Abstract
The present study aimed to investigate the functional properties of NMDA receptor coagonist release and to specifically evaluate whether light-evoked release mechanisms contribute to the availability of the coagonist d-serine. Two different methods were involved in our approach: (i) whole-cell recordings from identified retinal ganglion cells in the tiger salamander were used to study light adaptation with positive and negative contrast stimuli over a range of ± 1 log unit against a steady background illumination and (ii) the mechanisms for intensity encoding to a range of light intensities covering 6 log10 units were investigated. This latter study employed extracellular recordings of the proximal negative field potential, pharmacologically manipulated to generate a pure NMDA mediated response. For the adaptation study, we examined the light-evoked responses under control conditions, followed by light stimuli presented in the presence of d-serine, followed by light stimulation in the presence of dichlorokynurenic acid to block the coagonist site of NMDA receptors. For the brightness encoding studies, we examined the action of d-serine on each intensity used and then applied the enzyme d-serine deaminase to remove significant levels of d-serine. These studies provided new insights into the mechanisms that regulate coagonist availability in the vertebrate retina. Our results strongly support the idea that light-evoked coagonist release, a major component of which is d-serine, is needed to provide the full range of coagonist availability for optimal activation of NMDA receptors.
Key points
Activation of NMDA receptors (NMDARs) is essential for encoding visual stimuli into signals for the brain, although their over-activation can cause cell death. The recruitment of NMDARs is important for encoding light intensity in retinal ganglion cells.
d-serine binding is essential for proper activation of NMDARs, although its role in signal processing and the mechanisms that underlie its availability are not well understood.
In these light-evoked experiments, the addition of exogenous d-serine had a large effect on low contrast and low intensity NMDAR responses that decreased as the intensity was increased.
The degradation of endogenous d-serine decreased the responses more at higher intensities.
The results provide compelling evidence favouring a new interpretation of NMDAR recruitment in which light-evoked d-serine release serves an important regulatory control over the recruitment of NMDARs.
Introduction
NMDA receptors (NMDARs) are the only glutamate receptors that require a coagonist in addition to glutamate to properly gate the NMDAR ion channel: all other glutamate receptors can be opened by glutamate alone. When the requirement for a coagonist for NMDARs was first discovered, glycine quickly took centre stage as the leading candidate for what turned out to be an obligatory coagonist role (Johnson & Ascher, 1987). Shortly after glycine assumed its new status, studies revealed that other amino acids could substitute for glycine, among which were d-amino acids, including d-serine (Dingledine et al. 1990). Because d-amino acids were not considered to be part of the vertebrate amino acid repertoire, the report that d-serine is found in the brain in relatively high concentrations, especially in brain regions where NMDARs are enriched (Hashimoto et al. 1992), was unexpected. Subsequently, the main synthetic pathway for d-serine was revealed in the form of serine racemase (SR), an enzyme that converts l-serine to d-serine (Wolosker et al. 1999). Initially, SR was identified in astrocytes, although subsequent work revealed that it is also present in neurons, so that the cells responsible for d-serine synthesis and release became and remain an issue of some controversy (Kartvelishvily et al. 2006). More recent insights into the nature of d-serine synthesis have implicated astrocytes as a source of l-serine, whereas d-serine may be synthesized primarily by neurons and then taken up and released by astrocytes (Ehmsen et al. 2013). Whether this recent proposal accounts for d-serine synthesis and release in all regions of the nervous system remains to be explored. It should be added that studies in the retina strongly favour a glial source for the release of the d-serine (Sullivan & Miller, 2010) or at least the component that is evoked by the exogenous application of AMPA + cyclothiazide.
Shortly after the discovery of SR in the brain, work in the retina established both the presence of d-serine and its synthesizing enzyme SR (Stevens et al. 2003). Immunolabelling studies localized d-serine and SR to Müller cells and astrocytes, although more recent work has also identified retinal ganglion cells as a source of SR (Dun et al. 2008; Kalbaugh et al. 2009). There is evidence that d-serine may be developmentally regulated in the retina (Dun et al. 2008).
Physiological studies were carried out in the retina to identify the coagonist preference for NMDA receptors in retinal ganglion cells. This was especially critical because glycine, the competing NMDAR coagonist, subserves inhibition in the retina mediated by a subclass of glycinergic amacrine cells; thus, the inner retina has a cellular source of light-evoked release of glycine that could serve a coagonist function and work reported by Kalbaugh et al. (2009) suggested that this was the case.
Experiments carried out in the whole retina revealed that the coagonist NMDA receptor sites were not saturated under modest stimulus conditions because exogenous d-serine consistently enhanced NMDAR ionic currents. A powerful d-serine degrading bacterial enzyme, d-serine deaminase (DsdA), was used to significantly reduce d-serine levels, such that the NMDA receptor currents in ganglion cells were almost abolished and reduced to levels very close to those achieved by blocking the NMDA receptors with high levels of competitive antagonists, such as d,l-2-amino-phosphonoheptanoate (AP7) (Gustafson et al. 2007). What emerged from these studies was the idea that d-serine was probably the main and perhaps the only coagonist for NMDA receptors of retinal ganglion cells, at least those activated by light-evoked synaptic pathways. A recent report suggests that non-synaptic NMDA receptors in the retina may utilize glycine as a coagonist (Sullivan & Miller, 2012), an idea that has gained favour based on studies in the brain (Papouin et al. 2012).
Additional experiments were carried out to clarify the role of glycine as an NMDAR coagonist. It has been noted that, for d-serine to be effective as a coagonist, glycine, which is present in higher concentrations than d-serine (Reed et al. 2009), must be effectively reduced in concentration by local glycine uptake mechanisms, specifically the Na+-dependent glycine transporter type 1 (GlyT1) uptake system (Supplisson & Bergman, 1997). When GlyT1 transporters were blocked with N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)-propyl]sarcosine (Stevens et al. 2010), coagonist NMDAR sites in the retina became fully occupied because external levels of glycine were elevated. Furthermore, mice in which the GlyT1 transporter had been partially knocked out (GlyT1−/+) showed full occupancy of NMDA receptor coagonist sites (Reed et al. 2009), supporting the importance of this transport system in reducing local glycine levels, allowing d-serine to serve in this capacity. GlyT1 transporters are richly represented in the inner plexiform of the retina (Jager & Wassle, 1987; Pow & Hendrickson, 1999).
The uptake of d-serine in the retina appears to depend on Na+-dependent ASCT2 transporters (O'Brien et al. 2005; Dun et al. 2007). Although a high affinity, Na+-independent, Asc-1 transporter has been identified in the brain and may play a role in the release of glycine and d-serine (Rosenberg et al. 2013), its role in the retina has not been established, at least as an uptake transport mechanism, because d-serine uptake in the retina was blocked under Na+-free conditions, an environmental condition that should not block the uptake of d-serine by the Asc-1 transporter.
Additional studies comparing wild-type and serine racemase knockout mice raised the possibility that d-serine release was modulated by light activity (Sullivan & Miller, 2012). The experiments reported in the present study were undertaken to evaluate the possible light modulation of coagonist release. The fundamental issue addressed in the present study is related to the experimental observation in which exogenous d-serine enhances NMDAR currents (Stevens et al. 2003). This implies that the spatiotemporal distribution of light-evoked glutamate reaches NMDA receptors for which there is no coagonist available for channel gating. How then do the NMDARs find adequate coagonist when light stimulation requires a greater abundance of coagonist during times of enhanced NMDA receptor recruiting?
The present study was divided into two components; one of which studied light-adapted contrast sensitivity under (i) control conditions; (ii) elevated d-serine levels by exogenous d-serine application; and (iii) the application of dichlorokynurenate (DCK) used to block NMDA receptors by antagonism towards the coagonist binding site. This study involved whole-cell recordings from retinal ganglion cells that were identified as such in the tiger salamander retina.
We also studied a second, related function of the retina, which is the encoding of intensity information using light stimuli that covered a 6 log unit range of intensities against a dark background. This study, which required long duration recordings including pharmacological interventions, was not well suited for whole-cell recordings from retinal ganglion cells. For these experiments, we used extracellular recordings of the proximal negative field potential (PNFP), originally described as the proximal negative response (Burkhardt, 1970). Each of these studies converged to provide evidence that light-evoked coagonist release is required for full activation of the NMDA receptor population found in retinal ganglion cells.
Methods
Ethical approval
Animal maintenance and experimental protocols utilized in the present study were evaluated and approved by the University Institutional Animal Care and Use Committee.
Animals
Tiger salamanders (Ambystoma tigrinum) were purchased from a dealer (Charles D. Sullivan Co., Nashville, TN, USA) and maintained in circulated cold-water tanks (4°C) under a 12:12 h room light/dark cycle. Animals were killed by decapitation followed by double pithing.
Whole-cell recordings
Two unique retinal preparations from the tiger salamander were utilized for the ganglion cell studies in these experiments, including (i) an isolated flatmount retina (Stevens et al. 2003) and (ii) an intact retina-eyecup preparation that was newly developed during the course of these experiments. The flatmount preparation began by removing the cornea and lens and draining the vitreous (Miller & Dacheux, 1976). Next, the internal limiting membrane was removed either mechanically, by removing atomized alumina particles adherent to the surface of the retina with jeweler forceps, or by enzymatic digestion with collagenase and hyaluronidase (120 and 465 units ml–1). The retina was then removed from the retinal pigment epithelium using a blunted, fire-polished glass micropipette and placed, ganglion cells up, over a 1 mm hole in the centre of a 13 mm diameter nitrocellulose filter paper (Millipore, Billerica, MA, USA).
For the eyecup preparations, we modified previously described procedures (Burkhardt et al. 1989), beginning with enucleation of the eye and the placement of a crystal of dextran conjugated tetramethylrhodamine (10000 MW) onto the severed optic nerve. The eyecup was then half immersed, cornea up, in a 6 mm diameter, 2 mm deep well filled with warmed 4% agarose (Type IX-A) in normal amphibian Ringer solution containing (in mm) 110 NaCl, 2.5 KCl, 1.8 CaCl2, 1.0 Mg Cl2, 10 Hepes, 5 d-glucose (pH 7.8). After cooling, the cornea, lens and vitreous were removed, as above, and the internal limiting membrane was treated with collagenase and hyaluronidase (120 and 465 units ml–1). The eyecup (in agarose) was then placed in a 15 ml plastic centrifuge tube half filled with normal Ringer solution and secured in a light tight container on ice and bubbled with oxygen for 2–5 h, during which time the dye diffused into the ganglion cell bodies. The eyecups, still in agarose, were removed from the oxygenated Ringer solution, placed on a tissue slicer (Stolting, Chicago, IL, USA) and a vertical cut was made, which removed a side of the eyecup approximately one-quarter to one-third of the diameter of the intact eyecup. This allowed the recording micropipettes to access the retina under a water immersion objective.
At this point, the flatmount retina on filter paper and the eyecup in agarose were placed in custom designed chambers for use with water immersion objectives on an BX50WI microscope (Olympus, Tokyo, Japan) and connected to a gravity fed perfusion system at a flow rate of 1–1.5 ml min–1 with cooled (18ºC), oxygenated, normal amphibian Ringer solution. Each preparation had its own custom-built chamber; isolated retinas were transilluminated with infrared light (780 nm) using the microscope's built-in light source and IR filter. Eyecup preparations were visualized by fluorescence microscopy via the microscope's fluorescence filter cube. Each preparation was visualized with a CCD camera attached to a small LCD screen or video monitor. For the present study, we did not detect any differences in the results between the two preparations, and so the results from both were fused into a single dataset.
Whole-cell recordings were obtained from identified ganglion cells. Patch electrodes (5–15 MΩs) were pulled using a P-97 Flaming Brown Pipette Puller (Sutter Instruments, Novato, CA, USA) and filled with an intracellular solution containing (in mm): 98 KCH3SO4, 3.5 NaCH3SO4, 3.0 MgSO4, 1.0 CaCl2, 11 EGTA, 5.0 Hepes, 2.0 d-glucose, 1.0 glutathione, 1.0 MgATP, 0.5 NaGTP (pH 7.4). To visualize cells studied in the flatmount preparation, we added 5,6-carboxyfluorescein to the pipette solution. All recorded cells were identified as retinal ganglion cells with an axon clearly present. The pipette was held and manipulated with an MP-285 micromanipulator (Sutter Instruments). Dimpling of the fluorescently labelled ganglion cells in the eyecup preparation indicated the proximity of the pipette to the cell soma; we obtained whole-cell recordings only from ganglion cells that were clearly back labelled with the fluorescent dye.
After achieving a high resistance seal onto the cell, the capacitance of the pipette was compensated for by utilizing the automatic circuitry of the Axoclamp 700A amplifier (Molecular Devices, Sunnyvale, CA, USA) or manually with the Dagan 3900 (Dagan Corp., Minneapolis, MN, USA), each configured to record with a 10 kHz low pass Bessel filter at a sampling frequency of 10 kHz. Signals were digitized with a Digidata 1320 and recorded in PClamp 9.0 (Molecular Devices). Breakthrough to the whole-cell recording configuration was achieved using gentle negative pressure to the pipette. The cell was characterized in current clamp mode with input resistance determined by small negative current injections, whereas the light response of the cell was determined by stimulating with a 2 s spot (250 μm) or bar (100 μm) of light centered on the recording electrode (for details of the optical bench, see below). The solution was then switched to the control solution, which consisted of (unless noted otherwise) a nominally magnesium free version of the extracellular Ringer solution containing TTX (0.5 μm) to block action potentials and strychnine (10 μm) to block glycine receptors. Voltage-clamp studies were carried out after adjusting the clamp voltage so that zero current was established (average membrane potential = −64.9 ± 4.8 mV); series resistance was generally compensated by 50% to 95%. Because of the small size of the synaptic currents, the relatively low pipette resistance and the high input resistance of tiger salamander retinal ganglion cells (742.3 ± 183 MΩ), we did not correct for voltage errors associated with uncompensated series resistance. The reported voltages are also not corrected for the liquid junction potential (calculated at 10.1 mV more negative than those reported). All chemical reagants used in the present study were purchased from Sigma Chemical (St Louis, MO, USA), with the exception of hyaluronidase and collagenase (Worthington Chemical, Lakewood, NJ, USA), TTX (Alamone Labs, Juresalem, Israel), picrotoxinin (Fluka, Neu-Ulm, Switzerland) and KCH3SO4 (Pfaltz & Bauer, Westchester, PA); AP7, 5,7-dichlorokynurenic acid (5, 7-DCK) and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo-(F)-quinoxali-nedione (NBQX) were obtained from Tocris (Ellisville, MO, USA).
Light stimulation
Light stimulation was provided by a proprietary computer-controlled LCD projector system using a tungsten-halogen light source (Burkhardt et al. 1998) projected onto the retina through a 20× microscope objective (Olympus, Melville, NY, USA) and focused on the plane of the ganglion cell layer. A Fish-Schurman 6143 heat filter (Fish-Schurman, New Rochelle, NY, USA) and neutral density wedges (Kodak, Rochester, NY, USA) were used to attenuate the full intensity of the lamp to a background intensity of 9 cd m−2. Unless otherwise indicated, light stimuli were centered on the recording electrode and consisted of a 2 s, 250 μm diameter spot and a 1.0 log unit increase in contrast. Contrast is defined as log10F/B, where F is the light intensity during the flash and B is the steady background intensity (Fahey & Burkhardt, 2003). The interstimulus interval was 20 s for light stimuli. The diameter and the contrast were varied in a systematic fashion once a whole cell recording condition of stability was established (Fig.1). In all experiments, three or four data traces were signal averaged for each stimulus condition and voltage-clamp responses were evaluated as the total charge (integrated current) entering the cell during the 2 s light stimulus (ON response) and two seconds following the cessation of the stimulus (OFF response).
Figure 1. Contrast series.
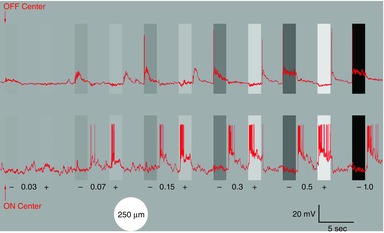
The two retinal ganglion cells (red traces; OFF-centre cell upper trace, ON-centre cell lower trace) were stimulated with a 2 s light stimulus, 250 μm in diameter at different contrast levels (0.03, 0.07, 0.15, 0.3, 0.5, and 1.0 log unit) against a steady background light of 9 lux. For each magnitude of the contrast displayed, the negative contrast (darker bar) precedes the positive contrast (lighter bar) stimulus. The first 5 s of each stimulus is displayed (of a 20 s interstimulus interval); the positive 1.0 log unit contrast is not shown. Arrows indicate the onset of the first stimulus because this low contrast step is difficult to discern from the background.
PNFP
Some of the experiments demanded long-term, stable recordings. To meet this requirement, we used a field potential of the inner retina first described by Burkhardt in the frog and referred to as the proximal negative response (1970). We have studied this response in the tiger salamander and renamed it the PNFP. This response is a reliable index of inner retina excitability and has representation among ganglion and amacrine cells. The PNFP is a very stable, light-evoked index of inner retinal activity and was the obvious choice for this component of the study, particularly because we have been able to generate a large, stable NMDA receptor signals based on pharmacological manipulations (Gustafson et al. 2007). For these experiments, we used a more conventional retina-eyecup preparation, as described previously (Gustafson et al. 2007). Briefly, bevelled glass microelectrodes, with tip resistances or a few megaohms, were filled with Ringer solution and inserted ∼50 μm into the retina. Responses to a small 110 μm spot of light, centered on the electrode, were measured using an Model 3000 amplifier (A-M Systems, Carlsborg, WA, USA), DigiData 1322 and pCLAMP software (Molecular Devices). A bright spot of varying intensities (reported as a relative value, with log I 6.5 = 250 ft-c or 2700 lux) alternated with a diffuse background of ∼0.01 ft-c or 0.1 lux. A control cocktail of (in μm) 10 NBQX, 10 strychnine, 50 picrotoxinin and 50 mecamylamine was used to isolate the NMDAR component of the PNR. The peak amplitude of the PNFP was used in the analysis.
DsdA
We initially obtained the bacterial enzyme d-serine deaminase as a gift from Herman Wolosker, who synthesized it (Shleper et al. 2005) using a DsdA clone from the CFT073 Escherichia coli strain. It was shipped in dry ice and stored in a freezer at −80°C and dissolved in physiological Ringer solution just before use. This enzyme is highly specific for d-serine, whereas l-enantiomers are not substrates under normal conditions. d-threonine is a weaker substrate for the enzyme. The enzyme is co-linked to pyridoxal phosphate that is essential for normal activity of the enzyme. This enzyme is orders of magnitude more effective than d-amino acid oxidase. DsdA converts d-serine into pyruvate and ammonia; the enzyme almost has no backward reaction. We have analysed this enzyme using capillary electrophoresis methods for d-serine detection. In previous experiments, we tested the sensitivity of DsdA versus D-amino acid oxidase (DAAO) by exposing a 10 μm level of d-serine and glycine to the enzymes. DsdA degraded d-serine below the detection limit of our capillary electrophoresis machine (3.4 nm) by 1 min, whereas DAAO exposure after 8 min left almost 50% of the original concentration (Gustafson et al. 2007). Neither enzyme degraded glycine. Thus, DsdA is a far more powerful enzyme than DAAO for d-serine degradation.
Statistical analysis
Electrophysiological data were analysed in Clampfit (Molecular Devices) to total charge; the data were averaged and graphed. Curve fitting and statistics were carried out in Origin, version 9.0 or 9.1 (OriginLab Corp., Northampton, MA, USA). All results are expressed as the mean ± SEM. A paired, one-tailed Student's t test was used to compare values between treatment groups. P < 0.05 was considered statistically significant.
Results
Table1 summarizes the results obtained with whole-cell recordings from two different salamander retina preparations used during the evolution of our adaptation study focused on retinal ganglion cells. No differences were observed between the results obtained in the two preparations, and so we combined the results for analysis. The input resistance measurements and the average values for the resting membrane potential are indicated. These input resistance values correspond well with those values for the tiger salamander originally reported from the intact retina-eyecup (Coleman & Miller, 1989).
Table 1.
Cell characteristics
| Preparation | n | Rin (MΩ) | Resting Vm (mV) | Light response | Axon visualized* |
|---|---|---|---|---|---|
| Flatmount | 53 | 537.4 ± 198 | −63.0 ± 5.3 | ON–OFF: 46 | 17/24 |
| Range: 257 to 1,256 | Range: −53 to −69 | ON: 4 | 2/3 | ||
| OFF: 3 | 3/3 | ||||
| Eyecup | 43 | 947.2 ± 205 | −66.8 ± 5.9 | ON–OFF: 37 | 37 |
| Range: 498 to 1,738 | Range: −58 to −72 | ON: 3 | 3 | ||
| OFF: 3 | 3 | ||||
| Total | 96 | 742.3 ± 183 | −64.9 ± 4.8 | ON–OFF: 83 | 54 |
| Range: 257 to 1,738 | Range: −53 to −72 | ON: 7 | 5 | ||
| OFF: 6 | 6 |
Biophysical properties of cells and method of cell identification: Two different preparations were used in the present study for whole-cell recordings of retinal ganglion cells; 96 ganglion cells comprised the cell population from which whole-cell recording data was obtained; 53 ganglion cell recordings were obtained from the isolated, perfused flatmount retina preparation, whereas 43 cell recordings were obtained from the eyecup preparation. RN: input resistance; Vm: membrane voltage; *For the flatmount experiments, the numerator indicates numbers of axons visualized for ON–OFF, ON, and OFF cells, whereas the denominator indicates the number of experiments where dye was included in the pipette. For the eyecup preparation, all experiments involved recording from cells labelled via the optic nerve backfill.
Figure1 illustrates whole-cell recordings from an OFF-center ganglion cell (upper record) and an ON-center ganglion cell (lower record) in response to light stimuli of increasing positive (light bars) and negative contrast (dark bars) against a steady background intensity of 9 lux. The stimulus consisted of a 250 μm diameter light flash of 2 s duration, with an interstimulus interval of 20 s for the responses to recover, of which only the first 5 s are displayed. Under these stimulus conditions, the OFF cell gave a minimal response to the negative contrast beginning with the 0.03 log value and responded to negative contrast levels with excitation at the onset and to positive contrast stimulation with OFF excitation, first evident at the 0.07 contrast level. The ON-cell responded to positive contrast with excitation, as first clearly evident at the 0.07 level, at which time, OFF excitation was also evident. An additional increase in both positive and negative contrast enhanced ganglion cell excitation at the onset of positive contrast stimuli and at the offset for negative contrast stimulation. This stimulation procedure was used in recordings from identified ganglion cells using the LCD panel to provide positive and negative contrast stimuli.
The stimulus protocol described above was used to study the role that d-serine plays in NMDA receptor activity in retinal ganglion cells. Figure2 illustrates a whole-cell voltage-clamp recording (Vhold −62 mV) from an ON–OFF ganglion cell in response to both positive (Fig. 2A) and negative (Fig. 2B) contrast steps of 1 log10 unit. Each stimulus lasted 2 s as indicated by the bar under each trace. A contrast series of six different values (0.03, 0.07, 0.15, 0.3, 0.5 and 1.0) was applied under control conditions (TTX + strychnine). Control responses (black) were obtained first, followed by an identical contrast series with the application of exogenous d-serine (100 μm; blue trace), after which the contrast series was repeated with DCK added to the bathing medium at 30 μm to block the coagonist site and prevent NMDA receptors from contributing to the response. Based on our recordings in the salamander retina, the IC50 for DCK was 1.5 μm (not shown), such that 30 μm completely blocked light-evoked NMDA receptor contributions to the light response, even at maximal light intensity. Thus, at this concentration of DCK, NMDA receptors were completely blocked, replicating the actions of applying an effective competitive antagonist for the glutamate binding site such as AP7.
Figure 2. Coagonist sensitivity to D-serine decreases as contrast is enhanced.
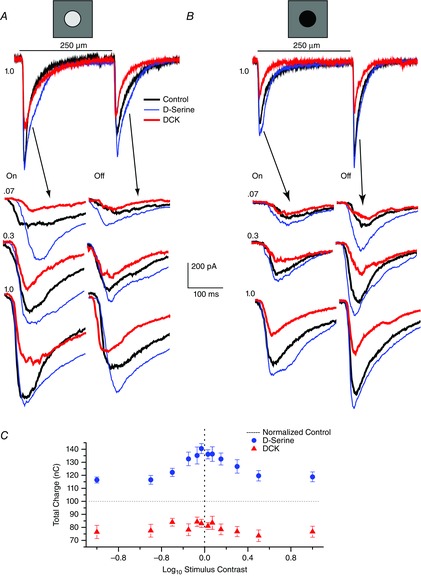
Both positive (A) and negative (B) contrast stimuli were acquired during each experimental run. A, whole-cell voltage-clamp recording (Vhold = −62 mV) of an ON–OFF retinal ganglion cell recorded from an eyecup preparation in response to a 2 s light stimulus (black bar) consisting of a one (1.0) log unit increase in illumination over the background in control Ringer solution (black trace), d-serine (100 μm, blue trace) and DCK (30 μm, red trace). ON and OFF responses to positive contrast steps of 0.07, 0.3 and 1.0 log units are shown below with an expanded timescale. B, same cell as in (A) but showing the results for negative contrast light steps. C, summary data from eight cells plotting the mean ± SEM charge integrated over 2 s for ON responses and 2 s for OFF responses. Control responses were normalized to 100, represented by a horizontal dashed line. d-serine (100 μm, blue dots) and DCK (30 μm, red triangles) to contrast steps of ±0.03, ±0.07, ±0.15, ±0.3, ±0.5 and ±1.0 log unit. ON and OFF responses from each cell were combined for the same intensity level before averaging.
The expanded traces illustrate the ON and OFF responses evoked at three different contrast steps listed as log10 units at the beginning of each trace. The delay differences in some of the traces reflect the fluctuation in triggering the LCD panel with external triggering procedures. For low contrast values, the enhancement associated with d-serine application is more dominant compared to the actions of DCK but, as the contrast intensity increased, d-serine was less effective in enhancing the response and the actions of DCK were more evident. The blocking action of DCK demonstrates that both NMDA and AMPA receptors were recruited to account for the light response. In the presence of DCK, the light response consists of a relatively pure AMPA receptor contribution.
Figure2C illustrates the relative magnitude differences in the integrated charge (derived by integrating the responses at the onset of the light stimulus for the 2 s duration of the light stimulus) for both positive and negative contrast steps. The application of d-serine and of DCK had similar effects on both the ON and OFF responses of each of the cells recorded; therefore, for each cell, we pooled the ON and OFF responses into a combined cell response. It is this cell response to the pharmacological manipulations that is then averaged and shown in Fig. 2C. The control data were normalized and are represented by the horizontal dashed line at 100%. d-serine (blue circles) enhanced the response especially for low contrast stimuli, whereas the actions of DCK (red triangles) showed a strong NMDA receptor component at each contrast level, albeit with less stimulus-dependent variation (from eight cells, ON and OFF responses combined, n = 14 for each). The d-serine effect at 0.03 contrast was significantly larger than at the 1.0 contrast for both positive and negative steps (P = 0.003 positive contrast, P < 0.001 for negative contrast).
Increasing the light stimulation diameter, at the same time as leaving the intensity constant, can also enhance the response of retinal ganglion cells over a wide range of stimulus diameters. We considered whether d-serine enhancement would also influence the light-evoked differences based on diameter, at the same time as keeping the stimulus intensity constant. Figure3 illustrates a whole cell recording from an ON retinal ganglion cell in which the diameter of the stimulus was changed, whereas the intensity remained fixed at 1.0 log unit above the background. TTX and strychnine continuously bathed the retina to eliminate impulse activity and reduce lateral inhibition. In addition, each cell was clamped at −60 mV, which approximates the reversal potential for chloride, thus eliminating chloride-dependent inhibition. In this example, the diameter of the light stimulus was changed progressively at the same time as keeping the light stimulus intensity constant. We used a discreet series of diameters (50, 100, 250, 600 and 1200 μm) generated on the LCD display with 1 log10 unit increase in contrast against a background of 9 lux. Figure3A–C illustrates the results that we obtained for three different diameters (50, 250 and 1200 μm) from a sustained ON ganglion cell (current clamp record in 3E), which had a prominent transient inward current, followed by a small, more sustained current. For each diameter of light stimulus, we obtained a control series (black) followed by an identical presentation of stimuli in the presence of 100 μm d-serine (blue) added to the bathing medium, after which DCK perfused the retina and the diameter series was repeated (red traces).
Figure 3. Increasing the stimulus spot diameter does not change the sensitivity to DCK or d-serine.
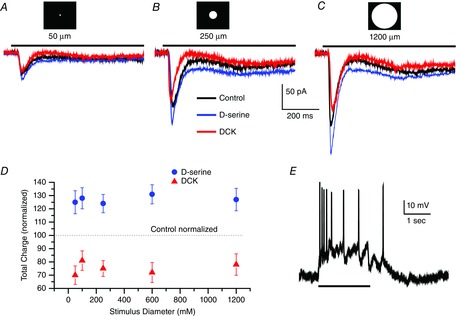
Whole-cell voltage-clamp recording (Vhold = −58 mV) of a sustained ON retinal ganglion cell in an eyecup preparation responding to a 50 μm (A), 250 μm (B) and 1200 μm (C) light stimulus 2 s in duration in control Ringer solution (black trace), d-serine (100 μm, blue trace) and DCK (30 μm, red trace). Scale bar under the trace in B applies to traces in A, B and C. D, summary data from eight cells plotting the mean ± SEM charge for responses studied for the range of diameters indicated. Recording in control Ringer solution was standardized to 100% as indicated by the dashed line. d-serine responses (blue dots) and responses recorded in DCK (red triangles). Light stimuli of 50, 100, 250, 600 and 1200 μm diameter were used to generate this data. ON and OFF responses were combined before calculating the mean. E, a current clamp record from an ON ganglion cell, light stimulus is represented by the black line at bottom.
Figure3D shows the normalized control responses (dotted horizontal line at 100), together with the measured integral (2 s) of the current to give total charge. Although these data clearly show that d-serine enhanced the response and DCK reduced the response amplitude, no systematic differences were evident as a function of diameter in terms of a clear trend towards enhancement by d-serine or suppression of the response by DCK. The data reflect the study of eight cells. In summary, the response enhancement in d-serine as well as the response decline in the presence of DCK were both relatively constant over the diameter range used in the present study. We conclude that stimulus intensity rather than stimulus diameter is the most important characteristic of the light stimulation parameters required to change the fraction of NMDA receptors recruited when d-serine was added to the bathing medium.
The ganglion cell studies provided a unique insight into how the participation of NMDA receptors might be modified for different contrast stimulation parameters. However, the device used to evoked positive and negative contrast stimuli was limited in its dynamic range and confined to ± 1 log10 units above and below the ambient background light.
We next embarked on a different series of experiments to examine a broader range of stimulus intensities, using the PNFP, first described by Burkhardt as the proximal negative response (Burkhardt, 1970). The PNFP is a localized response of the inner retina that reflects activity of third-order retinal neurons and provided us with a stable long-term recording condition such that we could study a broader range of stimulus conditions together with pharmacological modifications. A relatively pure NMDA receptor signal can be revealed with the PNFP via the application of a suitable cocktail of drugs, including NBQX (10 μm), strychinine (10 μm), picrotoxinin (50 μm) and mecamylamine (50 μm) (Gustafson et al. 2007). This cocktail was used throughout the PNFP recordings.
Figure4 illustrates the data obtained from an extensive series of PNFP recordings evaluated across a 6 log unit range of intensities. Figure4A illustrates recordings made at the indicated log intensities, for the control responses (black) and for those in the presence of 100 μm d-serine (grey); note the gain change between 1.5 log I and that for 3.5 and 6.5 log I. For low intensity light stimulation, d-serine application enhanced the response substantially more than was evident for 3.5 log I, whereas the 6.5 log I stimulus revealed saturation of the coagonist sites in the presence of 100 μm d-serine.
Figure 4. Flash intensity coding revealed through the PNFP illustrates that sensitivity to d-serine decreases as the flash intensity is increased.
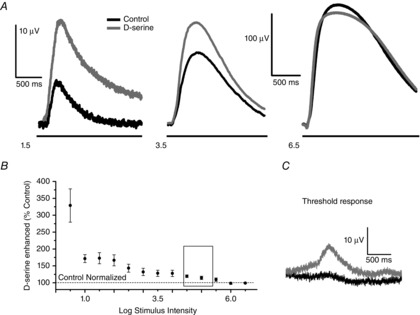
The PNFP was recorded over a range of 6 log units using 2 s flashes of light of increasing intensity; the interflash interval was 25 s. A, initial intensity series under control conditions (black traces) shown for intensities 1.5, 3.5 and 6.5, followed by an identical series in the presence of 100 μm d-serine (grey traces). B, for each intensity, the control was normalized to 100% (dashed line near bottom of the figure), whereas the response in the presence of d-serine is indicated by the filled circles, including the SEM (n = 6). Inset: range over which the adaption experiments explored the contrast sensitivity shown in Fig.2. C, example of a ‘threshold response’. In the presence of d-serine, a PNFP response was obtained at 0.5 log I when an insignificant response to light was observed under control conditions.
Figure4B graphically illustrates the broad range of stimulus intensities used in our PNFP experiments (cumulative data recorded at each stimulus intensity are presented in Table2). In this display, control responses were normalized for each intensity (dashed line at 100%), whereas the responses indicated by the filled circles were recorded in d-serine (100 μm) and show the responses compared to the control values. PNFP responses were enhanced by d-serine, although the augmentation depended heavily on the intensity of the light stimulus. Low light intensities showed the biggest enhancement with D-serine application. The trend for D-serine augmentation diminished as the intensity increased until the stimuli above 5.5 log I showed a full saturation of the response, indicating full occupancy of the NMDA receptor coagonist sites or maximum activation of the available pool of NMDA receptors under these stimulus conditions. The open rectangle illustrates the range of stimulus intensities that were achieved by the contrast stimulation method with the LCD panel; in the LCD panel experiments, d-serine elevated the response to values that were typically < 150% of control. Studies of the PNFP, however, allowed us to explore a broader range of stimulus intensities, the lowest of which was enhanced by d-serine to more than 300% of the control. In addition, we obtained several examples under our very lowest stimulus intensity that revealed almost no light response in control Ringer solution but, with the addition of d-serine, a significant response was evident. One such example is shown in Fig.4C (these data were not included in our totals because no control value was available with which to normalize the data). As the stimulus intensity increased (positive values), the addition of d-serine showed a progressive attenuation in its ability to enhance the PNFP and, at the intensity of 6 log units, the enhancing action of d-serine was eliminated entirely. The fact that d-serine can enhance the response substantially at low light levels argues against the Mg2+ block being the principal mechanism required to account for these responses and, instead, suggests a sufficient release of glutamate that cannot be matched by coagonist, such that the full complement of NMDA receptors for this low intensity range cannot be activated. However, as the intensity of the light increases, the enhancing action of exogenous d-serine diminishes because more coagonist is available, which must be released by light stimulation.
Table 2.
Effect of d-serine and DsdA on PNFP amplitude
| d-serine | DsdA | |||||
|---|---|---|---|---|---|---|
| Stimulus | ||||||
| intensity | % Control | SEM | P | % Control | SEM | P |
| 0.5 | 328.8 | 49.4 | 0.0012 | 109.7 | 4.47 | 0.096 |
| 1 | 171.6 | 11.8 | 0.0017 | 105.1 | 9.02 | 0.60 |
| 1.5 | 172.8 | 16.5 | 0.007 | 94.54 | 9.51 | 0.59 |
| 2 | 166.5 | 16.2 | 0.0094 | 81.12 | 5.33 | 0.017 |
| 2.5 | 143.2 | 11.9 | 0.015 | 78.04 | 2.58 | <0.001 |
| 3 | 132.2 | 10.9 | 0.032 | 72.27 | 4.33 | 0.0014 |
| 3.5 | 128.0 | 9.3 | 0.030 | 68.04 | 4.93 | 0.0013 |
| 4 | 127.8 | 9.2 | 0.030 | 62.62 | 5.32 | <0.001 |
| 4.5 | 119.4 | 4.0 | 0.0044 | 60.03 | 3.16 | <0.001 |
| 5 | 114.6 | 4.8 | 0.028 | 60.49 | 3.32 | <0.001 |
| 5.5 | 109.4 | 5.0 | 0.12 | 57.87 | 3.26 | <0.001 |
| 6 | 98.33 | 2.66 | 0.56 | 58.00 | 3.47 | <0.001 |
| 6.5 | 98.97 | 1.97 | 0.62 | 56.65 | 4.47 | <0.001 |
PNFP amplitude after d-serine and DsdA application was compared to control at 13 different stimulus intensities. % control was calculated as d-serine or DsdA/control × 100 at each of the log intensities. Standard errors and the P values calculated using Student's t test from six experiments are reported.
The idea that more coagonist is available for high intensity light stimulation poses the obvious question about the species of coagonist that provides this function. The two leading candidates are glycine and d-serine, each of which have been identified in the retina and postulated for coagonist function (Stevens et al. 2003; Kalbaugh et al. 2009). Figure5 illustrates an experimental strategy designed to address the question about the identity of the light-evoked coagonist. Figure5A illustrates single PNFP responses evoked by six different flash intensities, as first observed in the control environment (dark trace) followed by the introduction of d-serine deaminase (DsdA; red trace; 10 μg ml–1), which degrades d-serine into pyruvate and NH3. The DsdA application was followed by exogenous glycine (blue trace; 200 μm). It was necessary to use glycine rather than d-serine because residual enzyme in the retina and perfusion system continued to degrade d-serine. Glycine is not degraded by DsdA (Gustafson et al. 2007). Figure5B shows the cumulative results of six experiments in which DsdA was added to the bathing media. At the lowest intensities, no effect was seen on the PNFP amplitude. Beginning around the stimulus intensity of 1.5, there is gradual reduction in the PNFP versus control until this reduction plateaus around stimulus 4.5 (reduction of ∼40%) (Table2). These data suggest a role for d-serine that becomes more pronounced as the intensity of the light stimulus increases.
Figure 5. DsdA identifies d-serine as a major player in coagonist release.
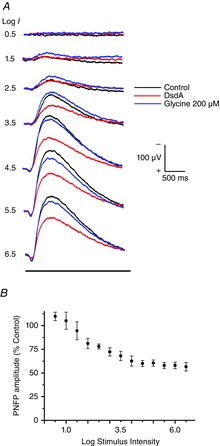
A, these recordings of the PNFP were generating by ascending flash intensities with a 25 s interval between flashes. The control intensity series (black trace) shows a progressive increase in amplitude. After the control series, DsdA enzyme (10 μg ml−1) was dissolved in the Ringer solution and constantly perfused the retina, during which time the intensity flash series was repeated. The DsdA response traces are shown in red. After obtaining the DsdA response series, the perfusion was changed to one that contained 200 μm glycine to observe recovery of the response (blue traces). B, cumulative normalized responses of the PNFP recorded in DsdA versus control (n = 6).
NMDA receptors of the retina play an essential role in expanding the dynamic range of retinal ganglion cell responses in the retina (Diamond & Copenhagen, 1993). To assess the role of d-serine in the light-dependent action, we compared the stimulus–response curves of the PNFP in control Ringer solution versus DsdA containing Ringer solution. Figure6 illustrates the segregated DsdA (grey circles) and control responses (black squares) from the range of stimulus intensities used in the present study and plotted as V/Vmax. For each curve, we fit a Boltzmann equation to the data (r2 > 0.99 for each fit). Notice that the curve for the DsdA responses reached a maximum at 4.5 log I, an intensity level at which the control still increased in its dynamic range. The truncated dynamic range of the DsdA curve reflects the failure for light-evoked coagonist release to fully compensate and provide sufficient coagonist to activate the full complement of NMDA receptors. We can view the difference in these two curves in a functional manner in which the presence of d-serine leads to an improved dynamic range by allowing more NMDA receptors to participate in the response, particularly at the higher intensity ranges. At the 4.5 log I stimulus, the DsdA responses have already reached their maximum value, whereas the control responses do not reach their full dynamic range until the very last stimulus intensity of 6.0 log units (cumulative data recorded at each stimulus intensity are presented in Table2).
Figure 6. The maximum PNFP response in DsdA occurs at a lower intensity than in control Ringer solution.
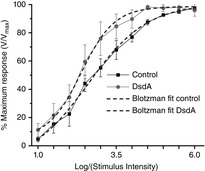
V/Vmax relationship for control (black squares) and DsdA (grey circles) PNFP responses over a range of 6 log units of flash intensity. Each response amplitude displays the mean ± SEM (n = 6). The data were fit by a Boltzmann curve for the control (black dashed line) and the DsdA responses (grey dashed line; r2 for the Boltzmann fit was 99). The main feature of this comparison illustrates how the DsdA responses plateau at 4.5 log I, whereas the control responses continue to increase over the entire intensity range studied.
Discussion
The results of the present study can be summarized simply: if a deficiency in coagonist occupancy is evident for low intensity light stimuli when NMDAR activation is minimal, how do the coagonist sites get more fully occupied when the stimulus is brighter, generating much larger NMDAR currents? The answer is that there must be a light-evoked release of coagonist to meet the demands of higher intensity light stimuli with sufficient coagonist. Indeed, the regulation of d-serine release could serve as one mechanisn by which NMDA receptors avoid being recruited under stimuli of low or modest intensity.
The present study has carried out an extensive analysis of light adaptation and stimulus intensity encoding mechanisms that provides new insights into how NMDA receptor responses are regulated and recruited under different stimulus conditions. Ganglion cell light adaption experiments revealed that, when the stimulus intensity was just above the background intensity, exogenous d-serine application enhanced the response far more effectively than when the contrast stimulus was brighter and closer to the maximum of ± 1 log10 units above background.
Another parameter of light stimulation that can affect the response amplitude of retinal ganglion cells is the stimulus diameter. When we experimented with stimuli of increasing diameter, keeping the intensity constant, the light-evoked currents of ganglion cells increased when d-serine was applied but this increase was not associated with differences in the sensitivity to exogenous d-serine, unlike the more prominent changes that we observed with responses of increasing contrast. Thus, it is the relative contrast of the light stimulus, and not its geometry, that modulates coagonist availability.
Studies of PNFP proved advantageous for the analysis of a much broader range of stimulus intensities and pharmacological conditions. The long-lasting, stable responses of this extracellular recording technique allowed us to study the effects of these manipulations within a single preparation, which would not be possible using whole-cell recordings from ganglion cells. In addition, the PNFP was especially useful because it can be pharmacologically modified to give a pure NMDA response evoked by focal light stimulation. In one series of experiments, we studied the action of d-serine and its enhancing actions on a broad range of stimulus intensities, covering a 6.5 log10 range. These studies clearly established that the level of coagonist binding is not constant in response to stimuli of different intensities. When the stimulus intensity was low, exogenous d-serine enhanced the response by many fold (as much as 300% at the very lowest stimulus intensity used in the present study) but, when the intensity of the stimulus was high, exogenous d-serine added little or nothing to the response amplitude, indicating that full occupancy of the coagonist site had been achieved. This means that low-level stimuli release sufficient glutamate to bind many more NMDA receptors than are bound by coagonist, whereas, at high intensities, the levels of glutamate and coagnist are more closely matched. What then was responsible for changing the occupancy state of the coagonist binding site?
We pursued the identity of the light-evoked coagonist by using the bacterial enzyme DsdA, taken from E. coli (EC 4.3.1.18). When this enzyme was added to the bathing medium, it significantly reduced the response amplitude by its well known behaviour of reducing the level of d-serine in the microenvironment. DsdA does not degrade glycine and its action on the PNFP can be overcome by application of exogenous glycine (Gustafson et al. 2007), and so the effects of this enzyme unequivocally establish d-serine as at least one of the modulating coagonists released by light stimulation. When we compared the two amplitude-intensity curves (control and DsdA) (Fig.6), we found that a Boltzmann fit best described the DsdA curve (reduced coagonist) and showed that the maximum response of the PNFP occurred at lower stimulus intensities compared to the control curve. In control Ringer solution, this maximum PNFP response resulted from a well-orchestrated combination of glutamate and coagonist release. It is no coincidence that the maximum response under control conditions was reached at the same intensity level at which the application of exogenous d-serine failed to further augment the PNFP response (Fig.4B). One interpretation of the graph shown in Fig.4B is that, because 100 μm of d-serine saturates the coagonist sites on all NMDA receptors, what we observe as the stimulus level increases is a continual glutamate-dependent activation of more and more NMDA receptors until the maximum activation occurs at the intensity of 6.0. What this means is that, at each progressive intensity, a larger amount of glutamate is being released. Presumably, this holds true when DsdA is included in the Ringer solution; more glutamate is released as the stimulus increases above 4.5 but, with no d-serine to bind to the NMDA receptors, the response fails to be augmented past this level. This suggests that one function of the release of coagonist is to provide a greater dynamic range of the response and allow for the recruitment of additional NMDARs. This behaviour of NMDA receptors was previously attributed to the voltage-dependent block by external Mg2+ (Diamond & Copenhagen, 1993), although the results of the present study indicate that light-evoked release of d-serine must be a contributing and perhaps more powerful factor for recruiting NMDA receptors.
The results of the present study have added additional clarity regarding the question of how coagonist availability is modulated by light. Our findings strongly suggest the presence of a light-evoked release of d-serine, based on the intensity of the light stimulus or its relative contrast when presented with a steady background light. In this way, the release of d-serine allows the retina to recruit more NMDA receptors. It is important to emphasize that, without a coagonist, glutamate alone cannot gate the NMDA receptor ion channel, and so the availability of the coagonist carries equal weight with the availability of glutamate. For low intensity light stimuli, a deficiency in coagonist is evident in that a larger NMDAR response can be observed when exogenous d-serine is added to the bathing medium; we found this relationship to be present regardless of whether we studied the PNFP or carried out whole-cell recordings from retinal ganglion cells. With higher intensity light stimulation, the glutamate and coagonist release appear to be better matched because the addition of 100 μm d-serine did not increase the response amplitude.
Sethuramanujam & Slaughter (2014) have recently clarified how NMDA receptors are recruited under different pharmacological conditions. They demonstrated that blocking inhibition resulted in a substantially greater increase in the NMDA receptor contribution to excitatory ganglion cell activity compared to the enhancement of the AMPA receptor component. This difference for the ON response of ON–OFF ganglion cells comprised more than a twenty-fold enhancement when contrasting NMDA with AMPA receptor activation. Undoubtedly, feedback inhibition depresses release of glutamate from bipolar cell terminals. What might comprise the mechanism that accounts for such a disparity in the NMDA to AMPA receptor activation ratio when presynaptic inhibitory influences are blocked? We suggest that the reduction in feedback inhibition not only gives rise to an increased release of glutamate, but also the NMDA receptor contribution is given more weight under these circumstances created by the increased release of d-serine from Müller cells via a Ca2+-dependent AMPA receptor mechanism (Sullivan & Miller, 2010; Sullivan & Miller, 2012).
There remain many challenges in understanding how d-serine is released in the retina. Although vesicles have been identified in Müller cells that immunostain for d-serine in human material (Diaz et al. 2007), a mechanism for vesicular release has not been established. d-serine release has been studied in the mouse retina using a mutant mouse line devoid of d-amino acid oxidase with an elevated level of d-serine (Sullivan & Miller, 2010). d-serine release evoked by AMPA + cyclothiazide was significantly reduced when the retina was treated with a gliotoxin. This Müller cell release pathway was mediated by Ca2+-permeable AMPA receptors.
Finally, we need to address the question of why DsdA did not influence the low intensity responses shown in Fig.5. Because the PNFP was under the constraint of the toxin cocktail used in the present study, the light-evoked responses were generated by NMDA receptors (Gustafson et al. 2007). We can only speculate as to why the responses at 2.0 log I and below were unaffected by the DsdA and what it means that DsdA blocked only 40% of the response at higher intensities. One possibility is that a portion of the NMDA receptor activation relies on glycine serving as the coagonist. Glycine is not degraded by DsdA. Stimulus-induced glycine release has been reported to act on NMDA receptors in the retina (Kalbaugh et al. 2009). Another aspect of NMDA receptor activation that still needs to be addressed fully is the role of coagonist on synaptic versus extrasynaptic NMDA receptors in the retina. Many NMDA receptors are located outside of glutamatergic synapses (Zhang & Diamond, 2006) and the identity of the active coagonist may be determined by the location of the receptors (Sullivan & Miller, 2012). In addition, although DsdA degrades extracellular d-serine, it does not degrade intracellular stores of d-serine, such as those that might be released via a vesicular pathway. Thus, d-serine released in close proximity to receptors could potentially survive without undergoing major degradation, allowing it to diffuse from the site of release to nearby NMDA receptors but not to those at more remote locations. At present, we do not know the pathway of the release mechanism or the position of Müller cell release sites as they relate to NMDA receptors on ganglion cells.
In summary, we have presented evidence strongly favouring the idea that a light-evoked release of d-serine is critical for activating the full population of NMDAR. The present evidence favours the view that d-serine is released, presumably by Müller cells, via a Ca2+-permeable AMPA receptor pathway (Sullivan & Miller, 2010). The release of this coagonist is meant to compensate for the increased reliance on NMDA receptors as the contrast of the stimulus is increased or the intensity of light stimulation is brighter (Sethuramanujam & Slaughter, 2014). Indeed, we have laid out clear evidence indicating that, for low intensity light stimulation, it is the absence of significant d-serine release that prevents the activation of a larger fraction of the NMDA receptor pool because there is already sufficient glutamate released to activate a larger pool of the NMDA receptor population.
Glossary
Abbreviations
- AP7
d,l-2-amino-phosphonoheptanoate
- DAAO
d-amino acid oxidase
- DCK
dichlorokynurenate
- DsdA
d-serine deaminase
- GlyT1
glycine transporter type 1
- NBQX
2, 3-dihydroxy-6-nitro-7-sulfamoyl-benzo-(F)-quinoxalinedione
- NMDAR
NMDAR receptor
- PNFP
proximal negative field potential
- SR
serine racemase
Additional information
Competing interests
The authors have no competing interests.
Author contributions
R.F.M. performed all of the experiments at the University of Minnesota. E.S.S. designed, performed and analysed the results of the whole-cell experiments. E.G.G. designed, performed and analysed the results of the PNFP experiments. All authors contributed to the drafting of the article and approved the final version submitted for publication.
Funding
This research was supported by National Eye Institute (NEI) Grant RO1 EY-003014 to R. F. Miller and NEI Training Grant T32 EY-07133 to E. R. Stevens and E. C. Gustafson.
References
- Burkhardt DA. Proximal negative response of frog retina. J Neurophysiol. 1970;33:405–420. doi: 10.1152/jn.1970.33.3.405. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA, Fahey PK. Sikora M. Responses of ganglion cells to contrast steps in the light-adapted retina of the tiger salamander. Vis Neurosci. 1998;15:219–229. doi: 10.1017/s0952523898152021. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA, Gottesman J. Thoreson WB. An eyecup slice preparation for intracellular recording in vertebrate retinas. J Neurosci Methods. 1989;28:179–187. doi: 10.1016/0165-0270(89)90034-4. [DOI] [PubMed] [Google Scholar]
- Coleman PA. Miller RF. Measurment of passive membrane parameters with whole-cell recording from neurons in the intact amphibian retina. J Neurophysiol. 1989;61:218–230. doi: 10.1152/jn.1989.61.1.218. [DOI] [PubMed] [Google Scholar]
- Diamond JS. Copenhagen DR. The contribution of NMDA and non-NMDA receptors to the light-evoked input-output characteristics of retinal ganglion cells. Neuron. 1993;11:725–738. doi: 10.1016/0896-6273(93)90082-3. [DOI] [PubMed] [Google Scholar]
- Diaz CM, Macnab LT, Williams SM, Sullivan RK. Pow DV. EAAT1 and D-serine expression are early features of human retinal development. Exp Eye Res. 2007;84:876–885. doi: 10.1016/j.exer.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Kleckner NW. McBain CJ. The glycine coagonist site of the NMDA receptor. Adv Exp Med Biol. 1990;268:17–26. doi: 10.1007/978-1-4684-5769-8_3. [DOI] [PubMed] [Google Scholar]
- Dun Y, Duplantier J, Roon P, Martin PM, Ganapathy V. Smith SB. Serine racemase expression and D-serine content are developmentally regulated in neuronal ganglion cells of the retina. J Neurochem. 2008;104:970–978. doi: 10.1111/j.1471-4159.2007.05015.x. [DOI] [PubMed] [Google Scholar]
- Dun Y, Mysona B, Itagaki S, Martin-Studdard A, Ganapathy V. Smith SB. Functional and molecular analysis of D-serine transport in retinal Muller cells. Exp Eye Res. 2007;84:191–199. doi: 10.1016/j.exer.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmsen JT, Ma TM, Sason H, Rosenberg D, Ogo T, Furuya S, Snyder SH. Wolosker H. D-Serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J Neurosci. 2013;33:12464–12469. doi: 10.1523/JNEUROSCI.4914-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey PK. Burkhardt DA. Center-surround organization in bipolar cells: symmetry for opposing contrasts. Vis Neurosci. 2003;20:1–10. doi: 10.1017/s0952523803201012. [DOI] [PubMed] [Google Scholar]
- Gustafson EC, Stevens ER, Wolosker H. Miller RF. Endogenous D-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J Neurophysiol. 2007;98:122–130. doi: 10.1152/jn.00057.2006. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T. Takahashi K. The presence of free D-serine in rat brain. FEBS Lett. 1992;296:33–36. doi: 10.1016/0014-5793(92)80397-y. [DOI] [PubMed] [Google Scholar]
- Jager J. Wassle H. Localization of glycine uptake and receptors in the cat retina. Neurosci Lett. 1987;75:147–151. doi: 10.1016/0304-3940(87)90288-6. [DOI] [PubMed] [Google Scholar]
- Johnson JW. Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kalbaugh TL, Zhang J. Diamond JS. Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells. J Neurosci. 2009;29:1469–1479. doi: 10.1523/JNEUROSCI.4240-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E. Wolosker H. Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281:14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- Miller RF. Dacheux RF. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. III. A model of ganglion cell receptive field organization based on chloride-free experiments. J Gen Physiol. 1976;67:671–690. doi: 10.1085/jgp.67.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KB, Miller RF. Bowser MT. D-Serine uptake by isolated retinas is consistent with ASCT-mediated transport. Neurosci Lett. 2005;385:58–63. doi: 10.1016/j.neulet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP. Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Pow DV. Hendrickson AE. Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci. 1999;16:231–239. doi: 10.1017/s0952523899162047. [DOI] [PubMed] [Google Scholar]
- Reed BT, Sullivan SJ, Tsai G, Coyle JT, Esguerra M. Miller RF. The glycine transporter GlyT1 controls N-methyl-D-aspartic acid receptor coagonist occupancy in the mouse retina. Eur J Neurosci. 2009;30:2308–2317. doi: 10.1111/j.1460-9568.2009.07020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, Foltyn VN, Inoue R, Mori H, Billard JM. Wolosker H. Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci. 2013;33:3533–3544. doi: 10.1523/JNEUROSCI.3836-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuramanujam S. Slaughter MM. Disinhibitory recruitment of NMDA receptor pathways in retina. J Neurophysiol. 2014;112:193–203. doi: 10.1152/jn.00817.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shleper M, Kartvelishvily E. Wolosker H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005;25:9413–9417. doi: 10.1523/JNEUROSCI.3190-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR. Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens ER, Gustafson EC. Miller RF. Glycine transport accounts for the differential role of glycine vs. D-serine at NMDA receptor coagonist sites in the salamander retina. Eur J Neurosci. 2010;31:808–816. doi: 10.1111/j.1460-9568.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SJ. Miller RF. AMPA receptor mediated D-serine release from retinal glial cells. J Neurochem. 2010;115:1681–1689. doi: 10.1111/j.1471-4159.2010.07077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SJ. Miller RF. AMPA receptor-dependent, light-evoked D-serine release acts on retinal ganglion cell NMDA receptors. J Neurophysiol. 2012;108:1044–1051. doi: 10.1152/jn.00264.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supplisson S. Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J Neurosci. 1997;17:4580–4590. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S. Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol. 2006;498:810–820. doi: 10.1002/cne.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]


