Abstract
The alterations exerted by neuromodulators on neuronal selectivity have been the topic of a vast literature in the visual, somatosensory, auditory and olfactory cortices. However, very few studies have investigated to what extent the effects observed when testing these functional properties with artificial stimuli can be transferred to responses evoked by natural stimuli. Here, we tested the effect of noradrenaline (NA) application on the responses to pure tones and communication sounds in the guinea-pig primary auditory cortex. When pure tones were used to assess the spectro-temporal receptive field (STRF) of cortical cells, NA triggered a transient reduction of the STRFs in both the spectral and the temporal domain, an effect replicated by the α1 agonist phenylephrine whereas α2 and β agonists induced STRF expansion. When tested with communication sounds, NA application did not produce significant effects on the firing rate and spike timing reliability, despite the fact that α1, α2 and β agonists by themselves had significant effects on these measures. However, the cells whose evoked responses were increased by NA application displayed enhanced discriminative abilities. These cells had initially smaller STRFs than the rest of the population. A principal component analysis revealed that the variations of parameters extracted from the STRF and those extracted from the responses to natural stimuli were not correlated. These results suggest that probing the action of neuromodulators on cortical cells with artificial stimuli does not allow us to predict their action on responses to natural stimuli.
Key points
Many studies have described the action of Noradrenaline (NA) on the properties of cortical receptive fields, but none has assessed how NA affects the discrimination abilities of cortical cells between natural stimuli. In the present study, we compared the consequences of NA topical application on spectro-temporal receptive fields (STRFs) and responses to communication sounds in the primary auditory cortex.
NA application reduced the STRFs (an effect replicated by the alpha1 agonist Phenylephrine) but did not change, on average, the responses to communication sounds.
For cells exhibiting increased evoked responses during NA application, the discrimination abilities were enhanced as quantified by Mutual Information.
The changes induced by NA on parameters extracted from the STRFs and from responses to communication sounds were not related.
Introduction
Noradrenaline (NA) is released in numerous brain areas including sensory cortices by varicosities originating from the locus coeruleus, a brainstem nucleus activated by presentation of sensory stimuli (Sara & Segal, 1991; Aston-Jones et al. 1997; Bouret & Sara, 2004). A vast literature has described the effects of noradrenergic activation on the functional properties of sensory cortex neurons. In vivo, two methodologies were used to study the noradrenergic effects – stimulation of the locus coeruleus and iontophoretic application – which led to different results because the size of the network affected by NA, as well as the NA concentration in the extracellular space, was quite different with these two approaches (reviewed by Edeline, 2012). Facilitatory effects have been described in the somatosensory cortex (Waterhouse & Woodward, 1980; Waterhouse et al. 1988, 1990), but in the auditory cortex (ACx) iontophoretic studies revealed that NA application increased the frequency selectivity of cortical neurons by depressing the tone-evoked responses both in anaesthetized and in unanaesthetized animals (Manunta & Edeline, 1997, 1999). In ACx, the decreases in tone-evoked responses were blocked by α1 receptor antagonists whereas the few cases of increases in evoked responses were blocked by β receptor antagonists (Manunta & Edeline, 1997, 2004). Some of these effects can be explained by a modulation of the intra-cortical inhibitory transmission. In vitro, in pyramidal cells from layer II/III, the inhibitory post-synaptic potential (IPSPs) evoked by stimulation in layer I were blocked by α1 adrenoreceptor activation, and the IPSPs evoked by stimulation in layer II/III were increased by α2 or β adrenoreceptor activation (Salgado et al. 2011b). In ACx slices, α2 or β adrenoreceptor activation induced an increase of IPSPs which relied on a presynaptic increase of GABA release probability by parvalbumin-positive interneurons (Salgado et al. 2012). Interestingly, this study also described an α1-dependent decrease of GABAergic currents in pyramidal cells.
Many hypotheses have been proposed to understand the functional role of the noradrenergic modulation in sensory cortices (reviewed by Hurley et al. 2004). Initially, several authors suggested that, whatever its effect on evoked responses (increases or decreases), NA enhances the signal-to-noise ratio, i.e. the ratio between evoked and spontaneous activity (Waterhouse et al. 1990; but see Manunta & Edeline, 1997 and Ego-Stengel et al. 2002 for a lack of such effect). It was also proposed that NA produces a gating effect, i.e. allows stimuli that initially generate subthreshold inputs to provide suprathreshold inputs (Waterhouse et al. 1988; but see Manunta & Edeline, 1998 for the opposite effect). However, if the neuromodulators in general, and NA in particular, play a key role in attention and other cognitive processes, they should impact the discriminative abilities of cortical neurons, especially when it is necessary to discriminate between natural stimuli that share common features (e.g. their spectral content). To the best of our knowledge, such a role has never been directly tested. In the best cases, this role has been inferred based on the effects of NA on the receptive fields of cortical neurons. In the visual cortex, the suppressive effect of NA was exerted with no significant sharpening of direction or orientation selectivity tuning (McLean & Waterhouse, 1994; Ego-Stengel et al. 2002), which can reflect a divisive effect (i.e. a gain control). In the ACx, the depressive effect of NA induced an increase in frequency selectivity as it could be expected from a subtractive effect (Manunta & Edeline, 1997, 1999).
Recently, it has been reported that the cortical responses to complex natural sounds, such as vocalizations, are quite different from cortical responses to pure tones. More precisely, the cortical responses to communication sounds display highly precise temporal organizations that carry more information about the vocalization identity than the firing rate does (Huetz et al. 2006, 2009; Schnupp et al. 2006; Liu & Schreiner, 2007; Wang et al. 2007; reviewed by Gaucher et al. 2013b). Moreover, algorithms trying to predict responses to vocalizations from responses to pure tones usually perform poorly (Machens et al. 2004; Laudanski et al. 2012). In concrete terms, the temporal organization of cortical responses to vocalizations relies on very precise timing of the action potential occurrence from one presentation to the next. In the sensorimotor cortex, studies have shown that locus coeruleus stimulation increased the temporal reliability of neuronal responses to tactile stimuli (Lecas, 2001, 2004). Thus far, similar studies do not exist in the ACx, although in some cases iontophoretic application of NA decreased the spike jitter in responses to pure tones (see figure 3 in Edeline, 2012). In fact, in the only study investigating the influence of NA on auditory cortical responses to communication sounds, only changes in firing rate have been described (Foote et al. 1975).
In the present study, topical applications of NA and of selective agonists of noradrenergic receptors were used to investigate the influence of NA on cortical responses to conspecific vocalizations and to pure tones. Our results confirm the dichotomy between the effects induced by α1 vs. α2/β receptor activation on responses to pure tones, and extend these effects to responses to vocalizations. Despite a lack of effect on the response to vocalizations in general, NA enhanced the neurons’ discriminative abilities (indexed by mutual information) for the recordings exhibiting the highest increase in evoked response, an effect that was not observed with any of the tested agonists.
Methods
Ethical approval
Experiments were performed under national licence A-91-557 and using procedures 32-2011 and 34-2012 validated by the Ethic committee no. 59 (Paris Centre et Sud). Surgical procedures were performed in compliance with the guidelines determined by the national (JO 887–848) and European (86/609/EEC) legislation on animal experimentation, which are similar to those described in the Guidelines for the Use of Animals in Neuroscience Research of the Society of Neuroscience.
Subjects
Neuronal activity was recorded in the primary ACx (area AI) of adult pigmented guinea pigs of either sex. Animals, weighing 450–950 g (3–7 months old), came from our own colony housed in a humidity- (50–55%) and temperature- (22–24°C) controlled facility on a 12/12 h light/dark cycle (lights on at 07:30 h) with free access to food and water. Two to 3 days before each experiment, the animal's audiogram was determined by testing auditory brainstem responses (ABRs) under isoflurane anaesthesia (2.5%), as previously described by Gourévitch et al. (2009). The ABRs were obtained by differential recordings between two subdermal electrodes (SC25; NeuroService, Etrechy, France) placed at the vertex and behind the mastoid bone. Averages of 500 responses were collected at nine frequencies (between 0.5 and 32 kHz) presented between 70 and 0 dB SPL to obtain a physiological audiogram. All the animals used in the present study showed an ABR audiogram in the range previously reported for healthy guinea pigs (Robertson & Irvine, 1989; Gourévitch et al. 2009; Gourévitch & Edeline, 2011).
Surgical procedures
Each animal was anaesthetized by an initial injection of urethane (1.2 g kg−1, i.p.) supplemented by additional doses (0.5 g kg−1, i.p.) when movements were observed after pinching the hind paw (usually four times during the recording session). A single dose of atropine sulphate (0.06 mg kg−1, s.c.) was given to reduce bronchial secretions. After placing the animal in a stereotaxic frame, a craniotomy was performed above the left temporal cortex. A local anaesthetic (xylocaine 2%, s.c.) was liberally injected in the wound and was re-injected at regular intervals. The skull opening was 8 mm wide (starting at the intersection point between parietal and temporal bones) and 8–10 mm high. The dura above the ACx was removed under binocular control and the cerebrospinal fluid was drained through the cysterna magna to prevent the occurrence of oedema. After surgery, a pedestal of dental acrylic cement was built to allow atraumatic fixation of the animal's head during the recording session. The stereotaxic frame supporting the animal was placed in a sound-attenuating chamber (model AC1; IAC, New York, USA). At the end of the recording session, a lethal dose of pentobarbital (>200 mg kg−1, i.p.) was administered to the animal.
Recording procedures
Extracellular multi-unit recordings were obtained from arrays of 16 tungsten electrodes (ø: 33 μm, impedance <1 MΩ) composed of two rows of eight electrodes separated by 1000 μm (350 μm between adjacent electrodes of the same row). A silver wire, used as ground, was inserted between the temporal bone and the dura mater on the contralateral side. The location of the primary ACx was estimated based on the pattern of vasculature observed in previous studies (Edeline & Weinberger, 1993; Manunta & Edeline, 1999; Wallace et al. 2000; Edeline et al. 2001). The raw signal was amplified 10,000 times (Medusa; Tucker-Davis Technologies (TDT), Alachua, FDL, USA) and was then processed by an RX5 multichannel data acquisition system (TDT). The signal collected from each electrode was filtered (610–10000 Hz) to extract multi-unit activity (MUA). The trigger level was set for each electrode to select the largest action potentials, and was the same before and after drug application. On-line and off-line examination of the waveforms suggests that the MUA collected here comprised action potentials generated by three to six neurons in the vicinity of the electrode. However, the result of a clustering based on the waveforms was not reliable enough to isolate single units. At the beginning of each experiment, we set the position of the electrode array in such a way that the two rows of eight electrodes sampled neurons responding from low to high frequency when progressing in the rostro-caudal direction (see examples of tonotopic maps recorded with such arrays in figure 1 of Gaucher et al. 2012; figure 2 in de Cheveigné et al. 2013).
Drug delivery
Topical application allows administration of pharmacological agents on cortical areas and has been previously used in several brain regions, including in the primary ACx (Riquimaroux et al. 1991, 1992; Jones & Barth, 2002; Caesar et al. 2003, 2008; Yu et al. 2008; Wang et al. 2009; Gaucher et al. 2013a). Here, this technique was used because we aimed to assess the consequences of noradrenergic receptor activation over the entire cortical map. During this manipulation, neuronal responses were simultaneously sampled from 16 different locations in the primary ACx. As described in a previous study (Gaucher et al. 2013a), we applied the drug only for a determined period via a filter paper placed in the immediate vicinity of the electrode array. More precisely, standardized pieces (≈0.7 × 2.5 mm) of filter paper immersed in the drug solution (at 38°C for at least 30 min before application) were delicately placed on the cortical surface under microscopic control (the filter paper was also removed under microscopic control). In a previous study (Gaucher et al. 2013a), we showed that such an application protocol could generate significant effects in the supragranular and granular layers in about 2 min and in all cortical layers in about 4 min (see figure 1 of Gaucher et al. 2013a). Based on our previous study, agonists of noradrenergic receptors (α1, phenylephrine; α2, clonidine; β, isoproterenol) were applied at a concentration of 10 μm for 4 min. Because of the reuptake and degradation processes occurring with NA, several pilot studies were performed to determine (i) the NA concentration to be used and (ii) the time course of the NA effect on ACx neurons. To that end, several combinations of NA concentration and application time were tested (Fig. 1), and the combination of 20 min of application/100 μm concentration was selected because it induced a consistent and systematic decrease of the spectro-temporal receptive field (STRF) parameters with a recovery within 30–40 min. All combinations of concentration and application duration led, on average, to shrinkage of the STRFs, except the 4 min/10 μm combination (which was probably too low to induce an effect). All drugs were freshly dissolved immediately before the experiment and kept at 38°C until application on the cortical surface.
Figure 1. Group data showing the effects of five combinations of NA concentration and application duration on three STRFs parameters.
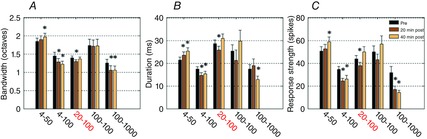
The bandwidth (A) corresponds to the sum in octaves of the frequency range of all the significant peaks in one STRF. The duration (B) is the difference (in ms) between the first and last spike times within the significant area of the STRF. The response strength (C) is the number of spikes in the significant area of the STRF. Values are mean ± SE. *Significant difference with control conditions (P < 0.05, paired t test). Each group of bars corresponds to a different combination of application duration (first number, in minutes) and concentration (second number, in μm). The combinations tested were 4 min/50 μm (three animals, 63 recordings), 4 min/100 μm (two animals, 33 recordings), 20 min/100 μm (five animals, 120 recordings), 100 min/100 μm (one animal, 23 recordings) and 100 min/1000 μm (three animals, 29 recordings). Note that NA reduced the STRF parameters for almost all combinations of concentration and duration of application. A 20 min application at 100 μm gave the most reliable effects.
Acoustic stimuli
Artificial stimuli
Acoustic stimuli were generated in MATLAB, transferred to an RP2.1-based sound delivery system (TDT) and sent to a Fostex speaker (FE87E). The speaker was placed at 2 cm from the guinea pig's right ear, a distance at which the speaker produced a flat spectrum (±3 dB) between 140 Hz and 36 kHz. Calibration of the speaker was made using noise and pure tones recorded by a Bruel & Kjaer 4133 microphone coupled to a B&K 2169 preamplifier and a Marantz PMD671 digital recorder. STRFs were determined using 97 or 129 gamma-tone frequencies, covering six (0.14–9, 0.28–18 or 0.56–36 kHz) or eight (0.14–36 kHz) octaves, respectively, and presented at 75 dB SPL. Each frequency was repeated eight times at a rate of 2.35 Hz in pseudorandom order. The duration of these tones over half-peak amplitude was 15 ms and the total duration of the tone was 50 ms, so there was no overlap between tones.
Natural stimuli
A set of three conspecific and one heterospecific vocalizations was used to assess the neuronal response to communication sounds. The conspecific vocalizations were recorded from animals of our colony. Pairs of animals were placed in the acoustic chamber and their vocalizations were recorded by a Bruel & Kjaer 4133 microphone coupled to a B&K 2169 preamplifier and a Marantz PMD671 digital recorder. A large set of vocalizations was loaded in the Audition software (Adobe audition 3) and representative examples of purr, chutter and whistle were selected. The heterospecific vocalization (a song of a Marsh Warbler Acrocephalus palustris) was selected to be strongly different from conspecific vocalizations. The heterospecific vocalization was downloaded from the Macaulay Library of the Cornell laboratory of Ornithology (see http://macaulaylibrary.org/index.do).
Experimental protocol
Insertion of a 16 electrode array in the cortical tissue almost systematically induces deformation of the cortex. A period of at least 30 min was allowed for the cortex to return to its initial shape, after which the array was slowly lowered. STRFs were used to assess the quality of the recordings and to adjust depth of the electrodes. The final recording depth was 500–1000 μm, which corresponds to layer III and the upper part of layer IV according to Wallace & Palmer (2008). However, as a result of the convex shape of the guinea-pig ACx, it is likely that the electrodes were not all exactly at the same cortical depth.
When a clear frequency tuning was obtained for at least 8 of the 16 electrodes, the stability of the tuning was assessed: we systematically required that each recording displayed at least three successive similar STRFs (each lasting 6 min) before starting to apply any pharmacological agents. When the stability was satisfactory (at least three similar STRFs obtained consecutively), the protocol started by presenting the acoustic stimuli in the following order: gamma-tones to determine the STRF at 75 dB SPL, followed by 1 min of spontaneous activity, followed by conspecific and hetereospecific vocalizations presented at 75 dB SPL. Each vocalization was repeated 20 times. Presentation of this entire stimulus set lasted 10 min and was followed by the 4 min period of drug application in the case of phenylephrine, clonidine and isoproterenol, or by a 20 min application period for NA. The entire set of stimuli was presented immediately after drug application in the same order. It was also repeated every 10 min as long as the neuronal activity was stable (usually 40–70 min after application). In the case of NA application, the two first presentations of the stimulus set were made during the 20 min of NA application.
Data analysis
Quantification of responses to pure tones
STRFs were obtained by constructing post-stimulus time histograms (PSTHs) for each frequency with 1 ms time bins. All spikes present in the averaging time window (starting at stimulus onset and lasting 100 ms) were counted. Thus, STRFs are matrices of 100 bins on the abscissa (time) multiplied by 97 or 129 bins on the ordinate (frequency). All STRFs were smoothed with a uniform 5 × 5 bin window.
For each STRF, the best frequency (BF) was defined as the frequency eliciting the highest firing rate. Peaks of significant response were automatically identified using the following procedure: a positive peak was defined as a contour of firing rate above the average level of baseline activity (estimated from the first 10 ms after tone onset, a latency too short for cortical responses) plus six times the standard deviation of the baseline activity. Three measures were extracted from the peaks: (i) the ‘bandwidth’ was defined as the sum of all peak widths in octaves, (ii) the ‘response duration’ was the time difference between the first and last spike of the significant peaks, and (iii) the ‘response strength’ was the total number of spikes occurring in the significant peaks.
Quantification of responses evoked by vocalizations
The responses to vocalizations were quantified using two parameters: (i) the evoked response, which corresponds to the firing rate during presentation of the vocalizations, and (ii) the spike-timing reliability coefficient (CorrCoef), which quantified the trial-to-trial reliability of the responses. This index was computed for each vocalization: it corresponds to the normalized covariance between each pair of action potential trains recorded during presentation of this vocalization and was calculated as follows:
where N is the number of trials and σxixj is the normalized covariance at zero lag between spike trains xi and xj where i and j are the trial numbers. Spike trains xi and xj were first convolved with a 10 ms Gaussian window. This value was chosen according to analysis of the temporal precision maximizing the mutual information (MI) (Schnupp et al. 2006; Huetz et al. 2009).
We investigated whether the CorrCoef was influenced by fluctuations of firing rate. In a first set of simulations, we computed the CorrCoef for 50 surrogate spike trains with various firing rates showing no spike-timing reliability (homogenous Poisson spike trains). The CorrCoef stayed around 0 for every firing rate tested. In a second set of simulations, we generated a spike train with a relatively high value of CorrCoef (≈0.4), then added spikes at random time points within this spike train to quantify putative variations of CorrCoef in a situation mimicking neuronal responses. Here again, this index did not increase with random insertions of action potentials, indicating that the CorrCoef index does not depend on the firing rate variations. These simulations also allowed us to compute the chance level of the CorrCoef value: 0.026 for a 0.01 confidence interval.
Systematic and meticulous examination of our database showed (as in a previous study, Gaucher et al. 2013a) that recordings exhibiting only a phasic, reliable, onset response generated a CorrCoef of at least 0.15, and that cells responding by tonic responses lacking in temporal organization generated a firing rate of at least 1.5 spikes s−1. To discard recordings not responding to any vocalization, we set a criterion of an evoked firing rate of at least 1.5 spikes s−1 or a CorrCoef of at least 0.15.
Peak detector
To assess the noradrenergic effects on the temporal organization of the responses, an automatic peak detector was applied on the response to vocalizations. The criterion used to define a peak was a level of activity of at least 50% of the maximal activity of the PSTH. Two peaks with less than 10 ms between them were considered as a single peak. PSTHs with peaks lasting more than 40 ms were classified as sustained activity. To determine if a peak detected in the PSTHs before application of a pharmacological agent corresponded to a peak detected after application, the criterion used was a temporal overlap of at least 25% of the duration of both peaks. If this criterion was not met, the two peaks were considered to be independent.
Mutual information
The method used here is exactly the same as in our previous study (Gaucher et al. 2013a), and only the main principles are summarized here. We used an indirect method (Schnupp et al. 2006) to quantify the amount of information (Shannon, 1948) contained in the responses to vocalizations obtained under control conditions and after application of pharmacological agents. This method allows us to quantify how well the vocalization's identity can be inferred from the neuronal responses recorded at a cortical site in response to the four vocalization used. Neuronal responses were represented using two time scales: 264 ms (firing rate) and 8 ms (precise temporal patterns), which allows us to analyse how much the spike timing contributes to the information. The method relies on a pattern recognition algorithm that is designed to ‘guess which stimulus evoked a particular response pattern’ by going through the following steps: from all the responses of a cortical site to the different stimuli, a single response (test pattern) is extracted and represented as a PSTH with a given bin size. Then, from the remaining responses (training set), a mean response pattern is computed for each stimulus class. The test pattern is then assigned to the stimulus class of the closest mean response pattern. This operation is repeated for all the responses, generating a confusion matrix where each response is assigned to a given stimulus class. From this confusion matrix, the MI is given by Shannon's formula:
where x and y are the rows and columns of the confusion matrix, or in other words, the values taken by the random variables ‘presented stimulus class’ and ‘assigned stimulus class’.
In our case, we used responses to four vocalizations and selected the first 264 ms of these responses to work on spike trains of the same duration (the shortest vocalization, the whistle, being 264 ms long). In a scenario where the responses do not carry any information, the assignment of each response to a mean response pattern will be equivalent to chance level (here 1/4 because we used four different stimuli and each stimulus was presented the same number of times) and the MI would be close to zero. In the opposite case where responses are very different between stimulus classes and very similar within a stimulus class, the confusion matrix would be diagonal and the mutual information would converge to log2(4) = 2 bits. Further information on this method can be found in Schnupp et al. (2006).
Principal component analysis (PCA)
PCA allows us to describe data where a great number of observations are coupled to a great number of variables. In this case, a scatter plot of all the observations and variables cannot be created. The PCA creates new axes (called principal component, PC) to represent the observations and the variables with maximized variance, each PC being orthogonal to the others. The contribution to the overall data variance of each PC can be used as a criterion to reduce the number of PCs involved in the new representation of the data. The PCA also allows us to visualize particular structures within the observations or particular relationships between the variables.
In our study, PCA was used to determine if the changes induced by NA application on several properties of neuronal responses varied in the same direction. For a given recording site, the difference between the control condition and post-application condition was computed for all the parameters extracted from the responses to pure tones (STRF bandwith, duration, response strength) and vocalizations (evoked firing rate, CorrCoef, peak duration and peak activity, and MI values computed from the firing rate (MIFR) and from the temporal patterns (MI8ms)). PCA is sensitive to differences in units or in magnitude of the variables, so all parameter variations were centred and scaled (z-score) before analysis. The PCA results are presented as a biplot of the observations in the three first PCs (i.e. with highest contribution to the overall variance) in red and the correlation of the variables with each PC in coloured vectors (see results and Fig. 8).
Figure 8. PCA for the NA-induced effects on STRF and responses to vocalizations.
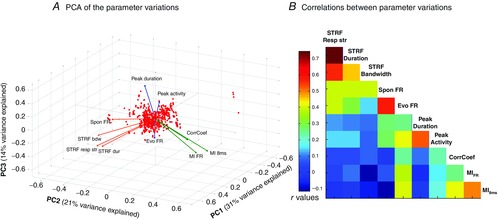
A, plot of the three first principal components of the PCA of the effect induced by NA application. These three components explain 31, 21 and 14% of the variance, respectively. Observations are represented as red dots and variables as coloured vectors (see Methods for details). The effect of NA analysed for each parameter corresponds to the value of a given parameter 20 min after application minus the value before application. Three clusters of parameters emerged: (i) the parameters extracted from STRFs and spontaneous firing rate (orange vectors), (ii) MIFR, MI8ms and CorrCoef (green vectors) and (iii) evoked firing rate, peak duration and peak activity (blue vectors). Note that the vector colours are illustrative and are not the result of mathematical clustering. B, correlation matrix of the effect induced by NA application. The correlation matrix confirms the PCA results: parameters extracted from STRFs are highly correlated, parameters indexing temporal reliability and information are highly correlated, and firing rate during the entire vocalizations vs. firing rate during the PSTH peaks are highly correlated.
Results
The results presented here are based on multi-unit recordings responsive to pure tones (as attested by significant peaks in the STRFs) and responsive to at least one vocalization (evoked firing rate > 1.5 spikes s−1 or CorrCoef > 0.15) before and after application of pharmacological agents. NA application was tested on five animals providing 120 stable recordings, application of phenylephrine was tested on three animals providing 64 recordings, clonidine was tested on three animals providing 80 recordings and isoproterenol was tested on three animals providing 73 recordings. These pharmacological agents had different effects on spontaneous activity: phenylephrine significantly reduced the spontaneous firing rate (P < 0.005) whereas clonidine and isoproterenol increased it (P < 0.005 and P < 0.05, respectively). On average, NA had no significant effect on spontaneous activity.
NA and α1 agonist reduced cortical STRFs
During NA application, the most common effect was a reduction of the STRFs in the spectral and temporal domain. As illustrated in Fig. 2A, this reduction affected (i) the bandwidth and duration of the STRFs and (ii) the response strength. It was reversible 40 min after application. Similarly, the application of the α1 adrenergic receptor agonist, phenylephrine, shrank the STRF (Fig.2B). In contrast, the activation of either α2 or β adrenergic receptors led to the opposite effect. Application of clonidine and isoproterenol increased the STRF bandwidth, duration and response strength, an effect that was usually maintained 40 min after application (Fig. 2C and D).
Figure 2. Examples of STRFs obtained before, and 20 and 40 min after application of NA (A), phenylephrine (B), clonidine (C) and isoproterenol (D).
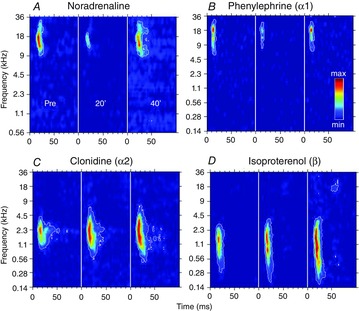
The colour code is proportional to the number of action potentials emitted during tone presentations. The same colour scale is used for the three time points and is normalized to the maximal evoked activity over the three time points. The area inside the white outline corresponds to a firing rate superior to the average baseline activity plus six times its standard deviation. Pre, before drug application; 20’, 20 min after drug application; 40’, 40 min after drug application. These examples show that NA and the α1 agonist phenylephrine induced a transient shrinkage of the STRFs (A, B) while α2 and β agonists induced an expansion of the STRFs.
On average, NA application induced a small, but significant, bandwidth reduction 20 min after application (Fig. 3A; paired t test, P < 0.001), which returned to control level 40 min later (paired t test, P = 0.5). STRF duration (Fig. 3B) was significantly reduced 20 min after application (P < 0.01) and returned to control level 40 min later (paired t test, P = 0.1). On average, the response strength (Fig. 3C) was also transiently reduced (20 min: P < 0.05; 40 min: P = 0.07). Phenylephrine triggered effects that were the closest to those triggered by NA: a significant reduction of STRF bandwidth (20 min: P < 0.001; 40 min: P < 0.05; Fig. 3A), and a transient reduction of STRF duration (20 min: P < 0.05; 40 min: P = 0.5; Fig. 3B) and of the response strength (20 min: P < 0.05; 40 min: P = 0.07; Fig. 3C). By contrast, the α2 and β agonists produced a significant increase in all STRF parameters, maintained up to 40 min after application. Clonidine increased the bandwidth (both at 20 and at 40 min: P < 0.001; Fig. 3A), the duration (both at 20 and at 40 min: P < 0.001; Fig. 3B) and the response strength (both at 20 and at 40 min: P < 0.001; Fig. 3C). Isoproterenol increased the bandwidth (both at 20 and at 40 min: P < 0.001), the duration (20 min: P < 0.01; 40 min: P < 0.001) and the response strength (both at 20 and at 40 min: P < 0.001). Thus, the effects detected here after topical application replicate the pharmacological profile described in ACx using iontophoretic application on single unit recordings (Manunta & Edeline, 1997, 2004).
Figure 3. Group data showing the effects of NA and noradrenergic receptor agonists on three STRFs parameters.
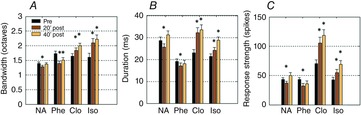
The bandwidth (A) corresponds to the sum in octaves of the frequency range of all the significant peaks in one STRF. The duration (B) is the difference (in ms) between the first and last spike times within the significant area of the STRF. The response strength (C) is the number of spikes in the significant area of the STRF. Values are mean ± SE. *Significant difference from control conditions (P < 0.05, paired t test). Note that both NA and phenylephrine decreased the STRF parameters whereas clonidine and isoproterenol increased the STRF parameters.
NA agonists, but not NA, had homogeneous effects on responses to vocalizations
The responses to vocalizations were analysed for recordings exhibiting an evoked firing rate of at least 1.5 spikes s−1 or a CorrCoef of at least 0.15 (see Methods). Figure 4 displays the raster plots of cortical responses before and after application of the three NA agonists. Phenylephrine decreased the evoked firing rate and disrupted some of the peaks of activity in the response (Fig. 4A). Clonidine increased the evoked firing rate and enlarged the response peaks (Fig. 4B). Similarly to clonidine, isoproterenol increased the evoked firing rate, but disrupted some of the response peaks (Fig. 4C). Group data confirmed these observations: phenylephrine application transiently decreased the evoked firing rate, whereas clonidine and isoproterenol application induced significant increases in evoked firing rate that lasted at least 40 min (Fig. 4D). These effects were coherent with those obtained on STRFs after application of these three pharmacological agents. Despite the fact that these agonists had opposite effects on the firing rate, they all slightly decreased the temporal reliability of the responses (quantified with CorrCoef, Fig. 4E). This decrease lasted at least 40 min, even in the case of phenylephrine application.
Figure 4. Modulation of vocalization-evoked responses by three NA agonists.
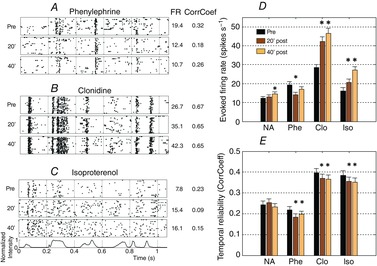
A–C, individual examples of rasters (20 trials; each dot represent the occurrence of an action potential) in response to the same vocalization before, and 20 and 40 min after application of phenylephrine (A), clonidine (B) and isoproterenol (C). The vocalization intensity as a function of time is displayed below the rasters. Two parameters were extracted from the responses to vocalizations: the evoked firing rate (noted FR) and the CorrCoef, values of which are presented to the right of each raster. D and E, the mean value (± SE) of the evoked firing rate (D) and the temporal reliability (E) are plotted as bar graphs. *Significant difference from control conditions (P < 0.05, paired t test). Phenylephrine decreased the firing rate whereas clonidine and isoproterenol increased it. The three NA agonists decreased the spike-timing reliability quantified by the CorrCoef index.
Surprisingly with regard to the strong effects induced by the three noradrenergic agonists, NA application induced relatively weak effects on responses to vocalizations. As shown in Fig. 4D and E, on average NA application induced almost no effect on the firing rate in response to vocalizations (a slight increase 40 min after application) and had no significant effect on the temporal reliability (as quantified with CorrCoef). The evoked firing rate and CorrCoef are two measures extracted from the PSTH in response to vocalizations. These PSTHs are usually organized in peaks of responses separated by periods with lower activity. To clarify the NA effect, a custom peak detector was used to isolate sharp increases in firing rate during the responses to vocalizations (see Methods). This allowed us to quantify a ‘real’ evoked activity, independently of periods reflecting spontaneous activity. We quantified the duration and the firing rate during the peaks isolated with this method. Based upon these peaks we distinguished two categories of responses: sustained responses and temporally organized responses. Temporally organized responses represented 82% of our recordings (sustained responses 18%). Occasionally, NA application changed the type of response, from sustained to temporally organized (11% of the cases) or the reverse (9% of the cases). For recordings exhibiting temporally organized responses before and after application, NA induced an increase in activity in 45% of the peaks and a decrease in 54% of the peaks. On average, the firing rate during the peaks and peak duration were not significantly changed (P = 0.9 and P = 0.7, respectively), indicating that the NA-induced increases and decreases of activity within the peaks had comparable amplitudes. This analysis showed that the lack of NA effect did not result from subtle, undetectable, effects in the PSTHs: NA induced both increases and decreases of evoked activity even when considering only the phasic peaks in the PSTHs.
To go further, we carefully examined the distribution of the NA effects on (i) the evoked firing rate and (ii) the temporal reliability in relation to changes in the STRF measures. Figure 5A1 shows that the change in evoked responses to vocalizations was strongly correlated with the change in STRF response strength (correlation coefficient: 0.59, P < 0.01). On this scattergram, the first and third quartiles of the STRF evoked responses are represented by the colour of the dot centre (red and green, respectively), and the quartiles of the responses to vocalizations are represented by the colour of the dot contours. The fact that, in many cases (>39%), the colour of dot centres matches the colour of the dot contours indicates that the largest changes were in the same directions in most of the cases. Figure 5A2 illustrates a strong decrease in evoked response to vocalizations associated with a strong shrinkage of the STRF; Fig. 5A3 illustrates an increase in evoked response to vocalizations associated with an increase in evoked response in the STRF.
Figure 5. Relationship between NA effects on STRFs and on responses to vocalizations.
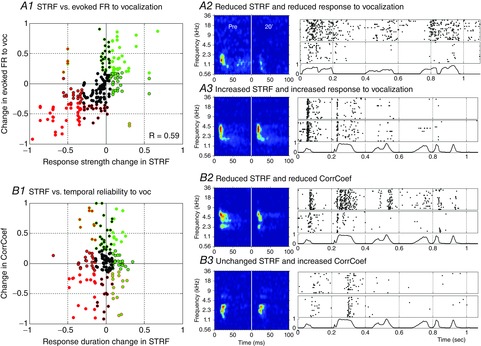
Scattergrams displaying the normalized NA effect on the STRF parameters vs. on parameters obtained from response to vocalizations. A1, the response strength during the STRF is plotted against the evoked firing rate in response to vocalizations. B1, the duration of the STRF is plotted against CorrCoef (which quantifies the spike timing reliability). In both cases, normalization between values in control conditions and 20 min after NA application is used: (value 20 min – control value)/(value 20 min + control value). The colour code corresponds to values inferior to the first quartile (red) or superior to the third quartile (green). The colour of the dot centre refers to the parameters extracted from the STRFs; the colour of the contour refers to the parameters extracted from responses to vocalizations. A2–3 and B2–3, examples of STRFs and rasters of response to vocalizations for the same recording site in control condition (Pre) and 20 min after application. The normalized vocalization intensity as a function of time is presented below the rasters. A2, example of a decrease in response strength and in evoked firing rate. A3, example of an increase in response strength and in response to vocalization. B2, example of a decrease in STRF duration and in CorrCoef. B3, example of an unchanged STRF duration and an increase in CorrCoef. The scattergrams and the individual examples show clearly that the NA effects on firing rate during STRFs and responses to vocalizations are strongly correlated (R = 0.59, P < 0.05), whereas the NA effects on STRF duration are not correlated with the temporal reliability of responses to vocalizations (R = 0.03, P = 0.69).
A similar analysis was performed in the time domain: we compared the NA effects on STRF duration and on temporal reliability during presentation of vocalizations. This revealed that there was no correlation between the changes in temporal reliability and the changes in STRF duration (Fig. 5B1, correlation coefficient: 0.03; P = 0.7). The examples in Fig. 5B2 and B3 illustrate that NA can decrease STRF duration while it increases or decreases CorrCoef. This clearly indicates that, in terms of temporal reliability of the neuronal responses, the NA effects differ between STRFs and responses to vocalizations, despite the fact that these effects are similar in terms of firing rate.
Noradrenergic and agonists effects on neuronal discriminability between vocalizations
As important variations were observed in the responses to vocalizations in terms of both firing rate and spike-timing reliability (Fig. 5), we investigated whether the information carried by neuronal activity in responses to vocalizations was affected by NA. MI quantifies how well the responses to vocalizations can be discriminated based on the overall firing rate (MIFR) or on temporal spike patterns (MI8ms, see Methods). On average, MIFR and MI8ms were not significantly changed after application of NA or of any of the agonists used here (data not shown, lowest P value = 0.15). As NA affects the evoked activity of cortical neurons in both directions (Fig. 5A1) we split our data into two populations based on the NA effect on the responses to vocalizations: we analysed independently the recordings showing the strongest increases (green contour dots in Fig. 5A1, labelled ‘Inc’ in Fig. 6) and the strongest decreases (red contour dots in Fig. 5A1; labelled ‘Dec’ in Fig. 6). MI8ms and MIFR were quantified on these two populations of recordings before and after NA, phenylephrine, clonidine and isoproterenol application (Fig. 6). For the recordings exhibiting the strongest increased responses (‘Inc’ in Fig. 6), the MI based on temporal patterns (MI8ms) was significantly increased after NA application (P < 0.005), an effect clearly visible on the scatterplot of the MI8ms values (Fig. 6E2). The noradrenergic agonists had no effect on this population of recordings, except phenylephrine, which decreased MIFR. For the recordings exhibiting the strongest decreased responses (‘Dec’ in Fig. 6), the MI was decreased in four out of eight conditions (after phenylephrine, clonidine or NA application) when computed either based on the firing rate or based on temporal patterns. These results demonstrate that the changes in MI did not systematically follow the changes induced by the different pharmacological agents on the evoked firing rate: an increase in firing rate did not necessarily lead to an increase in MI and vice versa. In addition, these results show that, on a particular cell population, NA increased the neuronal discriminative ability, an effect that was not replicated by any of the NA agonists.
Figure 6. Effects of NA and its agonists on mutual information for the largest increases and the largest decreases in response to vocalization.
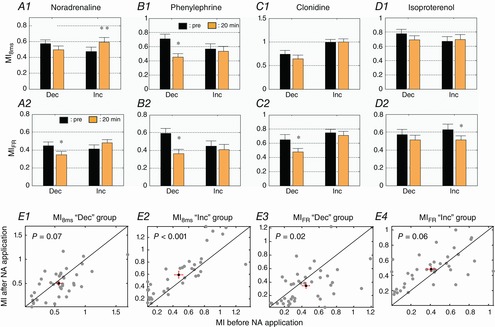
Based upon the first and last quartile of the distribution of the evoked firing rate variations (displayed in Fig. 4A1), two populations were identified: the strongest increases (Inc) and the strongest decreases (Dec). The mutual information (mean ± SE) based on firing rate (MIFR) and on temporal patterns (MI8ms) was quantified after NA (A1, A2), phenylephrine (B1, B2), clonidine (C1, C2) and isoproterenol (D1, D2) application. Scatterplots of the MI8ms (E1–2) and MIFR (E3–4) values before and after NA application are displayed for the ‘Inc’ and ‘Dec’ groups. Each grey dot represents a pair of MI values. In each scattergram, the black dot represents the mean values of MI before and after NA application; red bars represent the standard error. *Significant difference from control conditions (P < 0.05, paired t test). **Significant difference from control conditions (P < 0.005, paired t test). Cortical sites with increased evoked firing rate after NA application display increased discrimination abilities based on temporal patterns (MI8ms), an effect not replicated by the noradrenergic agonists.
Characteristics of recordings exhibiting increased discriminative abilities
In the two previous paragraphs, we showed that on a subpopulation of 38 recordings exhibiting the largest increased responses during NA application, the ability to discriminate communication sounds is significantly increased. But are there special characteristics of these recordings compared with the rest of the population? Figure 7A illustrate a typical recording of the ‘Inc’ population through the NA effect on its STRF (A1), raster of response to vocalization (A2) and classification performance based on temporal patterns (A3) and firing rate (A4). For this recording, NA increased STRF duration and response strength without modifying its frequency selectivity; the response to vocalization was also increased as well as MIFR and MI8ms. Figure 7B displays the main features of this subpopulation of recordings (‘Inc’) along with those of the rest of the population (‘All-Inc’), before and after NA application. Besides showing a twofold increase of evoked response to vocalizations, the ‘Inc’ population also displayed large increases in firing rate during the phasic peaks of responses to vocalizations (Peak activity, P < 0.001) and during the STRF tests (STRF resp str, P < 0.001). In contrast, for the ‘‘All-Inc’ population, the firing rate was significantly decreased during both the phasic peak of responses and the STRFs (both P < 0.001). The increase in MI8ms found for ‘Inc’ population probably relied on the fact that the CorrCoeff index was significantly enhanced for this population (P = 0.03), whereas it was unchanged for the ‘All-Inc’ population (P = 0.66). The recordings corresponding to the ‘Inc’ population had initially smaller STRFs both in the spectral and in the temporal domains: both tuning bandwidth (P = 0.03) and response duration (P = 0.03) were smaller than for the rest of the population. Note that despite these smaller STRFs, the firing rate within the response area of the STRFs was similar between these recordings and the rest of the population (P = 0.94). This ‘Inc’ population did not show a different range of BF, or a different response latency at the BF compared with the rest of the population (data not shown), suggesting that these two populations are intermingled in the primary ACx.
Figure 7. Spider chart of the NA effect on the largest increase (‘Inc’) vs. the rest of the recordings (‘All-Inc’).
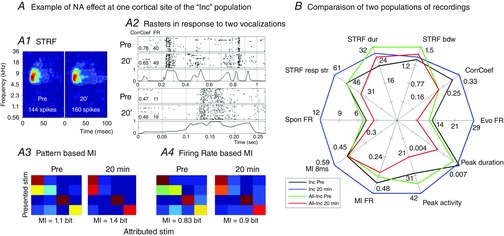
A, NA effect on one recording site belonging to the ‘Inc’ population. STRF duration and response strength are increased, but the bandwidth is not modified (A1). MI8ms and MIFR are increased, as illustrated by the confusion matrices (A3–4). Finally, the evoked activity is increased, but the temporal reliability is not affected, as visible in the raster plots of responses to two vocalizations (A2). B, spider chart displaying the mean value of each parameter for the cortical sites with the most increased activity vs. the rest of the population. These values are presented before (black line: Inc Pre; green line: All-Inc Pre) and 20 min after NA application (blue line: Inc 20 min; red line, All-Inc 20 min). Comparing the black and blue lines shows that NA significantly increased the parameters of the Inc group, except STRF bandwidth, MIFR and peak duration. In comparison, for the All-Inc group (green and red lines) NA significantly reduced the parameters, except CorrCoef and both MIFR and MI8ms. Before NA application, these two populations displayed similar values except for STRF bandwidth and duration, which were lower in the ‘Inc’ group than in the ‘All-Inc’ group (compare the values of the black and green lines for these parameters).
Weak correlation between STRF changes and response changes during vocalizations
NA application can increase or decrease evoked activity during tones and vocalizations. But is it possible to draw a coherent picture between parameters extracted from responses to artificial and natural stimuli? To analyse the potential co-variations between the NA effect on parameters extracted from the STRFs and from the responses to vocalizations, a PCA was performed (Fig. 8A). Only the three first components explain more than 10% of the data variance (31, 21 and 14%, respectively), and are therefore used as a new coordinate system to represent the data (Kaiser's criterion). The figure shows a representation of the observations (i.e. the normalized before–after differences) and the correlation of each parameter with the PCs. The representation of the observations (red dots) does not show any particular organization among our data. This result is not surprising as (i) the recording conditions are similar from one experiment to the next and (ii) multi-unit recording prevents the sampling of distinct neuronal populations. Parameters extracted from neuronal responses are represented as vectors starting at the origin of the coordinate system, the scalar product between two vectors being equal to the covariance between the parameters they represent. The effects on the parameters extracted from the STRFs (bandwidth, duration, response strength; STRFbdw, STRFdur, STRFresp str, respectively, on Fig. 8A) clearly form a cluster together with the NA effect on spontaneous activity (orange vectors). A second cluster involves the NA effect on parameters extracted from the reliability of neuronal responses to vocalizations (CorrCoef, MI8ms and MIFR, green vectors). These first two clusters are almost orthogonal, indicating very low values of covariance between these two groups of parameters. Finally, a third cluster, represented in blue, includes the NA effects on parameters directly dependent on the firing rate during the vocalizations (peak duration, peak firing rate and global response noted Evo FR in Fig. 8). The parameters comprising this third cluster are not systematically orthogonal to those from the two other clusters, but they present higher values of scalar products between themselves and much lower values with parameters from the other clusters. Note that the parameter correlations with the first PC are all positive, but the three-dimensional representation can be visually misleading. To extend this result, we analysed the correlations between the NA effects on these 10 parameters (Fig. 8B). In agreement with the conclusion of the PCA, the correlation coefficients between the NA-induced variations of the three parameters extracted from the STRFs are high (≥0.46); the correlations between these parameters and the variations of spontaneous firing rate are also high (>0.39). Correlations between NA effects on parameters extracted from STRFs and from responses to vocalizations are weaker (≤0.15). The NA effects on parameters from the blue PCA cluster (peak activity, peak duration and evoked firing rate) are not systematically highly correlated, but are >0.26. The NA effects on parameters from the green PCA cluster (CorrCoef, MI8ms and MIFR) display similar levels of correlations (>0.26). Interestingly, the variations in evoked firing rate display high levels of correlation with several other parameters, including STRF response strength and duration, peak duration and activity, and both MI8ms and MIFR. This analysis confirmed that the effects of NA application, although going in both directions, are coherent for groups of parameters, with a clear separation between the effect on parameters extracted from responses to pure tones and from vocalizations.
Discussion
In this study, we have quantified the effects of NA on the responses of ACx neurons to presentation of pure tones and communication sounds. When tested with pure tones, NA mainly triggered transient reductions of cortical STRFs both in the spectral and in the temporal domains. This effect was replicated by the α1 agonist phenylephrine, whereas α2 and β agonists produced an expansion of STRFs. When cortical neurons were tested with natural sounds, NA application induced either an increase or a decrease of the responses, its effects being related to those detected when testing the STRFs. Phenylephrine decreased the responses to communication sounds whereas clonidine (α2 agonist) and isoproterenol (β agonist) increased them. However, for a population of neurons displaying the largest increased responses during NA application, the discrimination between communication sounds (quantified by MI) was enhanced; this effect was not observed for any of the tested agonists. This population of neurons corresponded to recordings which initially displayed smaller receptive fields in both the spectral and the temporal domain. Although puzzling at a first glance, these effects are quite fundamental for understanding the functional role of NA in modulating the cortical processing of natural stimuli.
Effects on artificial stimuli and pharmacology of these effects
Using artificial stimuli, conflicting results have been reported with NA application in sensory cortices (reviewed by Edeline, 2012). Initial studies have pointed out facilitations of evoked responses (Waterhouse & Woodward, 1980; Waterhouse et al. 1981) whereas subsequent studies reported a dominant depressing effect (Armstrong-James & Fox, 1983; Videen et al. 1984). Even in the primary visual cortex, controversial results have been published: some studies reported an absolute increase in evoked activity (Kasamatsu & Heggelund, 1982; Waterhouse et al. 1988, 1990) while other studies failed to detect such an effect either with NA iontophoretic application or locus coeruleus stimulation (Videen et al. 1984; Sato et al. 1989; Ego-Stengel et al. 2002). In the visual cortex, these decreases promoted an improved velocity and direction selectivity, without modifying the orientation selectivity (McLean & Waterhouse, 1994; Ego-Stengel et al. 2002). In the ACx, the decreased responses induced by iontophoretic applications of NA increased the frequency selectivity and the acoustic threshold, as if the whole tuning curve was moved upward. This reduction of evoked response was mediated by α1 receptors, whereas β receptor activation increased cortical evoked activity (Manunta & Edeline, 1997, 2004). Similar results were obtained here using a different mode of NA application and multi-unit instead of single-unit recordings: α1 receptor activation or NA application reduced the STRF and mechanically increased the frequency selectivity of the recorded neurons, whereas α2 or β receptor activation extended the STRF, thus decreasing the response selectivity. In vitro studies have demonstrated that activation of cortical α1 receptors reduced the amplitude of excitatory post-synaptic currents recorded in layer II/III pyramidal cells (Dinh et al. 2009) and that activation of α2 or β also increased the amplitude of inhibitory post-synaptic currents evoked by stimulation of layer II/III (Salgado et al. 2011b). These in vitro results indicate that different mechanisms, involving potentially all types of adrenergic receptors, may contribute to the decreased evoked responses observed here in ACx. In a few studies performed in the somatosensory (Bassant et al. 1990) and auditory cortices (Foote et al. 1975; Manunta & Edeline, 1999) the effects of NA were tested in awake animals. In these studies, NA mainly reduced the evoked response to pure tones (Manunta & Edeline, 1999), vocalizations (Foote et al. 1975) and glutamate-evoked responses (Bassant et al. 1990). Furthermore, it was shown that the depressing effect of NA was present in different states of vigilance (awake, slow wave sleep). In fact, the proportion of neurons with significant effects (Manunta & Edeline, 1999) differed between states (wakefulness: 43%; slow wave sleep: 27%; anaesthesia: 62%) but the percentage of cells with decreased responses after NA application was similar in the three conditions (68% of cells in wakefulness, 70% in slow wave sleep, 80% under urethane anaesthesia). Therefore, although studies are needed to confirm this hypothesis, it seems reasonable to assume that the NA effects are independent of the vigilance state.
Noradrenergic effects on responses to vocalizations
On average, NA application had no significant effect on the firing rate in response to vocalizations, which is surprising given that here and in several studies performed in ACx NA produced a dominant depressive effect on cortical responses (Foote et al. 1975; Manunta & Edeline, 1997, 1998, 1999, 2004). In fact, when tested with pure tones, the increased reactivity induced by α2 and β receptors is masked by the prominent α1 depressive effect (Manunta and Edeline, 1997, 2004). So the question is, why is this not also the case with the responses to natural stimuli, i.e. why did the decreases triggered by the α1 receptors not surpass the increases triggered by the α2 and β receptors (Fig. 4D)? An explanation for this discrepancy may relate to the difference between the duration of the stimuli: the pure tones used to quantify the STRF lasted 50 ms (the half-peak amplitude is 15 ms) whereas the vocalizations lasted over hundreds of milliseconds, and in an in vitro study, it was shown that the duration of the stimulation changed the direction of the NA effect (Salgado et al. 2011a). In addition, testing the effects of a pharmacological agent during STRF determination is essentially testing its effects on onset responses. In contrast, as illustrated in Figs 4 and 5, during vocalizations, short periods of intense activation are separated by long periods with low, or no, firing rate. Thus, one can suspect that the firing rate changes observed over long temporal windows reflect changes in evoked and in spontaneous activity. Examination of the group data (Figs 3 and 4) indicates that the magnitude of increases and decreases induced by the NA agonists while testing the STRFs and the vocalizations were different, and favour the increases during the vocalization tests. For example, phenylephrine decreased the firing rate by 35% in STRF and by only 27% in vocalizations. By contrast, clonidine increased the activity by 39% in STRF and by 48% in vocalizations; isoproterenol increased the activity by 22% in STRF and by 29% in vocalizations. Quantifying spontaneous activity indeed showed that NA tended to increase it, potentially because the increase triggered by α2 and β receptors is quantitatively larger that the decrease produced by α1 receptor activation. This illustrates that the raw firing rate, although relevant for coding the frequency and intensity of pure tones (Micheyl et al. 2013), is not an appropriate metric to quantify the operations performed by cortical neurons on natural stimuli.
The temporal organization of responses to vocalizations was also quantified during our experiments. In a few studies it has been pointed out that noradrenergic activation can alter temporal patterns. For example, in the somatosensory cortex, locus coeruleus stimulations reduced the duration and increased the temporal precision of the response to simple tactile stimuli (Lecas, 2001, 2004). Similar observations have been made in the auditory system: in the cochlear nucleus, it was reported that the latency and the jitter latency of pure tone responses were decreased during NA iontophoretic application (Kössl & Vater, 1989), an effect that was also occasionally reported for some ACx neurons (figure 3 in Edeline, 2012). In the present experiment, there was no global change in the trial-to-trial reliability of the responses to vocalizations during NA application, despite the fact that large effects on the CorrCoeff index (increases or decreases) can be observed for some recordings (see scattergram in Fig. 5B1). One can argue that recording the activity of small groups of neurons, rather than of single neurons, may lead to difficulties in detecting changes in trial-to-trial reliability. However, we clearly detected small, but significant, changes produced by the three NA agonists tested (see Fig. 4E) despite the fact that they changed the firing rate in opposite directions. The fact that the activation of α2/β receptors increased spontaneous firing rate, i.e. the firing rate that was present between the phasic peaks of responses evoked by the vocalizations, might have masked the potential increases in spike timing induced by NA during the phasic responses.
The most interesting result of our study is probably that, despite its lack of a significant impact on the average firing rate, NA enhances the discriminative abilities for a population of cortical cells exhibiting the largest increase in evoked responses. Many hypotheses have been proposed to explain why NA can either increase or decrease evoked responses. The most widely discussed is that by Armstrong-James and Fox (1983) who pointed out that, in the somatosensory cortex, the direction of the noradrenergic effects was a function of both the cortical layer and of the NA concentration. In the ACx, we did not detect NA-induced increases at particular cortical depths (Manunta & Edeline, 1997, 1999) and here our recordings were all obtained between 500 and 1000 μm below pia. Also, as in previous iontophoretic studies, we did not observe biphasic effects (an initial increase followed by a decrease), leaving unresolved the reason why a population of cells showed increased evoked responses. However, we noted that in our data, these recordings initially displayed smaller STRFs than the rest of the population, potentially indicating that they received stronger intracortical inhibitory inputs. If true, it is interesting to point out that for these cells, NA did not alter STRF bandwidth but rather increased STRF duration as if NA was reducing feed-forward inhibition without modifying lateral inhibition.
Even if the increase in discriminative abilities (indexed by MI) was observed for a limited population, it is of importance because it points out that a neuromodulator that mostly depresses the responses to artificial sounds can facilitate the discrimination of natural stimuli for a subpopulation of cortical neurons. More generally, this suggests that inferring the modulation exerted by neuromodulatory systems on the basis of results obtained with artificial stimuli might be misleading and deceptive.
NA effects on ACx responses to different stimuli
Obviously, analysing the consequences of NA activation on the responses to natural stimuli is a complex task. In fact, the NA effects were strongly correlated between parameters extracted from the same type of stimuli (pure tones or vocalizations) and weakly correlated between parameters extracted from responses to different stimuli. Such results could be partially expected, as ACx responses are highly dependent of the stimulus used to probe them. For example, a study by Machens et al. (2004) has shown that in the ACx only 11% of the neuronal response to an unknown stimulus can be explained by the linear model underlying the STRF computation. Recent studies have demonstrated that even with artificial stimuli, the non-linearity of ACx responses did not allow good prediction of responses to an unknown stimulus (Ahrens et al. 2008; Bitterman et al. 2008). In fact, most of the recent studies agree that the cortical STRFs depend on the stimuli used to compute them, for artificial stimuli (Ahrens et al. 2008; Gourévitch & Eggermont, 2008) and natural stimuli (Laudanski et al. 2012). Our study – the first to compare the influence of neuromodulators on parameters extracted from responses to artificial and natural stimuli – indicates that the NA effect on responses to vocalizations cannot be predicted based upon effects on pure tones. In fact, the changes in parameters quantifying (i) the responses to pure tones (STRF) and (ii) the temporal organization of responses to vocalizations form two distinct clusters, illustrating the dual effect of NA in the ACx. Altogether, these results suggest that the networks activated by pure tones and by vocalizations differ, and more importantly that these networks can be differently modulated by an increase in NA concentration. It has been shown that acetylcholine differentially affects thalamo-cortical and cortico-cortical synapses (Hsieh et al. 2000; reviewed by Metherate & Hsieh, 2003, 2004) and a pilot study suggests that this might also be the case with NA (S. J. Cruikshank, J.-M. Edeline & R. Metherate, unpublished data). If we envisage that the responses to pure tones and to communication sounds preferentially involve thalamo-cortical and cortico-cortical networks, respectively, NA might be able to differentially affect the responses to different classes of stimuli. Further studies are obviously needed to support this hypothesis.
Acknowledgments
We thank C. Huetz and V. Ego-Stengel for insightful comments and careful reading of the manuscript. Special thanks to Nathalie Samson, Fabien Lerhicel, Céline Dubois, Pascale Leblanc-Veyrac for taking care of the guinea-pig colony.
Glossary
Abbreviations
- ABR
auditory brainstem response
- ACx
auditory cortex
- BF
best frequency
- CorrCoef
temporal reliability coefficient
- IPSP
inhibitory post-synaptic potential
- MI
mutual information
- MI8ms
mutual information based on temporal patterns
- MIFR
mutual information based on firing rate
- MUA
multi-unit activity
- NA
noradrenaline
- PC
principal component
- PCA
principal component analysis
- PSTH
post-stimulus time histogram
- STRF
spectro-temporal receptive field
Additional Information
Competing interests
None declared
Author contributions
Q.G. and J.-M. E. designed the experiments. Q.G. performed the experiments and analysed the data. Q.G. and J.-M.E. wrote the manuscript.
Funding
This work was supported by a grant from the National Research Agency (ANR2011 grant HearFin) to J.-M.E. Q.G. was supported by a fellowship from the Ministère de l'Education Nationale et de la Recherche (MENR).
References
- Ahrens MB, Linden JF. Sahani M. Nonlinearities and contextual influences in auditory cortical responses modeled with multilinear spectrotemporal methods. J Neurosci. 2008;28:1929–1942. doi: 10.1523/JNEUROSCI.3377-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M. Fox K. Effects of ionophoresed noradrenaline on the spontaneous activity of neurones in rat primary somatosensory cortex. J Physiol. 1983;335:427–447. doi: 10.1113/jphysiol.1983.sp014542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J. Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Bassant MH, Ennouri K, Lamour Y. Effects of iontophoretically applied monoamines on somatosensory cortical neurons of unanesthetized rats. Neuroscience. 1990;39:431–439. doi: 10.1016/0306-4522(90)90279-d. [DOI] [PubMed] [Google Scholar]
- Bitterman Y, Mukamel R, Malach R, Fried I. Nelken I. Ultra-fine frequency tuning revealed in single neurons of human auditory cortex. Nature. 2008;451:197–201. doi: 10.1038/nature06476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S. Sara SJ. Reward expectation, orientation of attention and locus coeruleus–medial frontal cortex interplay during learning. Eur J Neurosci. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Caesar K, Offenhauser N. Lauritzen M. Gamma-aminobutyric acid modulates local brain oxygen consumption and blood flow in rat cerebellar cortex. J Cereb Blood Flow Metab. 2008;28:906–915. doi: 10.1038/sj.jcbfm.9600581. [DOI] [PubMed] [Google Scholar]
- Caesar K, Thomsen K. Lauritzen M. Dissociation of spikes, synaptic activity, and activity-dependent increments in rat cerebellar blood flow by tonic synaptic inhibition. Proc Natl Acad Sci USA. 2003;100:16000–16005. doi: 10.1073/pnas.2635195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cheveigné A, Edeline J-M, Gaucher Q. Gourévitch B. Component analysis reveals sharp tuning of the local field potential in the guinea pig auditory cortex. J Neurophysiol. 2013;109:261–272. doi: 10.1152/jn.00040.2012. [DOI] [PubMed] [Google Scholar]
- Dinh L, Nguyen T, Salgado H. Atzori M. Noradrenaline homogeneously inhibits α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate- (AMPAR-) mediated currents in all layers of the temporal cortex of the rat. Neurochem Res. 2009;34:1896–1906. doi: 10.1007/s11064-009-9966-z. [DOI] [PubMed] [Google Scholar]
- Edeline J-M. Beyond traditional approaches to understanding the functional role of neuromodulators in sensory cortices. Front Behav Neurosci. 2012;6:45. doi: 10.3389/fnbeh.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Dutrieux G, Manunta Y. Hennevin E. Diversity of receptive field changes in auditory cortex during natural sleep. Eur J Neurosci. 2001;14:1865–1880. doi: 10.1046/j.0953-816x.2001.01821.x. [DOI] [PubMed] [Google Scholar]
- Edeline JM. Weinberger NM. Receptive field plasticity in the auditory cortex during frequency discrimination training: selective retuning independent of task difficulty. Behav Neurosci. 1993;107:82–103. doi: 10.1037//0735-7044.107.1.82. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Bringuier V. Shulz DE. Noradrenergic modulation of functional selectivity in the cat visual cortex: an in vivo extracellular and intracellular study. Neuroscience. 2002;111:275–289. doi: 10.1016/s0306-4522(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Foote SL, Freedman R. Oliver AP. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975;86:229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- Gaucher Q, Edeline J-M. Gourévitch B. How different are the local field potentials and spiking activities? Insights from multi-electrodes arrays. J Physiol Paris. 2012;106:93–103. doi: 10.1016/j.jphysparis.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Gaucher Q, Huetz C, Gourévitch B. Edeline J-M. Cortical inhibition reduces information redundancy at presentation of communication sounds in the primary auditory cortex. J Neurosci. 2013a;33:10713–10728. doi: 10.1523/JNEUROSCI.0079-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher Q, Huetz C, Gourévitch B, Laudanski J, Occelli F. Edeline J-M. How do auditory cortex neurons represent communication sounds? Hear Res. 2013b;305:102–112. doi: 10.1016/j.heares.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Gourévitch B, Doisy T, Avillac M. Edeline JM. Follow-up of latency and threshold shifts of auditory brainstem responses after single and interrupted acoustic trauma in guinea pig. Brain Res. 2009;1304:66–79. doi: 10.1016/j.brainres.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Gourévitch B. Edeline J-M. Age-related changes in the guinea pig auditory cortex: relationship with brainstem changes and comparison with tone-induced hearing loss. Eur J Neurosci. 2011;34:1953–1965. doi: 10.1111/j.1460-9568.2011.07905.x. [DOI] [PubMed] [Google Scholar]
- Gourévitch B. Eggermont JJ. Spectro-temporal sound density-dependent long-term adaptation in cat primary auditory cortex. Eur J Neurosci. 2008;27:3310–3321. doi: 10.1111/j.1460-9568.2008.06265.x. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ. Metherate R. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Res. 2000;880:51–64. doi: 10.1016/s0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- Huetz C, Del Negro C, Lebas N, Tarroux P. Edeline JM. Contribution of spike timing to the information transmitted by HVC neurons. Eur J Neurosci. 2006;24:1091–1108. doi: 10.1111/j.1460-9568.2006.04967.x. [DOI] [PubMed] [Google Scholar]
- Huetz C, Philibert B. Edeline JM. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J Neurosci. 2009;29:334–350. doi: 10.1523/JNEUROSCI.3269-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM. Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Jones MS. Barth DS. Effects of bicuculline methiodide on fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2002;88:1016–1025. doi: 10.1152/jn.2002.88.2.1016. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T. Heggelund P. Single cell responses in cat visual cortex to visual stimulation during iontophoresis of noradrenaline. Exp Brain Res. 1982;45:317–327. doi: 10.1007/BF01208591. [DOI] [PubMed] [Google Scholar]
- Kössl M. Vater M. Noradrenaline enhances temporal auditory contrast and neuronal timing precision in the cochlear nucleus of the mustached bat. J Neurosci. 1989;9:4169–4178. doi: 10.1523/JNEUROSCI.09-12-04169.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanski J, Edeline J-M. Huetz C. Differences between spectro-temporal receptive fields derived from artificial and natural stimuli in the auditory cortex. PloS One. 2012;7:e50539. doi: 10.1371/journal.pone.0050539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecas JC. Noradrenergic modulation of tactile responses in rat cortex. Current source-density and unit analyses. C R Acad Sci III. 2001;324:33–44. doi: 10.1016/s0764-4469(00)01276-2. [DOI] [PubMed] [Google Scholar]
- Lecas J-C. Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur J Neurosci. 2004;19:2519–2530. doi: 10.1111/j.0953-816X.2004.03341.x. [DOI] [PubMed] [Google Scholar]
- Liu RC. Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machens CK, Wehr MS. Zador AM. Linearity of cortical receptive fields measured with natural sounds. J Neurosci. 2004;24:1089–1100. doi: 10.1523/JNEUROSCI.4445-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manunta Y. Edeline JM. Effects of noradrenaline on frequency tuning of rat auditory cortex neurons. Eur J Neurosci. 1997;9:833–847. doi: 10.1111/j.1460-9568.1997.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Manunta Y. Edeline JM. Effects of noradrenaline on rate-level function of auditory cortex neurons: is there a “gating” effect of noradrenaline? Exp Brain Res. 1998;118:361–372. doi: 10.1007/s002210050290. [DOI] [PubMed] [Google Scholar]
- Manunta Y. Edeline JM. Effects of noradrenaline on frequency tuning of auditory cortex neurons during wakefulness and slow-wave sleep. Eur J Neurosci. 1999;11:2134–2150. doi: 10.1046/j.1460-9568.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- Manunta Y. Edeline J-M. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J Neurophysiol. 2004;92:1445–1463. doi: 10.1152/jn.00079.2004. [DOI] [PubMed] [Google Scholar]
- McLean J. Waterhouse BD. Noradrenergic modulation of cat area 17 neuronal responses to moving visual stimuli. Brain Res. 1994;667:83–97. doi: 10.1016/0006-8993(94)91716-7. [DOI] [PubMed] [Google Scholar]
- Metherate R. Hsieh CY. Regulation of glutamate synapses by nicotinic acetylcholine receptors in auditory cortex. Neurobiol Learn Mem. 2003;80:285–290. doi: 10.1016/s1074-7427(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Metherate R. Hsieh CY. Synaptic mechanisms and cholinergic regulation in auditory cortex. Prog Brain Res. 2004;145:143–156. doi: 10.1016/s0079-6123(03)45010-3. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Schrater PR. Oxenham AJ. Auditory frequency and intensity discrimination explained using a cortical population rate code. PLoS Comput Biol. 2013;9:e1003336. doi: 10.1371/journal.pcbi.1003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquimaroux H, Gaioni SJ. Suga N. Cortical computational maps control auditory perception. Science. 1991;251:565–568. doi: 10.1126/science.1990432. [DOI] [PubMed] [Google Scholar]
- Riquimaroux H, Gaioni SJ. Suga N. Inactivation of the DSCF area of the auditory cortex with muscimol disrupts frequency discrimination in the mustached bat. J Neurophysiol. 1992;68:1613–1623. doi: 10.1152/jn.1992.68.5.1613. [DOI] [PubMed] [Google Scholar]
- Robertson D. Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Salgado H, García-Oscos F, Dinh L. Atzori M. Dynamic modulation of short-term synaptic plasticity in the auditory cortex: the role of norepinephrine. Hear Res. 2011a;271:26–36. doi: 10.1016/j.heares.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H, Garcia-Oscos F, Martinolich L, Hall S, Restom R, Tseng KY. Atzori M. Pre- and postsynaptic effects of noradrenaline on γ-aminobutyric acid-mediated synaptic transmission in layer 2/3 of the rat auditory cortex. Synapse. 2012;66:20–28. doi: 10.1002/syn.20979. [DOI] [PubMed] [Google Scholar]
- Salgado H, Garcia-Oscos F, Patel A, Martinolich L, Nichols JA, Dinh L, Roychowdhury S, Tseng K-Y. Atzori M. Layer-specific noradrenergic modulation of inhibition in cortical layer II/III. Cereb Cortex. 2011b;21:212–221. doi: 10.1093/cercor/bhq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res. 1991;88:571–585. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- Sato H, Fox K. Daw NW. Effect of electrical stimulation of locus coeruleus on the activity of neurons in the cat visual cortex. J Neurophysiol. 1989;62:946–958. doi: 10.1152/jn.1989.62.4.946. [DOI] [PubMed] [Google Scholar]
- Schnupp JWH, Hall TM, Kokelaar RF. Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci. 2006;26:4785–4795. doi: 10.1523/JNEUROSCI.4330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27:379–423. [Google Scholar]
- Videen TO, Daw NW. Rader RK. The effect of noradrenaline on visual cortical neurons in kittens and adult cats. J Neurosci. 1984;4:1607–1617. doi: 10.1523/JNEUROSCI.04-06-01607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MN. Palmer AR. Laminar differences in the response properties of cells in the primary auditory cortex. Exp Brain Res. 2008;184:179–191. doi: 10.1007/s00221-007-1092-z. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG. Palmer AR. Identification and localisation of auditory areas in guinea pig cortex. Exp Brain Res. 2000;132:445–456. doi: 10.1007/s002210000362. [DOI] [PubMed] [Google Scholar]
- Wang L, Narayan R, Graña G, Shamir M. Sen K. Cortical discrimination of complex natural stimuli: can single neurons match behavior? J Neurosci. 2007;27:582–589. doi: 10.1523/JNEUROSCI.3699-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen G, Gao W. Ebner T. Long-term potentiation of the responses to parallel fiber stimulation in mouse cerebellar cortex in vivo. Neuroscience. 2009;162:713–722. doi: 10.1016/j.neuroscience.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Azizi SA, Burne RA. Woodward DJ. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during noradrenaline and serotonin microiontophoresis. Brain Res. 1990;514:276–292. doi: 10.1016/0006-8993(90)91422-d. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC. Woodward DJ. Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology. 1981;20:907–920. doi: 10.1016/0028-3908(81)90020-4. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Sessler FM, Cheng JT, Woodward DJ, Azizi SA. Moises HC. New evidence for a gating action of noradrenaline in central neuronal circuits of mammalian brain. Brain Res Bull. 1988;21:425–432. doi: 10.1016/0361-9230(88)90154-2. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD. Woodward DJ. Interaction of noradrenaline with cerebrocortical activity evoked by stimulation of somatosensory afferent pathways in the rat. Exp Neurol. 1980;67:11–34. doi: 10.1016/0014-4886(80)90159-4. [DOI] [PubMed] [Google Scholar]
- Yu H, Chen X, Sun C. Shou T. Global evaluation of contributions of GABAA, AMPA and NMDA receptors to orientation maps in cat's visual cortex. NeuroImage. 2008;40:776–787. doi: 10.1016/j.neuroimage.2007.12.014. [DOI] [PubMed] [Google Scholar]


