Abstract
To find useful tools to evaluate the prognosis in colorectal carcinoma (CRC) patients, we investigated the prognostic values of tumor-infiltrating T lymphocyte subsets according to intratumoral subsites as well as clinical or molecular characteristics. Immunohistochemistry for CD8, CD45RO, and FOXP3 was performed, and densities of the T cell subsets in each tissue microarray core (cells/mm2) were measured by image analysis. In the training set (n = 218) of CRC, T cell subset densities in the invasion front were more strongly associated with patient outcome than those in the tumor center. In the validation set (n = 549), T cell subset densities in the invasion front were evaluated. Univariate analysis showed that all three T cell subset densities were significantly associated with longer progression free survival and overall survival time (p < 0.001). In multivariate analysis, a high CD45RO density correlated independently with longer progression free survival (p = 0.011) and overall survival time (p = 0.007) in CRC patients, regardless of tumor location or adjuvant chemotherapy status. Our results showed that CD45RO density in the invasion front was the only independent prognostic factor regarding CRC. However, CD8 and FOXP3 densities were also independent prognostic factors in certain clinical settings. Thus, image analysis of tissue microarray cores in the invasion front of CRC could be used as a valid method for evaluating the prognostic significance of T cell subset densities.
Introduction
Colorectal cancer (CRC) is the second most common malignant tumor worldwide and is one of the most prevalent cancers in Western society [1,2]. Despite the high mortality of CRC [1], advance in accurate prediction of patient prognosis is discouraging. Therefore, identifying potential prognostic factors is essential in understanding tumor progression of CRC and identifying novel therapeutic targets of the disease [3].
Cancer cells are known to express tumor-associated antigens (TAAs) and chemokines, which become targets of the T cell-mediated immune response [4,5]. Tumor-infiltrating T lymphocytes (TILs) mediate adaptive immunity and are associated with prognosis in solid tumors, such as in liver, breast, lung, stomach, and colon [6–8]. T cells can be subdivided into functional subtypes, including cytotoxic CD8+ T cells (CTLs), memory CD45RO+ T cells [9–11], and regulatory T cells (Tregs; forkhead box P3 [FOXP3]) [12].
Regarding CRC, CD8+ T cells are essential in recognizing and lysing the malignant cells in microenvironment [13]. High infiltration of CD45RO+ T cells has been associated with increased expressions of T-helper1 (Th1) and cytotoxicity-related genes in early-stage CRC [14]. FOXP3+ T cells inhibit Th1 and T-helper2 (Th2) as well as T-helper17 (Th17) in CRC [15]. However, questions still remain as to which T cell subset in CRC bears the prognostic information [16].
Accumulating evidence suggests that cancer cells in different subsites within tumors, including the tumor center and the invasion front, express different molecular and pathological characteristics [16,17]. In CRC, tumor cells in the invasion front show more aggressive behavior than those in the tumor center [17,18]. It could thus be hypothesized that neoplastic cells might express different TAA and chemokine patterns according to intratumoral subsites. However, most previous studies did not distinguish the tumor center from the invasion front when evaluating the correlation between T cell subsets and patient survival in CRC [14,16,19–26]
The aforementioned findings prompted us to investigate which T cell subset is a potential marker of prognosis in CRC and which intratumoral subsite should be used for such a purpose. Therefore, the present study extended previous studies using immunohistochemical tissue microarray analysis and image analysis applied to a large number of CRC cases (n = 767) to elucidate the prognostic significance according to type of individual T cell subset (CD8+, CD45RO+, and FOXP3+ phenotypes) densities and intratumoral subsite (tumor center or invasion front). In addition, we examined clinical and molecular variables, including tumor location, adjuvant chemotherapy status, microsatellite instability (MSI), CpG island methylator phenotype (CIMP), KRAS mutation and BRAF mutation, to determine whether these characteristics act as confounding factors.
Materials and Methods
Ethics statement
The study was approved by the Institutional Ethics Committee of Seoul National University Hospital, which waived the requirement to obtain informed consent (approval No. H-1312-050-542).
Patient specimens
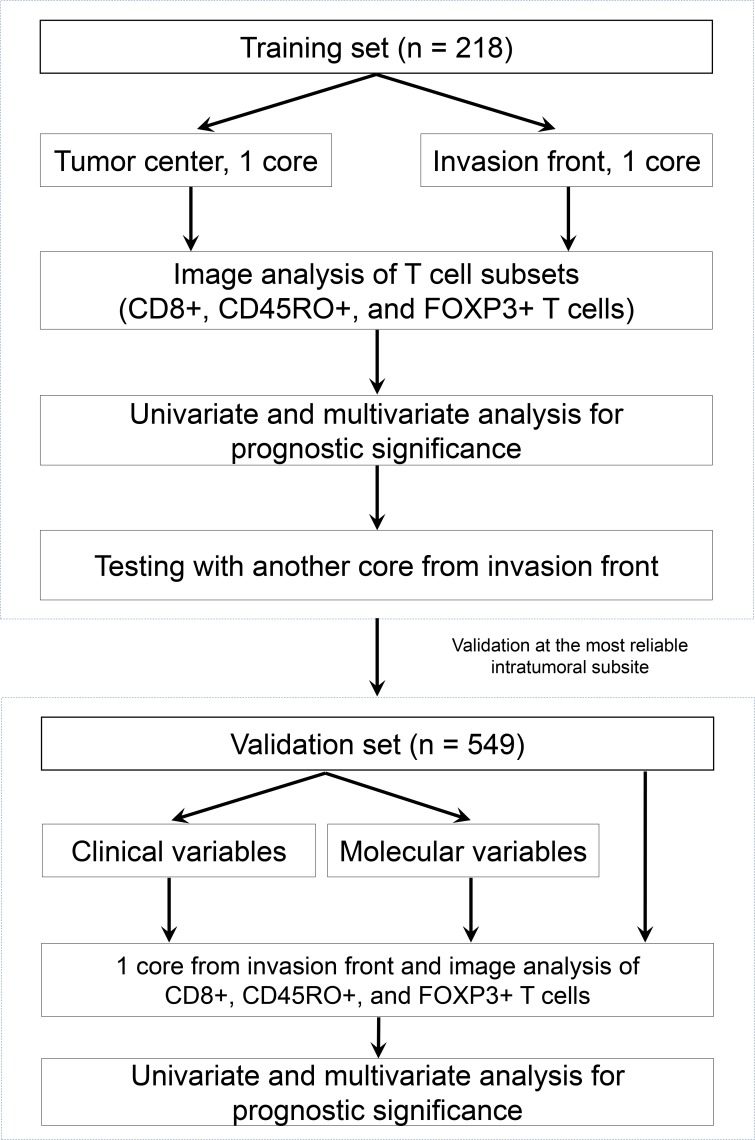
We retrospectively analyzed the clinicopathologic data of the 767 CRC patients who underwent tumor resection at Seoul National University Hospital from January 2004 to December 2006. We designed a training set, which consisted of 218 CRC cases, to identify which intratumoral subsite is more reliable to evaluate the association between T cell subset densities and patient outcome. Then, a validation set, which consisted of 549 cases, was designed. We split the entire cohort into the training set and the validation set randomly. The validation set excluded all patients in the training set. Among the 767 patients, 507 were treated with adjuvant chemotherapy. Patient information such as age, gender, pathologic tumor-node-metastasis (pTNM) stage, and tumor location was obtained from electronic medical records. According to the pTNM classification (of 6th version), the training set included 21 patients in stage I, 74 patients in stage II, 79 patients in stage III, and 44 patients in stage IV. The validation set included 93 patients in stage I, 174 patients in stage II, 192 patients in stage III, and 90 patients in stage IV.
Analysis of KRAS and BRAF mutations
Manually microdissected tissues were collected into microtubes and were incubated in a mixture of lysis buffer and proteinase K at 55°C for 2 days. Direct sequencing of KRAS codons 12 and 13, and allele-specific polymerase chain reaction (PCR) analysis of BRAF codon 600 were performed as described previously [27].
Analysis of MSI and CIMP
MSI status was determined by utilizing National Cancer Institute 5-marker scoring panel (BAT25, BAT26, D2S123, D5S345, and D17250). MSI-high was defined as the presence of instability in ≥ 40% of markers, MSI-low was defined as the presence of only one instable marker, and microsatellite stable (MSS) was defined as no instable markers.
Bisulfate DNA modification and MethyLight, a real-time PCR-based methylation assay, were performed as previously described [28]. CIMP status was determined by quantifying DNA methylation at eight markers—CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1. CIMP-positive was defined as the presence of methylation in more than four markers, and CIMP-negative was considered when four or fewer markers were methylated.
Tissue microarray (TMA) analysis
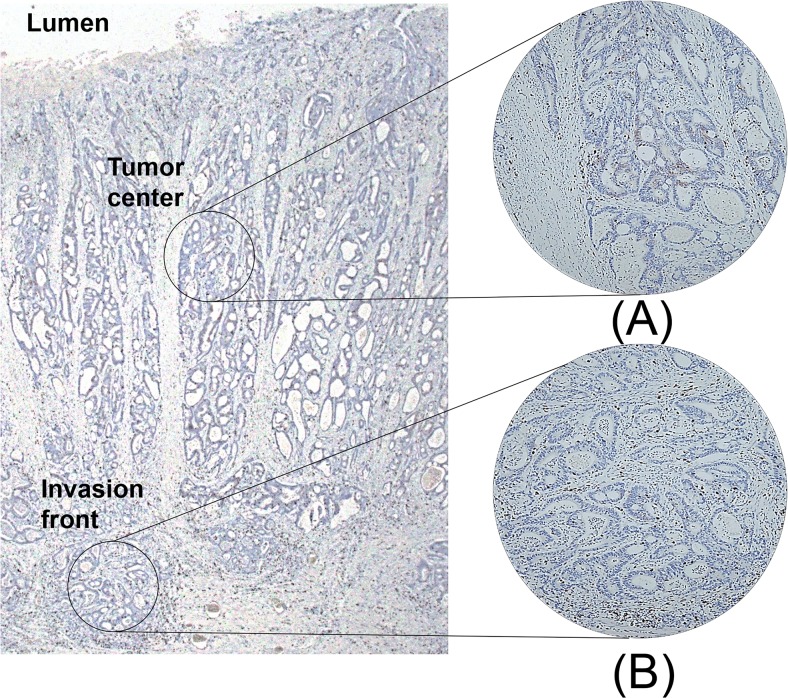
Through histologic examination, we marked portions that best represent the tumor. For the training set, one area that represents the tumor center and two areas that represent the invasion front (Fig 1) were extracted from each patient sample. For the validation set, one area that represents the invasion front was extracted from each patient sample. The cores extracted for the training set and the validation set were selected by two pathologists (YK and JMB). Core tissues (2 mm in diameter) were extracted from each paraffin-embedded CRC sample (donor block) and rearranged in a new recipient tissue microarray block using a trephine apparatus as described previously [29].
Fig 1. Depiction of intratumoral subsites where tissue cores were obtained.
Tissue microarray (TMA) core was constructed from tumor center (A) and invasion front (B), and immunohistochemistry for CD8 was performed.
Immunohistochemistry and image analysis
Formalin-fixed and paraffin-embedded sections of TMA were deparaffinized in xylene and were rehydrated with gradually decreasing concentrations of alcohol. Sections were immunostained after antigen retrieval using the Bond-max automated immunostainer (Leica Microsystems, Newcastle, UK). Primary antibodies used were the anti-CD8 polyclonal antibody (1:100, Neomarkers, Fremont, CA, USA), the anti-CD45RO monoclonal antibody (1:50, Neomarkers) and the anti-FOXP3 monoclonal antibody (1:50, Abcam, Cambridge, MA, USA). Antibody binding was detected by using the Bond Polymer Refine Detection kit (Leica Microsystems).
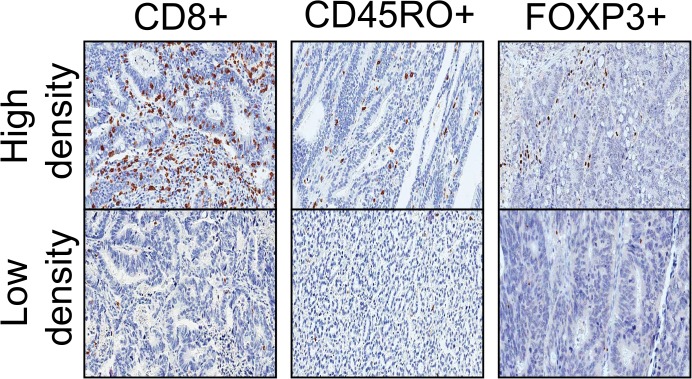
After immunohistochemical staining for each of the T cell markers, TMA slides were scanned by the Aperio image analysis system (Leica Biosystems, New Castle, UK) (Fig 2). The software counted the number of immunopositive nuclei in each tissue core. The average density (cells/mm2) of each lymphocyte subset was calculated in a whole TMA core. T cell subset densities were divided into two groups (high versus low) according to a median-split.
Fig 2. Photomicrographs of CD8, CD45RO, and FOXP3 immunohistochemistry in CRC.
The panels on the top show T cell subsets with high cell densities, and the panels on the bottom show T cell subsets with low cell densities.
Statistical analysis
All statistical analyses were performed using SPSS for Windows (version 21.0) (International Business Machines Corp., Armonk, NY, USA). The Mann-Whitney test was used to determine the relationship between T cell densities and clinicopathological parameters. We used Spearman’s correlation analysis to determine the relationship between T cell densities. The clinical database was last updated in August 2013. Progression-free survival (PFS) was calculated from the date of resection of CRC to the first date of documented recurrence or the date of death from any cause. Overall survival (OS) was measured from the date of operation to the date of death or the last clinical follow-up time before August 2013. PFS and OS were calculated according to the Kaplan-Meier method. The log-rank test was used to compare the survival distribution. Univariate and multivariate analysis were performed on the Cox regression model. Only variables that were significantly different in univariate analysis were entered into the next multivariate analysis. p values < 0.05 were considered statistically significant.
To avoid overfitting, twenty event per variable (EPV) was performed to limit the number of covariates in the multivariate analysis. In the training set, 76 deaths were observed, and three covariates could be used. Since up to two lymphocyte subset densities were significantly correlated with prognosis in the univariate analysis, only pTNM stage was included as the last covariate, although presence of lymphatic invasion and that of venous invasion were also significantly associated with worse prognosis (p < 0.001) in the training set. In the validation set, the number of death was 127, and six covariates could be used. Up to three lymphocyte subset densities were significantly correlated with prognosis in the univariate analysis, and three other covariates included pTNM stage, lymphatic invasion, and venous invasion.
Results
T cell subset density in CRC
Using automated image analysis, we calculated CD8, CD45RO, and FOXP3 densities (cells/mm2) in TMA cores from the tumor center and the invasion front (see flow chart in Fig 3). The densities of CD8, CD45RO, and FOXP3 in the cores of the tumor center were 119.9, 201.8, and 50.8, respectively, and those in the invasion front were 138.3, 177.7, and 58.6, respectively. The tumor-related stroma in the invasion front (48.84 ± 21.05%) was significantly more abundant than those of the tumor center (39.32 ± 18.96%) according to Mann-Whitney test (p < 0.001).
Fig 3. Flow chart of the study.
Correlation between T cell subset density and clinicopathologic characteristics in the training set
Table 1 shows the correlation between lymphocyte density and clinicopathologic variables in the tumor center and the invasion front. The median age of 218 CRC patients was 62.5 years (ranging from 28 to 85) and 61.0% of patients were male; 56.4% of tumors were pTNM stages I and II compared with 43.6% in stages III and IV. In the tumor center, none of the T cell subset densities showed significant correlation with the clinicopathological variables. In the invasion front, the CD8 density showed negative correlations with pTNM stages (p = 0.037) and distant metastasis (p = 0.002). The CD45RO density showed similar relationships (p = 0.012 and p < 0.001, respectively). In addition, the FOXP3 density was lower in colon cancer patients than in rectal cancer patients (p = 0.033)
Table 1. Relationship between T cell subset density and clinicopathological features in the training set of CRC.
| Tumor center | Invasion front | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | CD8 | p value | CD45RO | p value | FOXP3 | p value | CD8 | p value | CD45RO | p value | FOXP3 | p value | |
| Age | |||||||||||||
| <65 | 119 (54.6) | 120.0 | 0.886 | 204.0 | 0.910 | 54.0 | 0.267 | 138.1 | 0.798 | 177.5 | 0.385 | 57.4 | 0.927 |
| ≥65 | 99 (45.4) | 119.8 | 199.1 | 47.1 | 140.9 | 177.9 | 60.0 | ||||||
| Gender | |||||||||||||
| Male | 133 (61.0) | 126.3 | 0.371 | 209.3 | 0.875 | 56.4 | 0.071 | 135.9 | 0.627 | 178.2 | 0.740 | 62.5 | 0.087 |
| Female | 85 (39.0) | 109.3 | 189.7 | 42.2 | 142.1 | 177.0 | 52.5 | ||||||
| pTNM stage | |||||||||||||
| I or II | 123 (56.4) | 128.8 | 0.758 | 214.9 | 0.199 | 47.1 | 0.232 | 154.3 | 0.037 | 212.3 | 0.012 | 57.2 | 0.630 |
| III or IV | 95 (43.6) | 112.8 | 191.6 | 53.8 | 125.6 | 150.5 | 59.6 | ||||||
| Lymphatic invasion | |||||||||||||
| Yes | 107 (49.1) | 113.2 | 0.337 | 183.1 | 0.116 | 50.7 | 0.877 | 117.4 | 0.125 | 148.4 | 0.074 | 54.3 | 0.314 |
| No | 111 (50.9) | 126.1 | 219.5 | 51.0 | 158.1 | 205.4 | 62.7 | ||||||
| Distant metastasis | |||||||||||||
| Yes | 44 (20.2) | 101.5 | 0.133 | 199.0 | 0.208 | 51.9 | 0.965 | 88.1 | 0.002 | 104.4 | < 0.001 | 55.7 | 0.938 |
| No | 174 (79.8) | 124.5 | 202.6 | 50.6 | 150.1 | 195.6 | 59.3 | ||||||
| MSI status | |||||||||||||
| Positive | 8 (4.0) | 130.5 | 0.084 | 154.8 | 0.913 | 46.7 | 0.704 | 241.5 | 0.617 | 226.9 | 0.438 | 56.9 | 0.898 |
| Negative | 190 (96.0) | 119.0 | 205.6 | 51.4 | 137.1 | 178.4 | 5.9 | ||||||
| CIMP status | |||||||||||||
| Positive | 13 (6.0) | 111.7 | 0.843 | 263.4 | 0.227 | 68.2 | 0.130 | 105.2 | 0.249 | 164.5 | 0.540 | 56.7 | 0.927 |
| Negative | 205 (94.0) | 120.5 | 198.1 | 49.7 | 140.5 | 178.5 | 58.7 | ||||||
| Tumor location | |||||||||||||
| Colon | 158 (72.5) | 122.1 | 0.588 | 195.7 | 0.155 | 49.2 | 0.110 | 144.3 | 0.794 | 179.0 | 0.146 | 56.2 | 0.033 |
| Rectum | 60 (27.5) | 114.1 | 218.3 | 55.2 | 122.2 | 174.2 | 65.0 | ||||||
T cell subset densities represent median values for labelled cells per mm2; MSI: microsatellite instability; CIMP: CpG island methylator phenotype
Correlation between T cell subset densities in the training set
Table 2 shows the interrelations between the densities of T cell subsets in the tumor center and the invasion front. Spearman's correlation coefficients showed significant positive correlations between each other except for the correlation between the FOXP3 density in the tumor center and that in the invasion front (p = 0.165).
Table 2. Correlation between T cell subset density in the training set of CRC.
| Tumor center | Invasion front | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD8 | CD45RO | FOPX3 | CD8 | CD45RO | FOPX3 | |||||||
| Tumor center | ||||||||||||
| CD8 | r = 0.583 | r = 0.189 | r = 0.382 | r = 0.262 | r = 0.191 | |||||||
| p < 0.001 | p = 0.007 | p < 0.001 | p < 0.001 | p = 0.007 | ||||||||
| CD45RO | r = 0.345 | r = 0.278 | r = 0.420 | r = 0.162 | ||||||||
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |||||||||
| FOXP3 | r = 0.166 | r = 0.420 | r = 0.096 | |||||||||
| p = 0.016 | p = 0.009 | p = 0.165 | ||||||||||
| Invasion front | ||||||||||||
| CD8 | r = 0.382 | r = 0.278 | r = 0.166 | |||||||||
| p < 0.001 | p < 0.001 | p = 0.016 | ||||||||||
| CD45RO | r = 0.262 | r = 0.420 | r = 0.420 | r = 0.779 | ||||||||
| p < 0.001 | p < 0.001 | p = 0.009 | p < 0.001 | |||||||||
| FOPX3 | r = 0.191 | r = 0.162 | r = 0.096 | r = 0.506 | r = 0.425 | |||||||
| p = 0.007 | p < 0.001 | p = 0.165 | p < 0.001 | p < 0.001 | ||||||||
r, Spearman's correlation coefficient
Correlation between T cell subset density and patient prognosis in the training set
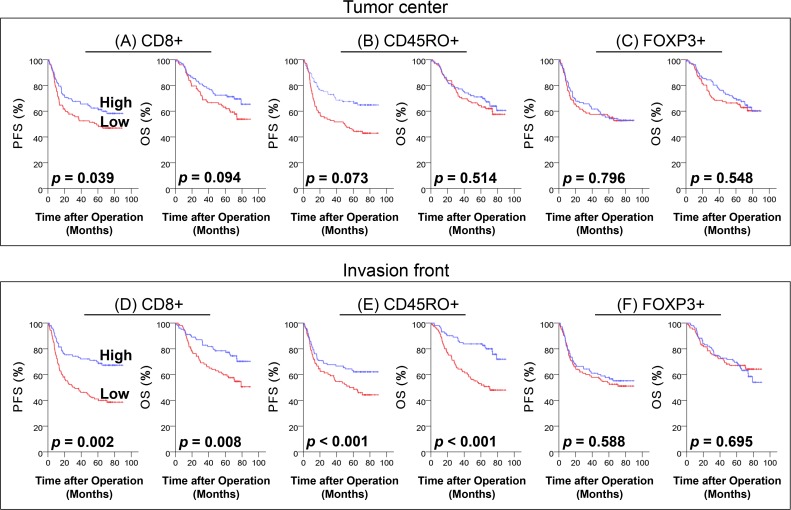
We examined the density of each T cell subset and patient survival. In Kaplan-Meier analysis with the log-rank test (Fig 4), patients with a high density of CD8 in the tumor center had significantly better PFS (p = 0.039; Fig 4A). In addition, high densities of CD8 (Fig 4D) and CD45RO (Fig 4E) in the invasion front were associated with favorable PFS (p = 0.002 and p < 0.001, respectively) and OS (p = 0.008 and p < 0.001, respectively).
Fig 4. Progression free survival (PFS) and overall survival (OS) in the training set of CRC.
Kaplan-Meier curves showing the prognostic significance of CD8 (A), CD45RO (B), and FOXP3 (C) densities in the tumor center and that of CD8 (D), CD45RO (E), and FOXP3 (F) densities in invasion front.
In multivariate Cox regression analysis (Table 3), a high density of CD8 in the tumor center, and high densities of CD8 and CD45RO in the invasion front were associated with better PFS (p = 0.035, p = 0.011 and p = 0.001, respectively). In addition, a high density of CD45RO in the invasion front was positively correlated with a longer OS than a low density of CD45RO (p < 0.001).
Table 3. T cell subset density and CRC patient mortality in the training set.
| Progression free survival | ||||||
| T cell subsets | Univariate HR | 95% CI | p value | Multivariate HR | 95% CI | p value |
| Tumor center | ||||||
| CD8 | 0.641 | 0.419–0.981 | 0.041 | 0.632 | 0.413–0.967 | 0.035 |
| CD45RO | 0.691 | 0.461–1.038 | 0.075 | |||
| FOXP3 | 0.948 | 0.636–1.416 | 0.796 | |||
| Invasion front | ||||||
| CD8 | 0.516 | 0.338–0.790 | 0.002 | 0.652 | 0.424–1.004 | 0.011 |
| CD45RO | 0.426 | 0.278–0.652 | < 0.001 | 0.603 | 0.388–0.938 | 0.001 |
| FOXP3 | 0.894 | 0.595–1.343 | 0.589 | |||
| Overall survival | ||||||
| T cell subsets | Univariate HR | 95% CI | p value | Multivariate HR | 95% CI | p value |
| Tumor center | ||||||
| CD8 | 0.668 | 0.415–1.075 | 0.096 | |||
| CD45RO | 0.860 | 0.546–1.354 | 0.515 | |||
| FOXP3 | 0.870 | 0.553–1.370 | 0.548 | |||
| Invasion front | ||||||
| CD8 | 0.527 | 0.325–0.855 | 0.009 | 0.572 | 0.352–0.928 | 0.055 |
| CD45RO | 0.356 | 0.216–0.586 | < 0.001 | 0.462 | 0.279–0.767 | < 0.001 |
| FOXP3 | 1.096 | 0.692–1.736 | 0.695 | |||
pTNM stage was adopted as the covariate in each multivariate analysis.
To eliminate bias caused by possible tumor heterogeneity, we constructed duplicate TMA cores from the invasion front of the same CRC specimens (n = 218). CD8 and CD45RO densities were independent prognostic factors regarding PFS (p = 0.023 and p = 0.003, respectively), whereas CD45RO density was an independent prognostic factor regarding OS (p = 0.008) (data not shown).
Correlation between T cell subset density and clinicopathologic characteristics in the validation set
In the training set, T cell subset densities in the invasion front showed stronger prognostic values than those in the tumor center. To gain stronger statistical power, the validation set was constructed with TMA cores from the invasive front of a larger CRC patient sample (n = 549) (see flow chart in Fig 3). Table 4 summarizes the correlation between the densities of the three T cell subsets and the clinicopathologic features of the 549 CRC patients included in the validation set. In this population, a high density of CD8 was associated with early pTNM stages (p < 0.001), absence of distant metastasis (p < 0.001), MSI-positivity (p < 0.001) and CIMP-positivity (p = 0.019). A high density of CD45RO was associated with early pTNM stages (p < 0.001) and absence of distant metastasis (p < 0.001). A high density of FOXP3 was associated with young age (p = 0.022), early pTNM stages (p < 0.001), absence of lymphatic invasion (p = 0.001) and absence of distant metastasis (p < 0.001).
Table 4. Relationship between T cell subset density and clinicopatholgical features in the validation set of CRC.
| N (%) | CD8 | p value | CD45RO | p value | FOXP3 | p value | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <65 | 321 (58.5) | 137.4 | 0.315 | 119.9 | 0.140 | 90.2 | 0.022 |
| ≥65 | 228 (41.5) | 130.5 | 105.5 | 78.9 | |||
| Gender | |||||||
| Male | 381 (60.3) | 130.7 | 0.063 | 123.5 | 0.395 | 80.8 | 0.074 |
| Female | 218 (39.7) | 131.2 | 128.4 | 67.1 | |||
| pTNM stage | |||||||
| I or II | 267 (48.6) | 159.4 | < 0.001 | 135.7 | < 0.001 | 104.4 | < 0.001 |
| III or IV | 282 (51.4) | 110.1 | 92.8 | 67.2 | |||
| Lymphatic invasion | |||||||
| Yes | 239 (43.5) | 124.5 | 0.065 | 103.66 | 0.177 | 69.9 | 0.001 |
| No | 310 (56.5) | 142.1 | 121.6 | 97.7 | |||
| Distant metastasis | |||||||
| Yes | 91 (16.6) | 96.1 | < 0.001 | 75.5 | < 0.001 | 59.6 | < 0.001 |
| No | 458 (83.4) | 142.0 | 121.7 | 90.5 | |||
| MSI status | |||||||
| Positive | 57 (10.6) | 210.4 | < 0.001 | 124.2 | 0.354 | 96.8 | 0.085 |
| Negative | 479 (89.4) | 124.9 | 112.3 | 84.0 | |||
| CIMP status | |||||||
| Positive | 35 (6.4) | 197.1 | 0.019 | 109.5 | 0.932 | 77.09 | 0.262 |
| Negative | 514 (93.6) | 130.4 | 114.24 | 86.1 | |||
| KRAS mutation | |||||||
| Positive | 140 (27.0) | 150.4 | 0.392 | 118.0 | 0.843 | 92.5 | 0.527 |
| Negative | 379 (73.0) | 130.2 | 116.4 | 85.6 | |||
| BRAF mutation | |||||||
| Positive | 25 (4.6) | 154.8 | 0.976 | 143.4 | 0.760 | 84.8 | 0.764 |
| Negative | 519 (95.4) | 133.5 | 122.9 | 85.9 | |||
| Tumor location | |||||||
| Colon | 381 (69.4) | 135.4 | 0.720 | 114.8 | 0.292 | 81.4 | 0.116 |
| Rectum | 168 (30.6) | 132.5 | 111.8 | 95.3 | |||
| Adjuvant chemotherapy | |||||||
| Yes | 351 (63.9) | 125.5 | 0.079 | 105.3 | 0.179 | 77.6 | 0.085 |
| No | 198 (36.1) | 149.9 | 129.0 | 99.3 |
T cell subset densities represent median values for labelled cells per mm2; MSI: microsatellite instability; CIMP: CpG island methylator phenotype
Correlation between T cell subset density and patient outcome in the validation set
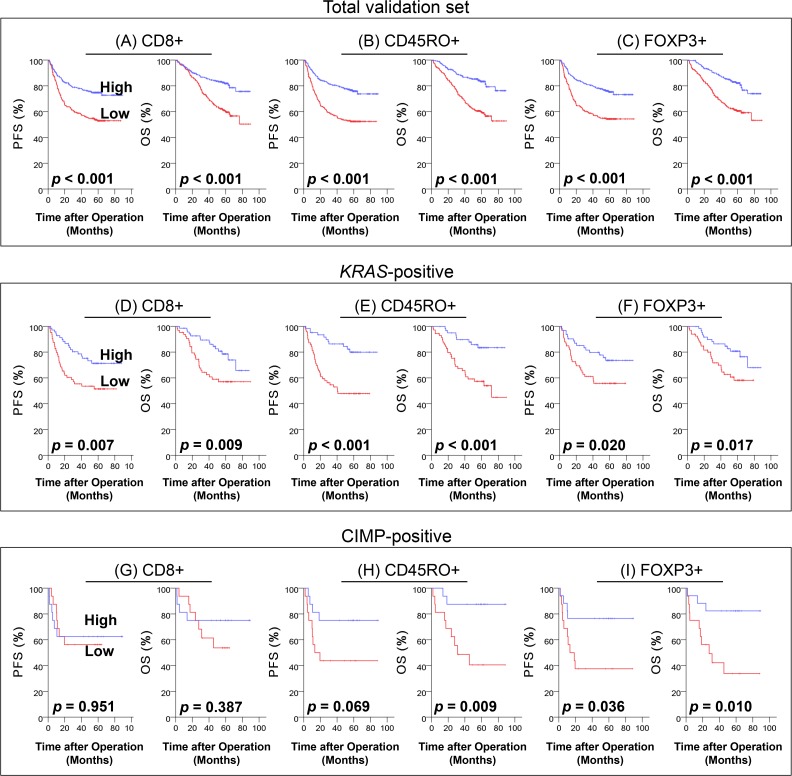
Kaplan-Meier analysis with the log-rank test showed significant correlations between all three T cell subset densities and PFS as well as OS for the total CRC patients (p < 0.001) (Fig 5A–5C). When patient samples were divided into two groups according to tumor location (colon versus rectum) or adjuvant chemotherapy status, all four groups showed positive correlations between all three T cell subset densities and PFS as well as OS (S1 Fig). Furthermore, patient samples were divided according to molecular variables (MSI, CIMP, KRAS mutation, and BRAF mutation). None of the three T cell subset densities were significantly associated with PFS and OS in MSI-high and BRAF-mutated samples (S2 Fig). By contrast, analysis of KRAS-mutated samples (Fig 5D–5F) showed that CD8+, CD45RO+, and FOXP3+ T cell subset densities were positively correlated with PFS (p = 0.007, p < 0.001 and p = 0.020, respectively) and OS (p = 0.009, p < 0.001 and p = 0.017, respectively). In CIMP-positive specimens (Fig 5G–5I), the FOXP3 density was positively correlated with PFS (p = 0.036), and CD45RO and FOXP3 densities were positively correlated with OS (p = 0.009 and p = 0.010, respectively).
Fig 5. Progression free survival (PFS) and overall survival (OS) in the validation set of CRC.
Kaplan-Meier curves showing the prognostic significance of CD8 (A, D and G), CD45RO (B, E and H), and FOXP3 (C, F and I) densities in invasion front from total CRC (A to C) (n = 549), KRAS-mutated (D to F) (n = 141), and CIMP-positive (G to I) (n = 36) patients
In multivariate analysis, the CD45RO density was positively correlated with PFS and OS in CRC patients (p = 0.011 and p = 0.008, respectively; Table 5). This was also true when the group was divided into colon cancer (p = 0.040 and p = 0.049, respectively) and rectal cancer patients (p = 0.035 and p = 0.002, respectively; S1 Table). In patients who did not receive postoperative chemotherapy, CD8, and FOXP3 densities showed positive correlations with OS (p = 0.011; S2 Table). The CD45RO density was an independent prognostic factor regardless of chemotherapy status (S2 Table). Although CD45RO density was significant for PFS (p = 0.003) and OS (p = 0.004) in KRAS-mutated specimens (S3 Table), other T cell subset densities were not independent prognostic factors in KRAS-mutated (S3 Table) and CIMP-positive specimens (S4 Table).
Table 5. T cell subset density and patient outcome in the validation set of CRC.
| Progression free survival | ||||||
| T cell subsets | Univariate HR | 95% CI | p value | Multivariate HR | 95% CI | p value |
| CD8 | 0.477 | 0.351–0.648 | < 0.001 | 0.851 | 0.568–1.277 | 0.436 |
| CD45RO | 0.420 | 0.307–0.576 | < 0.001 | 0.549 | 0.363–0.830 | 0.011 |
| FOXP3 | 0.453 | 0.329–0.623 | < 0.001 | 0.462 | 0.564–1.297 | 0.462 |
| Overall survival | ||||||
| T cell subsets | Univariate HR | 95% CI | p value | Multivariate HR | 95% CI | p value |
| CD8 | 0.426 | 0.300–0.606 | < 0.001 | 0.952 | 0.602–1.506 | 0.834 |
| CD45RO | 0.361 | 0.251–0.520 | < 0.001 | 0.470 | 0.293–0.753 | 0.007 |
| FOXP3 | 0.403 | 0.281–0.577 | < 0.001 | 0.704 | 0.448–1.109 | 0.130 |
pTNM stage, lymphatic invasion, venous invasion, and all three T cell subset densities were adopted as covariates in each multivariate analysis
Discussion
The present study investigated the prognostic significance of T cell subset (CD8, CD45RO, and FOXP3) densities using TMA cores and compared the difference between the tumor center and the invasion front of CRC. Furthermore, we used 2 mm diameter cores, which is at least 4 times larger than TMA cores (0.6 mm to 1 mm diameter) used in previous studies [11,19,21,26] to exclude the possible effects of tumor heterogeneity. Computerized image analysis software was used to minimize observer bias. In the training set composed of 218 CRC specimens, we found that T cell subset densities in the invasion front were stronger prognostic factors than those in the tumor center. In the validation set with 549 CRC specimens, all three T cell subset densities in the invasion front were associated with PFS and OS in univariate analysis. In multivariate analysis, only the CD45RO density in the invasion front was a prognostic factor in CRC, independent of pTNM stage, lymphatic invasion, venous invasion, and other T cell subset (CD8 and FOXP3) densities.
In previous reports, CD8+ T cells were correlated with prognosis in a univariate analysis of CRC patients [22,25], but not in a multivariate analysis. CD8/FOXP3, on the other hand, was proven to be an independent prognostic factor in a single study with a small sample size (n = 94) [30]. We found that, in multivariate analysis, a high CD8 density was correlated with good OS in chemotherapy-free CRC patients.
In CRC patients, a strong infiltration of CD45RO+ T cells was associated with good OS or disease free survival (DFS) in three published studies [19,22,31]. Among them, two studies were limited to early-stage CRC patients [19,31]. Meanwhile, Nosho et al. [22] did not stratify adjuvant chemotherapy status of the CRC patients. In the present study, we used CRC specimens from stage I to IV and found that the CD45RO density was an independent prognostic factor in patients with CRC, regardless of tumor location or adjuvant chemotherapy status. CD45RO is expressed on both memory and effector T cells. Since additional markers such as CCR7 are necessary to differentiate those populations, immunohistochemical staining using anti-CCR7 antibody was performed on the TMA for the training set. We found that CCR7 expression showed no significant correlation with patient prognosis in the univariate analysis (data not shown).
There have been a number of inconsistent reports regarding the relationship between the density of FOXP3+ T cells and patient survival in CRC. Several studies reported tumor infiltrating FOPX3+ T cell density as an unfavorable prognostic factor in CRC or advanced colon cancer [21,23]. Others claimed that FOXP3+ T cells was a favorable prognostic factor in CRC [32] and colon cancer [31,33] or was not an independent prognostic factor of CRC [22]. This discrepancy could be explained by hypothesizing an ambivalent relationship between FOXP3+ T cells and CRC. FOPX3+ T cells are known to suppress Th1-dependent antitumor immune response in several solid tumors; however, FOXP3+ T cells in septic environments induce an antitumor immune response by attenuating bacteria-stimulated Th17 cells [15]. If both of these reactions occur in CRC, inconsistency in the prognostic impact of FOXP3 might be caused by confounding factors affecting the role of FOXP3+ T cells in an antitumor immune response. Possible confounding factors include CD45RO+ T cells and adjuvant chemotherapy status. If this hypothesis is valid, the FOXP3 density might not be an appropriate prognostic factor in CRC.
In a recent meta-analysis study, Mei et al. [34] showed that CD8+ and FOXP3+ T cells were not significant prognostic markers in the tumor center of CRC. However, they could not evaluate CD45RO+ T cells because of the limited number of published studies. In addition, they suggested that clinical and molecular factors affecting the tumor microenvironment of CRC should be further evaluated. To this end, we adjusted clinical variables (tumor location and adjuvant chemotherapy) as well as key molecular variables associated with evolution of CRC (MSI, CIMP, KRAS mutation, and BRAF mutation) [35]. Unlike Nosho et al. [22], the present study did not show a prognostic significance of CD45RO in MSI-high patients but showed a prognostic significance in KRAS-mutated patients. This discrepancy might reflect different proportions of molecular variables in CRC, which commonly appears between Eastern and Western populations [28,36–39].
In conclusion, we found that among the three T cell subsets evaluated, only the CD45RO+ T cell density was significantly associated with longer survival in CRC patients, independent of tumor stage, lymphovascular invasion, and CD8+ and FOXP3+ T cell densities. However, CD8 and FOXP3 densities were also independent prognostic factors in certain clinical settings. Our results suggest that measuring T cell subset densities by automated image analysis of TMA cores in the invasion front, rather than those in the tumor center, is a more reliable strategy for predicting prognosis in CRC patients. Therefore, increasing T cell subsets within tumors through therapeutic intervention may benefit CRC patients with prolonged survival.
Supporting Information
Kaplan-Meier curves showing the prognostic significance of CD8 (A, D, G and J), CD45RO (B, E,H, and K), and FOXP3 (C, F, I, and L) densities in the invasion front from colon cancer (A to C), rectal cancer (D to F), chemotherapy-treated (G to I), and chemotherapy-free (J to L) patients.
(TIF)
Kaplan-Meier curves showing the prognostic significance of CD8 (A), CD45RO (B), and FOXP3 (C) densities in MSI-high and that of CD8 (D), CD45RO (E), FOXP3 (F) densities in BRAF-mutated CRC specimens.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by a grant from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2059080), a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI13C1804 & HI14C1277), and the NRF grant funded by the Ministry of Science, ICT and Future Planning (MSIP) (2011-0030049). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61: 212–236. 10.3322/caac.20121 [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63: 11–30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 3. Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol. 2003;46: 33–57. [DOI] [PubMed] [Google Scholar]
- 4. Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12: 337–365. [DOI] [PubMed] [Google Scholar]
- 5. Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67: 4725–4731. [DOI] [PubMed] [Google Scholar]
- 6. Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65: 2457–2464. [DOI] [PubMed] [Google Scholar]
- 7. Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH Jr, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107: 2866–2872. [DOI] [PubMed] [Google Scholar]
- 8. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29: 1949–1955. 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 9. Ostenstad B, Lea T, Schlichting E, Harboe M. Human colorectal tumour infiltrating lymphocytes express activation markers and the CD45RO molecule, showing a primed population of lymphocytes in the tumour area. Gut. 1994;35: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mackay CR. Dual personality of memory T cells. Nature. 1999;401: 659–660. [DOI] [PubMed] [Google Scholar]
- 11. Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71: 1263–1271. 10.1158/0008-5472.CAN-10-2907 [DOI] [PubMed] [Google Scholar]
- 12. deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18: 3022–3029. 10.1158/1078-0432.CCR-11-3216 [DOI] [PubMed] [Google Scholar]
- 13. Titu LV, Monson JR, Greenman J. The role of CD8(+) T cells in immune responses to colorectal cancer. Cancer Immunol Immunother. 2002;51: 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27: 5944–5951. 10.1200/JCO.2008.19.6147 [DOI] [PubMed] [Google Scholar]
- 15. Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60: 909–918. 10.1007/s00262-011-1046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ling A, Edin S, Wikberg ML, Oberg A, Palmqvist R. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer. 2014;110: 2551–2559. 10.1038/bjc.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Wever O, Pauwels P, De Craene B, Sabbah M, Emami S, Redeuilh G, et al. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130: 481–494. 10.1007/s00418-008-0464-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahlert C, Lahes S, Radhakrishnan P, Dutta S, Mogler C, Herpel E, et al. Overexpression of ZEB2 at the invasion front of colorectal cancer is an independent prognostic marker and regulates tumor invasion in vitro. Clin Cancer Res. 2011;17: 7654–7663. 10.1158/1078-0432.CCR-10-2816 [DOI] [PubMed] [Google Scholar]
- 19. Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353: 2654–2666. [DOI] [PubMed] [Google Scholar]
- 20. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 21. Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27: 186–192. 10.1200/JCO.2008.18.7229 [DOI] [PubMed] [Google Scholar]
- 22. Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyehardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222: 350–366. 10.1002/path.2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu W, Liu H, Song J, Fu HX, Qiu L, Zhang BF, et al. The appearance of Tregs in cancer nest is a promising independent risk factor in colon cancer. J Cancer Res Clin Oncol. 2013;139: 1845–1852. 10.1007/s00432-013-1500-7 [DOI] [PubMed] [Google Scholar]
- 24. Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137: 1270–1279. 10.1053/j.gastro.2009.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11: 19 10.1186/1471-2172-11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoon HH, Orrock JM, Foster NR, Sargent DJ, Smyrk TC, Sinicrope FA. Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One. 2012;7: e42274 10.1371/journal.pone.0042274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee DW, Kim KJ, Han SW, Lee HJ, Rhee YY, Bae JM, et al. KRAS Mutation is Associated with Worse Prognosis in Stage III or High-risk Stage II Colon Cancer Patients Treated with Adjuvant FOLFOX. Ann Surg Oncol. 2015;22: 187–94. 10.1245/s10434-014-3826-z [DOI] [PubMed] [Google Scholar]
- 28. Kim JH, Shin SH, Kwon HJ, Cho NY, Kang GH. Prognostic implications of CpG island hypermethylator phenotype in colorectal cancers. Virchows Arch. 2009;455: 485–494. 10.1007/s00428-009-0857-0 [DOI] [PubMed] [Google Scholar]
- 29. Park SY, Kim BH, Kim JH, Lee S, Kang GH. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med. 2007;131: 1561–1567. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, et al. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother. 2010;59: 653–661. 10.1007/s00262-009-0781-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee WS, Park S, Lee WY, Yun SH, Chun HK. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer. 2010;116: 5188–5199. 10.1002/cncr.25293 [DOI] [PubMed] [Google Scholar]
- 32. Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126: 2635–2643. 10.1002/ijc.24989 [DOI] [PubMed] [Google Scholar]
- 33. Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33: 435–441. 10.1097/CJI.0b013e3181d32f01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110: 1595–1605. 10.1038/bjc.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50: 113–130. [DOI] [PubMed] [Google Scholar]
- 36. Abe Y, Masuda H. Genetic alterations of sporadic colorectal cancer with microsatellite instability, especially characteristics of primary multiple colorectal cancers. J Surg Oncol. 2000;74: 249–256. [DOI] [PubMed] [Google Scholar]
- 37. Sugai T, Habano W, Jiao YF, Tsukahara M, Takeda Y, Otsuka K, et al. Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis: proposal for new molecular profile of colorectal carcinomas. J Mol Diagn. 2006;8: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang SC, Lin JK, Yang SH, Wang HS, Li AF, Chi CW. Relationship between genetic alterations and prognosis in sporadic colorectal cancer. Int J Cancer. 2006;118: 1721–1727. [DOI] [PubMed] [Google Scholar]
- 39. Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002;62: 6451–6455. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier curves showing the prognostic significance of CD8 (A, D, G and J), CD45RO (B, E,H, and K), and FOXP3 (C, F, I, and L) densities in the invasion front from colon cancer (A to C), rectal cancer (D to F), chemotherapy-treated (G to I), and chemotherapy-free (J to L) patients.
(TIF)
Kaplan-Meier curves showing the prognostic significance of CD8 (A), CD45RO (B), and FOXP3 (C) densities in MSI-high and that of CD8 (D), CD45RO (E), FOXP3 (F) densities in BRAF-mutated CRC specimens.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.