Abstract
The central amygdala (CeA) plays a central role in physiological and behavioral responses to fearful stimuli, stressful stimuli, and drug-related stimuli. The CeA receives dense inputs from cortical regions, is the major output region of the amygdala, is primarily GABAergic (inhibitory), and expresses high levels of pro- and anti-stress peptides. The CeA is also a constituent region of a conceptual macrostructure called the extended amygdala that is recruited during the transition to alcohol dependence. In this review, we discuss neurotransmission in the CeA as a potential integrative hub between anxiety disorders and Alcohol Use Disorder (AUD), which are commonly co-occurring in humans. Human imaging work and multi-disciplinary work in animals collectively suggest that CeA structure and function are altered in individuals with anxiety disorders and AUD, the end result of which may be disinhibition of downstream “effector” regions that regulate anxiety- and alcohol-related behaviors.
INTRODUCTION
Anxiety disorders and Alcohol Use Disorders (AUD) are highly co-morbid in humans. Anxiety disorders often precipitate alcohol abuse, and high anxiety is a hallmark symptom of alcohol dependence that manifests during withdrawal. Many anxiety disorders are marked by hyperactivity and/or hyperreactivity of the amygdala (1), as supported by neuroimaging data, although functional MRI and PET do not yet possess the resolution to reliably differentiate amygdaloid nuclei.
In healthy humans, amygdala activity is increased during fear conditioning (2, 3). Humans with post-traumatic stress disorder (PTSD) exhibit higher amygdala activity at rest (4), and hyper-reactivity of the amygdala to trauma-related stimuli (5) is predictive of symptom severity in PTSD patients (6, 7). Higher levels of amygdala activation are seen in generalized anxiety disorder (8, but see 9), social phobia (10), specific phobia (11, but see 12), and panic disorder (13, but see 14).
Alcohol withdrawal is defined by lasting increases in anxiety (15) that contribute to relapse (16, 17). Withdrawal-induced anxiety is attributable to recruitment of both neuroendocrine and extra-hypothalamic stress systems in humans and animals (18, 19). Alcohol-dependent humans exhibit reduced amygdala volume, which predicts alcohol craving and relapse (20, 21). Moderate-to-heavy non-dependent drinkers exhibit reduced amygdala activation during a risk-taking task (22). Individuals with a family history of alcohol dependence exhibit reduced amygdala volume (23) and reduced amygdala activation in response to fearful faces (24). PTSD patients that abuse alcohol exhibit altered amygdala blood flow relative to normal controls (4). In a cue-reactivity fMRI task, alcohol cues activate amygdala, striatum, and cortical regions (25, 26). Amygdala abnormalities may result in disinhibition of downstream brain regions that regulate physiology and behavior, as detailed below.
THE CENTRAL AMYGDALA (CeA)
The CeA functions as an integrative hub that converts emotionally-relevant sensory information about the external and internal environment into behavioral and physiological responses. The CeA is part of the extended amygdala (EA), a collection of limbic forebrain structures (including the lateral division of the bed nucleus of stria terminalis [BNST], and nucleus accumbens [NAc] shell [27]) that exhibit similar cytoarchitecture, overlapping afferents and efferents, and strong inter-connectivity (28, 29). The EA mediates negative affective states associated with stress and AUD (30, 31), and is densely populated by pro- and anti-stress neuropeptides (32). Here, we discuss CeA dysregulation in anxiety disorders and AUD, and the contribution of CeA peptides to these pathologies, with emphasis on the pro-stress peptide corticotropin-releasing factor (CRF) and the anti-stress peptide neuropeptide Y (NPY).
AMYGDALA CIRCUITRY
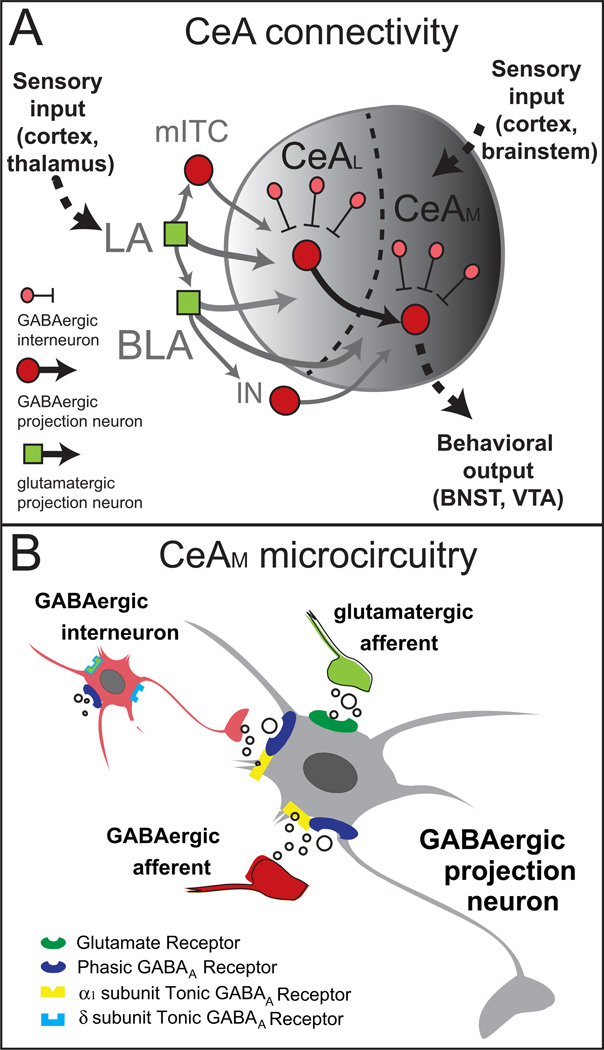
The amygdala is a collection of nuclei, including the lateral amygdala (LA), the basolateral amygdala (BLA), and the CeA, which contains lateral (CeAL) and medial (CeAM) subdivisions (Figure 1A). The amygdala exhibits a lateromedial flow of information from the LA/BLA to and through the intercalated cells (ITC), and into the CeA, which sends out information through amygdala efferents (33). The LA receives multi-sensory information from thalamus (34, 35), integrated sensory information from cortex (36), and noxious stimulus information from brainstem regions (37). The CeA also receives noxious stimuli information from brainstem regions (38, 39). Glutamatergic neurons in LA synapse onto glutamatergic BLA neurons, and onto GABAergic medial ITC cells (40) that separate BLA from CeA (41, 42). The LA/BLA sends dense glutamatergic projections to CeA, with the LA projecting only to CeAL, and the BLA projecting to both CeAL and CeAM (28, 43, 44). Projections out of BLA also synapse onto ITC GABA cells that in turn synapse on CeA neurons (45).
Figure 1.
(A) Schematic of amygdala circuitry showing inter- and intranuclear connectivity. Abbreviations: BLA- basolateral amygdala; CeAM- medial central amygdala, CeAL- lateral central amygdala; IN- main intercalated cell cluster; LA-lateral amygdala; mITC intercalated cell cluster. B. Microcircuitry of the CeAM illustrating excitatory transmission by glutamatergic afferents (green) and phasic and tonic inhibitory transmission by GABAergic afferents (dark red) and local interneurons (light red).
The CeAL and CeAM receive GABAergic afferents from other structures (46), contain local GABA interneurons and GABAergic projection neurons (47, 48) that may inhibit each other via axon collaterals (49; Figure 1B). The CeAL projects to CeAM, with no reciprocal projection from CeAM to CeAL (50). The CeAM is the major output nucleus of the amygdala and projects to regions that produce behavioral and physiological responses to emotionally relevant events (49, 50, 51), but recent data suggest the CeAL also sends GABAergic projections to behavioral and physiological effector regions (52).
Amygdala microcircuitry is critical for emotional processing, especially for interpretation of emotionally relevant stimuli or the attachment of emotional relevance to otherwise neutral stimuli (i.e., learning). Amygdala microcircuitry receives and integrates complex multi-modal information to produce behavioral responses. Amygdala dysfunction is implicated in both anxiety disorders (53) and substance abuse (30).
CeA AS A HUB FOR ANXIETY AND ALCOHOL CIRCUITS
Origins of Amygdala Afferents
Afferents from thalamus and cortex synapse in LA and ITC, which each project to CeA (Figure 1A). Medial prefrontal cortical (mPFC) inputs to amygdala have well-defined contributions to pathological behavioral states in humans and animals. Medial PFC pyramidal neurons send excitatory projections to the amygdala and are controlled by a complex network of GABA interneurons (54, 55). Human and animal studies suggest that alcohol and stress affect mPFC function and mPFC-amygdala functional connectivity.
The CeA integrates cortical/sensory inputs with innervation from “downstream” brainstem regions (50) including: 1) the ventral tegmental area (VTA), important for reward and synthesis of forebrain dopamine; 2) the locus coeruleus (LC) and nucleus of solitary tract (NTS), important for stress response, autonomic function and synthesis of brain norepinephrine; and 3) the periaqueductal gray (PAG), critical for pain processing. The CeA also receives input from the BNST (56), important for anxiety regulation, and is sensitized by glucocorticoid feedback following hypothalamic-pituitary adrenal (HPA) axis activation (57, 58), in contrast to glucocorticoid-mediated negative feedback in the PVN.
Effector Regions Targeted by CeA Efferents
The CeA integrates cortical, brainstem, and intra-amygdala afferents to coordinate behavioral and physiological responses via projections to downstream “effector” regions (Figure 1A). The target of specific CeAM projections determines the behavioral consequences of changes in amygdala activity, but evidence also exists for a subpopulation of CeAL neurons (i.e., oxytocin receptor-expressing neurons) with terminals in CeAM and ventral forebrain that dictate whether fear coping behaviors are passive (e.g., freezing) or active (e.g., exploratory/risk assessment) (59). Whether CeAM projection neurons exhibit mutually exclusive or overlapping targets and activation profiles is not fully understood. Basal amygdala projection neurons display anatomical and functional specificity in fear expression versus extinction conditions (60), raising the possibility that CeAM populations are likewise differentially activated by specific stimulus conditions.
Periaqueductal Gray (PAG)
The PAG is important for descending behavioral and physiological responses to fearful and painful stimuli (61). The CeAM sends dense and organized GABAergic projections to PAG (62) that co-localize CRF and substance P (63) and gate the anti-nociceptive pain response mediated by opioids in PAG (64, 65). The amygdala and PAG are each activated by unconditioned aversive stimuli, and this response is dampened by signals predictive of those stimuli (66).
Lateral Hypothalamus (LHA)
The LHA mediates autonomic responses to fearful stimuli (61), and houses dopamine (DA) fibers that project from VTA to forebrain and mediate brain reward function (67). The CeA sends dense GABAergic projections to the LHA (28, 68). Electrical kindling of the CeA increases the sensitivity of LHA to drug-induced facilitation of brain reward function (69), whereas CeA lesion reduces DA activity in LHA (70).
Paraventricular Hypothalamus (PVN)
The PVN regulates the neuroendocrine stress response via CRF projections to the pituitary that promote ACTH and cortisol/corticosterone production and release. The CeAM, but not the CeAL, sends monosynaptic (71) and disynaptic (72) projections to the PVN, which may function as a relay station to brainstem nuclei (73). Electrical CeA stimulation activates the HPA stress axis (74), and the CeA mediates pro-inflammatory cytokine-induced activation of the HPA axis (75).
Locus Coeruleus (LC)
The LC produces norepinephrine and regulates autonomic responses to stress (76). GABAergic projections from CeA to LC (77) often co-localize the pro-stress peptides, CRF and dynorphin (78), and synapse onto NE neurons in LC (79), creating a feed-forward loop that is activated during stress and alcohol withdrawal (80, 81). These CeA neurons also express glucocorticoid receptors, suggesting regulation by neuroendocrine feedback from the HPA axis (82).
Dorsal Vagal Complex (DVC)
The DVC is composed of the nucleus of the solitary tract (NTS) and the dorsal motor nucleus of the vagus (DMV), and is important for autonomic regulation. The CeAM sends GABAergic projections to NTS and DMV (83, 84) that mediate autonomic (i.e., parasympathetic) responses to aversive stimuli (85) and contribute to chronic stress-induced hypertension (86).
THE CeA IN ANXIETY AND ALCOHOL EFFECTS
CeA Neurotransmission in Regulation of Anxiety Responses
Amygdala activation mediates emotional responses to fearful or anxiety-provoking stimuli in healthy humans (87), and this response is specific to stimuli with a negative valence, even when the valence is not consciously registered (88). Humans with an anxiety disorder (e.g., PTSD) often exhibit hyperactive amygdala responses to these types of stimuli (89). Similarly, chronically stressed rats exhibit hyperexcitability of the LA (90) and CeA lesion blocks chronic stress-induced increases in anxiety-like behavior (91).
Within the amygdala, optical stimulation and inhibition of BLA-to-CeA projection neurons bi-directionally modulates anxiety-like behavior in rodents (53). This may explain the strong correlation between BLA and CeA activation, as measured by ERK phosphorylation, observed in previously stressed animals exposed to a stress reminder (92). Human and animal studies also suggest that individuals that exhibit high reactivity (i.e., poor coping) to traumatic stress exhibit heightened functional connectivity between PFC and amygdala nuclei (89, 92).
CeA Neurotransmission in Fear Conditioning
Rodent fear conditioning experiments have significantly contributed to our understanding of the circuitry mediating anxiety disorders. Plasticity in the LA has a central role in fear conditioning (37), but the CeA also has roles in acquisition, expression, generalization, consolidation, and extinction of conditioned fear (93–97). CeAL plasticity contributes to acquisition of conditioned fear, and CeAM output neurons are excited by fear stimuli in a manner that decays with extinction and that is sensitive to the activity of somatostatin-positive CeAL neurons (97–99).
Fear extinction relies heavily on descending projections from infralimbic cortex (ILC) to amygdala. Intercalated GABA cells are critical for mediating fear extinction via projections to CeA (100, 101). For example, CeAM neurons of fear-extinguished animals exhibit greater synaptic inhibition by ITC cells, likely due to increased excitatory drive from BLA onto ITC cells, an effect that is contingent on ILC activity during extinction (102). The net result of fear extinction is reduced inhibitory output from CeAM to brainstem effector regions, an effect due to either more inhibitory ITC input onto CeAM neurons, less inhibitory ITC input onto CeAL GABA neurons that project to CeAM, or both (40, 103).
CeA Neurotransmission in Acute Alcohol Effects
Relative to the fear and anxiety literature, less is known about the molecular identity and projection pattern of specific CeA circuits mediating alcohol effects. Acute alcohol increases GABAergic transmission in the BLA via increased pre-synaptic GABA release (104, 105), which has implications for downstream CeA neurons via dense excitatory projections to CeA (45). An emerging story has been the potentially overlapping role of cortico-amygdalar projections in conditioning/extinction processes related to cues and contexts associated with both fear and alcohol/drugs. Specifically, prelimbic projections to NAc core and BLA facilitate expression of cocaine-seeking behavior and fear, respectively, whereas infralimbic projections to NAc shell and CeA (via ITC) facilitate extinction of cocaine-seeking behavior and fear, respectively (106). Recent data suggest that prelimbic and infralimbic cortices regulate extinction and reinstatement of alcohol-seeking behavior (107), and it is possible that these effects are mediated by projections to amygdala.
CeA neurons display two types of inhibition: phasic, which involves inhibitory postsynaptic currents (IPSCs) that reflect ‘point to point’ transmission; and tonic, which involves persistent inhibitory currents resulting from ambient GABA acting at highly-sensitized GABAA receptors (108, 109). Tonic inhibition regulates neural network activity (110), and is modulated by both acute and chronic alcohol (111, 112). Acute alcohol dose-dependently and reversibly increases phasic GABA release in the CeA (113, 114), independent of GABABR blockade (113). Acute alcohol also increases phasic and tonic inhibition in a population of CeA neurons that synapse onto CeAM output neurons, resulting in disinhibition of CeA output to BNST (115).
CeA Neuroadaptations in Response to Chronic Alcohol
Offspring of alcohol-dependent humans exhibit reduced amygdala volume and reduced amygdala fMRI activation in response to fearful faces (116, 117). Furthermore, moderate-to-heavy drinking humans exhibit reduced amygdala activation during impulse control tasks (118). Alcohol-dependent humans that have endured more detoxifications and exhibit more loss-of-control over drinking also exhibit increased PFC-amygdala connectivity during attentional and executive function tasks (119).
Much of what is known about alcohol-induced neuroadaptations in CeA comes from studies on animals chronically exposed to intermittent bouts of alcohol with repeated withdrawal periods. This protocol accelerates the emergence of somatic, affective and motivational indices of alcohol dependence (120, 121). Relative to stress models, these dependence models may be most appropriately compared to findings from chronic stress studies. Indeed, alcohol dependence has been conceptualized in terms of a stress kindling process, in which CeA neuroadaptations play a central role (122).
Many studies on chronic alcohol effects on CeA neurotransmission utilize a chronic intermittent ethanol (CIE) vapor inhalation model in rodents (123). CIE augments spontaneous and evoked CeA GABA release via pre- and post-synaptic mechanisms (104, 114, 124). Alcohol-dependent rats exhibit increased GABA release in CeA during withdrawal, but do not exhibit tolerance to acute alcohol effects on CeA GABAergic transmission (114). Reductions in basal pre-synaptic GABABR activity during withdrawal may account for increased baseline CeA GABAergic transmission in alcohol-dependent rats (125). Gabapentin, a structural analog of GABA, facilitates evoked GABAergic transmission in alcohol-naïve rats, an effect that is blocked by a GABABR antagonist. Conversely, gabapentin decreases evoked CeA GABA transmission during alcohol withdrawal, suggesting that alcohol dependence-induced GABABR neuroadaptations may account for the differential behavioral effects of gabapentin in dependent versus non-dependent animals (125).
CeA STRESS PEPTIDES IN ANXIETY AND ALCOHOL DEPENDENCE
Role of CeA Pro- and Anti-Stress Peptides in Anxiety
Humans with anxiety disorders exhibit altered levels of pro- and anti-stress peptides in the CNS and periphery. Here, we discuss a few examples in the context of human PTSD, an anxiety disorder that can manifest following an acute traumatic stress event and that is highly comorbid with AUD (126). Positive coping and stress resilience in PTSD veterans are each predicted by higher plasma levels of the anxiolytic NPY (127). PTSD is also associated with polymorphisms and methylation levels for the genes encoding the anxiogenic pituitary adenylatecyclase-activating polypeptide (PACAP) and its receptor PAC1, and PAC1 mRNA is upregulated in the amygdala of fear-conditioned mice (128).
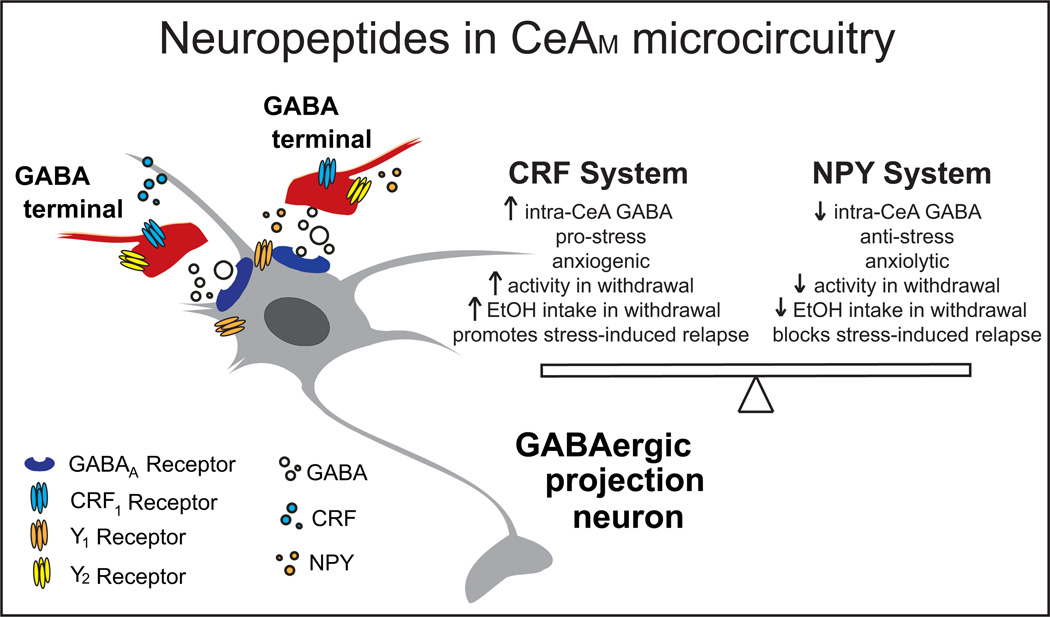
As illustrated in Figure 2, CRF and NPY co-localize in the CeA, where CRF promotes anxiety-like behavior (131) an NPY reduces anxiety-like behavior (132). The acoustic startle reactivity (ASR) test can be used to assess control by specific EA regions (CeA, BNST) over generalized anxiety-like or stimulus-specific fear behaviors. NPY dampens basal ASR and fear-potentiated startle in rats, and facilitates extinction of fear-potentiated startle, effects likely mediated by the CeA (133). CRF increases ASR (134) and mediates stress-induced enhancement of ASR via CRF1s in BNST (135). Acute restraint or footshock stress increases CRF mRNA in rat CeA (136, 137), and CeA CRF is critical for consolidation of fear memories (138). Intra-amygdala injection of a CRF agonist produces an aversive state resembling that elicited by an environmental stressor, and both effects are blocked by intra-amygdala injection of NPY (139). Following exposure to predator stress, rats with maximally dysregulated behavior (e.g., hyperarousal and high anxiety-like behavior) exhibit reduced NPY in the amygdala (140), and treatment with either NPY or a CRF1 antagonist reduces behavioral dysregulation in rodents following exposure to predator stress (140,141).
Figure 2.
Schematic illustrating location of neuropeptides and their receptors in the medial CeA synapse, and their proposed roles in stress, anxiety, and alcohol effects. Here and in the text, we focus on pro-stress pro-alcohol-drinking CRF and anti-stress anti-alcohol-drinking NPY systems, intended to provide a snapshot of what may be (or is) occurring with other stress peptides in CeA in response to stress and alcohol. Abbreviations: CeAM- medial central amygdala, CRF1- corticotropin-releasing factor receptor 1, NPY- neuropeptide Y, Y1 NPY Y1 receptor, Y2 - NPY Y2 receptor.
CeA Pro- and Anti-Stress Peptides in Alcohol Dependence
Alcohol withdrawal is defined by a negative emotional state mediated in part by the recruitment of pro- and anti-stress peptides in the EA (30). CRF and NPY in CeA play critical roles in mediating negative affect and excessive alcohol drinking in dependent rodents (Figure 2). CRF and NPY are both likely produced locally in the CeA (142, 143) and/or imported to CeA from distal projection neurons, but it is not yet clear which peptide pools are dysregulated by alcohol dependence (or stress) to produce heightened anxiety-like behavior and escalated alcohol drinking.
CRF increases GABA release in the CeA of rats (124) and mice (144) via activation of pre-synaptic CRF1s. These effects are exaggerated during withdrawal (124), along with concomitant increases in CRF and CRF1 mRNA levels, and increases in CRF release in the CeA of alcohol-dependent rats (124, 145). CRF1 antagonism reverses withdrawal-induced increases in drinking in alcohol-dependent rats and mice (146, 147) via effects in CeA (148), and chronic CRF1 antagonism prevents escalation of alcohol drinking during the transition to dependence (124). Binge-like drinking increases CRF immunoreactivity in the CeA of mice (149), and CRF1 antagonists reduce binge-like drinking without affecting non-binge-like alcohol intake (150–152). Interestingly, the ability of CRF to increase CeA GABAergic transmission is blunted in binge-like drinking mice (149), in contrast to the sensitized CRF effects observed in CeA of alcohol-dependent rats (124). Binge-like alcohol drinking may abolish CRF effects on GABAergic transmission in CeA via internalization of CRF1s in response to elevated CRF levels in binge alcohol drinkers (149), as seen in the dorsal raphe following stress (153).
The complex alcohol effects on local CeA microcircuitry are illustrated in the differential ethanol sensitivity of CRF1+ CeA neurons possessing an ethanol-insensitive ongoing tonic conductance and CRF1- CeA neurons possessing a tonic conductance that is enhanced by acute alcohol (115). Accordingly, acute alcohol decreases firing of CRF1-neurons, but increases firing of CRF1+ neurons, suggesting a local CeAM inhibitory microcircuit whose constituent neurons are differentially regulated by alcohol, similar to the CeAL inhibitory microcircuit described in the behavioral expression of conditioned fear (154). Future optogenetic studies will dissect the role of CeA microcircuitry in the behavioral consequences of alcohol dependence and as a locus for integration of anxiety and alcohol abuse.
NPY decreases GABAergic transmission in CeA, and also prevents and reverses acute alcohol-induced facilitation of evoked GABAergic transmission in the CeA of naïve and alcohol-dependent rats (155). Pharmacological experiments suggest that NPY exerts Y1 receptor (Y1R)-mediated post-synaptic effects on basal inhibitory transmission in CeA, whereas NPY blocks alcohol effects on GABA release in CeA via Y2 receptor (Y2R)-mediated pre-synaptic effects (155). Alcohol dependence produces neuroadaptations in CeA NPY systems, as evidenced by lower NPY levels in CeA of alcohol-dependent rats during withdrawal (156), and higher Y1R levels in CeA of chronic alcohol-drinking mice 48 hours into abstinence (157). NPY reduces GABAergic transmission in the CeA of binge-like alcohol drinking mice, but not in alcohol-naïve mice (158). Chronic ventricular infusion of NPY during withdrawals early in the transition to dependence prevents excessive alcohol drinking during subsequent withdrawals (155), and NPY infused into the CeA reduces excessive drinking by alcohol-dependent rats (159), suggesting that NPY may blunt excessive drinking by alcohol-dependent rats via modulation of CeA GABAergic neurotransmission.
In summary, CRF and NPY in CeA are recruited during stress and alcohol dependence, and exert opposite but convergent effects on anxiety-like behavior and escalated alcohol drinking, likely via modulation of CeA GABAergic transmission. CRF1s mediate withdrawal-induced increases in anxiety-like behavior (160), alcohol drinking (147), and sensitization of anxiety-like behavior over repeated withdrawals (161). In contrast, intra-CeA NPY reduces withdrawal-induced increases in anxiety-like behavior (155, 159). Y2R antagonism attenuates withdrawal-induced increases in anxiety-like behavior but not escalated alcohol drinking in dependent rats, suggesting that anxiolytic effects occur via Y2R autoreceptor modulation of NPY release, whereas effects on alcohol drinking occur via Y2R heteroceptor modulation of GABA release (162). Although NPY antagonizes the behavioral effects of CRF in the amygdala, the cellular interactions of NPY and CRF in the CeA remain uncharacterized.
CeA Pro- and Anti-Stress Peptides in Stress-Alcohol Interactions
Although humans report drinking alcohol to reduce anxiety (163), animal research has produced a complicated picture of stress effects on alcohol drinking. Studies report stress-induced increases, decreases, and null effects on alcohol drinking according to type/modality of stressor, intensity/frequency of stressor, time between stress and alcohol access, species and strain of animal tested, and other factors (164). One common procedure utilizes stress to reinstate previously-extinguished alcohol-seeking behavior in a session where operant responses do not produce alcohol deliveries. Until recently, there has been a lack of studies explicitly investigating the interaction between a PTSD-like state (which takes into account individual differences in stress reactivity) and alcohol self-administration (165). Recently, the stress-enhanced fear-learning (SEFL) model of PTSD was used to show that a single traumatic stress increases acquisition and maintenance of voluntary alcohol consumption in previously alcohol-naïve rats but does not alter drinking by rats previously trained to drink (166). Another study found that rats with high reactivity to predator odor stress exhibit escalated and compulsive-like alcohol drinking (167).
Evidence for the role of CeA stress peptides in stress-alcohol interactions comes from human genetics data showing that variation at the CRF1 locus contributes to increased stress sensitivity and may be associated with alcohol dependence susceptibility (167, 168). Alcohol-preferring rats exhibit increased stress sensitivity, increased extra-hypothalamic CRF1 signaling (169, 170), and increased basal spontaneous GABA release in CeA (171). Voluntary alcohol drinking by alcohol-preferring rats further increases GABA release and reduces sensitivity of the GABAergic system to CRF1 antagonism (171). Interestingly, stress-induced reinstatement of alcohol-seeking behavior is blocked by CRF receptor blockade and by NPY, but not by opioid receptor antagonists (172,173).
The CeA modulates autonomic and neuroendocrine responses to stress via reciprocal projections with LC and PVN (81, 174), suggesting that hypothalamic and extra-hypothalamic stress responses are coordinated (175). Alcohol-dependent humans exhibit a blunted HPA stress response (176, 177), and both alcohol-dependent rats and non-dependent drinkers exhibit blunted HPA response to an acute bolus alcohol injection (178). Unlike the PVN, abstinence in alcohol-dependent rats up-regulates GRs in CeA, thereby “sensitizing” extra-hypothalamic stress systems, which may provide new drug targets depending on the timing of therapeutic intervention (179). The critical determinant of whether glucocorticoids exert negative or positive feedback over specific brain regions remains unclear, but one possibility is that different splice variants of steroid coactivator (SRC)-1 work at GRs to negatively regulate gene expression in PVN, while increasing CRF gene expression in CeA (180,181).
CeA INTRACELLULAR SIGNALING PATHWAYS IN ANXIETY AND ALCOHOL DEPENDENCE
An emerging story is the role of stress peptide interactions with specific protein kinase C (PKC) isoforms in anxiety- and alcohol-related behaviors. Endogenous PKC epsilon (PKC) promotes anxiety-like behavior and expression of CRF mRNA and peptide in amygdala (182), and endogenous amygdalar PKC promotes alcohol consumption in mice (183). The PKC signaling pathway in CeA is activated by CRF1s, and the ability of acute alcohol to augment GABAergic transmission in CeA is contingent on the integrity of PKCε signaling pathways and the contribution of those pathways to vesicular GABA release (184, 185). Protein kinase A (PKA), which is activated by CRF1 (via Gs and Gq proteins), also plays an important role in facilitating CeA GABA release by acute alcohol (186), and PKA antagonists block CRF-induced increases in pre-synaptic CeA GABA release (186). CeAL neurons positive for PKC-δ appear to gate the output of CeAM neurons onto downstream effector regions (154), and NPY has been linked to PKC-δ signaling in brain (187). Future studies should examine potential crosstalk between PKA, PKCε and PKCδ pathways in regulating alcohol and peptide effects in CeA, especially as these pathways have been implicated in binge-like drinking and alcohol dependence (188, 189).
CONCLUSIONS
Anxiety disorders and AUDs are highly co-morbid in humans. A pre-existing anxiety disorder can precipitate alcohol abuse, and high anxiety is a hallmark symptom of alcohol dependence that manifests during withdrawal. Anxiety disorders and AUD in humans are both defined by altered amygdala structure and function, the end result of which may be disinhibition of downstream “effector” regions that regulate anxiety- and alcohol-related behaviors. Because the CeA is ascribed an important role in the aversive states and behavioral dysregulation associated with stress and alcohol dependence, it is critical to understand the overlapping and/or compounding effects of anxiety disorders and AUD on amygdala function. New research techniques combine traditional cellular, pharmacological, and anatomical approaches with sophisticated new genetic technologies, and will facilitate our understanding of how the amygdala is recruited in anxiety disorders and/or AUD, and in the tailoring of future treatment strategies.
ACKNOWLEDGEMNTS
This work was supported by NIH grants AA018400 (NWG), AA023305 (NWG), AA020430 (MAH), AA021491 (MR), AA017447 (MR), AA006420 (MR), AA013498 (MR) and AA015566 (MR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
BIBLIOGRAPHY
- 1.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behav Neurosci. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- 3.Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing vs response expression. Behav Neurosci. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF, Jr, Rugle L, et al. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- 5.Morey RA, Dolcos F, Petty CM, Cooper DA, Hayes JP, Labar KS, et al. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J Psychiatr Res. 2009;43:809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickie EW, Brunet A, Akerib V, Armony JL. An fMRI investigation of memory encoding in PTSD: influence of symptom severity. Neuropsychologia. 2008;46:1522–1531. doi: 10.1016/j.neuropsychologia.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Rauch SL, Whalen PJ, Shin LM, Mclnerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 8.Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, et al. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 11.Straube T, Mentzel HJ, Miltner WH. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 2006;59:162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Wright Cl, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
- 13.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- 14.Domschke K, Ohrmann P, Braun M, Suslow T, Bauer J, Hohoff C, et al. Influence of the catechol-O-methyltransferase val158met genotype on amygdala and prefrontal cortex emotional processing in panic disorder. Psychiatry Res. 2008;163:13–20. doi: 10.1016/j.pscychresns.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Hershon HI. Alcohol withdrawal symptoms: phenomenology and implications. Br J Addict Alcohol Other Drugs. 1973;68:295–302. doi: 10.1111/j.1360-0443.1973.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 16.Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- 17.Willinger U, Lenzinger E, Hornik K, Fischer G, Schonbeck G, Aschauer HN, et al. Anxiety as a predictor of relapse in detoxified alcohol-dependent patients. Alcohol Alcohol. 2002;37:609–612. doi: 10.1093/alcalc/37.6.609. [DOI] [PubMed] [Google Scholar]
- 18.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiefer F, Wiedemann K. Neuroendocrine pathways of addictive behavior. Addict Biol. 2004;9:205–212. doi: 10.1080/13556210412331292532. [DOI] [PubMed] [Google Scholar]
- 20.Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Kerich M, Schwandt ML, Rawlings RR, McKellar JD, Momenan R, et al. Smaller right amygdala in Caucasian alcohol-dependent male patients with a history of intimate partner violence: a volumetric imaging study. Addict. Biol. 2011 doi: 10.1111/j.1369-1600.2011.00381.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Yan P, Li CS. Decreased amygdala activation during risk taking in non-dependent habitual alcohol users: A preliminary fMRI study of the stop signal task. Am J Drug Alcohol Abuse. 2009;35:284–289. doi: 10.1080/00952990902968569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, et al. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- 24.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fryer SL, Jorgensen KW, Yetter EJ, Daurignac EC, Watson TD, Shanbhag H, et al. Differential brain response to alcohol cue distractors across stages of alcohol dependence. Biol Psychol. 2012;92:282–291. doi: 10.1016/j.biopsycho.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neurosci. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 28.Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- 29.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 30.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012;36:873–888. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- 33.Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990a;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci USA. 2007;104:14146–14150. doi: 10.1073/pnas.0704621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 37.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- 39.Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 41.McDonald AJ. Immunohistochemical identification of gammaaminobutyric acid-containing neurons in the rat basolateral amygdala. Neurosci Lett. 1985;53:203–207. doi: 10.1016/0304-3940(85)90186-7. [DOI] [PubMed] [Google Scholar]
- 42.Nitecka L, Ben-Ari Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol. 1987;266:45–55. doi: 10.1002/cne.902660105. [DOI] [PubMed] [Google Scholar]
- 43.Pitkänen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1995;356:288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- 44.Savander V, Go CG, LeDoux JE, Pitkänen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol. 1995;361:345–368. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]
- 45.Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Gal La Salle G, Paxinos G, Emson P, Ben-Ari Y. Neurochemical mapping of GABAergic systems in the amygdaloid complex and bed nucleus of the stria terminalis. Brain Res. 1978;155:397–403. doi: 10.1016/0006-8993(78)91037-5. [DOI] [PubMed] [Google Scholar]
- 47.McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neurosci. 1993;52:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- 48.Paré D, Smith Y. Distribution of GABA immunoreactivity in the amygdaloid complex of the cat. Neurosci. 1993;57:1061–1076. doi: 10.1016/0306-4522(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 49.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala. Oxford University Press; 2000. [Google Scholar]
- 51.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- 52.Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci. 2014;34:2432–2437. doi: 10.1523/JNEUROSCI.4166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Anderson S, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, González-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvárday Z, Kubota Y, Lewis DA, Marfn O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamás G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013 Mar;14(3):202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011 Aug;21(8):1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- 56.Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, Heimer L. The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience. 1998 Jun;84(4):967–996. doi: 10.1016/s0306-4522(97)00560-5. 1998. [DOI] [PubMed] [Google Scholar]
- 57.Cook CJ. Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol Behav. 2002 Apr l;75(4):455–464. doi: 10.1016/s0031-9384(02)00650-9. [DOI] [PubMed] [Google Scholar]
- 58.Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia U. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):12004–12009. doi: 10.1073/pnas.0803216105. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwarz AJ, et al. A neural switch for active and passive fear. Neuron. 2010;67:656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Luthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014 Jan 22;81(2):428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 61.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- 63.Gray TS, Magnuson DJ. Peptide immunoreactive neruons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- 64.Oliveira MA, Prado WA. Role of PAG in the antinociception evoked from the medial or central amygdala in rats. Brain Res Bull. 2001;54:55–63. doi: 10.1016/s0361-9230(00)00420-2. [DOI] [PubMed] [Google Scholar]
- 65.Xu W, Lundeberg T, Wang YT, Li Y, Yu L-C. Antinociceptive effects of calcitonin gene-related peptide in the central nucleus of the amygdala: activating opioid receptors through amygdala-periaqueductal gray pathway. Neurosci. 2003;118:1015–1022. doi: 10.1016/s0306-4522(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 66.Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nature Neurosci. 2010;13:979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- 68.Tsumori T, Yokota S, Qin Y, Oka T, Yasui Y. A light and electron microscopic analysis of the convergent insular cortical and amygdaloid projections to the posterior lateral hypothalamus in the rat, with special reference to cardiovascular function. Neurosci Res. 2006;56:261–269. doi: 10.1016/j.neures.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Kokkinidis L, Borowski TB. Sensitization of mesolimbic brain stimulation reward after electrical kindling of the amygdala. Brain Res Bull. 1991;27:791–796. doi: 10.1016/0361-9230(91)90210-b. [DOI] [PubMed] [Google Scholar]
- 70.Beaulieu S, Paolo TD, Barden N. Participation of the central amygdaloid nucelus in the response of adrenocorticotropin secretion to immobilization stress: opposing roles of the noradrenergic and dopaminergic systems. Neuroendocrinol. 1987;45:37–46. doi: 10.1159/000124701. [DOI] [PubMed] [Google Scholar]
- 71.Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinol. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- 72.Tsubouchi K, Tsumori T, Yokota S, Okunishi H, Yasui Y. A disynaptic pathway. 2007 doi: 10.1016/j.neures.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Petrov T, Krukoff TL, Jhamandas JH. Convergent influence of the central nucleus of the amygdala and the paraventricular hypothalamic nucleus upon brainstem autonomic neurons as revealed by c-fos expression and anatomical tracing. J Neurosci Res. 1995;42:835–845. doi: 10.1002/jnr.490420612. [DOI] [PubMed] [Google Scholar]
- 74.Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45:389–393. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 75.Xu Y, Day TA, Buller KM. The central amygdala modulates hypothalamicpituitary-adrenal axis responses to systemic interleukin-ip administration. Neurosci. 1999;94:175–183. doi: 10.1016/s0306-4522(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 76.Amaral DG, Sinnamon HM. The locus coeruleus: neurobiology of a central noradrenergic nucleus. Prog Neurobiol. 1977;9:147–196. doi: 10.1016/0301-0082(77)90016-8. [DOI] [PubMed] [Google Scholar]
- 77.Cedarbaum JM, Aghajanian Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978;178:1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- 78.Reyes BAS, Carvalho AF, Vakharia K, van Bockstaele EJ. Amygdalar peptidergic circuits regulating noradrenergic locus coeruleus neurons: linking limbic and arousal centers. Exp Neurol. 2011;230:96–105. doi: 10.1016/j.expneurol.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valentino RJ, van Bockstaele EV. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 82.Lechner SM, Valentino RJ. Glucocorticoid receptor-immunoreactivity in corticotrophin-releasing factor afferents to the locus coeruleus. Brain Res. 1999;816:17–28. doi: 10.1016/s0006-8993(98)00900-7. [DOI] [PubMed] [Google Scholar]
- 83.Danielsen EH, Magnuson DJ, Gray TS. The central amygdaloid nucleus innervation of the dorsal vagal complex in rat: a Phaseolus vulgaris leucoagglutinin lectin anterograde tracing study. Brain Res Bull. 1989;22:705–715. doi: 10.1016/0361-9230(89)90090-7. [DOI] [PubMed] [Google Scholar]
- 84.Saha S, Batten TFC, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neurosci. 2000;99:613–626. doi: 10.1016/s0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 85.Roozendaal B, Koolhaas JM, Bohus B. Central amygdala lesions affect behavioral and autonomic balance during stress in rats. Physiol Behav. 1991;50:777–781. doi: 10.1016/0031-9384(91)90017-i. [DOI] [PubMed] [Google Scholar]
- 86.Baklavadzhyan OG, Pogosyan NL, Arshakyan AV, Darbinyan AG, Khachatryan AV, Nikogosyan TG. Studies of the role of the central nucleus of the amygdala in controlling cardiovascular functions. Neurosci Behav Physiol. 2000;30:231–236. doi: 10.1007/BF02463163. [DOI] [PubMed] [Google Scholar]
- 87.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 88.Whalen PJ, Rauch SL, Etcoff NL, Mclnerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 90.Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, Pêgo JM. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci. 2013;7:32. doi: 10.3389/fnbeh.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barms M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Trans Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pare D, Quirk GJ, LeDoux JE. New Vistas on Amygdala Networks in Conditioned Fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 95.Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- 96.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 97.Andero R, Dias BG, Ressler KJ. A role forTac2, NkB, Nk3 Receptor in normal and dysregulated fear memory consolidation. Neuron. 2014;83:444–454. doi: 10.1016/j.neuron.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duvarci S, Popa D, Paré D. Central amygdala activity during fear conditioning. J Neurosci. 2011;31:289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amir A, Amano T, Paré D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol. 2011;105:3054–3066. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacol. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: The view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 105.Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]
- 106.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gass JT, Trantham-Davidson H, Kassab AS, Glen WB, Jr, Olive MF, Chandler LJ. Enhancement of extinction learning attenuates ethanol-seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci. 2014;34:7562–7574. doi: 10.1523/JNEUROSCI.5616-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Belelli D, Harrison NL. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 110.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326:475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mody I, Glykys J, Wei W. A new meaning for "Gin & Tonic": tonic inhibition as the target for ethanol action in the brain. Alcohol. 2007;41:145–153. doi: 10.1016/j.alcohol.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Subunit-specific tonic GABA currents and differential effects of ethanol within the central amygdala circuitry of CRF1 reporter mice. J Neurosci. 2013 doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001 Jun l;49(11):894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- 118.Yan P, Li CS. Decreased amygdala activation during risk taking in non- dependent habitual alcohol users: A preliminary fMRI study of the stop signal task. Am J Drug Alcohol Abuse. 2009;35:284–289. doi: 10.1080/00952990902968569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O'Daly OG1, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacol. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HTlA-receptor agonist. Neuropsychopharmacol. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- 122.Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a "kindling"/stress hypothesis. Psychopharmacol. 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008;9:29. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin Releasing Factor-Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence. Biol Psychiatry. 2010:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roberto M, Gilpin NW, O'Dell LE, Cruz MT, Morse AC, Siggins GR, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- 127.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 128.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Amarouchi K, et al. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol Psychiatry. 2012;71:309–316. doi: 10.1016/j.biopsych.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 130.Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 131.Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- 132.Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF. Anxiolytic-like action of neuropeptide Y: mediation by Yl receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacol. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- 133.Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28:12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinol. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 137.Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, Oleata CS, Heilig M, Roberto M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci. 2014;34:363–372. doi: 10.1523/JNEUROSCI.2400-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci. 2009;29:7379–7388. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in. 2006 doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- 140.Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathé AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacol. 2012;37:350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol. 2010;13:747–757. doi: 10.1017/S1461145709990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- 143.Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- 145.Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob G, Weiss F. Increase of extracellular corticotrophin-releasing factor-like immunoreactivity levels in the amygdale of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF. Corticotropin-releasing factor-1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, et al. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, et al. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav. 2012;100:522–529. doi: 10.1016/j.pbb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 Antagonist and CRF-2 Agonist Decrease Binge-Like Ethanol Drinking in C57BL/6J Mice Independent of the HPA Axis. Neuropsychopharmacol. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y Opposes Alcohol Effects on Gamma-Aminobutyric Acid Release in Amygdala and Blocks the Transition to Alcohol Dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- 157.Eva C, Mele P, Collura D, Nai A, Pisu MG, Serra M, Biggio G. Modulation of neuropeptide Y and Yl receptor expression in the amygdala by fluctuations in the brain content of neuroactive steroids during ethanol drinking discontinuation in YlR/LacZ transgenic mice. J Neurochem. 2008;104:1043–1054. doi: 10.1111/j.1471-4159.2007.05077.x. [DOI] [PubMed] [Google Scholar]
- 158.Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, Rinker JA, Jijon AM, Peňna J, Navarro M, Kash TL, Thiele TE. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Yl and Y2 receptors. Neuropsychopharmacol. 2012;37:1409–1421. doi: 10.1038/npp.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced decreases in alcohol drinking. Pharmacol Biochem Behav. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2003;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kallupi M, Vendruscolo LF, Carmichael CY, George O, Koob GF, Gilpin NW. Neuropeptide Y Y2 R blockade in the central amygdala reduces anxiety-like behavior but not alcohol drinking in alcohol-dependent rats. Addict Biol. 2013 May 3; doi: 10.1111/adb.12059. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38:953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- 164.Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacol. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Logrip ML, Zorrilla EP, Koob GF. Stress modulation of drug self- administration: implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacol. 2012;62:552–564. doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res. 2013;37:566–574. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Enoch MA, Goldman D. Genetics of alcoholism and substance abuse. Psychiatr Clin North Am. 1999 Jun;22(2):289–299. doi: 10.1016/s0193-953x(05)70077-0. viii. Review. [DOI] [PubMed] [Google Scholar]
- 168.Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- 169.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, et al. Variation at the rat Crhrl locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region- specific down-regulation of Crhrl gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007 Mar;12(1):30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 171.Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, Ciccocioppo R, Roberto M. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 2013 Apr;67:337–348. doi: 10.1016/j.neuropharm.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacol. 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- 174.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 175.Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- 176.Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin Exp Res. 2005;29:517–527. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- 178.Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self- administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, Meijer OC. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl AcadSciUSA. 2009;106:8038–8042. doi: 10.1073/pnas.0812062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinol. 2008;149:725–732. doi: 10.1210/en.2007-1234. [DOI] [PubMed] [Google Scholar]
- 182.Lesscher HM, McMahon T, Lasek AW, Chou WH, Connolly J, Kharazia V, et al. Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav. 2008;7:323–333. doi: 10.1111/j.1601-183X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 183.Lesscher HM, Wallace MJ, Zeng L, Wang V, Deitchman JK, McMahon T, et al. Amygdala protein kinase C epsilon controls alcohol consumption. Genes Brain Behav. 2009;8:493–499. doi: 10.1111/j.1601-183X.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci USA. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, et al. Protein Kinase C{epsilon} Regulates {gamma}-Aminobutyrate Type A Receptor Sensitivity to Ethanol and Benzodiazepines through Phosphorylation of {gamma}2 Subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- 186.Cruz MT, Bajo M, Maragnoli ME, Tabakoff B, Siggins GR, Roberto M. Type 7 Adenylyl Cyclase is Involved in the Ethanol and CRF Sensitivity of GABAergic Synapses in Mouse Central Amygdala. Front Neurosci. 2011;4:207. doi: 10.3389/fnins.2010.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kuo DY, Yang SF, Chu SC, Chen CH, Chen PN, Hsieh YS. The effect of protein kinase C-delta knockdown on anti-free radical enzyme and neuropeptide Y gene expression in phenylpropanolamine-treated rats. J Neurochem. 2010;114:1217–1230. doi: 10.1111/j.1471-4159.2010.06843.x. [DOI] [PubMed] [Google Scholar]
- 188.Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree Dl, Thompson AB, et al. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012;36:1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]