Abstract
Cortical sensory processing varies with cortical state and the balance of inhibition to excitation. Repetitive transcranial magnetic stimulation (rTMS) has been shown to modulate human cortical excitability. In a rat model, we recently showed that intermittent theta-burst stimulation (iTBS) applied to the corpus callosum, to activate primarily supragranular cortical pyramidal cells but fewer subcortical neurons, strongly reduced the cortical expression of parvalbumin (PV), indicating reduced activity of fast-spiking interneurons. Here, we used the well-studied rodent barrel cortex system to test how iTBS and continuous TBS (cTBS) modulate sensory responses evoked by either single or double stimuli applied to the principal (PW) and/or adjacent whisker (AW) in urethane-anaesthetized rats. Compared to sham stimulation, iTBS but not cTBS particularly enhanced late (>18 ms) response components of multi-unit spiking and local field potential responses in layer 4 but not the very early response (<18 ms). Similarly, only iTBS diminished the suppression of the second response evoked by paired PW or AW–PW stimulation at 20 ms intervals. The effects increased with each of the five iTBS blocks applied. With cTBS a mild effect similar to that of iTBS was first evident after 4–5 stimulation blocks. Enhanced cortical c-Fos and zif268 expression but reduced PV and GAD67 expression was found only after iTBS, indicating increased cortical activity due to lowered inhibition. We conclude that iTBS but less cTBS may primarily weaken a late recurrent-type cortical inhibition mediated via a subset of PV+ interneurons, enabling stronger late response components believed to contribute to the perception of sensory events.
Key points
Theta-burst stimulation (TBS) applied via transcranial magnetic stimulation is able to modulate human cortical excitability.
Here we investigated in a rat model how two different forms of TBS, intermittent (iTBS) and continuous (cTBS), affect sensory responses in rat barrel cortex.
We found that iTBS but less cTBS promoted late (>18 ms) sensory response components while not affecting the earliest response (8–18 ms). The effect increased with each of the five iTBS blocks applied.
cTBS somewhat reduced the early response component after the first block but had a similar effect as iTBS after four to five blocks.
We conclude that iTBS primarly modulates the activity of (inhibitory) cortical interneurons while cTBS may first reduce general neuronal excitability with a single block but reverse to iTBS-like effects with application of several blocks.
Introduction
Inhibitory systems are particularly important in regulating cortical excitability. Variations in the balance of excitation and inhibition appear to be involved in a multitude of physiological and pathophysiological mechanisms, such as the processing of sensory-motor responses (Isaacson & Scanziani, 2011; Merchant et al. 2012), cortical state changes (Freund, 2003), cortical development (Le Magueresse et al. 2013) and network plasticity (Hensch, 2005), as well as cognitive malfunction as in schizophrenia (Lewis et al. 2012). In particular, one class of interneurons, the parvalbumin-expressing (PV+), fast-spiking (FS) GABAergic interneurons appear to be very important in regulating cortical excitability because of their strong excitatory input from pyramidal cells on the one hand (Holmgren et al. 2003; Mateo et al. 2011; Avermann et al. 2012) and their strong inhibitory action on pyramidal cells via perisomatic (large basket cells) and axoaxonic (chandelier cells) synapses on the other (Markram et al. 2004). In this way, PV+ interneurons not only prevent pathological hyperactivity (Cammarota et al. 2013), they also regulate sensory activity in a manner of sparse coding in a state-dependent fashion (Petersen & Crochet, 2013).
Non-invasive brain stimulation methods such as repetitive transcranial magnetic (rTMS) and direct current stimulation are able to modify human cortical excitability, thereby having the potential to improve cortical function in a homeostatic fashion (Ridding & Ziemann, 2010; Pell et al. 2011) and to treat neurological (Schulz et al. 2013) and neuropsychiatric diseases (Demirtas-Tatlidede et al. 2013). In a rat model, we recently showed that theta-burst stimulation (TBS) applied via rTMS strongly reduced the number of cortical neurons expressing the 67 kDa isoform of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD67) and that of the calcium-binding proteins PV and calbindin (CB), indicative of reduced activity of inhibitory neurons (Trippe et al. 2009; Mix et al. 2010, 2013; Benali et al. 2011). The intermittent-type TBS protocol (iTBS) reported to enhance human cortical excitability (Huang et al. 2005; Di Lazzaro et al. 2008) was particularly effective in reducing the number of PV+ neurons (Mix et al. 2010; Benali et al. 2011).
To elaborate how rTMS affects cortical sensory processing, we repeatedly applied iTBS and continuous (cTBS) in a sham-controlled fashion to anaesthetized rats while recording neuronal activity from the barrel cortex evoked by whisker deflection. We were particularly interested in those response characteristics potentially modulated by cortical inhibition, such as the transience and sparseness of sensory responses (Petersen & Crochet, 2013) and the suppression of responses evoked by paired stimulation of one or two whiskers at short intervals, known as frequency adaptation (Simons, 1978; Kleinfeld et al. 2002; Garabedian et al. 2003; Khatri et al. 2004) and cross-whisker suppression (Simons 1985; Simons & Carvell 1989; Brumberg et al. 1996; Ego-Stengel et al. 2005). Following iTBS, but less after cTBS, we found an increase in late response components, probably controlled by intracortical connections, and a significantly weaker suppression of the second response evoked by paired whisker stimulation.
Methods
Ethical approval
All experiments were performed with permission of the government (Ref.: 9.93.2.10.32.07.057 & 87-51.04.2010.A097) and the local welfare committee. All procedures conformed to the guidelines of the animal welfare laws in Germany, the UK, the European Union and the ethical standards of The Journal of Physiology as reported by Drummond (2009).
Anaesthesia and recordings
Experiments were carried out on 32 adult (3 month) male Sprague Dawley rats delivered by Janvier Labs (Saint-Berthevin, France). Twelve rats were treated with iTBS, a further 12 with cTBS and eight received sham stimulation (see below). Initially, rats were deeply anaesthetized by an intraperitoneal bolus injection of urethane (2.0 g kg−1 body weight) to enable surgical procedures. Throughout the subsequent recording sessions anaesthesia was maintained by repeated urethane injections (0.2 g kg−1 body weight earliest after 4 h then about every 2 h), dissolved in 0.5 ml saline. An additional subcutaneous injection of 0.5 ml saline (without urethane) was given 2 h from onset of anaesthesia to support water and salt homeostasis. In previous experiments (see Funke & Benali, 2009) this procedure had been found to be adequate to achieve stable levels of anaesthesia for up to 12 h. Depth of anaesthesia was estimated by hind paw withdrawal and corneal reflex, by spontaneously occurring whisker movements and by visual inspection of the EEG with focus on the presence of slow waves. Offline, EEG status was determined by repeated calculation of the ratio of theta (4–7 Hz) versus delta band (1–3 Hz) power (see also below). If anaesthesia appeared insufficient – a most obvious sign were spontaneous whisker vibrations – a larger bolus injection of 0.5 g urethane per kg body weight was applied and experimental procedures continued as soon as whisker movements disappeared. Before and during the surgical procedures needed to get access to the surface of the brain (incision of the skin and trepanation of the skull), lidocaine hydrochloride (Xylocain gel 4%, AstraZeneca, London, UK) was topically applied to support analgesia. Body temperature was maintained between 37 and 37.5 °C using a feedback-controlled (rectal temperature) heating blanket (ATC1000, WPI, Berlin, Germany). Rats were fixed in a stereotaxic frame while recording multi-unit spiking activity (MUA) and local field potentials (LFPs) at three sites within D2 column of the barrel cortex using a bundle of three varnished tungsten electrodes (∼1 MΩ, 1–2 μm tip size; FHC, Bowdoin, ME, USA), glued together in a triangular arrangement with 100 μm spacing between the depth-aligned tips. After checking for the correct position to record from barrel D2 using a single tungsten electrode, the bundle was slowly (∼20 μm min−1) lowered towards layer 4 until first whisker-evoked spike responses occurred at a stimulus-response latency as short as 8–10 ms, indicating that upper border of layer 4 had been reached (Brumberg et al. 1999; Shimegi et al. 1999). The whisker eliciting the strongest responses at shortest latency was depicted as the principal whisker (PW). The cortical surface was then covered by agar and melted wax to stabilize the brain and the electrodes.
To enable long-term recordings from potentially the same group of neural units before, during and after rTMS, we used a special figure-of-eight coil (2 × 70 mm; The MagStim Company Ltd, Whitland, Dyfed, UK) equipped with a central tubing, allowing us to lower the electrode bundle at right angles through the plane of the coil to the site of recording. With this electrode orientation, and with all the wires attached to the electrodes and the animal kept distant from the coil and coursing at right angles to the coil wires, minimal electrical field and vibration is induced within the electrodes. A reference electrode (Ag–AgCl wire) was placed below the skin of the neck. In a first step, neuronal activity was amplified 1000× within a bandpass of 1–3000 Hz using a CyberAmp 380 (Axon Instruments, Molecular Devices, LLC, Sunnyvale, CA, USA). Records destined for LFP/EEG analysis were digitized at a sampling frequency of 866 Hz and stored on a PC hard drive for offline analysis using the CED power1401 interface and the Spike2 software (Cambridge Electronic Design, Cambridge, UK). The 50 Hz noise deriving from the power lines was efficiently eliminated using a ‘Hum Bug’ system (Quest Scientific, North Vancouver, Canada). In a second step, signals were further amplified (100×) and bandpassed at 300–3000 Hz for spike time detection with the aid of a window discriminator including a non-linear gain amplifier to improve spike detection via increasing signal-to-noise ratio even if spike amplitude varied across long-term recordings. With the positive and negative thresholds set at about +30% of the maximum noise events, noise within the window is amplified with a gain less than 1 while signals above threshold amplified with a gain larger than 1 (gain decreases or increases, respectively, with distance from threshold). A second threshold separated spikes from noise. Spikes were sampled and digitized at 8 kHz using the CED power1401 interface and the Spike2 software.
At the end of the experiment, about 3.5 h after the final rTMS block, rats were deeply anaesthetized using pentobarbital sodium (300 mg kg−1 body weight i.p., Narcoren, Rhone Merieux GmbH, Laubheim, Germany) and perfused transcardially with cooled saline followed by 4% paraformaldehyde to enable subsequent immunohistochemical analysis and verification of recording sites. The animals were painlessly killed by this procedure.
Whisker stimulation protocols
The PW (usually D2, occasionally D1) and the anterior adjacent whisker (AW, D3, occasionally D2) contralateral to the recording site (right hemisphere) were clipped to 1 cm length and inserted 5 mm into a stainless steel needle fixed to a piezo-driven bending actuator (Type P-871.127, Physik Instrumente GmbH and Co KG, Karlsruhe, Germany) and stabilized with melted wax. In this way, precise whisker deflection could be performed regarding amplitude, direction, velocity and timing. The anterior-to-posterior (A-P) deflection amplitude was always 150 μm, measured at 5 mm distance to the base of the whiskers, corresponding to an angle of 1.72 deg. Deflection amplitude was calibrated using an optical measurement system (optic microscopic scale plus light sensor voltammetry). Five different deflection velocities varying at steps of 200 deg s−1 from 200 to 1000 deg s−1 (8.6–1.72 ms in duration) were used to establish stimulus–response functions for the PW. Whisker deflections were performed in a ramp-like fashion with smoothed acceleration and deceleration phase to avoid spurious piezo vibrations (Ego-Stengel et al. 2005). In the case of single deflections of either the PW or the AW, the A-P ramp of different velocity was followed by a plateau phase of 500 ms before a slow posterior–anterior (P-A) ramp (150 deg s−1, 10.7 ms), eliciting almost no response, returned the whisker to its resting position. In the case of PW–PW or AW–PW stimulus pairs, the A-P ramp of 800 deg s−1 was directly followed by the slower P-A ramp to enable stimulus intervals of 20 ms. To test for recurrent intracortical inhibition within a single barrel or between neighbouring barrels either the PW was stimulated twice at an interval of 20 or 100 ms, or the AW was stimulated at 20 or 100 ms prior to the PW, respectively. Both whiskers were also stimulated simultaneously to test for fast feedforward-type (inhibitory) interactions between adjacent barrels. Piezo action was controlled by the digital-to-analog output of the CED power1401 with a temporal resolution of 0.01 ms and by the two-channel piezo-amplifier E-651.2S (Physik Instrumente). The different whisker stimulation protocols were applied in three blocks with each stimulus occurring 30 times in a quasi-random order. Block 1, lasting 5 min, included the stimuli for deflection of the PW at five different velocities. Block 2, lasting 4 min, included the single and paired PW stimuli (fixed velocity of 800 deg s−1). Block 3, lasting 8 min, included the single AW stimulation and the pairing with PW (also with deflection in P-A direction, not presented here because of effects very similar to A-P direction). The different stimuli of each block were presented in a quasi-random order and at intervals of 2 s (0.5 Hz) to avoid interference between subsequent stimuli.
TMS
TMS was applied using a MagStim Rapid device equipped with four boosters and a 70 mm figure-of-eight coil (The MagStim Company). Positioning of the coil was identical to previous experiments (Trippe et al. 2009; Benali et al. 2011), aiming at stimulation of the callosal axons to induce supragranular cortical activity without stimulating other parts of the brain (Funke & Benali, 2011). Using a mediolateral orientation of the coil allows us to stimulate the long axons of the corpus callosum at a lower stimulus intensity (24–28% of maximum machine output (MSO), corresponding to an induced electric field strength of 37–50 V m−1 at the level of subcortical white matter, including corpus callosum) than is needed for direct stimulation of the cortex (and subcortical white matter) if using a rostrocaudal coil orientation as has been done by others. For example, a stimulus intensity of 80–100% MSO is needed to evoke motor responses directly within rat motor cortex when using A-P orientation of the coil (Rotenberg et al. 2010). The stimulus strength we used was subthreshold for inducing peripheral motor activity as is also done in human studies in the case of high-frequency stimulation to avoid discomfort and induction of epileptiform activity (Rossi et al. 2009). The low stimulation intensity also reduces the likelihood of stimulating deeper brain structures (Funke & Benali, 2011). Coil distance to brain surface was about 8 mm in the case of verum stimulation and about 60 mm for sham stimulation (maximum distance possible with electrodes of 100 mm length). Due to the exponential decay of magnetic field strength with distance from the coil surface, the induced electric field strength will be about one order of magnitude lower than at 8 mm distance.
TBS protocols were applied according to the study of Huang et al. (2005), with one iTBS block consisting of 20 trains of ten 50 Hz bursts (three pulses) repeated at 5 Hz (lasting 192 s with 10 s intervals between trains) and one cTBS block being a single 40 s train of bursts repeated at 5 Hz. Each type of stimulation block (iTBS, cTBS or iTBS-sham) containing 600 pulses was applied five times at intervals of about 20 min during the course of each experiment.
Histochemistry
Immunohistochemical analysis was done according to earlier experiments (extensively described elsewhere, e.g. Trippe et al. 2009; Benali et al. 2011). Immunohistochemical quantification of marker proteins was performed on 30 μm thick frontal sections of the left hemisphere, opposite to the recorded hemisphere to avoid impaired staining quality due to the micro-lesions caused by the electrodes. Our previous studies revealed no asymmetric effects of rTMS when the corpus callosum had been stimulated centrally (Trippe et al. 2009; Mix et al. 2010, 2013; Benali et al. 2011; Hoppenrath & Funke, 2013). As regions of interest (ROI), sections of motor cortex (MC, 1.2 mm anterior to bregma) and somatosensory cortex (SC, 1.8 mm posterior to bregma) including the barrel field were chosen for analysis. Histological verification of the recording sites was performed on flatted horizontal sections of the right hemisphere. Electrode tracks were visualized by immunohistochemical detection of extravagated serum protein (biotinylated rabbit-anti rat antibody, 1:1000, BA4000, Vector Labs, Burlingame, CA, USA) while barrels within layer 4 were visualized via cytochrome oxidase staining according to Wong-Riley (1979), using Cytochrome C Typ III (Sigma C-2506). Image reconstruction and alignment of horizontal sections through layers 3 and 4 were done with the Neurolucida system (MicroBrightField Europe e.K., Magdeburg, Germany). The following primary antibodies were used for staining neuronal marker proteins: GAD67 (monoclonal, 1:2000, clone 1G10.2; Millipore, Billerica, MA, USA), PV (monoclonal, 1:1000, clone 234; Swant, Bellinzona, Switzerland), CB (monoclonal, 1:1000, clone 300; Swant), zif268 protein (rabbit anti-Erg-1, polyclonal, 1:1000; Santa Cruz Biotech., Santa Cruz, CA, USA), c-Fos protein (polyclonal, 1:1000; Santa Cruz Biotech.) and NeuN (neuron-specific nuclear antigen, monoclonal, 1:1000, clone A60; Millipore). The neuronal marker NeuN served as a control to rule out neuronal cell loss as a possible reason for changes in the number of cells labelled for one of the marker proteins, and to normalize numbers of labelled cells with regard to differences in the total number of neurons within the ROI if needed. Visualization of specific labelling was done using 3,3′-diaminobenzidine (DAB) as chromogen.
Experimental design, data analysis and statistics
The first pre-TMS measurements were started when spike recordings were stable about 30 min after the electrodes had reached layer 4. The complete set of whisker stimulation protocols was applied before TMS (pre), after each of the five TMS blocks (TMS1, TMS2, …, TMS5) and a further 60 and 120 min (post1, post2) after the last TMS block (see Fig.1). Analysis of neuronal activity was done for the number of spikes evoked by whisker stimulation and for LFPs. For every stimulus protocol and time point tested, 30 measurements were averaged. The earliest reliably evoked spikes occurred within layer 4 at 8 ms after stimulus and initial transient response terminated at about 18 ms. Therefore, this early response part was quantified within these 10 ms. Because later response components became evident after iTBS, we analysed a second time window between 19 and 39 ms, called the late response. Recordings from electrodes showing no stimulus-modulated activity or abnormal high spike counts due to triggering noise or artefacts were excluded from analysis (less than 10% of the recordings). As it is highly unlikely to record spiking activity from identical units for many hours, we decided not to analyse single units but analysed population spiking activity of multi-units (MUA). Spike counts of early and late response components were separately analysed ANOVA with factors TIME (pre, TMS1, TMS2, TMS3, TMS4, TMS5, post1, post2), followed by post hoc Dunnett's test, or Tukey test when including factor TMS (iTBS, cTBS, sham) in ANOVA. A P value less than 0.05 had been considered as a statistical significant sample difference rejecting the null hypothesis.
Figure 1. Study design.
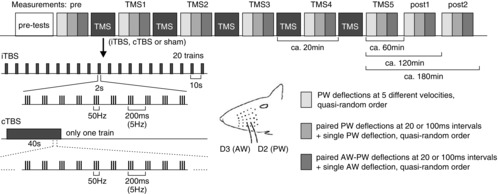
The first block ‘pre-tests’ included recordings with a single metal electrode for finding upper layer 4 of the cortical barrel corresponding to the principal whisker (PW) and repeated recordings via the triple electrode bundle placed at the same site until spiking activity stabilized. Then, three blocks of measurements were performed (pre) using PW deflections at five different velocities (light grey), testing paired PW deflections at either 20 or 100 ms intervals (middle grey) and combined AW–PW stimulation also at intervals of 20 or 100 ms intervals (dark grey), mixed with single PW and AW deflections in a quasi-random order, before the first iTBS, cTBS or sham stimulation block had been applied (TMS). Identical measurement blocks were repeated after each TMS block (TMS1 to TMS5) and a further 60 and 120 min after the last TMS block (post1, post2). iTBS and cTBS were applied according to Huang et al. (2005). For further description of TMS, see Methods.
LFPs established by 30 stimulus repetitions were statistically analysed by comparing post-TMS measurements (TMS1, TMS5) with the pre-TMS condition using a non-parametric permutation based t test (two-sided) of the EEGLAB-Toolbox (Delorme & Makeig, 2004), including correction for multiple measurements according to Dunnett's test.
Spike counts of MUA per 30 stimuli were first quantified via peri-stimulus time histograms (PSTHs), followed by calculating the mean spike rate (and standard error of the mean, SEM) for the early (8–18 ms) and late (19–39 ms) response component for each experimental time point. LFPs are also analysed as grand average means as done for MUA.
The spectral composition of the EEG background activity at each recording site and for each experimental phase (pre, TMS1–5, post1 and post2) was determined using the fast Fourier transformation algorithm (FFT) of the Spike2 program. The ratio of the power of the theta to delta bands was calculated as a measure of anaesthetic depth as power of the delta band but less that of the other frequency bands has been shown to be a valid predictor of anaesthetic depth (Xu et al. 2005). Normalization to theta band power was performed to compensate for variations in signal strength due to variations in electrode impedance (Van Luijtelaar & Coenen, 1984; Funke & Benali, 2009).
Cell counts were performed on images of histological sections obtained with a Leitz Wetzlar Dialux 20 microscope (Leica, Wetzlar, Germany) equipped with a colour video camera (CCD KH 609, Heimann; Metamorph, Universal Imaging, Downingtown, PA, USA) at a magnification of 100×. Cell counts were performed in a semi-automatic, and thus blinded, fashion by setting the same threshold of staining intensity for all experimental groups and ROIs for a particular marker and then applying automatic software-based cell counting to the images (Neurolucida, MicroBrightField). For the CB we had to set a threshold clearly above the fainter staining of pyramidal cells and neuropile to count only the strongly stained cells verified to be interneurons (Kawaguchi & Kubota, 1993). Cells were counted in equally sized areas of four frontal sections through sensorimotor cortex of left hemisphere spanning all cortical layers from pia mater to white matter (1.44 × 0.72 mm). All results are shown as group means of cell counts (and SEM) and statistically analysed using Student's t test.
Results
Multi-unit spiking activity
In total, eight experiments were performed with sham rTMS, 12 with verum iTBS and a further 12 with verum cTBS. By using a bundle of three electrodes we were able to record reliable whisker deflection evoked MUA from 23 sites in the case of the sham-stimulation experiments, from 28 sites in the case of the iTBS experiments and from 34 sites in the case of the cTBS experiments.
Findings on single whisker deflection
Deflection of the PW (800 deg s−1) evoked a transient spiking response in this anaesthetized preparation which was largely restricted to the time window of the early response component (8–18 ms). With iTBS also a late response component evolved which strongly grew with each iTBS block (see PSTHs of Fig.2A). Differently, cTBS induced only a small increase in this late response, which was first evident after the fourth to fifth cTBS block. During sham stimulation, no late response evolved during the course of the experiment. Responses of similar dynamics were evoked with deflection of the rostrally adjacent whisker (AW) but the response amplitudes were smaller (20–30% of the PW response, not shown as PSTHs). Effects of iTBS and cTBS on sensory responses were very similar for AW and PW deflection. Statistical analysis using ANOVA with factor TIME (pre to post2) revealed a significant effect of iTBS on the late PW and AW response components (19–39 ms) and for the late AW response in the case of cTBS (see Table1 for a summary of all F- and P-values of ANOVA with factor TIME). No significant effect of TIME was evident for the late response in the case of sham stimulation. Post hoc comparison of all post-rTMS data with the pre-rTMS condition by applying Dunnett's test revealed a significant increase of the late PW response after the second, fourth and fifth iTBS block (see Fig.3, right side, TMS2, TMS4, TMS5) and after the fourth and fifth iTBS blocks, and also for the two post-rTMS measurements in the case of the late AW responses. No significant changes were detected for the sham and cTBS conditions. ANOVA with factor TMS revealed a significant difference between iTBS and sham for the late PW (F2,82 = 13.84, P = 0.004) and AW response (F2,82 = 6.89, P = 0.014) but no effect of cTBS in both cases. Post hoc comparison of the different TMS conditions using the Tukey test revealed a significantly stronger increase of the late PW and AW responses after the second or third iTBS block compared to sham and cTBS (see Fig.3 for details).
Figure 2. Changes in the dynamics of sensory MUA after theta-burst TMS.
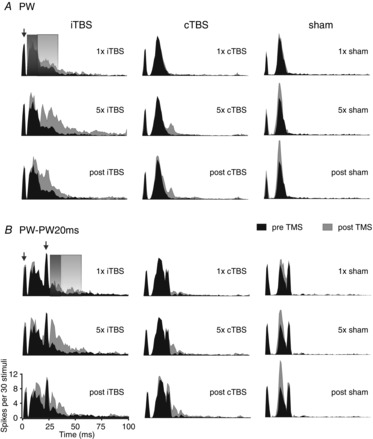
Peri-stimulus time histograms (PSTHs) show grand average MUA of sensory responses for experiments with either iTBS application (left column, mean of 28 recording sites), cTBS (middle column, 34 recording sites) or sham stimulation (right column, 23 recording sites). Black areas always show pre-TMS activity while grey areas show responses after one block of stimulation (upper panels, ‘1 × iTBS, cTBS or sham’), after five blocks of stimulation (middle panels, ‘5× …’) or 60–120 min after the last TMS block had been applied (lower panels, ‘post …’; post1 and post2 recording sessions averaged). A, responses obtained with a single deflection of the principal whisker (PW); B, responses obtained with 2-fold stimulation of the PW at 20 ms interval (PW–PW20ms). The grey-shaded rectangles indicate the time windows used for quantification of the early (8–18 ms) and late (19–39 ms) components of the sensory responses. Note that for PW–PW20ms the two analysis time windows refer to the early and late responses evoked by the second PW deflection. PW deflection was always performed with a velocity of 800 deg s−1. The ordinate of the PSTHs (spikes per 30 stimuli per bin) refers to the number of spikes accumulated within each 1 ms bin with repetition of 30 identical stimuli. The arrows point to the artefacts resulting from the voltage step needed to move the piezoelectric actuator.
Table 1.
Results of the uni-factorial ANOVA with factor TIME for single and double stimulation of the principal (PW) and the anterior adjacent (AW) whisker
| PW single | AW single | PW–PW20ms | AW–PW20ms | AW–PW0ms | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | |
| (a) Early response (8–18 ms) | ||||||||||
| Sham | 4.026 | 0.000 | 7.236 | 0.000 | 0.604 | 0.752 | 1.610 | 0.135 | 1.526 | 0.161 |
| iTBS | 0.960 | 0.462 | 0.604 | 0.752 | 3.043 | 0.005 | 3.115 | 0.004 | 0.679 | 0.69 |
| cTBS | 0.966 | 0.457 | 1.342 | 0.231 | 2.807 | 0.554 | 1.675 | 0.115 | 1.599 | 0.136 |
| (b) Late response (19–39 ms) | ||||||||||
| Sham | 1.144 | 0.337 | 1.063 | 0.39 | 1.125 | 0.440 | 0.624 | 0.735 | 1.677 | 0.117 |
| iTBS | 2.639 | 0.013 | 3.331 | 0.002 | 2.164 | 0.039 | 1.694 | 0.112 | 1.907 | 0.069 |
| cTBS | 1.441 | 0.190 | 2.382 | 0.023 | 0.841 | 0.554 | 1.433 | 0.192 | 0.238 | 0.976 |
Statistically significant cases are shown in bold. Degrees of freedom: sham, 7180; iTBS, 7220; cTBS, 7268.
Figure 3. Statistics of iTBS and cTBS effects on sensory responses evoked by single PW or AW deflection.
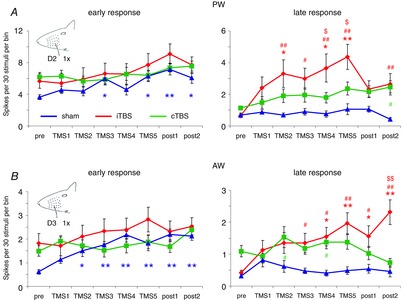
Diagrams show mean sensory response amplitudes (±SEM) before (pre), after one to five TMS blocks (TMS1, TMS2 … TMS5) and 60 and 120 min after the fifth TMS block (post1, post2) separately for the early (left column) and late (right column) response component. Spikes evoked by 30 stimuli of the same kind were summed within 8–18 ms after whisker deflection (800 deg s−1) in the case of the early response and between 19 and 39 ms in the case of the late response. The number of spikes was then divided by the number of integrated bins (1 ms) to achieve the same scaling as used for the PSTHs in Fig.2 (spikes per 30 stimuli per bin). Data from sham stimulation experiments are shown in blue, iTBS in red and cTBS in green. Asterisks (*P < 0.05, **P < 0.01) indicate statistically significant differences from the ‘pre’ condition for each experimental condition (post hoc Dunnett's test, note colour code); hash symbols (#P < 0.05, ##P < 0.01) indicate statistically significant differences between iTBS and sham (red) or cTBS and sham (green) (post hoc Tukey test); and dollar symbols ($P < 0.05, $$P < 0.01) indicate statistically significant differences between iTBS and cTBS (post hoc Tukey test).
In the early response component, ANOVA revealed no effect of iTBS or cTBS with factor TIME but a significant change during the sham condition. The early responses steadily increased over time and the results of Dunnett's test indicated a significant increase of early PW and AW responses from phase TMS4 or TMS3 on, respectively (Fig.3, left side). As it is possible that response amplitudes vary with changes in cortical state due to variations in anaesthetic depth, we analysed the EEG and calculated the power ratio of the theta and delta band for every experimental condition. A comparison between the three different experiments (sham, iTBS, cTBS, see Fig.4A) using ANOVA with factors TMS and TIME revealed a significant effect of factor TMS (F2,767, P < 0.01), but no significant correlation (Pearson test) between the EEG power ratio and the spike rates of the early PW response was evident (sham: r = −0.088, θ/δ = 0.231; iTBS: r = 0.088, θ/δ = 0.229; cTBS: r = 0.061, θ/δ = 0.332; see also Fig.4B–D).
Figure 4. No correlation between changes in spectral power of the EEG and sensory response rates.
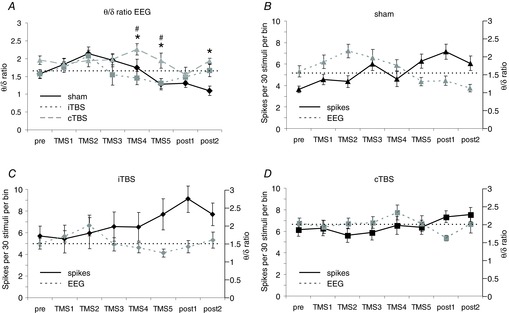
A, means (±SEM) of the ratio of the power of the theta and delta frequency band of the EEG (θ/δ ratio) for the different time points (pre to post2) of the three experimental conditions. B–D, comparisons of changes in EEG power ratio with changes of sensory response spike rates (PW early response) for the different experimental conditions (B, sham; C, iTBS; D, cTBS). The dotted horizontal line gives a reference to the ‘pre’ θ/δ power ratio of the EEG. Statistically significant differences (P < 0.05) are shown between cTBS and sham condition (*) or between cTBS and iTBS (#, post hoc Tukey test).
Single whisker deflection – response gain
To examine if rTMS may change response dynamics with respect to response gain, we tested the same conditions as described above for five different velocities of PW deflection (200–1000 deg s−1). The rTMS effects were in principle the same as described above; iTBS significantly increased the late response component independent of PW deflection velocity while cTBS did not (see Table2 for a summary of ANOVA results with factor TIME). The increase in early response amplitude during the sham condition was also evident in this data sample but also a more pronounced suppressive effect of cTBS than in the data sample described above (see Table2). Figure5 plots the means of the early and late response components versus PW deflection velocity for the conditions pre-TMS, TMS1 and TMS5 for the three kinds of experiments (sham, iTBS, cTBS). In all experiments, the early response shows a clear stimulus–response relationship with increasing response amplitude as deflection velocity increases. No such relationship was evident for the late response component. Curves shifted up or down depending on TMS condition but did not show changes in slope except for the region of response saturation of the early response, which could be achieved at 800 or 1000 deg s−1 depending on the vertical position of the curves. A weak positive slope evolved for the late response when it was strongly increased after the fifth iTBS block.
Table 2.
Results of uni-factorial ANOVA with factor TIME for PW whisker deflections of different velocity
| 200 deg s−1 | 400 deg s−1 | 600 deg s−1 | 800 deg s−1 | 1000 deg s−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | |
| (a) Early response (8–18 ms) | ||||||||||
| Sham | 2.877 | 0.005 | 3.951 | 0.000 | 2.343 | 0.020 | 2.097 | 0.037 | 1.822 | 0.075 |
| iTBS | 0.396 | 0.922 | 0.651 | 0.734 | 0.749 | 0.648 | 1.269 | 0.260 | 0.974 | 0.456 |
| cTBS | 2.755 | 0.006 | 3.421 | 0.001 | 1.553 | 0.139 | 2.492 | 0.012 | 1.648 | 0.111 |
| (b) Late response (19–39 ms) | ||||||||||
| Sham | 1.522 | 0.151 | 2.009 | 0.046 | 1.055 | 0.396 | 2.074 | 0.040 | 2.228 | 0.027 |
| iTBS | 2.882 | 0.004 | 2.521 | 0.012 | 2.220 | 0.027 | 2.598 | 0.010 | 3.013 | 0.003 |
| cTBS | 1.570 | 0.133 | 1.492 | 0.159 | 1.198 | 0.300 | 0.803 | 0.600 | 0.814 | 0.590 |
Statistically significant cases are shown in bold. Degrees of freedom: sham, 7180; iTBS, 7220; cTBS, 7268.
Figure 5. No TMS effect on response gain.
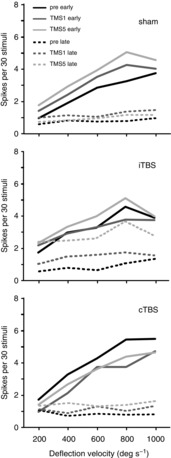
For each of the three experimental conditions (sham, iTBS, cTBS) the diagrams show mean early (continuous lines) and late (dashed lines) response rates evoked by five different whisker deflection velocities during the pre (black), TMS1 (dark grey) and TMS5 (light grey) conditions. The error bars were excluded here for better readability of the diagrams. Both iTBS and cTBS mainly induced vertical shifts of the curves without a change in slope. While early response components showed a clear stimulus–response relationship, late responses did not except when strongly increased after the fifth iTBS block. Only at deflection velocities of 800–1000 deg s−1 did varying response saturation change the slope of the curves.
Findings on paired whisker deflections
Paired whisker deflections with either the PW stimulated twice at 20 or 100 ms intervals, or first the anterior AW and then the PW at 20 or 100 ms intervals had been conducted to test if intracortical recurrent activity, probably including inhibition, contributes to the well-known suppression of the second response. In addition, we tested the simultaneous stimulation of PW and AW. Whisker deflection velocity was 800 deg s−1 in any case. Description of the results obtained with 100 ms intervals are omitted because effects were similar to those with 20 ms intervals, albeit weaker. Double PW stimulation at 20 ms intervals (PW–PW20ms) resulted in a strong suppression of the second response in all experimental series (ratio 2nd/1st: sham 0.05, iTBS 0.17, cTBS 0.09). Suppression of the PW response by paired AW–PW stimulation (AW–PW20ms) resulted in a similar but somewhat weaker suppression of the PW response. The first iTBS block increased the early component of the second response to 52% of the first response and a further increase up to 75% of the PW response occurred after the next four iTBS blocks. The second response in the pair was still enhanced (69% of PW) 2 h after the last iTBS block (post2, Fig.6A, B). Also, the late component of the second response increased to a similar degree. A post-iTBS increase of the second response was also evident for the AW–PW20ms stimulation protocol (Fig.6C, D).
Figure 6. Changes in the amplitude of the second response with paired whisker stimulation following theta-burst TMS.
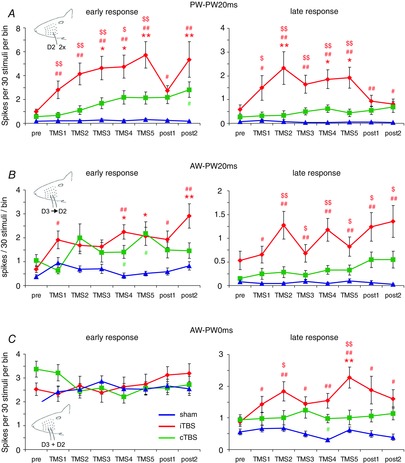
Early and late response components of the second response evoked by paired whisker stimulation, as compared to Fig.3 (A, PW twice at 20 ms interval; B, AW–PW stimulation at 20 ms interval; C, simultaneous AW–PW stimulation). As in Fig.3, sham experiments are shown in blue, iTBS experiments in red and cTBS experiments in green. Asterisks (*P < 0.05, **P < 0.01) indicate statistically significant differences from the pre condition for each experimental condition (post hoc Dunnett's test, note colour code); hash symbols (#P < 0.05, ##P < 0.01) indicate statistically significant differences between iTBS and sham (red) or cTBS and sham (green) (post hoc Tukey test); and dollar symbols ($P < 0.05, $$P < 0.01) indicate statistically significant differences between iTBS and cTBS (post hoc Tukey test).
For both PW–PW20ms and AW–PW20ms, ANOVA with factor TIME revealed a significant effect of iTBS on the early response component of the second response, and also for the late response in the case of PW–PW20ms, but no effect was evident with cTBS or sham stimulation, (Table1). As indicated in Fig.6, the increase in response amplitude reached statistical significance with pair-wise post hoc comparison (Dunnett's test) after two to four iTBS blocks. A much weaker effect was found with cTBS: ANOVA with factor TIME indicated a significant effect on the early part of the second response in the PW–PW20ms condition (Table1), but post hoc testing revealed no significant difference between pairs of pre- and post-cTBS episodes. No effect at all was evident in the sham conditions. ANOVA with factor TMS further revealed a significant difference between iTBS and sham condition in the case of PW–PW20ms both for the early and for the late response components of the second sensory response (iTBS vs. sham, early: F2,82 = 9.86, P = 0.000; late: F2,82 = 7.06, P = 0.000; iTBS vs. cTBS early: F2,82 = 8.85, P = 0.001; late: F2,82 = 6.35, P = 0.001) and for the late response in the case of AW–PW20ms (iTBS vs. sham: F2,82 = 3.8, P = 0.003; iTBS vs. cTBS: F2,82 = 3.45, P = 0.009).
The responses elicited by simultaneous deflection of AW and PW (AW–PW0ms) were smaller than those evoked by a single PW stimulation (∼50%) but larger than those evoked by a single AW stimulation (20–30% of PW, early response part), indicating that afferent input of both whiskers did not simply summate but may be controlled by fast feedforward inhibition. The effects of TMS were similar to those seen with single PW and AW stimulation. The early but not the late response components increased slightly over time in the sham condition (but differences not being significant with factor TIME of ANOVA and post hoc testing). The increase of the late response component after iTBS was weaker, reaching statistical significance only after the fifth iTBS block (post hoc Dunnet's test, see Fig.6E, F), while no effect of cTBS and sham condition was evident. However, ANOVA with factor TMS indicated a significant difference between iTBS and sham condition (F2,82 = 6.89, P = 0.02).
Local field potentials
Analysis of LFPs recorded from the border region of layer 3/4 generally supported the findings obtained with MUA analysis. LFP waveforms showed little change during the sham stimulation experiments but effects of one or five iTBS or cTBS on LFP waveform differed. LFPs evoked by single PW deflection showed a sequence of two positive and two negative peaks (Fig.7A). A first positive peak occurred between 10 and 13 ms (P1), followed by a negative peak around 20 ms (N1). A slow positive wave peaking between 30 and 50 ms (P2) passed into a long negative wave of low amplitude peaking around 150 ms (N2). P1 largely corresponds to the time window of the early spiking response between 8 and 18 ms while P2 coincides with the late spiking response (19–39 ms) with N1 located at the transition between these response components. Primarily, the late N1–P2 complex changed after TMS. N1 increased with each iTBS block shifting also P2 to more negative values (significant after the first, fourth and fifth iTBS block, P < 0.05, t test-based Dunnett's test). The effect of cTBS differed from that of iTBS in one important aspect: the first cTBS block caused a significant positive shift of the N1 and P2 wave. First after the fifth block, cTBS caused a similar although somewhat weaker (not significant) negative shift of the N1–P2 complex similar to iTBS.
Figure 7. Changes in LFP waveforms evoked by single and paired PW stimulation after TBS.
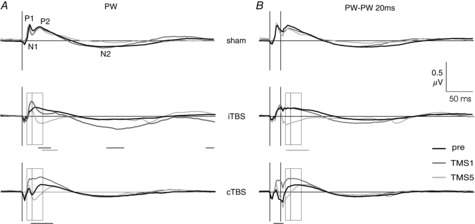
The diagrams show grand average LFPs obtained from 24 recording sites for the sham experiments (top row) and 36 recording sites in the iTBS and cTBS experiments (middle and bottom row, respectively). A, LFPs evoked by single PW stimulation; B, LFPs evoked by paired PW stimulation (PW–PW20). Shown are LFPs of the pre-TMS state (black), after one iTBS or cTBS block (dark grey) and after five iTBS or cTBS blocks (light grey). Open rectangles indicate the early (8–18 ms) and late (19–39 ms) time windows chosen for statistical analysis of MUA (compare with Fig.2). All LFP measurements were performed at the same time as the MUA recordings. Horizontal bars below the diagrams indicate statistically significant differences of LFP waveforms between TMS1 and pre (black) or TMS5 and pre (grey) at P < 0.03 (non-parametric permutation-based t test, two-sided, corrected for multiple measurements by Dunnett's test).
LFPs evoked by paired PW stimulation at 20 ms intervals (see Fig.7B) had a waveform very similar to those elicited by single PW stimulation (Fig.7A), thus confirming the suppression of the second sensory response seen with MUA also for neuronal population activity. Also in this case, TMS primarily affected the N1–P2 complex. iTBS progressively shifted it to negative values (significant after the fourth block) while cTBS first caused a significant shift to positive values (TMS1) before a weaker (not significant) shift to negative values occurred during the next four cTBS blocks.
LFPs evoked by single AW and paired AW–PW stimulation (AW–PW20ms) were similar in shape to single PW and PW–PW20ms stimulation, respectively, and also the TMS effects were principally the same (data not shown).
Verification of recording sites and immunohistochemical analysis of neuronal markers
In three animals of the sham group and in each of the four animals of the iTBS and cTBS groups we obtained immunohistochemical serum protein and cytochrome oxidase (COX) stains of sufficient quality to localize the recording sites. COX staining of the remaining material did not allow us to visualize the complete barrel field. In all cases, termination of electrode tracks was found at the border region between layer 3 and layer 4, superficial to barrel D2. Figure8 shows one example of combined serum protein and COX staining in horizontal sections through sensorimotor cortex of the right hemisphere. Figure9 summarizes the results of the immunohistochemical analysis of the neuronal activity markers PV, CB, GAD67, c-Fos and zif268 obtained for the sensorimotor cortex after the recording sessions. Immunohistochemical analysis of the brain could not be performed in all animals due to death before perfusion or inadequate brain perfusion. As described previously (Trippe et al. 2009; Benali et al. 2011), iTBS stronger than cTBS reduced the PV and GAD67 expression while cTBS primarily reduced the CB expression, indicating that inhibitory systems had been differently modulated by the two stimulation protocols. Opposite effects of iTBS and cTBS were found for the general neuronal activity markers c-Fos and zif268, which primarily reflect excitatory activity. We found an increase in the number of c-Fos+ and zif268+ neurons after iTBS but a decrease after cTBS. Significant differences from sham-treated rats and between iTBS and cTBS groups are indicated in Fig.9C (Student's t test). The reduction of PV, CB and GAD67 expression was quantitatively weaker than described in our previous studies (Trippe et al. 2009; Benali et al. 2011). One reason could be a partial recovery of protein expression during the longer survival time of the animals between stimulation and perfusion as compared to the previous studies (∼4 h vs. ∼1–2 h). Sensory activity elicited by the frequent whisker stimulation during this episode may have forced this recovery. In a previous study we found that PV expression partially recovered in rats which were forced to use their whiskers in a tactile discrimination task following iTBS, while rats housed in a standard cage after stimulation did not show a recovery of PV expression (Mix et al. 2010).
Figure 8. Histological verification of a recording site.
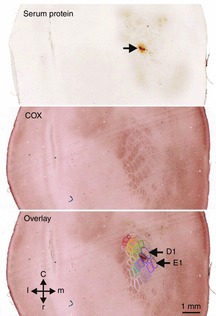
The top image shows serum protein staining surrounding the electrode track (arrow) within layer 3 close to layer 4 of the right hemisphere. The middle image shows the barrel layout visualized by cytochrome oxidase (COX) staining within layer 4 about 120–150 μm deeper. The bottom image shows an overlay of the top and middle images and graphical assignment of the barrels. Accordingly, the track of the bundle of the three electrodes terminated close to the upper border of barrel D2.
Figure 9. TMS-induced changes of neuronal activity markers.
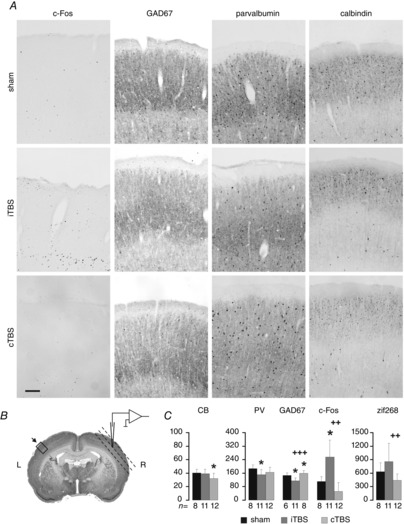
A, example images of histological sections through sensorimotor cortex showing cells labelled via antibodies directed to c-Fos, GAD67, parvalbumin and calbindin and subsequent diaminobenzidine staining for animals of the sham, iTBS and cTBS series. Shown are only the upper cortical layers (1–4, pial side on top) which showed the strongest effects. Scale bar = 100 μm. B, Nissl-stained frontal section at the level of sensorimotor cortex including representation of the vibrissal pad showing the location of the region of interest (ROI) for counting labelled cells (rectangle, about 1 mm2 reaching from pia mater to white matter, 0.720 mm width × 1.44 mm height, at 1.2–1.8 mm from bregma). Horizontal sections through the same area of the right hemisphere (planar to cortical surface, dashed lines) were prepared to verify the electrode tracks (see Fig.8). C, bar graphs showing the mean number (+SEM) of labelled neurons counted within the ROI of four sections obtained from each animal with successful immunohistochemistry (number of animals indicated below the bar diagrams). PV, parvalbumin; CB, calbindin; GAD67, acid decarboxylase; c-Fos, zif268, immediate early gene products. *P < 0.05 relative to sham, ##P < 0.01, ###P < 0.001 iTBS vs. cTBS (Student's t test).
Discussion
Our findings demonstrate that sensory responses of rat barrel cortex are differently affected if five blocks of either iTBS or cTBS are applied at intervals of 20 min. Each block of iTBS continuously increased the amplitude of a late sensory response component (>18 ms) and reduced the suppression of the second response in paired PW or AW–PW stimulation at 20 ms intervals, which temporally coincides with the time window of the late first response. It is thus likely that iTBS progressively weakened a process that suppresses late response components as would be the case with intracortical recurrent inhibition. Differently, cTBS had a discontinuous effect by first slightly suppressing primarily the early response after the first block but causing a weaker increase of the late response as seen with iTBS after the fourth to fifth block. This difference between cTBS and iTBS was particularly evident for changes in LFP waveform.
The study had been performed in a sham stimulation controlled fashion to gather also changes in sensory responses possibly occurring independent of the TMS procedure during the course of the experiments. Indeed, a steady increase in the early but not late response component was evident in all experiments, reaching statistical significance in the sham series. Changes in anaesthetic depth or synaptic plasticity due to repeated stimulation of the same whiskers are possible reasons. As we found no significant correlation between the ratio of EEG theta to delta band power and the response spike rates, we can largely exclude anaesthetic depth as a reason for the steady increase in response amplitude. Furthermore, theta-to-delta power ratio went up and down during the course of the experiments while responses continuously increased. We cannot explain the reasons for this increase in MUA activity but responses may increase because of the isolated stimulation of one whisker or associative stimulation of two neighbouring whiskers while the others are actually deprived during the experiment as even no whisker movement was present due to anaesthesia (see Holtmaat & Svoboda, 2009). Repeated associative stimulation of one whisker in combination with a foot shock actually resulted in a reduced number of active units within mouse barrel cortex but these units showed higher responses, a matter of sparse coding (Gdalyahu et al. 2012).
The different changes in sensory response dynamics obtained after the first iTBS or cTBS block are thus comparable with the principal findings obtained in human studies using TBS: one block of iTBS increased motor cortex excitability while one cTBS block reduced it (Huang et al. 2005). Recent human TMS studies revealed that the TBS effects are highly variable, depending on the current state and history of activity within the cortical network (Hamada et al. 2013). In this respect, rat studies may have the advantage that individuals grow up under almost identical conditions and that sensory and motor experience will be less variable as in humans with different biography. Interestingly, the cTBS effect almost reversed if several blocks of stimuli had been repeated. Changes in the polarity of TBS effects have also been observed in human studies, either when changing the number of pulses within one stimulation block (Gentner et al. 2008; Gamboa et al. 2010) or when applying two conventional blocks at different intervals (Gamboa et al. 2001). The number of stimuli within one train and the pattern of stimulation with breaks of different length appear to be important parameters in addition to the current cortical state. In our study, we found no polarity reversing effect for iTBS – the effects of each additional block accumulated over time – but cTBS showed a reversal after the first or second block approaching an effect similar to iTBS. A recent human TMS study demonstrated that repetition of iTBS blocks caused a stronger increase in motor cortex excitability than a single iTBS block (Nettekoven et al. 2014). It can thus be suggested that repetition of stimulus trains with breaks of considerable length, as with iTBS, typical high-frequency rTMS (>4 Hz) and biomimetic high-frequency stimulation (BHFS; Rodger et al. 2012), more probably induce facilitation of cortical excitability than continuous stimulation trains, as with cTBS and 1 Hz rTMS. Repeating cTBS blocks with intervals of a couple of minutes may thus lead to similar changes of cortical activity as a single iTBS block.
We analysed cortical responses to single and paired whisker deflection with the intention to learn more about the network mechanisms of rTMS. Is it modulating the intrinsic excitability of neurons or is it affecting interneuron networks controlling the excitability of other neurons? We found that the early response components evoked by single stimuli, which are probably evoked by direct thalamocortical inputs and feedforward inhibition (Bruno & Simons, 2002; Gabernet et al. 2005; Sun et al. 2006; Higley & Contreras, 2007) (see Fig.10, connection 1), were not affected by iTBS but the later components, which are probably under the control of recurrent cortical connections (connections 2–4 in Fig.10). If we applied a second stimulus during this late response, then the early component of the second response also increased after iTBS. It is conceivable that recurrent cortical activity (and lateral inter-column projections in the case of paired AW–PW stimulation) mediated via interneurons affects not only late response components but also limits the intervals at which a second response can occur. We thus conclude that iTBS primarily modulated intracortical connections. The current and our previous studies (Trippe et al. 2009; Benali et al. 2011; Hoppenrath & Funke, 2013) demonstrate that iTBS reduces PV expression in cortical interneurons, while simultaneously enhancing the expression of the neuronal activity markers c-Fos and zif268, indicating that activity of cortical interneurons of the fast-spiking type may have been reduced by iTBS. If iTBS enhances neuronal excitability in general terms, also early responses driven by thalamocortical synapses can be expected to increase. The first cTBS block, however, caused a suppression of the early response (partially significant with the series of different whisker deflections performed earlier after a cTBS block), probably related to other mechanisms of neuronal excitability than recurrent inhibitory control.
Figure 10. Scheme of cortical connections affected by TMS.
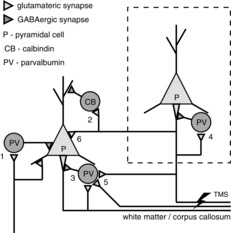
Stimulation of the axons of the corpus callosum (lightning symbol) results not only in antidrome activation of the pyramidal cells of origin, but also in orthodrome activation of collateral synapses at both inhibitory interneurons (CB- and PV-type neurons, numbers 2–5) and other pyramidal cells (number 6). All these connections are involved in intra- and inter-areal cortical processing. Recurrent perisomatic inhibition can be either local (numbers 3 and 4) or via a long loop involving pyramidal cells of other cortical layers or areas (indicated by the dashed rectangle, connection number 5). It is assumed that different populations of PV-type interneurons exist, one receiving primarily cortical input and being affected by TMS, and another population primarily receiving thalamic input (number 1) and supposed not to be (or less) affected by TMS (see Staiger et al. 2009 for different populations of PV-type interneurons).
Our findings are in line with our previously raised hypothesis that rTMS primarily activates the axons of layer 2/3 pyramidal cells equipped with axon collaterals projecting to the corpus callosum (Funke & Benali, 2011). It can be assumed that just layer 2/3 pyramids are involved in spatiotemporal integration of sensory activity, including the regulatory momentum of recurrent inhibition (Derdikman et al. 2003). Stimulation of afferent input to barrel cortex slices results in more widespread activity within layer 2/3 than layer 4 (Feldmeyer et al. 2002; Petersen et al. 2003) associated with the activation of diverse interneurons (Beierlein et al. 2003), including those mediating feedback to layer 4 (Helmstaedter et al. 2009). In this way, also suppression of activity elicited by stimulation of whiskers adjacent to the PW may be mediated. A morphological study has demonstrated that two different populations of PV+ interneurons can be distinguished in layer 4, one group receiving primarily thalamic synapses probably mediating forward inhibition (see connection 1 in Fig.10), the other group receiving more numerous synaptic input of cortical origin, predestining this group for mediating recurrent cortical inhibition (Staiger et al. 2009). Recurrent inhibition may be controlled locally but also by remote pyramids belonging to different layers or areas (connections 3–5 in Fig.10). The probable coexistence of thalamocortical feedforward and intracortical recurrent inhibition within layer 4 biased the decision to analyse rTMS-induced changes in cortical inhibition at this site but close to layer 3.
Interestingly, a recent study by Sachidhanandam et al. (2013) demonstrated that PV+ interneurons of the rat barrel cortex are able to suppress late sensory response components following the initial transient. Even more interestingly, this late response is essential for awareness of stimulation. The failure rate of rats responding to whisker stimulation in a behavioural task was significantly enhanced when the late sensory response was suppressed due to optogenetic stimulation of PV+ interneurons at topographically corresponding cortical sites. Strikingly similar, also human perception of visual stimuli at threshold level is related to a late cortical response component in an all-or-none fashion (Sekar et al. 2013): subjects perceived a visual stimulus if a visually evoked potential peaking around 240 ms at temporoparietal sites was present, while it was not perceived if the late response was absent. The initial response evoked around 100 ms did not correlate with perception. Late sensory activity may evolve by a combination of local and remote (top-down) activity but the local activity of PV+ interneurons may finally control the amplitude of late sensory responses (see connections 3–5 of Fig.10).
Our findings indicate that rTMS, and particularly the iTBS protocol, may be able to modulate those components of sensory cortical processing, being relevant for awareness and spatiotemporal integration of sensory activity. Indeed, human studies have shown that rTMS can improve perception and cognitive functions in healthy subjects (for a review see Luber & Lisanby, 2014). Ragert et al. (2008) demonstrated that humans receiving iTBS above primary somatosensory cortex transiently improved in tactile performance, associated with diminished paired-pulse suppression of the second cortical response (N20/P25) evoked by median nerve stimulation at intervals of 30 ms. Similarly, as we found with paired whisker stimulation, only the second response evoked in a pair but not the first response showed considerable increase in amplitude. Di Lazzaro et al. (2008) found that iTBS increased particularly the late waves of intracortical activity (I-waves 2 and 3) evoked by a single TMS pulse directed to primary motor cortex. Also, these findings indicate that particularly recurrent cortical activity increased after iTBS, probably due to a suppression of recurrent cortical inhibition. Targeted attenuation of cortical inhibition in conjunction with other therapeutic procedures appears to be a promising strategy to normalize cortical activity in neurological and neuropsychiatric diseases (as reviewed by Demirtas-Tatlidede et al. 2013; Schulz et al. 2013).
Limitations of the study
Measurements had to be performed in anaesthetized animals because documentation of acute and lasting rTMS effects required the continuous recording of neuronal activity for several hours and with the animal restrained to perform precise whisker deflections. We used urethane for anaesthesia because it has fewer effects on inhibitory and excitatory synaptic transmission as compared with ketamine and pentobarbital, respectively, but causes an unspecific reduction in evoked firing rates due to an increase of a potassium leak current (Sceniak & MacIver, 2006). The balance between excitatory and inhibitory processes may thus be preserved. Nevertheless, late sensory response components may be weaker than in conscious animals, possibly leading to overestimation of cortical inhibition. Also, the suppression of responses evoked at short intervals (frequency adaptation) was weaker in rats anaesthetized with isoflurane (Ewert et al. 2008) and appears to represent more probably sensory processing in the awake state. Effect of rTMS may also differ in more natural stimuli such as active whisking and with vibrations induced when touching objects.
Furthermore, we could not distinguish between spikes of excitatory and inhibitory neurons contributing to MUA. Because of the long recording time (>5 h) and the likelihood of small drifts of the electrodes, MUA may comprise different cells at the beginning and the end of the experiments. Therefore, we made no attempts to analyse spike responses at single unit level. Our study was not intended to analyse effects on particular types of neurons but to analyse more general changes in the state and dynamics of cortical information processing first. MUA and LFPs recorded from 23 to 34 sites in each experimental series should be large enough to represent a general effect on layer 3/4 sensory activity.
Sham stimulation was performed with a coil to brain distance of about 60 mm. A larger distance could not be achieved due to guidance of the electrodes through the central tubing of the coil. Although the induced electrical field strength will be about one order of magnitude lower at this distance as if the coil is close to the brain, we cannot exclude neuronal effects even at this low field intensity. It is less likely that action potentials will be induced at this field strength but low-intensity magnetic stimulation has been shown to affect neuronal morphology, development and survival, probably due to release of calcium ions from intracellular stores (Rodger et al. 2012; Makowiecki et al. 2014; Grehl et al. 2015). Our sham condition, however, is largely comparable to human rTMS studies when coil surface was orientated 90 deg from brain surface. Our TMS conditions further differ from those in humans as we aimed to induce cortical activity via the axon collaterals of supragranular pyramidal cells. Effects in humans may differ if, in addition, infragranular neurons are directly activated with stimulus intensities close to motor threshold.
Conclusions
Our findings indicate that iTBS applied to rat neocortex – and with repetition of stimulation blocks also cTBS – primarily affects intracortical processing but less so thalamocortical inputs. The latter finding also indicates that excitability of neurons is not generally modified but that neuronal responsiveness is more probably controlled by recurrent cortical connections including inhibition. The increase in the late response components could be the result of reduced inhibition mediated by PV+ interneurons. These interneurons appeared to be affected by the activity induced in axons of supragranular pyramidal cells according to stimulation of the corpus callosum via TMS.
Acknowledgments
The authors gratefully acknowledge the help of Dimitrula Winkler, Christina Liebig and Ute Neubacher with the histochemical techniques, Ellen Kloosterboer for Neurolucida-based reconstruction of electrode tracks and the support of Manuel Machado Lemos Rodrigues with the statistical analysis of the LFP data. Thanks are given also to Alia Benali for proofreading the manuscript.
Glossary
Abbreviations
- AW
adjacent whisker
- BHFS
biomimetic high frequency stimulation
- CB
calbindin
- c-Fos
cellular DNA-binding proteins encoded by the c-fos genes
- cTBS
continuous TBS
- FS
fast-spiking cell
- GAD65/GAD67
65 and 67 kDs isoforms of the glutamic acid decarboxylase
- iTBS
intermittent TBS
- LFP
local field potential
- MUA
multi-unit activity
- PV
parvalbumin
- PW
principal whisker
- rTMS
repetitive transcranial magnetic stimulation
- TBS
theta-burst stimulation
- zif268
zinc finger transcription factor
Additional information
Competing interests
The authors have no conflicts of interest to declare.
Author contributions
A.T. conducted the experiments and largely analysed the data as part of his MD thesis. The experiments were carried out in the Department of Neurophysiology, Ruhr-University Bochum. K.F. designed the study as part of the SFB874 project A4, proofed data analysis and statistics and wrote the manuscript. Both authors have read and approved the final submission.
Funding
This work was supported by a fund of the Deutsche Forschungsgemeinschaft (DFG) to K.F. (SFB874, TP A4).
References
- Avermann M, Tomm C, Mateo C, Gerstner W. Petersen CC. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J Neurophysiol. 2012;107:3116–3134. doi: 10.1152/jn.00917.2011. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR. Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, Eysel UT, Erdmann R. Funke K. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011;31:1193–1203. doi: 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ. Simons DJ. Spatial gradients and inhibitory summation in the rat whisker barrel system. J Neurophysiol. 1996;82:130–140. doi: 10.1152/jn.1996.76.1.130. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ. Simons DJ. Cortical columnar processing in the rat whisker-to-barrel system. J Neurophysiol. 1999;82:1808–1817. doi: 10.1152/jn.1999.82.4.1808. [DOI] [PubMed] [Google Scholar]
- Bruno RM. Simons DS. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci. 2002;22:10966–10975. doi: 10.1523/JNEUROSCI.22-24-10966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Losi G, Chiavegato A, Zonta M. Carmignoto G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J Physiol. 2013;591:807–822. doi: 10.1113/jphysiol.2012.238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A. Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Vahabzadeh-Hagh AM. Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–578. doi: 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D, Hildesheim R, Ahissar E, Arieli A. Grinvald A. Imaging spatiotemporal dynamics of surround inhibition in the barrels somatosensory cortex. J Neurosci. 2003;23:3100–3105. doi: 10.1523/JNEUROSCI.23-08-03100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA. Rothwell JC. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586:3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in the Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Mello e Souza T, Jacob V. Shulz DE. Spatiotemporal characteristics of neuronal sensory integration in the barrel cortex of the rat. J Neurophysiol. 2005;93:1450–1467. doi: 10.1152/jn.00912.2004. [DOI] [PubMed] [Google Scholar]
- Ewert TA, Vahle-Hinz C. Engel AK. High-frequency whisker vibration is encoded by phase-locked responses of neurons in the rat's barrel cortex. J Neurosci. 2008;28:5359–5368. doi: 10.1523/JNEUROSCI.0089-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer DL, Lübke J, Silver RA. Sakmann B. Synaptic connections between layer 4 spiny neuron – layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J Physiol. 2002;538:803–822. doi: 10.1113/jphysiol.2001.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Funke K. Benali A. Short-latency afferent inhibition varies with cortical state in rat somatosensory cortex. Neuroreport. 2009;20:1313–1318. doi: 10.1097/WNR.0b013e32832e9b3b. [DOI] [PubMed] [Google Scholar]
- Funke K. Benali A. Modulation of cortical inhibition by rTMS – findings obtained from animal models. J Physiol. 2011;589:4423–4435. doi: 10.1113/jphysiol.2011.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M. Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Gamboa OL, Antal A, Moliadze V. Paulus W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res. 2010;204:181–187. doi: 10.1007/s00221-010-2293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa OL, Antal A, Laczo B, Moliadze V, Nitsche MA. Paulus W. Impact of repetitive theta burst stimulation on motor cortex excitability. Brain Stimul. 2001;4:145–151. doi: 10.1016/j.brs.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Garabedian CE, Jones SR, Merzenich MM, Dale A. Moore CI. Band-pass response properties of rat SI neurons. J Neurophysiol. 2003;90:1379–1391. doi: 10.1152/jn.01158.2002. [DOI] [PubMed] [Google Scholar]
- Gdalyahu A, Tring E, Polack PO, Gruver R, Golshani P, Fanselow MS, Silva AJ. Trachtenberg JT. Associative fear learning enhances sparse network coding in primary sensory cortex. Neuron. 2012;75:121–132. doi: 10.1016/j.neuron.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D. Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cerebr Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA. Nicolelis MA. Nonlinear processing of tactile information in the thalamocortical loop. J Neurophysiol. 1997;78:506–510. doi: 10.1152/jn.1997.78.1.506. [DOI] [PubMed] [Google Scholar]
- Grehl S, Viola H, Fuller-Carter PI, Carter KW, Dunlop SA, Hool L, Sherrard RM. Rodger J. Cellular and molecular changes to cortical neurons following low intensity repetitive magnetic stimulation at different frequencies. Brain Stimul. 2015;8:114–123. doi: 10.1016/j.brs.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M. Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013;23:1593–1605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Sakmann B. Feldmeyer D. Neuronal correlates of local, lateral, and translaminar inhibition with reference to cortical columns. Cereb Cortex. 2009;19:926–937. doi: 10.1093/cercor/bhn141. [DOI] [PubMed] [Google Scholar]
- Hensch TK( Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Higley MJ. Contreras D. Cellular mechanisms of suppressive interactions between somatosensory responses in vivo. J Neurophysiol. 2007;97:647–658. doi: 10.1152/jn.00777.2006. [DOI] [PubMed] [Google Scholar]
- Holmgren C, Harkany T, Svennenfors B. Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A. Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hoppenrath K. Funke K. Time-course of changes in neuronal activity markers following iTBS-TMS of the rat neocortex. Neurosci Lett. 2013;536:19–23. doi: 10.1016/j.neulet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP. Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindin D28 k immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Khatri V, Hartings JA. Simons DJ. Adaptation in thalamic barreloid and cortical barrel neurons to periodic whisker deflections varying in frequency and velocity. J Neurophysiol. 2004;92:3244–3254. doi: 10.1152/jn.00257.2004. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Sachdev RN, Merchant LM, Jarvis MR. Ebner FF. Adaptive filtering of vibrissa input in motor cortex of rat. Neuron. 2002;34:1021–1034. doi: 10.1016/s0896-6273(02)00732-8. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C. Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR. Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber B. Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS) Neuroimage. 2014;3:961–970. doi: 10.1016/j.neuroimage.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowiecki K, Harvey AR, Sherrard RM. Rodger J. Low-intensity repetitive transcranial magnetic stimulation improves abnormal visual cortical circuit topography and upregulates BDNF in mice. J Neurosci. 2014;34:10780–10792. doi: 10.1523/JNEUROSCI.0723-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G. Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mateo C, Avermann M, Gentet LJ, Zhang F, Deisseroth K. Petersen CC. In vivo optogenetic stimulation of neocortical excitatory neurons drives brain-state-dependent inhibition. Curr Biol. 2011;21:1593–1602. doi: 10.1016/j.cub.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Merchant H, de Lafuente V, Peña-Ortega F. Larriva-Sahd J. Functional impact of interneuronal inhibition in the cerebral cortex of behaving animals. Prog Neurobiol. 2012;99:163–178. doi: 10.1016/j.pneurobio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Mix A, Benali A, Eysel UT. Funke K. Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur J Neurosci. 2010;32:1575–1586. doi: 10.1111/j.1460-9568.2010.07425.x. [DOI] [PubMed] [Google Scholar]
- Mix A, Benali A. Funke K. Strain differences in the effect of rTMS on cortical expression of calcium binding proteins in rats. Exp Brain Res. 2013;232:435–442. doi: 10.1007/s00221-013-3751-6. [DOI] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Kutscha M, Pool EM, Rehme AK, Eickhoff SB, Fink GR. Grefkes C. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34:6849–6859. doi: 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell GS, Roth Y. Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2011;93:59–98. doi: 10.1016/j.pneurobio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Petersen CC. Crochet S. Synaptic computation and sensory processing in neocortical layer 2/3. Neuron. 2013;78:28–48. doi: 10.1016/j.neuron.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Petersen CCH, Grinvald A. Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci. 2003;23:1298–1309. doi: 10.1523/JNEUROSCI.23-04-01298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Franzkowiak S, Schwenkreis P, Tegenthoff M. Dinse HR. Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. Exp Brain Res. 2008;184:1–11. doi: 10.1007/s00221-007-1073-2. [DOI] [PubMed] [Google Scholar]
- Ridding MC. Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J, Mo C, Wilks T, Dunlop SA. Sherrard RM. Transcranial pulsed magnetic field stimulation facilitates reorganization of abnormal neural circuits and corrects behavioral deficits without disrupting normal connectivity. FASEB J. 2012;26:1593–1606. doi: 10.1096/fj.11-194878. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, Muller PA, Vahabzadeh-Hagh AM, Navarro X, López-Vales R, Pascual-Leone A. Jensen F. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin Neurophysiol. 2010;121:104–108. doi: 10.1016/j.clinph.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidhanandam S, Sreenivasan V, Kyriakatos A, Kremer Y. Petersen CC. Membrane potential correlates of sensory perception in mouse barrel cortex. Nat Neurosci. 2013;16:1671–1677. doi: 10.1038/nn.3532. [DOI] [PubMed] [Google Scholar]
- Sceniak MP. Maciver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol. 2006;95:3865–3874. doi: 10.1152/jn.01196.2005. [DOI] [PubMed] [Google Scholar]
- Schulz R, Gerloff C. Hummel FC. Non-invasive brain stimulation in neurological diseases. Neuropharmacology. 2013;64:579–587. doi: 10.1016/j.neuropharm.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Sekar K, Findley WM, Poeppel D. Llinás RR. Cortical response tracking the conscious experience of threshold duration visual stimuli indicates visual perception is all or none. Proc Natl Acad Sci USA. 2013;110:5642–5647. doi: 10.1073/pnas.1302229110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimegi S, Ichikawa T, Akasaki T. Sato H. Temporal characteristics of response integration evoked by multiple whisker stimulations in the barrel cortex of rats. J Neurosci. 1999;19:10164–10175. doi: 10.1523/JNEUROSCI.19-22-10164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissae units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Simons DJ( Temporal and spatial integration in the rat S1 vibrissal cortex. J Neurophysiol. 1985;54:615–635. doi: 10.1152/jn.1985.54.3.615. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Zuschratter W, Luhmann HJ. Schubert D. Local circuits targeting parvalbumin-containing interneurons in layer IV of rat barrel cortex. Brain Struct Funct. 2009;214:1–13. doi: 10.1007/s00429-009-0225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR. Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–1230. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippe J, Mix A, Aydin-Abidin S, Funke K. Benali A. Theta-burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009;199:411–421. doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar ELJM. Coenen AML. An EEG averaging technique for automated sleep-wake stage identification in the rat. Physiol Behav. 1984;33:837–841. doi: 10.1016/0031-9384(84)90056-8. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytchrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Xu J, Zheng C, Liu X, Pei X. Jing G. Detecting brain activity variation of rat during anesthesia by spectral entropy. Conf Proc IEEE Eng Med Biol Soc. 2005;7:6985–6988. doi: 10.1109/IEMBS.2005.1616113. [DOI] [PubMed] [Google Scholar]


