Abstract
Background
To determine if extracranial venous structural and flow abnormalities exist in patients with multiple sclerosis (MS).
Methods
Magnetic resonance imaging was used to assess the anatomy and function of major veins in the neck in 138 MS patients and 67 healthy controls (HC). Time-of-flight (TOF) MR angiography (MRA) was used to assess stenosis while 2D phase contrast flow quantification (PCFQ) was used to assess flow at the C2/C3 and C5/C6 levels. Venous flow was normalized to the total arterial flow. The MS patients were divided into stenotic and non-stenotic groups based on MRA assessment, and each group was compared to the HC group in anatomy and flow.
Results
The MS group showed lower normalized internal jugular vein (IJV) blood flow (tIJV/tA) than the HC group (p < 0.001). In the MS group, 72 (52%) evidenced stenosis (ST) while 66 (48%) were non-stenotic (NST). In the HC group, 11 (23%) showed a stenosis while 37 (77%) were non-stenotic. The ST-MS group had lower IJV flow than both HC and NST-MS groups.
Conclusion
After categorizing the MS population into two groups based upon anatomical stenosis determined from an absolute quantification of IJV cross-section, clear differences in IJV flow between the stenotic MS and HC samples became evident. Despite the unknown etiology of MS, abnormal venous flow was noted in a distinct group of MS patients compared to HC.
Keywords: venous flow, flow quantification, phase-contrast MRI, vessel cross sectional areas, stenosis
Background
Recent findings suggest that structural and flow abnormalities in the extra-cranial vasculature may be comorbid in patients with multiple sclerosis (MS) [1]. Diagnosis of MS relies on both clinical symptoms and the appearance of demyelinating, inflammatory plaques on magnetic resonance imaging (MRI) of the central nervous system [2]. Despite significant progress in pharmaceutical intervention, treatment of MS patients remains a challenge without a clear understanding of the disease etiology.
Since the seminal finding by Charcot [3], attention has been drawn to the venocentricity of MS lesions[4–6]. However, the role of the venous system and its abnormalities in MS remains unclear. In one of the earliest studies of its kind, treatment of stenotic veins improved the condition of patients affected by paraplegia or quadriplegia [7]. More recently, reduced venous blood flow in major extracranial veins, such as the internal jugular (IJV), vertebral, and azygous, has been called cerebrospinal venous insufficiency (CCSVI). This condition was first documented using ultrasound [8] but has since been inconsistently observed, in all likelihood due to the poor reliability of the method [9–13]. Further, imaging the entire vasculature simultaneously is not possible with ultrasound.
These shortcomings can be alleviated by MRI, which can provide a user-independent means to assess the entire vasculature and to quantify flow in MS patients. The goal of this paper was to quantify and evaluate the anatomy and flow characteristics of the IJV in MS patients and healthy controls (HC), and to further identify differences related to stenosis. We hypothesized that MRI would provide a set of reliable indices of venous flow that would discriminate MS patients (or a subgroup with stenosis) from HC.
Methods
MRI data were collected at two imaging sites with institutional review board approval. MS subjects were recruited at site 1 prior to seeking potential vascular intervention and HC were recruited at site 2. All MS patients were imaged on a 3T TRIO Scanner (Siemens, Erlangen, Germany) and all HC were imaged on a 3T VERIO Scanner (Siemens, Erlangen, Germany) both with a 16 channel head/neck coil. The imaging technicians had 14 years and 22 years of experience at site 1 and site 2, respectively. The sample consisted of 138 MS patients, mean age 48.6 years [standard deviation (SD) = 11.5 years] and 67 HC, mean age 39.0 years (SD = 13.2 years). The MS group was older than the HC group, t(203) = −5.35, p < 0.001, although the HC were sex-matched with the MS patients (χ2 = 2.59, p = 0.1). Disease duration for the MS group was 12.4 years (SD = 9.1 years) and similar between types of MS, F(1, 32) = 1.17, p = 0.27. The MS patients were clinically classified as follows: 110 relapsing remitting (RR-MS), 17 secondary progressive (SP-MS), and 11 primary progressive (PP-MS). Additional information about subject demographics and MS type appears in Table 1.
Table 1.
Subject demographic descriptors.
| All MS | NST-MS | ST-MS | HC | |
|---|---|---|---|---|
| Number of Subjects (number of females) |
138 (96) | 66 (53) | 72 (43) | 67 (39) |
| Female : male ratio | 2.3: 1 | 4.1:1 | 1.5:1 | 1.4: 1 |
| Age range in years | 20–72 | 26–71 | 20–72 | 18–71 |
| Mean age (SD) in years | 48.6 (11.5) | 47.9 (11.7) | 49.3 (11.3) | 39 (13.2) |
| Mean disease duration (SD) in years | 12.4 (9.0) | 12.9 (9.0) | 12 (9.2) | |
Self-referred MS patients seeking possible treatment for CCSVI were included in this study between January, 2012 and March, 2013 at site 1. MS was diagnosed by the patient’s primary care physician or by neurologist via clinical examination, prior neurological imaging, or spinal tap. Inclusion criteria for the HC and MS subjects were: males and females between 18 and 90 years of age capable of comprehending the nature of the study, including the risks and benefits and the ability to execute an informed consent. MS subjects were excluded if they had any contra-indicated implant(s), history of hypertension, previous vascular intervention, presence of hypercoagulable state as well as special populations, including: prisoners, pregnant women, and cognitively-impaired individuals who were unable to provide informed consent. HC were excluded if they had a history of diabetes, chronic renal disease, a prior known psychiatric or neurological disorder, or substance abuse; currently receiving chemotherapy, on dialysis or any known contraindication to MRI such as a pacemaker or implanted device; or if females were pregnant or nursing. Some HC were part of a study which did not include contrast: for the HC who underwent contrast-enhanced venography, subjects were excluded if they were allergic to MRI contrast, or have moderate to severe kidney disease with impaired ability to filter contrast agents (serum creatinine > 1.8 mg/dL);
Imaging
The MR scanning protocol for the HC and MS patients included conventional imaging and a comprehensive evaluation of the head and neck vasculature. The latter included coronal 3D time-resolved contrast-enhanced (CE) MR arteriovenography, transverse 2D time-of-flight (TOF) venography and 2D phase-contrast (PC) flow quantification. Imaging sequence parameters for Siemens scanners have been published [1, 14]. The PC-MRI images were acquired perpendicular to the IJVs at two levels: the C2/C3 level, which is at the disc between C2 and C3 vertebrae, superior to the carotid bifurcation and intersects the IJV inferior to the jugular foramen; and the cervical vertebrae 5–6 (C5/C6) level, which is at the disc between the C5 and C6 vertebrae, superior to the confluence of the IJV with the subclavian veins and inferior to the common carotid artery (CCA) bifurcation. It is also necessary to position the C2/C3 slice such that the vertebral arteries (VA) do not run in-plane with the slice. A maximum velocity encoding of 50 cm/sec was used at both sites. The head was secured with head coil restraints resting on the subject’s forehead and a neck pad/pillow positioned to subject’s comfort. Out of the 67 HC, 19 were part of a study that collected only PC-MRI flow data and did not have venography data.
To test the consistency of phase contrast flow measurements, PC-MRI for one subject was collected by acquiring the data with the head tilted laterally at the following angles: 0°, ± 10° and ± 20°. The neutral position (0°) was collected multiple times to validate consistency. The same procedure was collected four times with a one day separation between sittings.
Data Processing
Data were processed retrospectively by two analysts trained in MRI signal processing with four years of experience each. SPIN (Signal Processing in NMR, Detroit, MI, USA) software was used to evaluate the presence and cross sectional area (CSA) of suspected IJV stenosis on axial 2D TOF MRV, or reformatted axial 3D CE TWIST if 2D TOF MRV was not available. Vessel boundaries were delineated automatically using a full-width half maximum region growing threshold method [15] with manual modification applied when appropriate. Vessels were labeled stenotic if the CSA was less than 25 mm2 at or caudal to the C3 level and less than 12.5 mm2 cranial to the C3 level [1, 16]. The criterion of 25 mm2 was chosen assuming 70% stenosis in an average IJV diameter of 1 cm, as well as from values in previous research [17, 18]. Lack of visibility of the IJV in all imaging modalities with clear visualization of surrounding vasculature in a segment was determined to be atresia, and lack of visibility observed throughout the entire vessel length was determined to be aplasia. Diffuse stenosis is characterized by stenosis through the length of the entire vessel.
For the PC-MRI images, our in-house software written in Matlab (Mathworks, Natick, MA, USA) was used to quantify flow [1, 16, 19] as six measures: three vessels for right and left sides. This included the IJV and VA at both C2/C3 and C5/C6 levels, the internal carotid artery (ICA) at the C2/C3 level, and the CCA at the C5/C6 level. and. Phase unwrapping was performed when the flow velocity exceeded 50 cm/sec. IJV flow was normalized to the arterial sum (tA) of the CCA and VA at the C5/C6 level and the ICA and VA at the C2/C3 level. This normalization was done in order to account for variations in total flow into and out of the brain among subjects. Of the two IJV, the IJV carrying the higher flow was considered the dominant jugular (dJ), and the IJV carrying lower flow was considered the subdominant jugular (sdJ). The individual arterial vessel flow measurements were normalized to tA to show their relative input.
Individual vessel flow and the sum (right + left) of vessel flow were compared between processors. Prior to data collection, rater reliability was assessed by an intra-class correlation coefficient computed under a random raters assumption (ICC2) [20]. The method evidenced high reliability with ICC(2) ≥ 0.85 for left- and right-side measures and 0.90 for bilateral total, of each vessel.
Statistical Analyses
Flow indices including bilateral IJV flow normalized to total arterial flow (tIJV/tA) at both the C2/C3 and C5/C6 cervical levels served as dependent variables. A general linear model (GLM) was used to differentiate normalized IJV flow between groups (ST-MS, NST-MS, and HC), controlling for age and sex. Post-hoc univariate GLM and paired t-tests further investigated significant group differences. In addition, a receiver operating characteristic (ROC) curve analysis was performed to determine the utility of these measures in discriminating among healthy controls, NST-MS and ST-MS. Optimum ROC sensitivity and specificity was determined as the point with the least distance from coordinate (0,1) with sensitivity as the y-axis and 1-specificity as the x-axis. The ROC thresholds for each cervical level were applied to the RR-MS, PP-MS, and SP-MS subtypes. A chi-square test for proportion was used to test number of ST between the groups with significance at p < 0.05. Nominal significance of all tests was set at p < 0.05. A chi-square test for proportion was also used to test group differences in the percentage of people who were dominant in the right or left jugular.
Results
Differences in Flow Attributable to Change in Head Positioning
The results for the HC subject scanned four times on consecutive days revealed a normalized IJV flow of 0.85 (SD = 0.06) for 0° and 0.79 (SD = 0.06) if all angles were included. There were no significant differences of position by side (right vs. left), F(6, 21) = 2.29, p = 0.07.
Classification of Stenosis
More than half the MS group 72/138 (52%) showed stenosis compared to 11/48 (23%) HC participants (χ2 = 12.01, p < 0.001). Various forms of stenoses were observed in the MS and HC groups (Figure 1) and the breakdown by stenosis type and location are presented in Table 2. Stenosis breakdown for the MS types was as follows: 60/110 (55%) for RR-MS, 6/11 (55%) for PP-MS, and 6/17 (35%) for SP-MS. In the HC group, 2/48 (4%) showed atresia and no aplasia cases were observed; however, 16/138 (12%) of the MS group had atresia and 2/138 (1.4%) had aplasia. NST-MS and ST-MS groups significantly differed in normalized IJV flow in the right (both p < 0.001) and the left (both p < 0.001) at both the C2/C3 and C5/C6 levels. A histogram showing the percentage of each group that fell under specified CSA ranges is shown in Figure 4. Therefore, additional analyses considered differences between HC and all MS patients, and then did further comparisons between HC, NST-MS, and ST-MS.
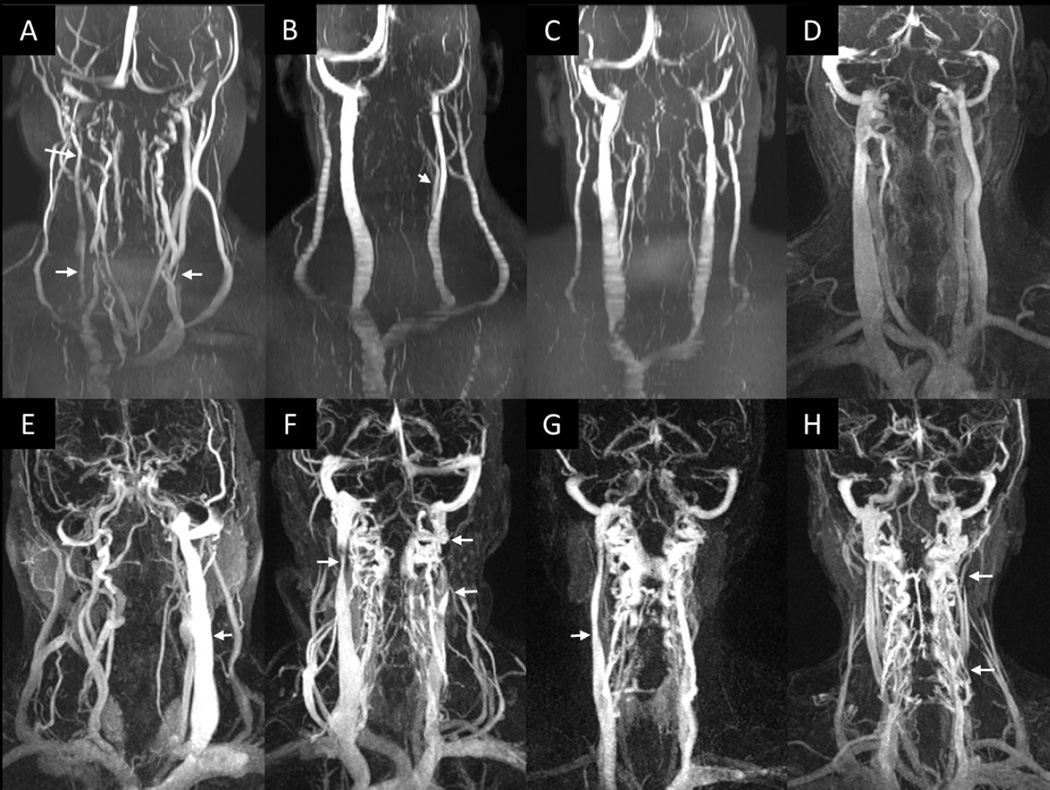
Figure 1.
2D TOF MRV (A–C) 3D CE TWIST MRAV (D–H). CE images were chosen at the time point where the veins appear the brightest. Top row: HC (A–D); bottom row: MS patients (E–H). Various venous abnormalities are noted. Image A shows a stenotic RIJV in the upper and lower neck levels, as well as a stenotic LIJV in the lower neck level. Image B shows a stenotic case, where the CFV branches off at a low level of the neck which would reduce the IJV caliber Images C and D are without anomaly but one individual has clear external jugulars (C) while the other does not (D). Image E shows an aplastic RIJV, the LIJV is present (arrow). Image F shows upper neck level stenosis of the RIJV (upper left arrow), and atresia of the LIJV between the arrows. Image G shows an aplastic LIJV, RIJV is present (arrow). Image H shows multiple atresias in the LIJV (arrows).
Table 2.
Results from anatomic assessment. The type of stenosis and the location are presented in terms of both total number and percentage within each group.
| MS (138) | HC (48) | |||
|---|---|---|---|---|
| N | Percent | N | Percent | |
| LL RIJV Stenosis | 16 | 11.6% | 5 | 10.4% |
| UL RIJV Stenosis | 21 | 15.2% | 1 | 2.1% |
| LL LIJV Stenosis | 29 | 21.0% | 7 | 14.6% |
| UL LIJV Stenosis | 31 | 22.5% | 2 | 4.6% |
| LL RIJV atresia | 0 | 0.0% | 0 | 0.0% |
| UL RIJV atresia | 6 | 4.3% | 0 | 0.0% |
| LL LIJV atresia | 3 | 2.2% | 0 | 0.0% |
| UL LIJV atresia | 8 | 5.8% | 2 | 4.6% |
| RIJV diffuse stenosis | 1 | 0.7% | 0 | 0.0% |
| LIJV diffuse stenosis | 1 | 0.7% | 0 | 0.0% |
| RIJV aplasia | 1 | 0.7% | 0 | 0.0% |
| LIJV aplasia | 1 | 0.7% | 0 | 0.0% |
| RIJV only stenosis | 3 | 2.2% | 2 | 4.6% |
| LIJV only stenosis | 53 | 38.4% | 6 | 12.5% |
| Bilateral stenosis | 5 | 3.6% | 3 | 6.3% |
Note: LL: Lower level, UL: Upper level.
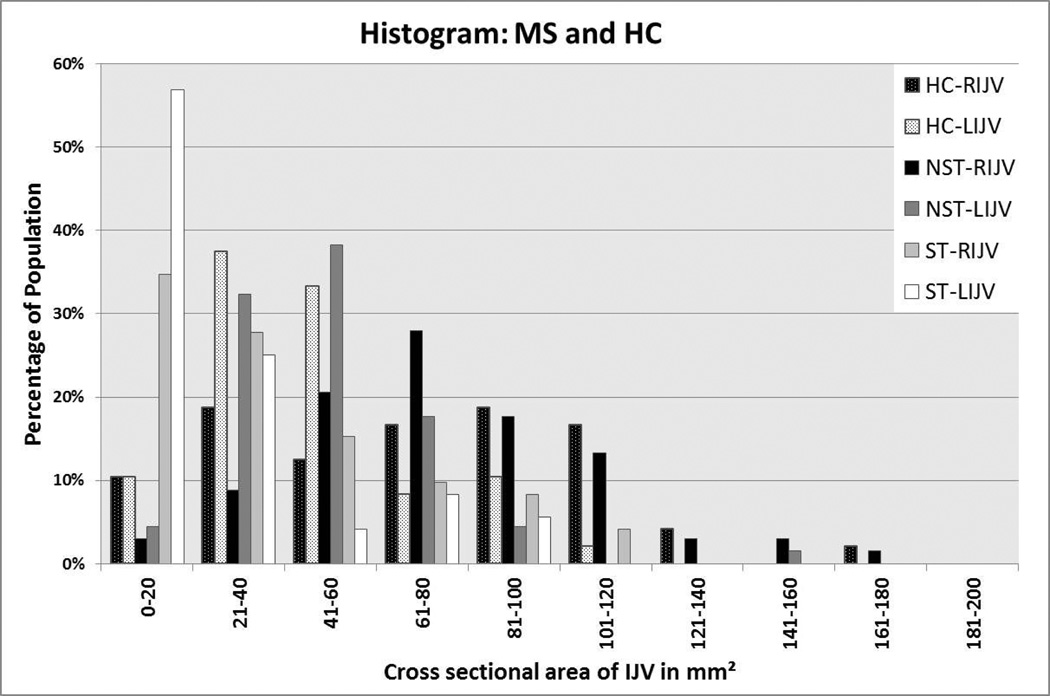
Figure 4.
Histogram of RIJV and LIJV for MS and HC groups showing the percentage of those samples within the given CSA ranges. This data set comes from the C5/C6 neck level using 2D TOF MRV (and if no signal was present, then the 3D CE MRAV was used). There are 48 cases here as 19 did not have venography collected.
To identify possible differences in flow by cervical level and side (left or right), group differences in normalized IJV flow were tested in a 2 (cervical level) × 2 (side) repeated measure GLM. Across all participants, normalized IJV flow was larger at the C2/C3 level compared to the C5/C6 level, F(1, 201) = 25.19, p < 0.001; and flow on the right side was larger than the left, F(1, 201) = 24.12, p < 0.001. However, the extent of these differences did not vary by group (all Fs < 0.54, ps > 0.59), nor were there lateralized group differences (group × side interaction: F(1,201) = 0.54, p = 0.46). Therefore, additional analyses of group differences included normalized flow averaged across bilateral C2/C3 and C5/C6 measures.
Group Differences in IJV flow
Total arterial flow did not differ between site 1 and site 2, t(203), 1.35, p = 0.18. After controlling for age, a non-significant trend towards a difference in average normalized flow between the three groups was observed, F(1, 200) = 3.50, p = 0.06. The trend reflected an isolated difference in the ST-MS group. Follow-up paired t-tests identified that average normalized IJV flow did not differ between HC (M = 0.74, SD = 0.14) and NST-MS (M = 0.75, SD = 0.11; t(131) = 0.87, p = 0.38). Critically, average normalized flow in the ST-MS group (M = 0.52, SD = 0.20) was significantly lower than that of NST-MS, t(136) = 8.31, p < 0.001, and HC, t(137) = −7.15, p < 0.001. Across groups, there were no age: (F (1, 200) = 1.00, p = 0.32) or sex: (F (1, 200) = 0.10, p = 0.75) differences.
Normalized IJV flow in the MS group as a whole differed from flow in the HC group at both cervical levels, t(203) = −3.70, p < 0.001. There was an age × group interaction, F (1,200) = 4.93, p = 0.03, due to the wider age range of the HC [(b = 4.3 × 10−5, R2 = 2.0 × 10−5; 95% CI(−2.6 × 10−3, 2.7 × 10−3)] compared to the MS group [(b = 2.2 × 10−4, R2 = 1.7 × 10−4; 95% CI(−2.7 × 10−3, 3.1 × 10−3)], however, it was controlled in the group model. Group differences were limited to IJV flow, as MS and HC had similar tA, t(203), 1.35, p = 0.18. The flow measures at each cervical level are presented in Table 3. To confirm that age differences between the groups did not group differences in flow, a second set of analyses were performed with HC in the same age range as MS. In these secondary analyses, normalized IJV flow in the MS (n = 138, M = 0.63, SD = 0.20) group as a whole differed from flow in the HC (n = 55, M = 0.73, SD = 0.14) group at both cervical levels, t(191) = −3.70, p < 0.001. The ST-MS group (M = 0.52, SD = 0.20) was lower than that of the HC, t(125) = −6.57, p < 0.001; while the NST group (M = 0.75, SD = 0.11) showed similar flow to the HC, t(119) = 1.02, p = 0.16.
Table 3.
Normalized flow measurements at the C2/C3 and C5/C6 neck level for all MS, non-stenotic MS (NST), stenotic MS (ST) and healthy controls (HC).
| HC | NST-MS | ST-MS | All MS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| C2/C3 | |||||||||
| HR (/s) | 65.05 | 12.01 | 66.24 | 12.14 | 67.84 | 9.43 | 67.07 | 10.8 | |
| FRn | |||||||||
| LICA/tA | 0.36 | 0.04 | 0.37 | 0.05 | 0.38 | 0.05 | 0.37 | 0.05 | |
| RICA/tA | 0.36 | 0.04 | 0.36 | 0.05 | 0.36 | 0.05 | 0.36 | 0.05 | |
| LVA/tA | 0.14 | 0.05 | 0.15 | 0.05 | 0.14 | 0.06 | 0.15 | 0.05 | |
| RVA/tA | 0.14 | 0.04 | 0.11 | 0.05 | 0.12 | 0.05 | 0.12 | 0.05 | |
| LIJV/tA | 0.26 | 0.17 | 0.29 | 0.17 | 0.19 | 0.21 | 0.24 | 0.2 | |
| RIJV/tA | 0.51 | 0.19 | 0.51 | 0.2 | 0.36 | 0.29 | 0.44 | 0.26 | |
| tIJV/tA | 0.78 | 0.16 | 0.81 | 0.13 | 0.55 | 0.24 | 0.68 | 0.23 | |
| sdJ/dJ | 0.41 | 0.26 | 0.52 | 0.29 | 0.22 | 0.27 | 0.36 | 0.32 | |
| sdJ/tA | 0.21 | 0.12 | 0.25 | 0.14 | 0.08 | 0.1 | 0.16 | 0.14 | |
| C5/C6 | |||||||||
| HR (/s) | 65.18 | 12.56 | 67.27 | 12.3 | 67.92 | 10.27 | 66.17 | 12.35 | |
| FRn | |||||||||
| LCCA/tA | 0.39 | 0.03 | 0.4 | 0.04 | 0.41 | 0.03 | 0.41 | 0.04 | |
| RCCA/tA | 0.4 | 0.04 | 0.4 | 0.03 | 0.4 | 0.04 | 0.4 | 0.05 | |
| LVA/tA | 0.11 | 0.04 | 0.11 | 0.03 | 0.11 | 0.04 | 0.11 | 0.05 | |
| RVA/tA | 0.1 | 0.04 | 0.09 | 0.04 | 0.08 | 0.04 | 0.09 | 0.05 | |
| LIJV/tA | 0.25 | 0.13 | 0.26 | 0.13 | 0.18 | 0.15 | 0.22 | 0.15 | |
| RIJV/tA | 0.44 | 0.17 | 0.43 | 0.15 | 0.31 | 0.21 | 0.37 | 0.19 | |
| tIJV/tA | 0.69 | 0.17 | 0.69 | 0.12 | 0.49 | 0.19 | 0.59 | 0.19 | |
| sdJ/dJ | 0.43 | 0.24 | 0.55 | 0.27 | 0.29 | 0.28 | 0.42 | 0.3 | |
| sdJ/tA | 0.2 | 0.1 | 0.23 | 0.11 | 0.1 | 0.09 | 0.16 | 0.12 | |
Note. Flow rates (mL/s) are normalized as a fraction of total arterial (tA) flow are shown. HR=heart rate; FRn= normalized flow rate; sdJ=sub dominant IJV; dJ= dominant IJV. Because we are interested in flow to the cranium, the vessels ICA and VA are chosen as the vessels in the C2/C3 normalization calculation. Though other arteries exist such as the external carotids which supply the facial tissue, they do not supply flow to the brain.
The means and standard deviations of the normalized individual and total IJV flow observed here are compared to Feng and colleagues’ [16] findings in Table 4. Further, the normalized flow measurements for the LIJV (t(459), t = 0.05, p = 0.96), RIJV (t(459), t = 0.41, p = 0.68 two tailed), and total IJV flow (t(459), t = 0.42, p = 0.34) are also in high agreement with each other.
Table 4.
Normalized individual and total IJV flow comparing present measures of the MS group to that reported for MS in Feng et al.[16]. Reported values are the normalized flow averaged across C2/C3 and C5/C6 cervical levels. Results show no differences between the groups.
| Left IJV |
Right IJV |
Total IJV |
||
|---|---|---|---|---|
| Reported sample | M | 0.23 | 0.40 | 0.63 |
| SD | 0.03 | 0.04 | 0.05 | |
| Feng et al. | M | 0.23 | 0.39 | 0.62 |
| SD | 0.02 | 0.03 | 0.03 | |
No Group Differences in Lateralized IJV Flow Dominance
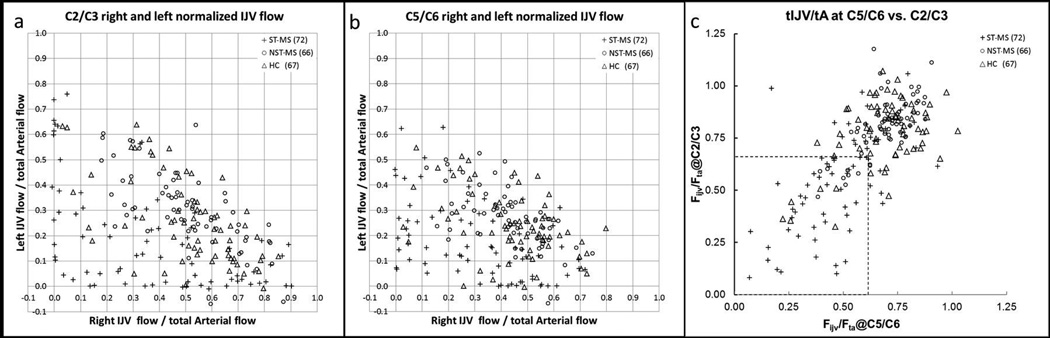
For the HC group, 52/67 (78%) subjects were right IJV dominant, and 15/67 (22%) were left dominant. For the MS group, 94/138 (68%) subjects were right IJV dominant, and 34/138 (32%) were left dominant. Figure 2 illustrates the normalized right and left IJV flows at both the C2/C3 (a) and C5/C6 (b) levels. There were no group differences in the percentage of people who were right IJV dominant or left IJV dominant (χ2 = 1.98, p = 0.16). The total IJV flow rates are plotted in Figure 3 to better illustrate the overall functioning of the IJV at both cervical levels and both IJVs.
Figure 2.
a) Plot of right versus left C2/C3 normalized IJV flow. b) Plot of right versus left C5/C6 normalized IJV flow. c) IJV flow normalized to arterial flow for the C5/C6 neck level plotted against the C2/C3 neck level. Legend: triangles: HC; open circles: NST-MS; and plus signs: ST-MS. Threshold lines of Fijv/TA at C6 < 0.62 and Fijv/TA atC2 < 0.66 are drawn (dotted lines).
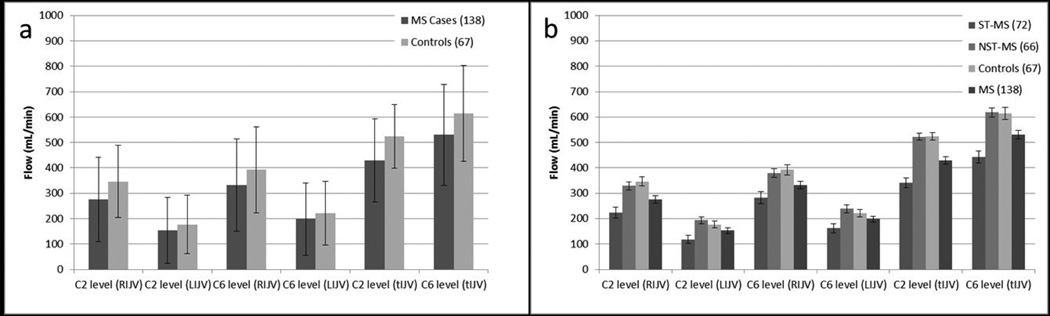
Figure 3.
a) Comparison of mean flow (mL/min) between MS and HC groups at both the C2/C3 and C5/C6 neck levels for LIJV, RIJV and total IJV flow (one standard deviation error bars of the population distribution are shown). This plot is an attempt to replicate the data from the study by Rodger and colleagues [23]. b) Comparison of mean flow between the ST-MS group, NST-MS group, and HC group at both C2/C3 and C5/C6 neck levels for LIJV, RIJV and total IJV flow. In this chart, the bars represent the standard error of the mean. As discussed in the text, there are significant differences between the ST-MS group and the HC for total IJV flow.
IJV Flow Discriminates between Groups
Based upon the ROC analysis, differences in normalized IJV flow discriminated between ST-MS and HC. The largest difference between the ST-MS and HC groups was observed at 0.66 at the C2/C3 cervical level and 0.62 at the C5/C6 cervical level (Figure 2c). When these thresholds were applied to the HC and MS groups at both cervical levels, 4/67 (6%) of the HC group and 51/138 (37%) of the MS group as a whole meet the criteria. This included 44/72 (61%) ST-MS and 7/68 (11%) NST-MS subjects. We further classified the type of MS of cases that fell below the criterion of 0.62 at each cervical level. At C2/C3, 43/110 (39%) RR-MS, 5/11 (45%) PP-MS, and 2/17 (13%) SP-MS fell below this threshold; and at C5/C6, 58/110 (53%) RR-MS, 6/11 (54%) PP-MS, and 6/17 (35%) SP-MS fell below this threshold.
Discussion
This is the fourth study of its kind to evaluate structural and flow abnormalities of the IJV in MS patients using MR angiography and PCFQ [1, 16, 19]. However, previous studies included few, if any HC cases. Although the previous results suggested a difference in structural and flow behavior between MS patients and HC, there was insufficient evidence to suggest a criterion. Here, we show in an ROC analysis that a criterion difference of 62–66% in normalized IJV flow distinguishes ST-MS patients from HC. The classification of MS patients into ST and NST groups based solely on the anatomical information led to the additional conclusion that the flow in HC and ST-MS subjects differs while the HC and NST-MS subjects appear to have the same flow properties.
Much of the discussion of whether or not CCSVI is present more in disease states than in a healthy population has been based on the original criteria proposed by Zamboni and colleagues [8]. These disagreements may stem, in part, from the inconsistency of ultrasound operators across sites, as well as the validity of the five conditions he proposed. To avoid this potential bias, we present a different means of assessing vascular abnormalities that are independent of the methods proposed by the Zamboni group [8]. These measures include the presence or absence of stenosis and the tIJV/tA measurement. As demonstrated here, these two methods distinguished a subgroup of MS patients with stenosis that had different CSA and flow distributions compared to HC. Further, we have provided reliability data while the Zamboni group did not [8], which also may account for the lack of data replicability.
It is important to note that, for veins, the definition of stenosis is an absolute and not a relative (i.e., percentage) one. Generally, the relative definition is used in evaluating carotid arteries, which have geometrically consistent CSA from the subclavian to the bifurcation; while the IJV can flare out at the base near the subclavian vein and can vary in size throughout its course. Zamboni originally proposed 0.30 cm2 as a cutoff [8] whereas we chose 0.25 cm2 at or below C3 and 0.125 cm2 above C3 to be consistent with previous work [1]. A recent study suggested no difference in stenosis rates between HC and MS patients [21], with a stenosis rate of 74% in MS patients and 70% in HC. The study, however, defined IJV stenosis as a narrowing of more than 50% of the widest vessel segment below the mandible. If we were to do the same analysis using this percentage method, we would have found 83% of MS patients and 44% of HC being stenotic suggesting that the percentage definition of stenosis for veins is unrealistic.
Figure 4 shows a percentage of the MS and HC groups who were stenotic using a variable CSA at the C5/C6 neck level. At a CSA ≤ 25 mm2, none of the HC was classified as stenotic for the RIJV, while 4% of the HC met this criterion for the LIJV. In the MS group, 24% of the patients were classified as stenotic for the RIJV, and 38% meet this criterion for the LIJV. In general, more MS patients were classified as stenotic even using a higher CSA cutoff value. As shown in Table 2, various types of stenosis are observed among MS patients. Particularly, cases with atresia, diffuse stenosis, and aplasia were only seen in the MS group.
In addition to differences in the method of stenosis measurement, previous MR studies conducted by other groups have evaluated a limited number of patients [11, 13, 22], have not combined anatomic and flow information [9] or have not fully analyzed the data as presented herein. A recent paper gives a limited evaluation of the flow data, examining only left and right IJVs but not total flow and without further evaluation of differences related to stenosis [23]. As shown in Figure 3a, measures of total IJV flow distinguished MS patients from HC. Figure 3b shows a greater difference in total IJV flow between the ST-MS and the HC and NST-MS groups. Only by virtue of this analysis did we identify a difference between MS and HC, which is possibly why previous studies that relied on lateralized histogram analyses did not.
It is of interest to note that despite the use of a different MR system (Siemens versus General Electric) and certain dissimilarity in population base, that the results for arterial flow between our study and the work of Feng and colleagues [16] are in high agreement. The convergence of evidence across these two independent studies supports the notion that venous flow abnormalities are present in the MS population, and may therefore be a meaningful aspect of the disease etiology.
This study has several limitations. One is a potential systematic bias between the sites; i.e., absolute flows measured from the images collected at two different sites may not be the same. Although the HC and MS patients were scanned on different scanners, they were scanned with the same sequence parameters on machines built by the same manufacturer. As demonstrated, arterial flow measures were similar across all participants. Therefore, by normalizing IJV to arterial flow, the concern about systematic scanner differences is mitigated.
The second concern about the data quality is the presence of white noise in all flow and CSA measurements. Because these noise-related errors are similar in HC and MS groups, this should introduce only a negligible bias in the present analyses. In Appendix I, relative errors from the scanners were estimated based on the work of Wolf et al. using average values from all cases in the site [24].
The third concern relates to possible differences attributable to head position and use of neck cushions, which could potentially compress the IJV and artificially alter flow. Here we partly address the potential confound with repeated measurements of a single participant across days with different head positions, and we find no significant differences. However, we cannot presently comment on the effects of neck pillows or head constraints. Future flow studies should investigate the effects of age with respect to CSA measurements and blood flow.
Finally, the method used here to identify a subgroup of MS patients can provide valuable insights into other vascular and neurological conditions. Indeed, Parkinson’s disease has been studied with this method already [25]. Other conditions characterized by impaired venous outflow, such as idiopathic intracranial hypertension and traumatic brain injury [25–27] may also benefit from this approach.
Acknowledgements
Funding: The authors would like to thank MR Innovations India, Robert Loman, MD, and Imran Saqib for assistance with data processing; Paul Kokeny for assistance with statistics; Dr. Phil Levy from Wayne State University for usage of healthy control data; and the Annette Funicello Research Fund and the Center for Neurological Diseases for assistance with funding. This work was supported in part by a grant from the National Institute on Aging, R37-AG011230 to NR.
Appendix I
Errors in PCFQ stem from two main causes: noise, and partial volume effect (PV). The following assumptions were made in order to estimate PV effects: vessels are circular, flow is laminar, resolution is 1 mm3, average partial volume fraction is constant 0.7, and all PV pixels were included in the segmentation. In work by Wolf et al. [24], the models examining intravoxel phase dispersion and PV effects were based on the simplifying assumption of uniform magnitude of isochromats within a vessel; that is, flowing isochromats were always assumed to have experienced only one RF pulse as they passed through the imaging slice. In reality, isochromats near the edge of the vessel will have decreased magnitude due to decreased flow-related enhancement.
| Error Type | VA | CCA/[ICA] | IJV | tA | tIJV/tA | |
|---|---|---|---|---|---|---|
| HC-C5/C6 | PV | 1.33% | 0.44% | −0.16% | 0.29% | |
| HC-C5/C6 | SNR | 4.18% | 3.07% | 7.04% | 3.30% | |
| HC-C5/C6 | Total | 4.38% | 3.10% | 7.05% | 3.31% | 7.78% |
| MS-C5/C6 | PV | 1.48% | 0.53% | −0.14% | 0.34% | |
| MS-C5/C6 | SNR | 4.21% | 2.75% | 8.09% | 3.03% | |
| MS-C5/C6 | Total | 4.46% | 2.80% | 8.09% | 3.05% | 8.64% |
| HC-C2/C3 | PV | 1.37% | [0.84%] | −0.67% | 0.45% | |
| HC-C2/C3 | SNR | 4.04% | [2.78%] | 4.91% | 3.13% | |
| HC-C2/C3 | Total | 4.26% | [2.90%] | 4.96% | 3.17% | 5.88% |
| MS-C2/C3 | PV | 1.44% | [0.84%] | −0.72% | 0.46% | |
| MS-C2/C3 | SNR | 4.36% | [2.88%] | 5.34% | 3.27% | |
| MS-C2/C3 | Total | 4.59% | [3.00%] | 5.39% | 3.30% | 6.31% |
References
- 1.Haacke EM, Feng W, Utriainen D, Trifan G, Wu Z, Latif Z, Katkuri Y, Hewett J, Hubbard D. Patients with multiple sclerosis with structural venous abnormalities on MR imaging exhibit an abnormal flow distribution of the internal jugular veins. J Vasc Interv Radiol. 2012;23:60–68. doi: 10.1016/j.jvir.2011.09.027. e1-3. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charcot J-M. Histologie de la sclérose en plaques. 1868 [Google Scholar]
- 4.Cruveilhier J. Pathological anatomy of the human body. Paris (France): Billière; 1829. [Google Scholar]
- 5.Rindfleisch E. Histological detail to the gray matter degeneration of brain and spine. Arch Path Anat Physiol Klin Med. 1863;26:474. [Google Scholar]
- 6.Haacke EM. Chronic cerebral spinal venous insufficiency in multiple sclerosis. Expert Rev Neurother. 2011;11:5–9. doi: 10.1586/ern.10.174. [DOI] [PubMed] [Google Scholar]
- 7.Aboulker J, Bar D, Marsault C, Khouadja F, Redondo A, Garel L, Nahum H. Intraspinal venous hypertension caused by muliple abnormalities of the caval system: a major cause of spinal cord problems. Chirurgie. 1977;103:1003–1015. [PubMed] [Google Scholar]
- 8.Zamboni P, Galeotti R, Menegatti E, Malagoni AM, Tacconi G, Dall'Ara S, Bartolomei I, Salvi F. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. 2009;80:392–399. doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zivadinov R, Lopez-Soriano A, Weinstock-Guttman B, Schirda CV, Magnano CR, Dolic K, Kennedy CL, Brooks CL, Reuther JA, Hunt K, Andrews M, Dwyer MG, Hojnacki DW. Use of MR venography for characterization of the extracranial venous system in patients with multiple sclerosis and healthy control subjects. Radiology. 2011;258:562–570. doi: 10.1148/radiol.10101387. [DOI] [PubMed] [Google Scholar]
- 10.Doepp F, Wurfel JT, Pfueller CF, Valdueza JM, Petersen D, Paul F, Schreiber SJ. Venous drainage in multiple sclerosis: a combined MRI and ultrasound study. Neurology. 2011;77:1745–1751. doi: 10.1212/WNL.0b013e318236f0ea. [DOI] [PubMed] [Google Scholar]
- 11.Sundström P, Wåhlin A, Ambarki K, Birgander R, Eklund A, Malm J. Venous and cerebrospinal fluid flow in multiple sclerosis: A case-control study. Annals of neurology. 2010;68:255–259. doi: 10.1002/ana.22132. [DOI] [PubMed] [Google Scholar]
- 12.Menegatti E, Genova V, Tessari M, Malagoni A, Bartolomei I, Zuolo M, Galeotti R, Salvi F, Zamboni P. The reproducibility of colour Doppler in chronic cerebrospinal venous insufficiency associated with multiple sclerosis. Int Angiol. 2010;29:121–126. [PubMed] [Google Scholar]
- 13.Hojnacki D, Zamboni P, Lopez-Soriano A, Galleotti R, Menegatti E, Weinstock-Guttman B, Schirda C, Magnano C, Malagoni AM, Kennedy C, Bartolomei I, Salvi F, Zivadinov R. Use of neck magnetic resonance venography, Doppler sonography and selective venography for diagnosis of chronic cerebrospinal venous insufficiency: a pilot study in multiple sclerosis patients and healthy controls. Int Angiol. 2010;29:127–139. [PubMed] [Google Scholar]
- 14.Utriainen D, Feng W, Elias S, Latif Z, Hubbard D, Haacke EM. Using magnetic resonance imaging as a means to study chronic cerebral spinal venous insufficiency in multiple sclerosis patients. Tech Vasc Interv Radiol. 2012;15:101–112. doi: 10.1053/j.tvir.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Kokeny PJJ, Haacke EM. International Society for Magnetic Resonance in Medicine 2013. Salt Lake City UT, USA: 2013. Assessing the Effects of Vessel Segmentation Boundary Size on Flow Quantification Error and Comparing Multiple Automatic Segmentation Algorithms. [Google Scholar]
- 16.Feng W, Utriainen D, Trifan G, Elias S, Sethi S, Hewett J, Haacke EM. Characteristics of flow through the internal jugular veins at cervical C2/C3 and C5/C6 levels for multiple sclerosis patients using MR phase contrast imaging. Neurological research. 2012;34:802–809. doi: 10.1179/1743132812Y.0000000079. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa SNT, Sakaguchi I, Nishi K. The diameter of the internal jugular vein studied by autopsy. Rom J Leg Med. 2010:125–128. [Google Scholar]
- 18.Tartiere D, Seguin P, Juhel C, Laviolle B, Malledant Y. Estimation of the diameter and cross-sectional area of the internal jugular veins in adult patients. Critical care. 2009;13:R197. doi: 10.1186/cc8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng W, Utriainen D, Trifan G, Sethi S, Hubbard D, Haacke EM. Quantitative flow measurements in the internal jugular veins of multiple sclerosis patients using magnetic resonance imaging. Rev Recent Clin Trials. 2012;7:117–126. doi: 10.2174/157488712800100206. [DOI] [PubMed] [Google Scholar]
- 20.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 21.Traboulsee AL, Knox KB, Machan L, Zhao Y, Yee I, Rauscher A, Klass D, Szkup P, Otani R, Kopriva D, Lala S, Li DK, Sadovnick D. Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case-control study. Lancet. 2013;383:138–145. doi: 10.1016/S0140-6736(13)61747-X. [DOI] [PubMed] [Google Scholar]
- 22.Wattjes MP, van Oosten BW, de Graaf WL, Seewann A, Bot JC, van den Berg R, Uitdehaag BM, Polman CH, Barkhof F. No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow-quantification study. Journal of neurology, neurosurgery, and psychiatry. 2011;82:429–435. doi: 10.1136/jnnp.2010.223479. [DOI] [PubMed] [Google Scholar]
- 23.Rodger IW, Dilar D, Dwyer J, Bienenstock J, Coret A, Coret-Simon J, Foster G, Franchetto A, Franic S, Goldsmith CH, Koff D, Konyer NB, Levine M, McDonald E, Noseworthy MD, Paulseth J, Ribeiro L, Sayles MJ, Thabane L. Evidence against the involvement of chronic cerebrospinal venous abnormalities in multiple sclerosis. A case-control study. PLoS One. 2013;8:e72495. doi: 10.1371/journal.pone.0072495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf RL, Ehman RL, Riederer SJ, Rossman PJ. Analysis of systematic and random error in MR volumetric flow measurements. Magn Reson Med. 1993;30:82–91. doi: 10.1002/mrm.1910300113. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, Xu H, Wang Y, Zhong Y, Xia S, Utriainen D, Wang T, Haacke EM. Patterns of chronic venous insufficiency in the dural sinuses and extracranial draining veins and their relationship with white matter hyperintensities for patients with Parkinson's disease. Journal of vascular surgery. 2014 doi: 10.1016/j.jvs.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomschar A, Koerte I, Lee S, Laubender RP, Straube A, Heinen F, Ertl-Wagner B, Alperin N. MRI evidence for altered venous drainage and intracranial compliance in mild traumatic brain injury. PLoS One. 2013;8:e55447. doi: 10.1371/journal.pone.0055447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedelmann M, Kaps M, Mueller-Forell W. Venous obstruction and jugular valve insufficiency in idiopathic intracranial hypertension. J Neurol. 2009;256:964–969. doi: 10.1007/s00415-009-5056-z. [DOI] [PubMed] [Google Scholar]