Abstract
Inherited syndromes provide unique opportunities to identify key regulatory mechanisms governing human disease. We previously identified germline loss-of-function DICER1 mutations in a human syndrome defined by the childhood lung neoplasm, pleuropulmonary blastoma (PPB), which arises during lung development. DICER1 regulates many biological processes critical in development and disease pathogenesis. Significant challenges in defining the role of DICER1 in human disease are identifying cause-effect relationships and generating manipulatable systems that model the complexity of organ development and disease pathogenesis. Herein we report generation of a murine model for PPB and demonstrate that precise temporal and cell type-specific Dicer1 ablation is necessary and sufficient for development of cystic lungs that histologically and phenotypically model PPB. Dicer1 ablation in the distal airway epithelium during early stages of lung development resulted in a cystic lung phenotype indistinguishable from PPB whereas DICER1 function was not required for development of the proximal airway epithelium or during later stages of organogenesis. Mechanistic studies demonstrate that Dicer1 loss results in epithelial cell death, followed by cystic airway dilation, accompanied by epithelial and mesenchymal proliferation. These studies define precise temporal and epithelial cell type-specific DICER1 functions in the developing lung and demonstrate that loss of these DICER1 functions is sufficient for development of cystic PPB. These results also provide evidence that PPB arises through a novel mechanism of non-cell-autonomous tumor initiation, wherein the genetic abnormality initiating the neoplasm does not occur in the cells that ultimately transform, but rather occurs in a benign-appearing epithelial cell component that predisposes underlying mesenchymal cells to malignant transformation.
Keywords: Dicer1, Lung Development, Pleuropulmonary Blastoma
Introduction
Pleuropulmonary blastoma (PPB) is the most common primary malignant lung neoplasm in childhood. PPB is diagnosed in children under six years of age and can arise during fetal development. It presents in three distinct clinicopathologic forms that represent tumor progression. Type I PPB is the earliest form of tumor development, being detected as early as 23 weeks’ gestation, with a median age at diagnosis of eight months. Type I PPB is characterized by cysts lined by benign-appearing epithelium resting on septae containing undifferentiated mesenchymal cells. Types II and III PPB represent progressive overgrowth of the cysts by a high grade sarcoma, and are diagnosed in children with median ages of 35 and 41 months, respectively. The clinicobiologic progression from Type I to Type III PPB is reflected in the median age at diagnosis from eight months to 41 months as well as documented clinical and pathological disease progression in individual patients [1,2]. Pathologic type is the only known statistically significant predictor of outcome [3]. It is currently unclear if all Type II/III PPBs result from progression of early cystic Type I PPB. At the other end of the spectrum, not all Type I PPBs are destined to progress to Type II-III [4]. We have identified children and even adults with lung cysts with the unique Type I PPB architecture, but without the expanded mesenchymal tumor cells characteristic of PPB presenting in younger patients. Similar cysts can also be seen in PPB patients with multiple lesions, where one or more of the cysts show signs of progression and other lesions contain features of regression [4]. These benign multicystic lesions are thought to represent Type I PPBs that failed to progress to the sarcomatous stage.
PPB is diagnosed in utero, providing evidence that PPB initiates during lung development. Proper development of mammalian tissues requires precise spatiotemporal control of gene expression. MicroRNAs (miRNAs) are important post-transcriptional regulators of gene expression and play a critical role during embryonic development and tumorigenesis [5,6]. DICER1 is a key endonuclease in the highly conserved miRNA regulatory pathway and is required for the final cleavage step to produce mature miRNAs that target mRNAs for destruction or inhibit their translation. miRNAs regulate crucial cellular processes including cellular proliferation, survival and differentiation [7]. Significant challenges in defining the role of miRNAs in disease pathogenesis are identifying cause-effect relationships between molecular alterations and human diseases, and generating manipulatable systems that model the complexity of organ development and disease pathogenesis. We identified germline loss-of-function DICER1 mutations in a human syndrome defined by inherited predisposition to PPB. This represents the first described human disease with germline DICER1 mutations, making PPB an important model for studying how loss of DICER1, and the miRNAs it generates, manifests biologically in human disease.
Heterozygous germline DICER1 mutations are detected in 66% of PPB cases, demonstrating that DICER1 loss promotes PPB pathogenesis [8]. The precise roles of DICER1 in PPB initiation and progression, however, have not been defined. Additionally, it is unclear whether the distinctive childhood occurrence of PPB is due to loss of temporally specific DICER1 functions critical in lung organogenesis, the loss of which sets the stage for PPB initiation. In the current studies, we directly demonstrate that Dicer1 loss targeted to the lung epithelium is sufficient for development of a PPB phenotype. Moreover, we demonstrate that DICER1 has temporal and cell type specific functions during lung organogenesis that when lost result in a cystic lung phenotype modeling Type I PPB.
Materials and methods
Mice
SPC-rtTA; tetCre or CC10-rtTA; tetCre mice were crossed with Dicer1f/f mice obtained from The Jackson Laboratory (B6.Cg-Dicer1tm1Bdh/J) [9–11]. Recombination of the Dicer1f/f allele produces a null allele, as evidenced by germline cre-mediated recombination in homozygous Dicer1f/f mice resulting in the same phenotype as germline Dicer1-null mice [11]. Pregnant dams were treated with doxycycline food (Modified RMH 1500 with 0.0625% doxycycline, Purina/Cinti Lab Supply) at the designated gestational times. Genotypes were determined by PCR analysis as previously described [9–11]. Double transgenic Dicer1f/f or Dicer1w/w wild type mice were bred with the previously described ROSA26 reporter strain [12]. All mice were cared for according to IACUC guidelines.
Histology and immunohistochemistry
Tissues were fixed in 4% paraformaldehyde or 10% formalin, paraffin embedded and analyzed by hematoxylin and eosin staining. Immunohistochemistry was performed using Vectastain Elite ABC and DAB Substrate kits (Vector Laboratories) or the automated BenchMark XT IHC/HIS Staining Module. Methanol/hydrogen peroxide pretreatment and serum blocking were performed, and some antibodies were applied after microwave 10 mmol/L citrate, EDTA, or protease antigen retrieval. Antibodies and dilutions are detailed in Supplemental methods. Slides were counterstained with nuclear fast red or hematoxylin.
Analysis of cell death and proliferation
Cell death was assessed using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore). Cell proliferation was assessed by immunohistochemistry for phosphorylated histone H3 (pHH3) (US Biological H5110-14B, 1:1000). BrdU incorporation was assessed by injecting pregnant dams intraperitoneally with 300 uL Cell Proliferation Labeling Reagent solution (RPN201, GE Healthcare Life Sciences) 2 hours before embryo harvest, followed by fixation in 4% paraformaldehyde and immunohistochemistry on paraffin sections using BrdU Staining Reagent (93–3943, Invitrogen). Quantifications represent analysis of 184–325 cells representing at least two lung lobes per animal.
Whole mount β-galactosidase staining
Lungs were washed in cold PBS (5 minutes X3), fixed in 2% paraformaldehyde/0.2% glutaraldehyde (SPI Supplies) on ice for 2 hours, washed in buffer (5mM EGTA, 0.01% sodium deoxycholate, 0.02% NP-40, 2mM MgCl2 in 1X PBS (pH7.3)), incubated in staining solution (5mM K3Fe(CN)6, 5mM K4Fe(CN)6, 5mM EGTA, 0.01% sodium deoxycholate, 0.02% NP-40, 2mM MgCl2 and 1mg/ml X-Gal (Promega) in PBS, (pH 7.3)) for 16 hours at 30°C, rinsed in cold PBS, post-fixed in 2% paraformaldehyde/2% glutaraldehyde in 0.1M sodium cacodylate buffer (pH7.3) for 10 minutes on ice, rinsed in cold PBS (5 minutes X3) and stored in 70% ethanol at 4°C.
Quantitative real time PCR analysis
Total RNA was isolated from whole lungs or E13.5 lung epithelium and mesenchyme separations prepared as previously described [13] using Trizol reagent (Invitrogen) followed by cDNA generation using iScript (Bio-Rad) or Taqman miRNA RT Kit (4366596). Quantitative real time PCR (qRT-PCR) was performed using Taqman Real-Time PCR Gene Expression System (Applied Biosystems) and probe sets specified in Supplemental methods. At least triplicate samples were analyzed using 7300 Real-Time PCR System and software.
Northern blot miRNA analysis
Total RNA was extracted using Trizol reagent (Invitrogen) and quantified using Nanodrop. Total lung RNA (1ug) was run on 15% TBE-urea gels and transferred onto positively charged nylon membranes (11209299001, Roche) that were subsequently cross-linked with 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide EDC solution (E7750, Sigma Aldrich) for 1 hour at 60°C, washed, prehybridized for 30 minutes and hybridized at 50°C overnight, washed and blocked with DIG block buffer (11585762001, Roche), incubated with hsa-miR-17 LNA probe (38461-15, Exiqon) diluted in block buffer for 30 minutes, and then washed and developed in CDP-Star developing solution (12041677001, Roche).
Statistics
Chi-square analyses, Kaplan Meier log-rank test, unpaired t-tests and one-way ANOVA followed by Tukey’s multiple comparison tests using GraphPad Prism version 5.0d software were used for data analysis. Statistical significance was defined as p < 0.05. Data is represented as mean ± SEM
Results
Dicer1 ablation targeted to the developing lung epithelium results in a lung phenotype mimicking cystic PPB in neonates
We previously reported loss of DICER1 immunohistochemical staining in PPB-associated epithelium, despite retention of staining in the septal mesenchymal component, in six of seven families that developed Type I/II PPBs [14]. Assessment of additional Type I/II PPBs showed loss of or diminished DICER1 staining in the epithelial component in 31% (20/64) of cases, with retention of DICER1 staining in the underlying septal mesenchymal cells in all cases (Fig. 1a). Areas of absent DICER1 staining in the tumor epithelium were segmental and focal in the majority of cases. PPB-associated DICER1-deficient epithelium was morphologically benign in all cases, distinguishing PPB from pulmonary blastoma, which has a malignant epithelial component.
Figure 1. Dicer1 ablation targeted to the developing lung epithelium results in neonatal death and a PPB-like phenotype.
(a) PPB resected from a 10 day old infant (left panel) showing cystic spaces separated by septae with expanded mesenchyme (arrows) adjacent to normal bronchiole (arrowheads) and alveoli. In the normal neonatal lung (center panel), DICER1 expression is detected in the bronchiolar (arrowheads) and alveolar (arrows) epithelium as well as in the mesenchymal cells by immunohistochemistry. In PPB (right panel), DICER1 expression is lost in the epithelial (arrows) but not mesenchymal component of the neoplasm (200×, inset 400×). (b) Dicer1 ablation targeted to the lung epithelium in SPC-rtTA/Cre double transgenic Dicer1f/f mice (Dicer1 Ablated, n=8) resulted in death by postnatal day 5, in contrast to surviving double transgenic Dicer1w/f mice (Dicer1 haploinsufficient, n=16) and control littermates including double transgenic Dicer1w/w (n=16) and Dicer1f/f, Dicer1w/f or Dicer1w/w mice lacking one or both transgenes required for Dicer1 recombination (n=51). (c) Recombination of Dicer1f/f alleles (Rec) by PCR analysis of lung DNA from E18.5 Dicer1-ablated but not double transgenic Dicer1w/w littermates. The wild type Dicer1 allele (WT) was detected in lung DNA from Dicer1w/w mice. (d) Dicer1 mRNA levels were decreased in lungs from E18.5 Dicer1-ablated as compared to double transgenic Dicer1w/w controls by quantitative RT-PCR. Data represented as mean ± SEM (n=5–6 per group; ****p<0.0001). (e) Normal human neonatal lung and adjacent Type I PPB (top row), and E18.5 lung from Dicer1-proficient double transgenic Dicer1w/w control and littermate with Dicer1-ablated lung epithelium (bottom row) showing cysts and expanded mesenchyme morphologically similar to Type I PPB. Cyst lining epithelial cells stain for pancytokeratin by immunohistochemistry (human lung 40×, PPB 40×, 1000×, 100×, inset 400×; mouse lung 100×, Dicer1-ablated lung 100×, 1000×, 200×, inset 1000×).
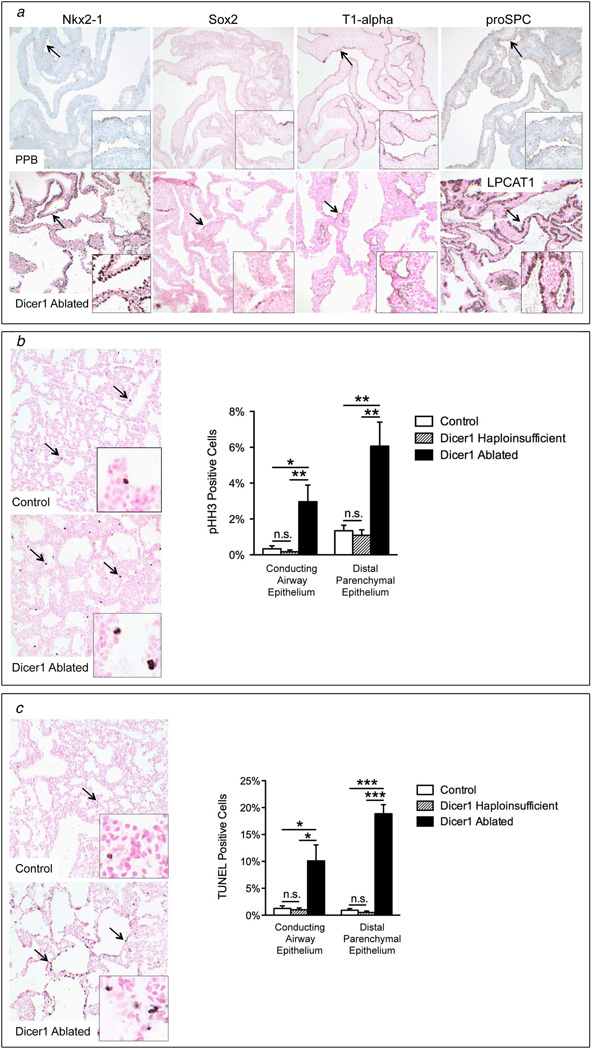
To directly test whether Dicer1 loss in the developing lung epithelium is sufficient to induce PPB, Dicer1 ablation was targeted to the developing pulmonary epithelium by generating mice with floxed Dicer1 alleles (Dicer1f/f) and two transgenes: 1) the reverse tetracycline-responsive transactivator under control of the human surfactant protein C (SPC, Sftpc) promoter (SPC-rtTA), and 2) Cre recombinase under control of the tet operator (mice hereafter designated Dicer1 ablated) [9,11]. Epithelial-specific Dicer1 ablation induced throughout lung development resulted in neonatal death (Fig. 1b–d). Dicer1-deficient embryonic day (E) 18.5 lungs had multiple epithelium-lined cysts separated by thickened septae containing primitive mesenchymal cells, morphologically resembling Type I PPBs in neonates (Fig. 1e) [4]. The cyst-lining epithelial cells in both PPB and DICER1-deficient lungs had a distal respiratory type I and type II cell phenotype. Specifically, cyst-lining epithelial cells were positive for the general lung epithelial cell marker, Nkx2-1 (thyroid transcription factor1, Ttf1) in addition to the type I cell marker, Type I alpha (T1-alpha), and type II cell markers, proSPC and lysophosphatidylcholine acyltransferase (LPCAT1) (Fig. 2a) [15–17]. Only rare cells stained positive for the proximal conducting airway marker, Sox2. Cyst-lining epithelial cells were hyperproliferative with ectopic apoptosis (Fig. 2b–c). Dicer1 haploinsufficency in the lung epithelium was not sufficient for initiation of the PPB phenotype. Mice with haploinsufficent lung epithelium had normal survival rates to one year of age and were without lung abnormalities (n=14) (Fig. 1b, 2b–c and data not shown). The finding that haploinsufficiency alone is not sufficient for PPB initiation is consistent with human carriers of DICER1 mutations not developing PPB and heterozygous germline Dicer1 null mice being viable and without lung abnormalities [18–20]. These data demonstrate that Dicer1 ablation, but not haploinsufficiency, in the developing lung epithelium is sufficient to induce a phenotype that morphologically and phenotypically mimics cystic Type I PPB.
Figure 2. Cyst lining cells in PPB and Dicer1-ablated lungs have a distal respiratory epithelial cell phenotype, increased proliferation and ectopic apoptosis.
(a) Epithelial cells lining cysts in PPB (top row) and E18.5 Dicer1-ablated lungs have positive immunohistochemical staining for Nkx2-1 and type I (Type I (T1)-alpha) and type II (proSPC and LPCAT1) cell markers. Only rare epithelial cells stain for the conducting airway epithelial cell marker, Sox2. Arrows and insets highlight positive staining (PPB 100×, insets 400×; Dicer1-ablated 200×, insets 1000×). (b) Epithelial cell proliferation and (c) apoptosis were increased in conducting and distal parenchymal epithelia in Dicer1-ablated lungs as compared to Dicer1-haploinsufficient (Dicer1f/w) and Dicer1-proficient (Dicer1w/w) double transgenic controls by immunohistochemical staining for phosphorylated histone H3 (pHH3) and TUNEL staining, respectively (arrows and inset). (n=5–7 per group; *p<0.05, **p<0.01, ***p<0.001, n.s. = not significant; 200×, insets 1000×).
Mice with Dicer1 ablation targeted to the developing proximal conducting airway epithelium are viable, with preserved lung organogenesis
DICER1-deficient lungs were noted to have more substantive abnormalities in the distal as compared to the conducting airways, which were not cystically dilated and have less marked aberrant epithelial cell proliferation and apoptosis (Fig. 2b–c). To directly assess for potential region-specific DICER1 functions, Dicer1 deletion was targeted to the developing conducting airway epithelium by generating double transgenic Dicer1f/f mice under control of the rat Clara cell secretory protein (CCSP, Scgb1a1 or Clara cell-specific 10-kDa protein, CC10) (hereafter designated Dicer1CCSPcKO) [10]. Interestingly, Dicer1CCSPcKO mice survived at the expected ratios (15/63 (23%) Dicer1CCSPcKO obtained vs. 25% expected; p=0.9065) and lungs from E18.5 Dicer1CCSPcKO pups were morphologically similar to DICER1-proficient controls (Fig. 3a). Successful targeting of Dicer1 ablation to conducting airways was demonstrated by crossing mice into the ROSA26 reporter strain, wherein cells with functional Cre activity and their descendants are marked by β-galactosidase expression [12]. The conducting airway epithelium of E18.5 Dicer1CCSPcKO and double transgenic Dicer1w/w lungs was diffusely targeted along with rare parenchymal cells (Fig. 3b). Dicer1 recombination was also detected in Dicer1CCSPcKO lungs but not in littermate control lungs. These data demonstrate that DICER1 has regional and cell type-specific functions in the developing lung epithelium, and that distal respiratory epithelial cells are more dependent upon DICER1 function, providing an explanation for the peripheral parenchymal location of PPB.
Figure 3. Lungs with Dicer1 ablation targeted to the developing proximal conducting airway are morphologically similar to Dicer1-proficient control lungs.
(a) Dicer1 ablation targeted to the developing conducting airway epithelium using the rat CCSP promoter driving the rt-TA (Dicer1CCSPcKO) resulted in lungs with morphology similar to Dicer1-proficient littermates lacking one or both transgenes (Control) (top row, 100×, bottom row, 400×). (b) Conducting airway epithelial cells were targeted in double transgenic Dicer1w/w (Dicer1 WT) and Dicer1CCSPcKO lungs treated with doxycycline throughout gestation as indicated by whole mount and immunohistochemical β-galactosidase staining of lungs from mice containing the ROSA26 reporter locus (+) but not in control lungs from mice lacking the Cre transgene (C). Whole mount staining of lung lobes from two Dicer1CCSPcKO and two control mice are shown (whole mounts 8×, microscopic images 200×, inset 1000×). Recombination of Dicer1f/f alleles (Rec) was detected by PCR analysis of lung DNA from E18.5 Dicer1CCSPcKO (Ablated) but not Dicer1-proficient littermate controls lacking one or both transgenes (Control). PCR controls include wild type (w, WT) and recombined (r, Rec) Dicer1 alleles. NS=nonspecific band.
Dicer1 ablation targeted to the developing lung epithelium results in ectopic epithelial apoptosis followed by increased proliferation
DICER1-deficient lungs were examined at sequential developmental time points (E12.5–E18.5) to identify mechanisms underlying the PPB phenotype. The initial morphologic abnormality was epithelial apoptosis detected at E13.5 (Fig. 4a,f). Dicer1 mRNA levels were reduced in the distal epithelium but not mesenchyme of E13.5 lungs, consistent with the temporal and epithelial-specific targeting characteristics of the SPC promoter (Fig. 4b) [21,22]. Distal epithelial cell apoptosis was further increased in E15.5 DICER1-deficient lungs and was associated with distal tubule dilation (Fig. 4c,f). DICER1 function was assessed in E15.5 lungs by determining expression of miR-17, a miRNA with preferential high expression in the epithelium during the pseudoglandular stage [23]. Mature miR-17 was significantly reduced in DICER1-deficient lungs as compared to controls (Fig. 4d–e). In contrast, levels of unprocessed miR-17 were unchanged, consistent with DICER1 functioning to generate mature miRNAs. Increased epithelial cell proliferation followed the apoptotic phenotype, being initially detected at E16.5 and sustained at E18.5 (Figs. 2b, 4g).
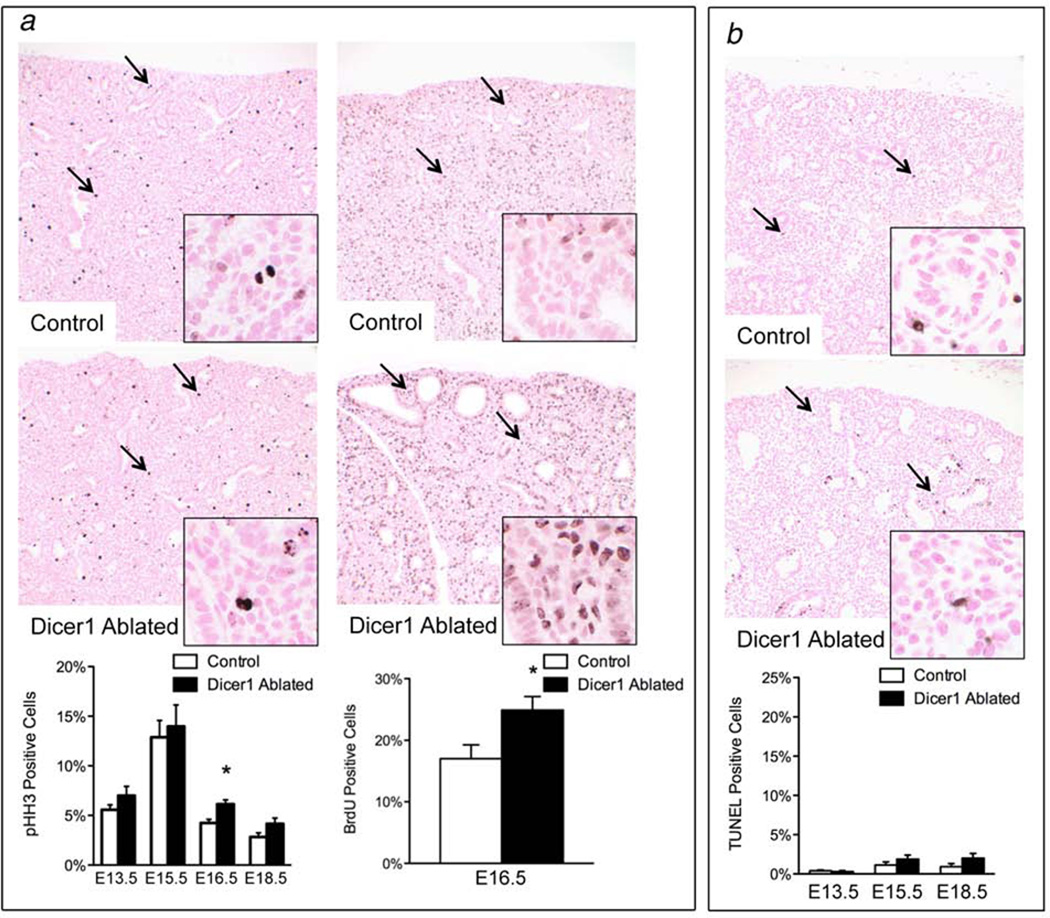
Figure 4. Dicer1 loss targeted to the lung epithelium results in ectopic cell death and cystic airway dilation followed by increased cellular proliferation.
(a) E13.5 double transgenic SPC-rtTA/Cre Dicer1f/f lungs (Dicer1-ablated) but not Dicer1-proficient controls lacking one or both transgenes (Control) showed morphologic evidence of apoptosis in distal airway epithelial cells (arrows) (top row 100×, bottom row 1000×). (b) Dicer1 mRNA levels were decreased in distal airway epithelial cells but not in the surrounding mesenchyme obtained from E13.5 Dicer1-ablated lungs as compared to Dicer1-proficient controls by quantitative RT-PCR (n=3–6 per group; **p<0.01). (c) E15.5 Dicer1-ablated lungs had dilated distal airways (arrowheads) and morphologic evidence of apoptosis in epithelial cells lining distal airways (arrows) (top row 40×, bottom row 1000×). (d) Mature, but not primary, miR-17 was decreased in Dicer1-ablated E15.5 lungs as compared to Dicer1-proficient controls by quantitative RT-PCR (n=4 per group; *p<0.05). (e) Northern blot analysis showed decreased mature miR-17 in Dicer1-ablated as compared to Dicer1-proficient E15.5 lungs. Relative Dicer1 levels as determined by quantitative RT-PCR and miR-17 densitometry values relative to matched littermates are shown. Densitometry values were normalized to the U6 loading control. Levels of pre-miR-17 and a 23 bp RNA oligonucleotide marker indicating the migration position of mature miR-17 are also shown. Low level miR-17 expression is detected in Dicer1-ablated lungs since miR-17 is expressed in both mesenchymal and epithelial cells with relatively higher expression in the epithelium. (f) Apoptosis was increased in distal airway epithelial cells (arrows) in Dicer1-ablated E13.5 and E15.5 lungs as compared to Dicer1-proficient controls by TUNEL analysis (n=3–10 per group; *p<0.05, ****p<0.0001; E15.5 lungs shown, 200×, insets 1000×). (g) Distal epithelial cell proliferation was similar in Dicer1-ablated and control E13.5 and E15.5 lungs but increased in Dicer1-ablated E16.5 lungs as compared to Dicer1-proficient controls by immunohistochemical staining for pHH3 (arrows) (n=4–10 per group; ***p<0.001; E16.5 lungs shown, 200×, insets 1000×).
Mesenchymal proliferation was assessed to determine whether enhanced proliferation was the underlying mechanism for the mesenchymal expansion. A transient increase in proliferation was detected in E16.5 DICER1-deficient lungs but was not sustained in E18.5 lungs (Fig. 5a). Enhanced mesenchymal cell survival was not an underlying mechanism contributing to the mesenchymal expansion as shown by similar numbers of TUNEL-positive cells in DICER1 deficient and proficient lungs (Fig. 5b). Additionally, mesenchymal cells in the DICER1 deficient lungs were not anaplastic, as is seen in the solid component of Type II-III PPB. Thus, DICER1 function is essential for epithelial cell survival and cell cycle regulation during organogenesis, and Dicer1 loss in the developing lung epithelium promotes mesenchymal expansion by inducing a transient increase in proliferation.
Figure 5. Dicer1 loss targeted to the lung epithelium results in a transient increase in mesenchymal proliferation.
(a) Proliferation of mesenchymal cells surrounding the distal airways was transiently increased in Dicer1-ablated E16.5 lungs as compared to Dicer1-proficient controls by immunohistochemical staining for pHH3 and BrdU (arrows and insets). (b) Distal mesenchymal cell apoptosis (arrows and insets) was similar in E13.5, E15.5 and E18.5 Dicer1-ablated and proficient control lungs by TUNEL analysis. (n=4–10 per group; *p<0.05; E15.5 lungs shown for TUNEL analysis, 200×, insets 1000×)).
Timing of Dicer1 ablation dictates phenotypic outcomes
Expression of epithelial factors that control mesenchymal growth is under precise temporal control in the developing lung, leading us to hypothesize that timing of Dicer1 loss is a critical determinant of phenotypic outcomes [16,17]. To test this hypothesis, Dicer1 ablation was targeted to the entire lung epithelium with the SPC promoter at specified developmental stages, by treating pregnant dams with doxycycline throughout development (E0.5–E18.5), in early development (E0.5–E14.5) or during late development (E14.5–E18.5). Animal survival was dependent upon timing of Dicer1 ablation. No viable double transgenic mice were obtained when Dicer1 ablation was induced throughout development and one viable mouse was obtained when Dicer1 ablation was induced during early development (1/30 (3%) vs. 25% expected; p=0.001). In contrast, the expected ratio of double transgenic mice was obtained when Dicer1 ablation was induced during late development (10/27 (37%) vs. 50% expected; p=0.178). Double transgenic Dicer1f/f E18.5 pups were obtained at the expected ratios for all three treatment groups, demonstrating that Dicer1 ablation in the lung epithelium resulted in early postnatal death, consistent with death being due to a lung phenotype. Lungs from E18.5 pups with Dicer1 ablation throughout development or during early development had similar histologic features, consisting of epithelium-lined lung cysts with primitive mesenchymal cells in the septal walls underlying the epithelium (Fig. 6a). Morphologic features of apoptosis were present in the cyst lining epithelium and nuclear debris was present in the airspaces. In contrast, lungs from E18.5 pups with Dicer1 ablation induced during late development were similar to Dicer1-proficient controls, with the exception of scattered epithelial cells with morphologic features of apoptosis in a subset of animals. Similar diffuse lung epithelial cell targeting was verified in all three treatment groups, and Dicer1 recombination and reduced Dicer1 mRNA expression were confirmed (Fig. 6b–c). Thus, the distinct lung phenotypes seen after Dicer1 ablation during early versus late lung development are due to timing of Dicer1 loss rather than differences in extent or cell types targeted for Dicer1 ablation.
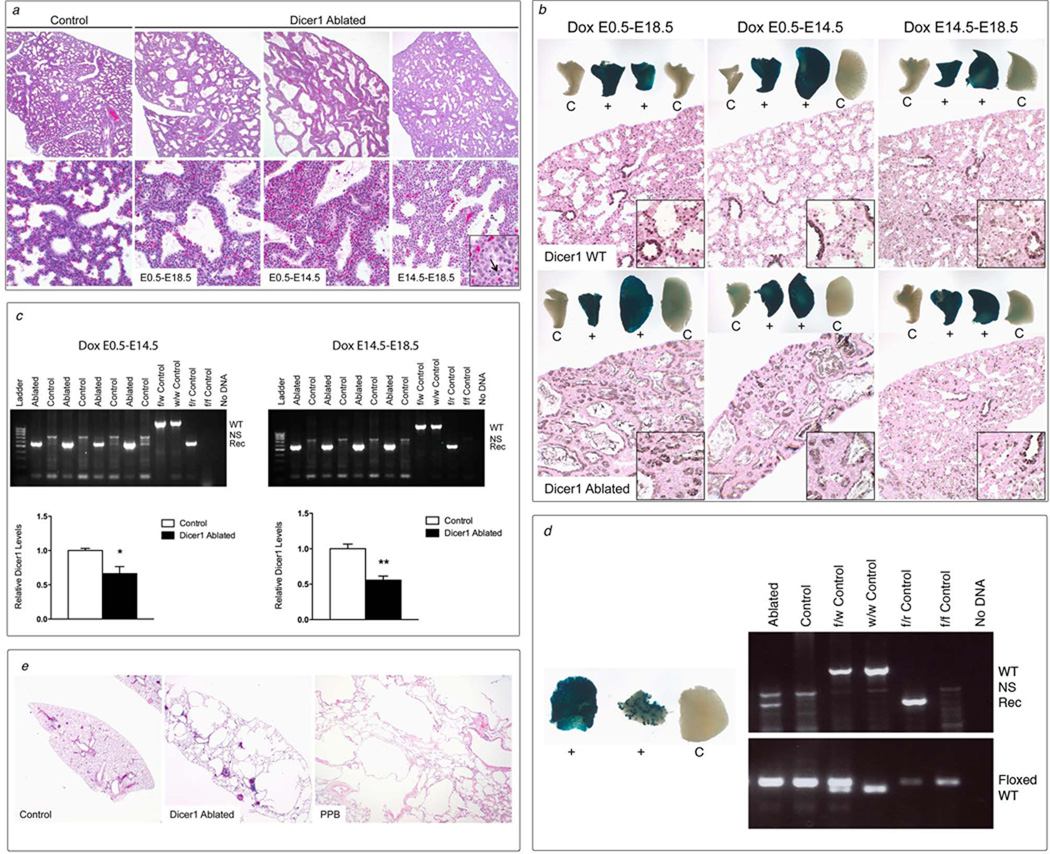
Figure 6. Dicer1 ablation at different stages of lung development results in distinct phenotypes.
(a) Dicer1-ablated and proficient control E18.5 lungs treated with doxycycline throughout development (E0.5–E18.5), during early development (E0.5–E14.5) or during late development (E14.5–E18.5). Dicer1 ablation throughout lung development and in early lung development resulted in cystic lungs with expanded mesenchyme resembling Type I PPB. Dicer1 ablation during late development resulted in lungs that were morphologically similar to Dicer1-proficient controls expect for rare epithelial cells with morphologic features of apoptosis (inset, arrow) (top row 100×, bottom row 400×, inset 1000×). (b) Conducting airway and parenchymal epithelial cells were targeted in control double transgenic Dicer1w/w (Dicer1 WT) and Dicer1f/f (Dicer1 Ablated) lungs treated with doxycycline (dox) throughout development as well as during early and late development by whole mount and immunohistochemical β-galactosidase staining of lungs from mice containing the ROSA26 reporter locus. Whole mount staining of lung lobes from two double transgenic (+) and two Cre transgene negative control (C) mice are shown (whole mounts 8×, microscopic images 200×, inset 1000×). (c) Recombination of Dicer1f/f alleles (Rec) was detected by PCR analysis on lung DNA from E18.5 Dicer1-ablated (Ablated) but not Dicer1-proficient littermate controls lacking one or both transgenes (Control) treated with dox during early and late development. PCR controls include the wild type (w, WT) and recombined (r, Rec) Dicer1 alleles. NS=nonspecific band. Dicer1 mRNA levels were decreased in lungs from Dicer1-ablated as compared to control lungs from mice treated with dox during early and late development by quantitative RT-PCR. (n=4 per group; *p<0.05, **p<0.01). (d) Whole mount beta-galactosidase staining of lung lobes from three littermates containing the ROSA26 reporter locus and treated with dox during early development showing typical diffuse conducting airway and parenchymal epithelial staining of lungs from double transgenic Dicer1f/f mice (+, left) and a rare lung with less uniform staining (+, right). No specific staining was detected in lungs from littermate controls lacking the Cre transgene (C) (8×). Recombination of Dicer1f/f alleles (Rec) was detected by PCR analysis on lung DNA from the single surviving Dicer1-ablated adult mouse treated with doxycycline during early development (Ablated) but not in lung DNA from a littermate control lacking the Cre transgene (Control). Dicer1f/f alleles were detected in both Dicer1-ablated and control lungs. PCR controls include wild type (w, WT), recombined (r, Rec) and floxed (f, Floxed) Dicer1 alleles. NS=nonspecific band. (e) Lungs from surviving Dicer1-ablated adult mouse and Dicer1-proficient littermate control showing that Dicer1 ablation resulted in cystic lungs lacking the mesenchymal expansion seen in E18.5 lungs morphologically identical to regressed or non-progressing cystic Type I PPB (20×).
One viable mouse was obtained when Dicer1 ablation was induced during early lung development. Less diffuse Dicer1 ablation may account for the survival of this animal, since a rare lung showed less uniform epithelial targeting (Fig. 6d). The surviving adult mouse was dissected at 19.3 months of age and the presence of Dicer1-deficient cells in the lung was confirmed by detection of recombined Dicer1 alleles. The lungs contained multiple macrocysts characterized microscopically as parenchymal cysts with thin walls associated with a mild chronic inflammatory infiltrate (Fig. 6e). No cambium layer or mesenchymal expansion was present. This pathology is similar to Type I PPBs with the multicystic architecture but lacking expanded mesenchyme characteristic of non-progressive or regressed lesions in older siblings and relatives of kindreds with germline DICER1 mutations (Fig. 6e) [4]. Although these findings represent a single animal to date, the results further support that Dicer1 ablation is not sufficient to induce sustained mesenchymal proliferation characteristic of PPB progression. Taken together, the spectrum of lung phenotypes resulting from Dicer1 ablation indicates that DICER1 has temporal-specific functions in the lung epithelium and that ablation of Dicer1 function prior to completion of the pseudoglandular stage is required for development of the PPB phenotype.
Discussion
The current studies investigated the functional significance of temporal and cell type-specific loss of DICER1 function in PPB pathogenesis. Using in vivo genetic analyses, we demonstrate that Dicer1 ablation targeted to the developing lung epithelium results in a lung phenotype indistinguishable from cystic Type I PPB. Interestingly, the mesenchymal cell condensation underlying the epithelial layer that defines the cambium layer in PPB was not seen in Dicer1-deficient lungs. The lack of a cambium layer correlates well with pathologic features of Type I PPB in neonates, wherein the mesenchymal cellularity and composition are more uniform than the same tumors in children [4]. Together, these data demonstrate that Dicer1 ablation in the developing airway epithelium results in cystic lungs that model the earliest stage of PPB. Thus we have generated a clinically relevant PPB model that genetically and phenotypically mimics early PPB, to uncover mechanisms underlying PPB pathogenesis.
Our observations in human PPB and DICER1-deficient murine lungs provide evidence that DICER1 loss in the developing lung epithelium is sufficient for PPB initiation, but not for mesenchymal cell progression to sarcoma. DICER1 expression was lost in PPB-associated epithelium but retained in the tumor mesenchyme, and Dicer1 ablation targeted to the developing lung epithelium resulted in a cystic PPB phenotype, providing direct evidence that epithelial-specific Dicer1 loss is sufficient for PPB initiation. Although Dicer1 ablation in the lung epithelium induced mesenchymal expansion, epithelial Dicer1 loss did not lead to sustained mesenchymal proliferation, septal overgrowth and cellular anaplasia that indicate sarcomatous progression. Furthermore, multicystic lungs from a rare surviving adult mouse with Dicer1 deficient lung epithelium lacked primitive mesenchymal cells; a morphology reminiscent of cystic PPB lesions that did not progress to solid PPB detected in adult family members of children with PPB [4]. Of note, the mesenchyme in the current mouse models is not haploinsufficient for DICER1; thus the mice may be less susceptible to sarcomatous transformation than humans with heterozygous germline loss of function DICER1 mutations. However, mesenchymal transformation to high grade sarcoma in both hereditary and sporadic PPB is associated with biallelic DICER1 mutations in mesenchymal cells, consisting predominantly of germline loss of function mutations and somatic missense mutations in the RNase IIIb domain, that lead to defects in 5p miRNA generation [24,25]. Biallelic p53 inactivation is also found in the majority of cases. These data support a model for PPB pathogenesis wherein DICER1 loss in the developing lung epithelium results in PPB initiation, followed by acquisition of additional genetic mutations altering DICER1 RNase IIIb and/or p53 function in the mesenchymal cells, resulting in progression to sarcoma.
DICER1-dependent functions critical in lung organogenesis and PPB pathogenesis are likely to be relevant to development of other tumors associated with the DICER1 tumor predisposition syndrome, namely renal cystic nephroma and ovarian Sertoli-Leydig cell tumors [20,26–29]. The biologic reason for organ-specific phenotypes, despite murine models demonstrating critical DICER1 functions in a wider spectrum of tissues, is not yet clear, but may relate to cell type-specific DICER1 functions and/or critical epithelial-mesenchymal signaling shared by the affected organs. Germline DICER1 loss of function mutations and somatic ‘hotspot’ missense mutations in the DICER1 RNase IIIb domain seen in PPB are also detected in cystic nephromas and Sertoli-Leydig cell tumors, supporting similar tumor pathogenesis [8,24,25,30–32]. Cystic nephroma is also analogous to PPB, in that the neoplastic process appears to arise during specific stages in kidney development, with progression to sarcomas resembling the sarcomatous component of Types II-III PPBs [4,8,33]. Additionally, like the lung, renal development requires reciprocal inductive events between epithelial and mesenchymal tissues, and mouse models demonstrate similar cell type-specific DICER1 functions in the developing kidney [34–43]. Interestingly, Dicer1 ablation in the developing ureteric bud and collecting duct epithelia results in collecting duct cysts with apoptosis preceding cyst formation and accompanying cyst progression, similar to that seen in the current lung studies [39,40]. These findings support common DICER1-dependent pathways critical for organogenesis and tumor suppression in the lung and kidney.
Temporal analysis of DICER1-dependent lung phenotypes provide mechanistic insights by identifying key biologic processes that require DICER1 function, as well as temporal and cell type-specific requirements for DICER1 control of lung organogenesis. The initial phenotype induced by Dicer1 loss was epithelial apoptosis, followed by distal airspace dilation, epithelial hyperplasia and a transient increase in mesenchymal proliferation leading to septal expansion. This phenotypic progression differs from the lung phenotype reported after Dicer1 ablation induced by Sonic Hedgehog (Shh)-promoted Cre, that consisted of arrested branching morphogenesis resulting in large pouches lined by a heterogeneous epithelium detached from the underlying mesenchyme and characterized as stratified, pseudostratified and simple columnar epithelium, suggesting divergent proximal conducting and distal respiratory epithelial differentiation [19]. Additionally, the initial Shh-Cre-induced morphologic phenotypes were present prior to an increase in epithelial apoptosis, leading to the proposal that DICER1 plays a specific role in regulating lung epithelial morphogenesis independent of its requirement for cell survival. In contrast, DICER1-deficient lungs in the current studies show a less severe defect in branching morphogenesis, uniform distal respiratory epithelial cell lining of the cysts that remained attached to the underlying mesenchyme, and apoptosis preceding development of the morphologic abnormalities. Differences in the two models could account for the differing phenotypic outcomes. Shh-induced Dicer1 ablation is not restricted to the lung and occurs earlier in foregut development, before lung initiation [19,44]. Additionally, Dicer1 ablation in the Shh model is accompanied by loss of one wild-type Shh allele. Although heterozygous Shh+/− mice do not have lung abnormalities, it is unclear whether decreased SHH levels alter DICER1-dependent phenotypes, since SHH is known to have a critical role in lung development [45]. Phenotypic differences in these distinct murine models are likely due, at least in part, to loss of temporal-specific DICER1 functions, given our results directly demonstrating that timing of Dicer1 loss plays a fundamental role in determining phenotypic outcomes.
The current studies directly demonstrate that DICER1 plays an essential role in controlling lung development during the pseudoglandular period but that DICER1 is dispensable during later stages of lung organogenesis. Early lethality of germline Dicer1−/− embryos combined with the failure to observe Dicer1 null tumors and homozygous DICER1 loss-of-function alterations in murine models and human tumors, respectively, led to the notion that DICER1 is essential for tumor formation and may be required for cell survival. Contrary to these observations, restricted cell lineages survive in the absence of DICER1 function, and DICER1 is not essential for sarcoma tumor cell survival or proliferation in culture or xenograft formation in vivo [11,46,47]. The current studies extend these findings by demonstrating that DICER1 functions are developmental stage-specific and not essential for survival, proliferation and differentiation of subpopulations of epithelial cells during lung organogenesis.
Our genetic discoveries in PPB combined with the current studies in mouse models demonstrate that DICER1 loss is critical in PPB pathogenesis, and that DICER1 loss in the developing lung epithelium is sufficient for initiating a cystic PPB phenotype. These data provide evidence that PPB can arise through a novel mechanism of non-cell autonomous tumor initiation, wherein the genetic abnormality initiating tumorigenesis (Dicer1 loss) does not occur in the mesenchymal cells that ultimately transform, but rather in the benign epithelial component. Consistent with this novel mechanism of oncogenesis, our pathologic and clinical observations in PPBs and murine models provide evidence that DICER1 loss is not sufficient for progression to sarcoma, but rather that early stage PPB may remain benign cystic lesions as lung maturation continues. Thus, the currently generated mouse models provide mechanistic insights into how DICER1 controls organogenesis and manifests biologically in human disease and will be valuable for elucidating events that cooperate with DICER1 loss to promote tumor progression.
Supplementary Material
Acknowledgments
We thank Betsy Dipasquale and Paula Blair for immunhistochemical staining, Daniel Yu for assistance with animal studies, Michael S. Burhans for technical expertise, Veterinary Services for excellent animal care, and the International PPB Registry and patients who generously give of themselves to promote our research. This work was supported by the St. Baldrick’s Foundation, Society for Pediatric Pathology and National Institute of Health (NIH) (HL109265) (K.A.W.-B.), NIH F32 CA189685 and NIH T32 CA117846 (P.K.W.), and NIH T32 HL007752 (M.A.G.).
Footnotes
Conflict of interest statement: No conflicts of interest were declared.
Author contribution statement
D.A.H. and K.A.W-B. conceived the project; P.K.W., M.A.G., X.M, M.C. and K.A.W.-B. performed and analyzed the experiments; D.A.H., Y.H.M. and L.P.D. provided and/or pathologically evaluated PPB samples including DICER1 immunohistochemistry; J.M.S. provided expertise in lung epithelial and mesenchymal separations; S.E.W. provided immunohistochemical staining expertise; P.K.W., M.A.G., D.A.H., L.P.D and K.A.W-B. wrote and/or revised the manuscript.
References
- 1.Priest JR, McDermott MB, Bhatia S, et al. Pleuropulmonary blastoma: a clinicopathologic study of 50 cases. Cancer. 1997;80:147–161. [PubMed] [Google Scholar]
- 2.Wright JR., Jr Pleuropulmonary blastoma: A case report documenting transition from type I (cystic) to type III (solid) Cancer. 2000;88:2853–2858. doi: 10.1002/1097-0142(20000615)88:12<2853::aid-cncr28>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Priest JR, Hill DA, Williams GM, et al. Type I pleuropulmonary blastoma: a report from the International Pleuropulmonary Blastoma Registry. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4492–4498. doi: 10.1200/JCO.2005.05.3595. [DOI] [PubMed] [Google Scholar]
- 4.Hill DA, Jarzembowski JA, Priest JR, et al. Type I pleuropulmonary blastoma: pathology and biology study of 51 cases from the international pleuropulmonary blastoma registry. Am J Surg Pathol. 2008;32:282–295. doi: 10.1097/PAS.0b013e3181484165. [DOI] [PubMed] [Google Scholar]
- 5.Medina PP, Slack FJ. microRNAs and cancer: an overview. Cell Cycle. 2008;7:2485–2492. doi: 10.4161/cc.7.16.6453. [DOI] [PubMed] [Google Scholar]
- 6.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nature reviews Molecular cell biology. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Doros LA, Rossi CT, Yang J, et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014 doi: 10.1038/modpathol.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson DS, Mason-Richie NA, Gettler CA, et al. Retinoblastoma family proteins have distinct functions in pulmonary epithelial cells in vivo critical for suppressing cell growth and tumorigenesis. Cancer Res. 2009;69:8733–8741. doi: 10.1158/0008-5472.CAN-09-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wikenheiser-Brokamp KA. Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development. 2004;131:4299–4310. doi: 10.1242/dev.01232. [DOI] [PubMed] [Google Scholar]
- 11.Harfe BD, McManus MT, Mansfield JH, et al. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 13.Shannon JM, Nielsen LD, Gebb SA, et al. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Developmental dynamics : an official publication of the American Association of Anatomists. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Hill DA, Ivanovich J, Priest JR, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridges JP, Ikegami M, Brilli LL, et al. LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice. The Journal of clinical investigation. 2010;120:1736–1748. doi: 10.1172/JCI38061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiological reviews. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 17.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Developmental cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein E, Kim SY, Carmell MA, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 19.Harris KS, Zhang Z, McManus MT, et al. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slade I, Bacchelli C, Davies H, et al. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. Journal of medical genetics. 2011;48:273–278. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 21.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–29. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- 22.Perl AK, Wert SE, Nagy A, et al. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carraro G, El-Hashash A, Guidolin D, et al. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Developmental biology. 2009;333:238–250. doi: 10.1016/j.ydbio.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pugh TJ, Yu W, Yang J, et al. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene. 2014;0 doi: 10.1038/onc.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki M, Yoshida K, Shiraishi Y, et al. Biallelic DICER1 mutations in sporadic pleuropulmonary blastoma. Cancer Res. 2014;74:2742–2749. doi: 10.1158/0008-5472.CAN-13-2470. [DOI] [PubMed] [Google Scholar]
- 26.Boman F, Hill DA, Williams GM, et al. Familial association of pleuropulmonary blastoma with cystic nephroma and other renal tumors: a report from the International Pleuropulmonary Blastoma Registry. The Journal of pediatrics. 2006;149:850–854. doi: 10.1016/j.jpeds.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 27.Priest JR, Watterson J, Strong L, et al. Pleuropulmonary blastoma: a marker for familial disease. The Journal of pediatrics. 1996;128:220–224. doi: 10.1016/s0022-3476(96)70393-1. [DOI] [PubMed] [Google Scholar]
- 28.Schultz KA, Pacheco MC, Yang J, et al. Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1 mutations: a report from the International Pleuropulmonary Blastoma Registry. Gynecologic oncology. 2011;122:246–250. doi: 10.1016/j.ygyno.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rio Frio T, Bahubeshi A, Kanellopoulou C, et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA : the journal of the American Medical Association. 2011;305:68–77. doi: 10.1001/jama.2010.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anglesio MS, Wang Y, Yang W, et al. Cancer-associated somatic DICER1 hotspot mutations cause defective miRNA processing and reverse-strand expression bias to predominantly mature 3p strands through loss of 5p strand cleavage. The Journal of pathology. 2013;229:400–409. doi: 10.1002/path.4135. [DOI] [PubMed] [Google Scholar]
- 31.de Kock L, Plourde F, Carter MT, et al. Germ-line and somatic DICER1 mutations in a pleuropulmonary blastoma. Pediatric blood & cancer. 2013;60:2091–2092. doi: 10.1002/pbc.24692. [DOI] [PubMed] [Google Scholar]
- 32.Heravi-Moussavi A, Anglesio MS, Cheng SW, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. The New England journal of medicine. 2012;366:234–242. doi: 10.1056/NEJMoa1102903. [DOI] [PubMed] [Google Scholar]
- 33.Priest JR, Williams GM, Hill DA, et al. Pulmonary cysts in early childhood and the risk of malignancy. Pediatric pulmonology. 2009;44:14–30. doi: 10.1002/ppul.20917. [DOI] [PubMed] [Google Scholar]
- 34.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michos O. Kidney development: from ureteric bud formation to branching morphogenesis. Current opinion in genetics & development. 2009;19:484–490. doi: 10.1016/j.gde.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu JY, Sims-Lucas S, Bushnell DS, et al. Dicer function is required in the metanephric mesenchyme for early kidney development. American journal of physiology Renal physiology. 2014 doi: 10.1152/ajprenal.00426.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey SJ, Jarad G, Cunningham J, et al. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. Journal of the American Society of Nephrology : JASN. 2008;19:2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho J, Ng KH, Rosen S, et al. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. Journal of the American Society of Nephrology : JASN. 2008;19:2069–2075. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagalakshmi VK, Ren Q, Pugh MM, et al. Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney international. 2011;79:317–330. doi: 10.1038/ki.2010.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastorelli LM, Wells S, Fray M, et al. Genetic analyses reveal a requirement for Dicer1 in the mouse urogenital tract. Mammalian genome : official journal of the International Mammalian Genome Society. 2009;20:140–151. doi: 10.1007/s00335-008-9169-y. [DOI] [PubMed] [Google Scholar]
- 41.Sequeira-Lopez ML, Weatherford ET, Borges GR, et al. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. Journal of the American Society of Nephrology : JASN. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi S, Yu L, Chiu C, et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. Journal of the American Society of Nephrology : JASN. 2008;19:2159–2169. doi: 10.1681/ASN.2008030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei Q, Bhatt K, He HZ, et al. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. Journal of the American Society of Nephrology : JASN. 2010;21:756–761. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harfe BD, Scherz PJ, Nissim S, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annual review of physiology. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- 46.Cuellar TL, Davis TH, Nelson PT, et al. Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc Natl Acad Sci U S A. 2008;105:5614–5619. doi: 10.1073/pnas.0801689105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravi A, Gurtan AM, Kumar MS, et al. Proliferation and tumorigenesis of a murine sarcoma cell line in the absence of DICER1. Cancer cell. 2012;21:848–855. doi: 10.1016/j.ccr.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.