test the difference between groups. The Cox model was used to estimate covariate values, such as gender, classified age, elderly (≥65 years), AF, hypertension, DM, and dyslipidemia. Finally, both Score and Wald test were considered to verify that these Cox models with parameters can appropriately be estimated from the sample in NHIRD. The criterions for model selection, such as Akaike information criterion (AIC) and Bayesian information criterion (BIC), were adopted for selecting the outcome models from a set of candidate models as well. Data were calculated as means, standard deviations, percentages and confidence intervals. All analyses were conducted by using SAS statistical software, Version 9.3 (SAS Institute Inc., Cary, North Carolina). A p-value < 0.05 was considered statistically significant.
Results
The prevalence and incidence of AF in Taiwanese adults increases with age in both men and women (Table 1). After exclusion, a total of 704,225 participants were identified who did not have previous CV events and AF, compared to a total of 3,737 participants who developed newly diagnosed AF without pre-existing CV events (Table 2). Before matching, the differences of baseline characteristics—gender, age, hypertension, DM and dyslipidemia—were significantly more in the AF group (P<0.0001) than the non-AF group. The incidences of future MACE and mortality were also predominant in the AF group (P<0.0001). After matching at a ratio of 4:1, the differences of the baseline characteristics were identical in both groups (P = 1), while future MACE and mortality remained more frequent in the AF group (P = 0.0113 to P<0.0001), and most of the deaths were non-stroke related in both groups. Among these MACE, HF was the most frequent developed event in AF group (27.1%) followed by stroke (21.9%). However, in non-AF group, stroke was the most frequent developed event (19.5%) instead of HF (7.81%). Moreover, both MACE and mortality were significantly different between AF and non-AF groups over time (Fig 2, both Log-rank P<0.0001) when analyzed by using Kaplan-Meier method.
Table 1. The prevalence and incidence of atrial fibrillation (AF) between 2006 and 2010 in Taiwan.
| Population | Prevalence of AF | Incidence of AF | ||||
|---|---|---|---|---|---|---|
| Age | Female | Male | Female | Male | Female | Male |
| 18–55 | 6,701,788 | 6,776,865 | 9,363 | 17,115 | 5,200 | 9,237 |
| (%) | (0.14) | (0.25) | (0.08) | (0.14) | ||
| 55–65 | 1,325,396 | 1,274,414 | 12,589 | 19,412 | 5,709 | 8,262 |
| (%) | (0.95) | (1.52) | (0.43) | (0.65) | ||
| 65–75 | 735,413 | 650,323 | 25,441 | 29,831 | 8,840 | 10,085 |
| (%) | (3.46) | (4.59) | (1.20) | (1.55) | ||
| 75–85 | 438,191 | 423,123 | 33,535 | 40,625 | 8,720 | 11,101 |
| (%) | (7.65) | (9.27) | (1.99) | (2.62) | ||
| 85+ | 125,778 | 115,065 | 16,145 | 13,042 | 3,557 | 3,181 |
| (%) | (12.8) | (11.3) | (2.83) | (2.76) | ||
| Total | 9,326,566 | 9,239,790 | 97,073 | 120,025 | 32,026 | 41,866 |
| (%) | (0.10) | (0.13) | (0.34) | (0.45) | ||
Note: Source from Department of Household Registration Affairs, Ministry of the Interior, ROC. 2010. The official website is http://sowf.moi.gov.tw/stat/year/list.htm.
Table 2. Matching by Propensity Score method in participants with and without newly diagnosed atrial fibrillation (AF).
| Before matching | After matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Data Source | Without AF | With AF | P Value | Without AF | With AF | P Value | ||||
| (n = 704,225) | (n = 3,737) | (n = 14,948) | (n = 3,737) | |||||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||
| Gender | <0.0001 | 1.0000 | ||||||||
| Female | 354,651 | (50.4) | 1,659 | (44.4) | 6,636 | (44.4) | 1,659 | (44.4) | ||
| Male | 349,574 | (49.6) | 2,078 | (55.6) | 8,312 | (55.6) | 2,078 | (55.6) | ||
| Elder | 89,044 | (12.6) | 2,345 | (62.8) | <0.0001 | 9,380 | (62.8) | 2,345 | (62.8) | 1.0000 |
| HTN | 176,682 | (25.1) | 2,316 | (62.0) | <0.0001 | 9,264 | (62.0) | 2,316 | (62.0) | 1.0000 |
| DM | 91,949 | (13.1) | 945 | (25.3) | <0.0001 | 3,780 | (25.3) | 945 | (25.3) | 1.0000 |
| Dyslipidemia | 138,514 | (19.7) | 899 | (24.1) | <0.0001 | 3,596 | (24.1) | 899 | (24.1) | 1.0000 |
| MACE | 62,962 | (8.94) | 1,753 | (46.9) | <0.0001 | 4,006 | (26.8) | 1,753 | (46.9) | <0.0001 |
| MI | 7,270 | (1.03) | 219 | (5.86) | <0.0001 | 502 | (3.36) | 219 | (5.86) | <0.0001 |
| PCI | 4,378 | (0.62) | 149 | (3.99) | <0.0001 | 252 | (1.69) | 149 | (3.99) | <0.0001 |
| CABG | 985 | (0.14) | 34 | (0.91) | <0.0001 | 64 | (0.43) | 34 | (0.91) | 0.0003 |
| HF | 14,996 | (2.13) | 1,013 | (27.1) | <0.0001 | 1,167 | (7.81) | 1,013 | (27.1) | <0.0001 |
| Stroke | 46,119 | (6.55) | 818 | (21.9) | <0.0001 | 2,911 | (19.5) | 818 | (21.9) | 0.0010 |
| Malignant arrhythmia | 2,804 | (0.40) | 132 | (3.53) | <0.0001 | 186 | (1.24) | 132 | (3.53) | <0.0001 |
| Thrombolysis | 4,378 | (0.62) | 149 | (3.99) | <0.0001 | 252 | (1.69) | 149 | (3.99) | <0.0001 |
| Cardiogenic shock | 692 | (0.10) | 52 | (1.39) | <0.0001 | 54 | (0.36) | 52 | (1.39) | <0.0001 |
| Pulmonary embolism | 625 | (0.09) | 21 | (0.56) | <0.0001 | 37 | (0.25) | 21 | (0.56) | 0.0020 |
| Deep vein thrombosis | 1,947 | (0.28) | 38 | (1.02) | <0.0001 | 94 | (0.63) | 38 | (1.02) | 0.0113 |
| Mortality | 151 | (0.02) | 60 | (1.61) | <0.0001 | 8 | (0.15) | 60 | (1.61) | <0.0001 |
| Stroke death | 18 | (11.9) | 10 | (16.7) | <0.0001 | 0 | (0.0) | 10 | (16.7) | <0.0001 |
| Non-stroke death | 133 | (88.1) | 50 | (83.3) | <0.0001 | 8 | (100.0) | 50 | (83.3) | <0.0001 |
Elder = the people are over 65 years old; AF = atrial fibrillation; CABG = coronary artery bypass grafting; DM = diabetes mellitus; HF = heart failure; HTN = hypertension; MACE = major adverse cardiovascular events; MI = myocardial infarction; PCI = percutaneous coronary intervention.
Fig 2. Mortality and MACE between AF and non-AF.
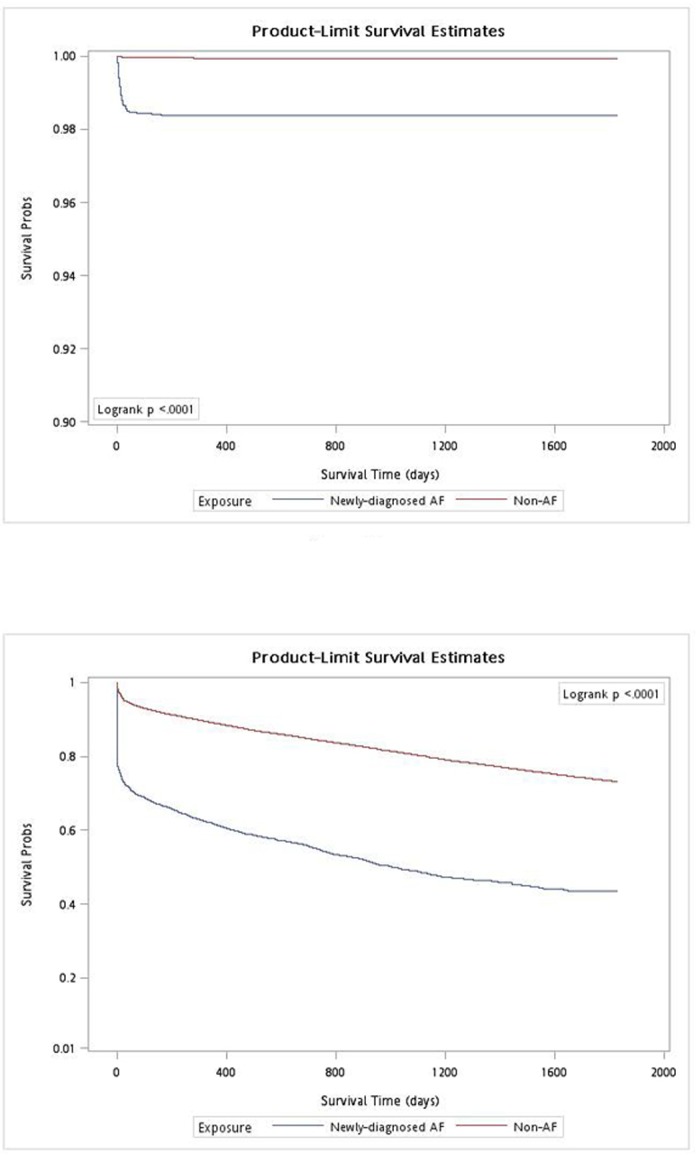
The Kaplan-Meier estimated cumulative all-cause death in non-atrial fibrillation (AF, red) and newly diagnosed AF (blue). A. Mortality; B. Major adverse cardiovascular events (MACE).
Univariate Cox regression analysis revealed that female, elderly, AF, hypertension, DM and dyslipidemia patient tended to develop more MACE statistically (Table 3). In order to describe the effects of age level in MACE, it was organized into category (18–55, 55–65, 65–75, 75–85, and 85+ years) and binary (over 65 years or not) data to depict in multivariate model 1 and 2, respectively. In multivariate models with satisfying the criteria of model selection, similar results were obtained for elderly (hazard ratio, HR 2.98–9.77, P<0.0001 in Model 1 and HR 3.4, P<0.0001 in Model 2), AF (HR 3.11–3.51, P<0.0001), hypertension (HR 1.85–2.03, P<0.0001) and DM patients (HR 1.32–1.33, P<0.0001) (Table 4). However, males developed more future MACE (HR 1.11–1.12, P<0.0001) in multivariate analysis, and there was statistically insignificant in dyslipidemia in Model 2 (HR 1.04, P = 0.1661). The multivariate Cox modeling and model selection were showed in Table 5, which showed both models were appropriately fitted for the sample in statistical significance. We further demonstrated the average time between the incident of AF and MACE (Table 6), which showed the earliest developed MACE after newly diagnosed AF were MI and HF (0.49 year, 95% CI 0.36 to 0.62 and 0.44 to 0.54, respectively). The latest developed event was malignant arrhythmia (1.09 year, 95% CI 0.89 to 1.29).
Table 3. Univariate Cox regression analysis for factors and 2x2 tables associated with MACE.
| Variables | MACE | Univariate analysis | |||||
|---|---|---|---|---|---|---|---|
| No | (%) | Yes | (%) | HR | 95%CI | P value | |
| Gender | |||||||
| Female | 5,671 | (68.4) | 2,624 | (31.6) | 1.0 | ||
| Male | 7,255 | (69.8) | 3,135 | (30.2) | 0.95 | 0.90–1.00 | 0.0424 |
| Age | |||||||
| 18–55 | 4,752 | (92.7) | 375 | (7.31) | 1.0 | ||
| 55–65 | 1,323 | (72.2) | 510 | (27.8) | 4.65 | 4.07–5.31 | <0.0001 |
| 65–75 | 4,249 | (68.1) | 1,992 | (31.9) | 5.01 | 4.49–5.59 | <0.0001 |
| 75–85 | 2,175 | (50.4) | 2,143 | (49.6) | 9.00 | 8.06–10.0 | <0.0001 |
| 85- | 427 | (36.6) | 739 | (63.4) | 13.1 | 11.6–14.8 | <0.0001 |
| Elder | |||||||
| No | 6,075 | (87.3) | 885 | (12.7) | 1.0 | ||
| Yes | 6,851 | (58.4) | 4,874 | (41.6) | 3.84 | 3.58–4.13 | <0.0001 |
| AF | |||||||
| No | 10,942 | (73.2) | 4,006 | (26.8) | 1.0 | ||
| Yes | 1,984 | (53.1) | 1,753 | (46.9) | 3.40 | 3.20–3.60 | <0.0001 |
| HTN | |||||||
| No | 5,875 | (82.7) | 1,230 | (17.3) | 1.0 | ||
| Yes | 7,051 | (60.9) | 4,529 | (39.1) | 2.58 | 2.42–2.74 | <0.0001 |
| DM | |||||||
| No | 10,162 | (72.8) | 3,798 | (27.2) | 1.0 | ||
| Yes | 2,764 | (58.5) | 1,961 | (41.5) | 1.67 | 1.59–1.77 | <0.0001 |
| Dyslipidemia | |||||||
| No | 9,992 | (70.4) | 4,198 | (29.6) | 1.0 | ||
| Yes | 2,934 | (65.3) | 1,561 | (34.7) | 1.21 | 1.14–1.28 | <0.0001 |
Elder = the people are over 65 years old; AF = atrial fibrillation; DM = diabetes mellitus; HR = hazard ratio; HTN = hypertension; MACE = major adverse cardiovascular events.
Table 4. Multivariate Cox models for factors associated with MACE.
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender | ||||||
| Female | 1.0 | 1.0 | ||||
| Male | 1.12 | 1.06–1.18 | <0.0001 | 1.11 | 1.06–1.17 | <0.0001 |
| Age (year) | ||||||
| 18–55 | 1.0 | 1.0 | ||||
| 55–65 | 2.98 | 2.60–3.41 | <0.0001 | |||
| 65–75 | 4.21 | 3.77–4.71 | <0.0001 | 3.40 | 3.16–3.66 | <0.0001 |
| 75–85 | 6.76 | 6.05–7.56 | <0.0001 | |||
| 85+ | 9.77 | 8.61–11.1 | <0.0001 | |||
| AF | ||||||
| No | 1.0 | 1.0 | ||||
| Yes | 3.11 | 2.93–3.30 | <0.0001 | 3.51 | 3.31–3.72 | <0.0001 |
| HTN | ||||||
| No | 1.0 | 1.0 | ||||
| Yes | 1.85 | 1.73–1.97 | <0.0001 | 2.03 | 1.90–2.17 | <0.0001 |
| DM | ||||||
| No | 1.0 | 1.0 | ||||
| Yes | 1.32 | 1.25–1.40 | <0.0001 | 1.33 | 1.25–1.41 | <0.0001 |
| Dyslipidemia | ||||||
| No | 1.0 | 1.0 | ||||
| Yes | 1.13 | 1.06–1.20 | 0.0001 | 1.04 | 0.98–1.11 | 0.1661 |
AF = atrial fibrillation; DM = diabetes mellitus; HR = hazard ratio; HTN = hypertension; MACE = major adverse cardiovascular events.
Table 5. Multivariate Cox modeling and model selection.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Modeling | ||||
| Coefficient | P value | Coefficient | P value | |
| Score | 4,925 | <0.0001 | 4,196 | <0.0001 |
| Wald | 4,020 | <0.0001 | 3,734 | <0.0001 |
| -2 log L. | 106,147 | 106,813 | ||
| Model selection | ||||
| AIC | 106,165 | 106,825 | ||
| BIC | 106,225 | 106,865 | ||
-2 log L. = -2 log Likelihood; AIC = Akaike information criterion; BIC = Bayesian information criterion; Score and Wald are testing global hull hypothesis by Chi-square test with 9 and 6 degree of freedom in model 1 and 2, respectively.
Table 6. The average time between the incident of AF and MACE.
| AF (n = 3,737) | |||
|---|---|---|---|
| MACE (n = 1,753) | n | years | 95%CI |
| MI | 219 | 0.49 | 0.36–0.62 |
| PCI | 149 | 0.61 | 0.47–0.75 |
| CABG | 34 | 1.06 | 0.55–1.57 |
| HF | 1,013 | 0.49 | 0.44–0.54 |
| Stroke | 818 | 0.58 | 0.52–0.64 |
| Malignant arrhythmia | 132 | 1.09 | 0.89–1.29 |
| Thrombolysis | 149 | 0.60 | 0.47–0.73 |
| Cardiogenic shock | 52 | 0.59 | 0.36–0.82 |
| Pulmonary embolism | 21 | 0.91 | 0.34–1.48 |
| Deep vein thrombosis | 38 | 0.57 | 0.35–0.79 |
AF = atrial fibrillation; MACE = major adverse cardiovascular events; MI = myocardial infarction; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; HF = heart failure; CI = confidence interval.
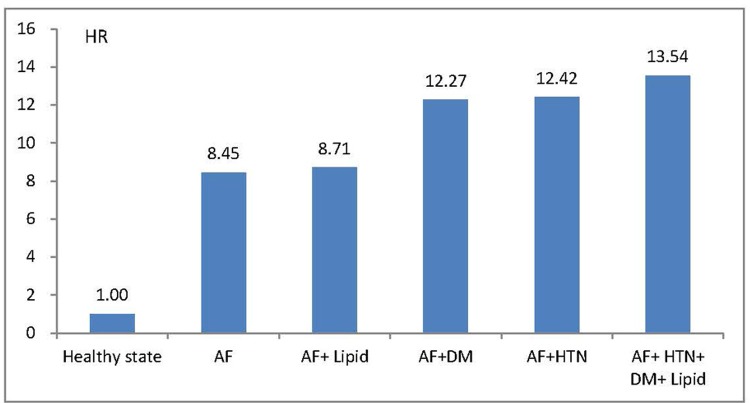
Moreover, compared to healthy participants without any CV risk factors, newly diagnosed AF was associated with 8.45 times the risk of developing future MACE (95% CI 7.37 to 9.68, P<0.0001) (Fig 3). In addition to AF, the rate of future MACE increased when patients had more associated CV comorbidities (HR 8.71, 95% CI 6.55 to 11.58, P<0.0001 in AF and dyslipidemia; HR 12.27, 95% CI 9.51 to 15.82, P<0.0001 in AF and DM; HR 12.42, 95% CI 10.94 to 14.09, P<0.0001 in AF and hypertension; and HR 13.54, 95% CI 11.29 to 16.22, P<0.0001 in AF with hypertension, DM and dyslipidemia).
Fig 3. HR for future MACE.
The hazard ratios (HR) of atrial fibrillation (AF) and other cardiovascular risk factors compared to healthy participants in developing future major adverse cardiovascular events (MACE). Lipid = dyslipidemia; DM = diabetes mellitus; HTN = hypertension.
Discussion
In previous studies, the influence of AF on future MACE or premature death did not exclude co-existing CV diseases. The present work is the first large-scale study to show that newly diagnosed AF patients without prior CV diseases associate with a higher risk of MACE, compared to patients without AF. After age, gender, hypertension, DM and dyslipidemia matching, newly diagnosed AF remains an independent risk factor for future MACE. In addition, AF is also a strong predictor of mortality over time.
In a subgroup analysis of the Candesartan in heart failure—assessment of reduction in mortality and morbidity (CHARM) program, AF in patients with HF experienced a higher risk of worsening HF and stroke regardless of baseline ejection fraction [10]. In the same study, newly onset of AF patients with HF was also associated with worse CV outcomes. Similar results were also demonstrated in a recently published study [13]. The relationship between long-term outcome and AF in patients after acute MI complicated by HF was also examined in a subgroup analysis of Valsartan in acute myocardial infarction trial (VALIANT) [8]. Both previously known and newly diagnosed AF were associated with more CV events than those without AF. However, in another population-based study in Scotland, AF was associated with more stroke and HF events but not acute MI [6]. In our study, we excluded pre-existing CV diseases in the new onset of AF, which is different from previous studies. Our study demonstrated a higher risk not only for HF and stroke but also for MI, PCI, CABG, malignant dysrhythmia, thrombolysis and cardiogenic shock.
As expected, age, hypertension and DM were also associated with higher risk of MACE in our multivariate analysis. Similar results have been well established in previous studies [6, 11, 20, 21], and treating such factors can significantly reduce future events [22]. However, the impact of gender on MACE is uncertain. For stable angina but normal coronary arteries, the MACE rate was equal among men and women [23]. In two population-based studies, cardiovascular events and mortality were more frequently developed in women in one [6] but not in the other [11]. In current study, we demonstrated that men associated with a higher MACE rate than women. We suspect the uncertainty may come from different selected cohorts and population.
AF is an independent factor for mortality and future thromboembolic events [2–4]. On the other hand, previous study showed the majority of deaths in AF patients were not related to stroke [24]. In our study, we also demonstrated most of the mortality were non-stroke related in both AF and non-AF groups. Mortality was higher in permanent and first detected AF compared to paroxysmal and persistent AF [25]. In the past, AF management concepts have changed, such as the increased use of antiarrhythmic drugs, control underlying comorbidities and increased aggressiveness in anticoagulation and AF ablation. Mortality trends have improved over time in some studies [26–28] but not in others [9]. A community-based study concluded that newly diagnosed AF was associated with high mortality risk, especially within the first four months, but the mortality trend has not significantly changed over the past 21 years [9]. The authors presumed that AF per se may act as a “risk marker” rather than a “risk factor”. The worse outcome resulted from underlying diseases or pathologic changes, and the treatment toward AF itself may have not influenced the mortality trend. Similar points of view were also proposed by another study [11] in which patients who had new-onset AF died earlier than the general population but mainly due to non-CV causes. The mortality trend was constant for both in patients with or without previous heart diseases. The authors concluded that AF itself may be only a minor component of excess mortality. In our study, newly diagnosed AF without pre-existing CV diseases was associated with higher mortality rate than in patients without AF. We speculate that the cause of premature death is multifactorial, and new-onset AF itself may be not the direct cause but represent a higher severity of underlying diseases, which results in higher mortality. Treatment of the primary medical illnesses that lead to new-onset AF may be the first priority to reduce the risk of premature death.
Potential limitations of this study should be noted. First, the diagnosis of AF was made by the ICD-9 code from NHIRD but not directly by documented electrocardiography. Although we compared the results with the patients without such coding, there may exist some misclassified cases in either group. In addition, the sustaining pattern of newly developed AF was not recorded in our database, and therefore we could not evaluate the different impacts among permanent, persistent and paroxysmal AF patients. Second, several unmeasured confounders, such as body mass index, smoking, blood pressure level or low density lipoprotein level, which are associated with MACE and CV mortality, were not included in our database. Third, the incidence of MACE was also collected from ICD-9 and operation codes, where the details of such events were not available, such as ST-segment elevation MI or non-ST-segment elevation MI, systolic or diastolic HF, and the numbers of coronary arteries that intervened. Although the NHI system includes almost all of the hospitals and clinics in Taiwan, it is possible that some cases of MACE or mortality did not seek any medical attention. Finally, the study was a population-based cohort study in Taiwan in which the results may not be applicable to other ethnic and racial groups.
In conclusion, newly diagnosed AF without pre-existing MACE is an independent factor for future CV events even after matching with other associated CV risk factors. Compared to patients without AF, newly diagnosed AF is also associated with a higher mortality risk. In addition to AF, more associated CV comorbidities increase the rate of future MACE. Further interventional studies to treat newly onset AF itself are needed to determine whether AF is a primary cause or a risk marker of premature death.
Acknowledgments
We thank Michael Wu’s critical reading of the current paper.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Dr. Chu P-H is supported by the Ministry of Science and Technology (99-2314-B-182A-106-MY3 and 102-2314-B-182A-060-MY2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Majeed A, Moser K, Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart. 2001;86(3):284–8. Epub 2001/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. The American journal of medicine. 1995;98(5):476–84. Epub 1995/05/01. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Archives of internal medicine. 1987;147(9):1561–4. Epub 1987/09/01. [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke; a journal of cerebral circulation. 1991;22(8):983–8. Epub 1991/08/01. [DOI] [PubMed] [Google Scholar]
- 5. Lin LY, Lee CH, Yu CC, Tsai CT, Lai LP, Hwang JJ, et al. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation—a nation wide database analysis. Atherosclerosis. 2011;217(1):292–5. Epub 2011/04/26. 10.1016/j.atherosclerosis.2011.03.033 [DOI] [PubMed] [Google Scholar]
- 6. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. The American journal of medicine. 2002;113(5):359–64. Epub 2002/10/29. [DOI] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52. Epub 1998/09/16. [DOI] [PubMed] [Google Scholar]
- 8. Kober L, Swedberg K, McMurray JJ, Pfeffer MA, Velazquez EJ, Diaz R, et al. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. European journal of heart failure. 2006;8(6):591–8. Epub 2006/03/02. [DOI] [PubMed] [Google Scholar]
- 9. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. Journal of the American College of Cardiology. 2007;49(9):986–92. Epub 2007/03/06. [DOI] [PubMed] [Google Scholar]
- 10. Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. Journal of the American College of Cardiology. 2006;47(10):1997–2004. Epub 2006/05/16. [DOI] [PubMed] [Google Scholar]
- 11. Patel PJ, Keating RJ, Gersh BJ, Hodge DO, Hammill SC, Shen WK. Outcome of patients with newly diagnosed atrial fibrillation at the Mayo Clinic and residing in that area. The American journal of cardiology. 2004;94(11):1379–82. Epub 2004/11/30. [DOI] [PubMed] [Google Scholar]
- 12. Vidaillet H, Granada JF, Chyou P, Maassen K, Ortiz M, Pulido JN, et al. A population-based study of mortality among patients with atrial fibrillation or flutter. The American journal of medicine. 2002;113(5):365–70. Epub 2002/10/29. [DOI] [PubMed] [Google Scholar]
- 13. McManus DD, Hsu G, Sung SH, Saczynski JS, Smith DH, Magid DJ, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. Journal of the American Heart Association. 2013;2(1):e005694 Epub 2013/03/26. 10.1161/JAHA.112.005694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin YS, Tang CH, Yang CY, Wu LS, Hung ST, Hwa HL, et al. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. The American journal of cardiology. 2011;107(2):325–30. Epub 2011/01/08. 10.1016/j.amjcard.2010.08.073 [DOI] [PubMed] [Google Scholar]
- 15. Tang CH, Wu CS, Lee TH, Hung ST, Yang CY, Lee CH, et al. Preeclampsia-eclampsia and the risk of stroke among peripartum in Taiwan. Stroke; a journal of cerebral circulation. 2009;40(4):1162–8. Epub 2009/02/21. 10.1161/STROKEAHA.108.540880 [DOI] [PubMed] [Google Scholar]
- 16. Wu LS, Tang CH, Lin YS, Lin CP, Hung ST, Hwa HL, et al. Major Adverse Cardiovascular Events and Mortality in Systemic Lupus Erythematosus Patients After Successful Delivery: A Population-Based Study. The American journal of the medical sciences. 2012. Epub 2012/12/21. [DOI] [PubMed] [Google Scholar]
- 17. Lu JF, Hsiao WC. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff (Millwood). 2003;22(3):77–88. Epub 2003/05/22. [DOI] [PubMed] [Google Scholar]
- 18. Chiang TL. Taiwan's 1995 health care reform. Health Policy. 1997;39(3):225–39. Epub 1997/02/06. [DOI] [PubMed] [Google Scholar]
- 19. D'Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. Epub 2008/01/24. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 20. Kempler P. Learning from large cardiovascular clinical trials: classical cardiovascular risk factors. Diabetes research and clinical practice. 2005;68 Suppl1:S43–7. Epub 2005/06/16. [DOI] [PubMed] [Google Scholar]
- 21. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes care. 1993;16(2):434–44. Epub 1993/02/01. [DOI] [PubMed] [Google Scholar]
- 22. Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA: the journal of the American Medical Association. 1996;276(23):1886–92. Epub 1996/12/18. [PubMed] [Google Scholar]
- 23. Sedlak TL, Lee M, Izadnegahdar M, Merz CN, Gao M, Humphries KH. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. American heart journal. 2013;166(1):38–44. Epub 2013/07/03. 10.1016/j.ahj.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 24. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128(20):2192–201. Epub 2013/09/11. 10.1161/CIRCULATIONAHA.112.000491 [DOI] [PubMed] [Google Scholar]
- 25. Nieuwlaat R, Prins MH, Le Heuzey JY, Vardas PE, Aliot E, Santini M, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. European heart journal. 2008;29(9):1181–9. Epub 2008/04/10. 10.1093/eurheartj/ehn139 [DOI] [PubMed] [Google Scholar]
- 26. Frost L, Engholm G, Moller H, Husted . Decrease in mortality in patients with a hospital diagnosis of atrial fibrillation in Denmark during the period 1980–1993. European heart journal. 1999;20(21):1592–9. Epub 1999/10/26. [DOI] [PubMed] [Google Scholar]
- 27. Frost L, Vestergaard P, Mosekilde L, Mortensen LS. Trends in incidence and mortality in the hospital diagnosis of atrial fibrillation or flutter in Denmark, 1980–1999. International journal of cardiology. 2005;103(1):78–84. Epub 2005/06/01. [DOI] [PubMed] [Google Scholar]
- 28. Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, et al. Improving survival for patients with atrial fibrillation and advanced heart failure. Journal of the American College of Cardiology. 1996;28(6):1458–63. Epub 1996/11/15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.