Abstract
Human skin color, i.e. pigmentation, differs widely among individuals as do their responses to various types of ultraviolet radiation (UV) and their risks of skin cancer. In some individuals UV-induced pigmentation persists for months to years in a phenomenon termed long-lasting pigmentation (LLP). It is unclear whether LLP is an indicator of potential risk for skin cancer. LLP seems to have similar features to other forms of hyperpigmentation, e.g. solar lentigines or age spots, which are clinical markers of photodamage and risk factors for precancerous lesions. To investigate what UV-induced molecular changes may persist in individuals with LLP, clinical specimens from non-sunburn-inducing repeated UV exposures (UVA, UVB or UVA+UVB) at 4 months post-exposure (short-term LLP) were evaluated by microarray analysis and dataset mining. Validated targets were further evaluated in clinical specimens from 6 healthy individuals (3 LLP+ and 3 LLP-) followed for more than 9 months (long-term LLP) who initially received a single sunburn-inducing UVA+UVB exposure. The results support a UV-induced hyperpigmentation model in which basal keratinocytes have an impaired ability to remove melanin that leads to a compensatory mechanism by neighboring keratinocytes with increased proliferative capacity to maintain skin homeostasis. The attenuated expression of SOX7 and other hemidesmosomal components (integrin α6β4 and plectin) leads to increased melanosome uptake by keratinocytes and points to a spatial regulation within the epidermis. The reduced density of hemidesmosomes provides supporting evidence for plasticity at the epidermal-dermal junction. Altered hemidesmosome plasticity, and the sustained nature of LLP, may be mediated by the role of SOX7 in basal keratinocytes. The long-term sustained subtle changes detected are modest, but sufficient to create dramatic visual differences in skin color. These results suggest that the hyperpigmentation phenomenon leading to increased interdigitation develops in order to maintain normal skin homeostasis in individuals with LLP.
Keywords: hemidesmosome, skin, pigmentation, ultraviolet radiation, sunburn
Introduction
Human skin is not a passive barrier, but rather acts as a dynamic barrier constantly responding to stimuli. One prominent input is the proportion of ultraviolet radiation (defined from here on as UV) that individuals encounter on a daily basis. The long-term effects that may or may not be involved in carcinogenic progression in skin malignancies are caused by pigmentary, molecular and morphological changes that develop over time from solar or artificial UV exposure over years to decades.
To maintain skin homeostasis, keratinocytes undergo terminal differentiation as they move up through the epidermis to the surface of the skin. The epidermal-dermal junction is a boundary of undulating epidermal rete ridges and dermal ridges forming an interdigitated surface area that reinforces cohesion between the epidermis and dermis. This junction remodels itself throughout its life and adapts to disruptions of its boundary during wound repair when cells lose hyperadhesiveness to gain mobility based on their Ca2+ dependent state [1]. Pigmented spots, i.e. solar lentigines, that develop a hyperpigmented basal layer with an increased number of elongated club-shaped protrusions into the dermis have long been considered a visual indicator of the accrual of photodamage. Degradation of extracellular matrix that leads to premature photoaging has already been indicated to develop after physiologically relevant UV exposure [2]. Therefore, this measure of chronic UV exposure is related to an elevated risk for skin cancer [3].
An important question to be addressed is: what are the long-term consequences of different types of UV exposures? Any potential skin morphological changes after sunburn- or non-sunburn-inducing UV doses would be predicted to occur at long-term time points after the initial UV exposure. Typically, UV-induced skin pigmentation fades over time, however we recently identified a pigmentation phenomenon termed long-lasting pigmentation (LLP) where the skin pigmentation persists in the UV-exposed areas in some individuals and lasts for months to years [4,5]. Very little is known about LLP, except that it is a phenomenon predisposed in subjects with a higher UV sensitivity threshold, where increasing age and UV dose facilitate its induction. It is unclear whether LLP is an indicator of potential risk for skin cancer and/or provides a protective effect. The goal of this study was to determine if there were changes in the skin of LLP+ individuals at multiple levels, including: pigmentation, melanin content, cell proliferation, gene transcription profiles and/or skin morphology. The results show that there were no overt long-term changes but there were subtle changes that led to the spatial disruption of hemidesmosomal components after a single sunburn-inducing UV dose. These modest changes at the basement membrane lead to sustained pigmentation increases potentially through feedback from the basement membrane components relaying signals to the nucleus to affect gene expression.
Materials and Methods
Clinical studies and tissue culture
Skin specimens from two sets of clinical studies [6,7] were used in this study. Both clinical studies were approved by the FDA and Beiersdorf Research Involving Human Subjects Committees (FDA#99-002-R; BDF37378) and were conducted according to Helsinki guidelines. The original clinical studies recruited healthy human subjects from their respective geographical areas by having them screened by a dermatologist after providing informed consent for their participation. General information about the study subjects’ UV sensitivity, phototype, gender, age and propensity to develop pigmentation are summarized in Table S1.
The first set of clinical specimens studied were acquired to investigate delayed UV-induced changes in areas of the skin that received the highest single erythemal UVA+UVB exposures (corresponding to 4, 2.8 or 2 minimal erythema doses) on 6 healthy individuals (3 LLP+ and 3 LLP-) of phototypes between 2-5 followed >9 months after the initial UV exposure. The second set of clinical specimens were acquired at 4 months post-UV exposure from single or repetitive suberythemal types of UV (UVA, UVB and/or UVA+UVB) over 2 weeks from 7 healthy human subjects of phototypes 2-3. siRNA knockdown assay, melanosome isolation, melanosome uptake assay and cells cultured to generate protein lysates used for immunoblotting are described in the Supplementary materials and methods. Details on the microarray dataset and statistical analysis are described in the Supplementary materials and methods.
Immunohistochemistry, proximity ligation assays and antibodies
For details, see Supplementary materials and methods. Briefly, for melanocyte identification, mouse monoclonal antibodies against MITF (Lab Vision Neomarkers, Fremont, CA; 1:100), MART1 (Lab Vision Neomarkers; 1:100) and a rabbit polyclonal antibody to TYR (αPEP7h, 1:750) (Jiménez et al., 1991), were used for immunofluorescence. For identification of proliferating cells, a mouse monoclonal antibody to PCNA (Dako, Carpinteria, CA 1:200) was used. Other UV-responsive factors tested by immunofluorescence included mouse monoclonal antibodies to SOX7 (Novus Biologicals, Littleton, CO; 1:50) and integrin β4 [M126] (Abcam; Cambridge, MA 1:100) and a rabbit polyclonal antibody to plectin (Abcam; 1:100). Microscopy and quantification assays are described in detail in the Supplementary materials and methods.
Electron microscopy of paraffin sections
Long-term LLP specimens and unexposed controls for LLP+ (#S100, S101) and LLP- (#S36, S81) were prepared for electron microscopy by deparaffinizing formalin-fixed paraffin-embedded samples as previously described [8]. Approximately 90 nm sections were cut using a Leica EM UC6 Ultra-microtome and were collected on 1 mm wide slot grids where they were counter-stained with uranyl acetate and lead citrate. Those specimens were then imaged together by electron microscopy with a Gatan 1 × 1k cooled CCD camera on a FEI CM120 transmission electron microscope with a beam energy of 120 keV (FEI Company, Hillsboro, OR, USA).
Results
Pigmentation, melanocyte density, melanin content and distribution
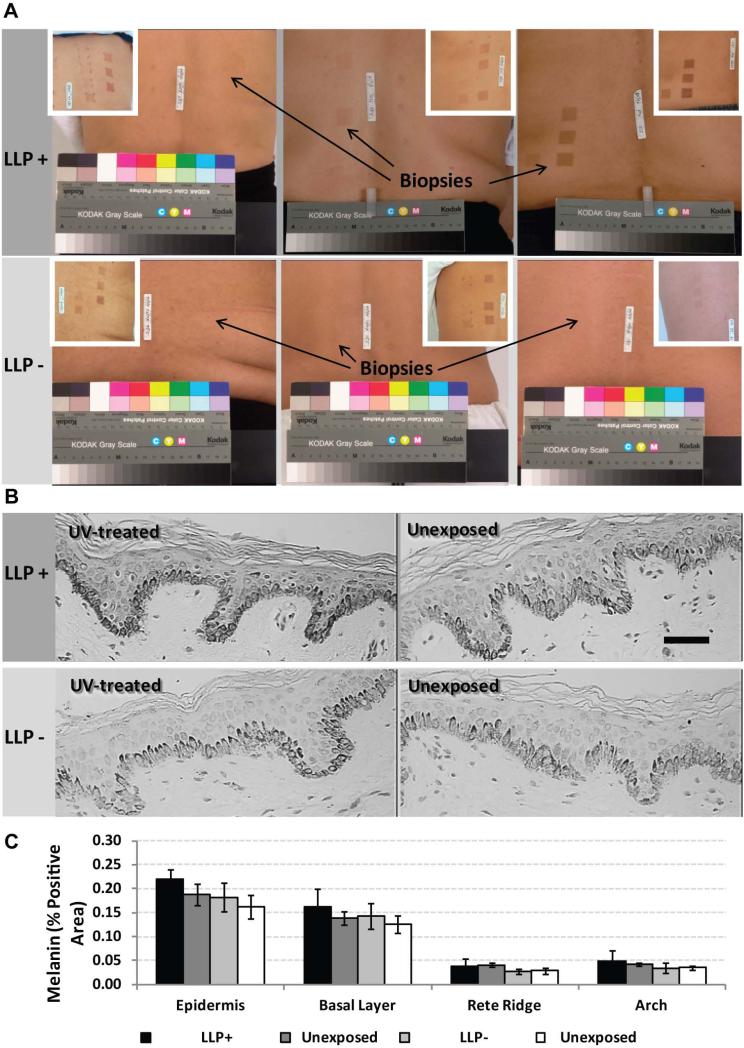
To evaluate potential long-term changes in the epidermis, pigmentation was characterized in skin biopsy specimens from a sunburn-inducing clinical study (Table S1). Visually, the long-term LLP+ individuals showed increased pigmentation and a hyperpigmented basal layer compared to their unexposed control and LLP- counterparts (individuals who received a sunburn-inducing UV dose but did not develop long-term LLP) (Figure 1A). Skin biopsies acquired from the most pigmented areas (black arrows in Figure 1A) that had received UV more than 9 months earlier showed elevated levels of melanin positive areas both in the entire epidermis as well as predominantly in the basal layer in the long-term LLP+ individuals compared to the unexposed controls and LLP- individuals (Figure 1B and 1C). Although not statistically significant, the trend suggests that a hyperpigmented basal layer is present after the initial single UV treatment > 9 months earlier.
Figure 1.
Long-term LLP hyperpigmentation increases levels of melanin in the basal layer of human skin. (A) Images of skin after a single erythemal UV treatment at > 9 months post-exposure are depicted for the 3 LLP+ and 3 LLP- subjects from which biopsy specimens used for immunohistochemistry were acquired from the most pigmented UV-exposed area versus the adjacent unexposed control (insets show pigmentation in the UV-treated areas after 2 weeks). (B) Melanin contents of specimens detected by Fontana-Masson staining; representative micrographs are shown for #S100. scale bar = 50 μm. (C) Melanin content (% Positive Area) and distribution quantified for the total epidermis, the basal layer, the rete ridge area and the arch area in the epidermis for the 3 LLP+ and 3 LLP- UV-exposed samples and the adjacent unexposed controls; no significant differences detected. Data are from 15 random micrographs across 2 sections per specimen ± SD.
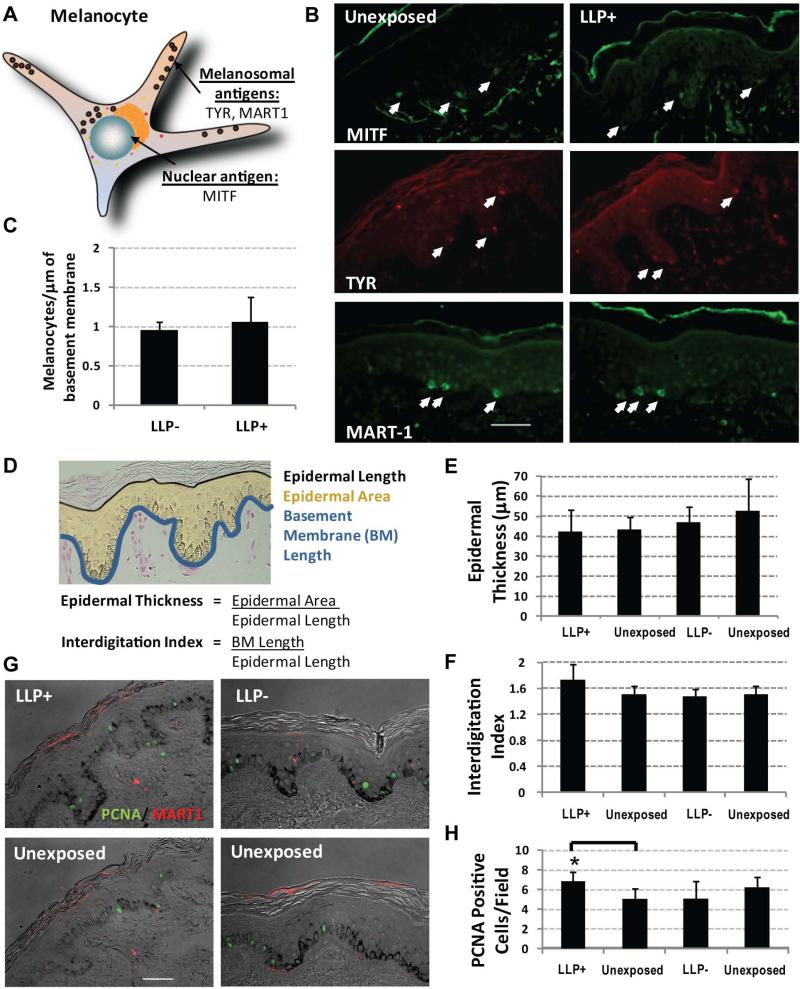
To determine if the elevated melanin levels were a function of increased numbers of melanocytes, several melanocyte-specific markers (TYR, MART1 and MITF) were used to identify melanocytes at the epidermal-dermal junction (Figure 2A). The fact that the number of melanocytes is not statistically different among LLP+ subjects versus LLP- subjects in the long-term LLP group suggests that there is no proliferation of melanocytes occurring at this time point (Figure 2B and 2C). In a study evaluating the UV responses in the skin of subjects representing different racial/ethnic groups, no statistically significant increases in melanocyte density at 1 week post-UV exposure were seen in individuals receiving a single sunburn-inducing dose of UVA+UVB from which these archival samples were derived [6]. There has been some indication of certain individuals showing increased melanocyte density in other long-term studies, but those individuals received increasing repetitive UV doses and with the exception of one individual did not show any increase in visible pigmentation [9]. Taken together, these markers provide a consistent representation of the comparable number of melanocytes found in the skin [6,10]. The results suggest that the hyperpigmentation of the basal layer may not be due to increased melanin synthesis from an increased number of melanocytes.
Figure 2.
Melanocyte density and epidermal thickness remains the same while keratinocyte proliferation and interdigitation increase in long-term LLP > 9 months. (A) Schematic of melanocyte-specific targets identified at the epidermal-dermal junction by immunofluorescence for TYR (tyrosinase), MITF (microphthalmia transcription factor) and MART1 (melanoma antigen recognized by T-cells). (B) Micrographs of staining for MITF, TYR and MART1 in long-term LLP+ skin and in unexposed control skin; representative micrographs are shown for #S100 (scale bar = 50 μm). (C) Quantification of melanocyte density assessed using the 3 melanocyte-specific markers quantified per basement membrane length and normalized to the respective adjacent unexposed control; data taken from 5 random micrographs across 2 sections for each marker ± SD; no significant differences detected. (D) Epidermal thickness and interdigitation index were calculated as defined in the diagram in tissue specimens using the epidermal area, epidermal length and basement membrane length as endpoints. (E) No changes in epidermal thickness occurred between LLP+ skin and adjacent unexposed controls; data taken from 5 random micrographs across 2 sections per specimen ± SEM; no significant differences detected. (F) Only the interdigitation index increased in LLP+ skin; data taken from 5 random micrographs across 2 sections per specimen ± SEM; no significant differences detected. (G) Proliferation evaluated by immunofluorescence staining for PCNA (green) in LLP+ individuals along with a melanocyte marker MART1 (red). (H) Number of PCNA-positive cells increased in LLP+ samples (P<0.05) versus unirradiated control skin, but that trend was not seen in LLP- subjects; data taken from 5 random micrographs across 2 sections for each individual.
Epidermal morphology characteristics
To evaluate potential long-term changes in the skin, epidermal thickness and interdigitation were characterized to detect subtle morphological changes that might occur (Figure 2D). In LLP+ and LLP- individuals, epidermal thickness remained unchanged (Figure 2E). Although not statistically significant, there was a trend towards an increased interdigitation of the dermis and epidermis as indicated by the 14% increase in mean interdigitation index in LLP+ individuals compared to the unexposed control that was not seen in LLP- subjects (Figure 2F). This suggests a greater degree of rete ridge and dermal ridge formation in LLP prone individuals.
Cell proliferation characterization
To further evaluate any abnormal cell proliferation in LLP+ individuals, that parameter was assessed using PCNA as a marker. In LLP+ individuals, PCNA-positive staining was not seen in melanocytes using MART1 as a marker (Figure 2G). This corroborated the results of the comparable melanocyte density in LLP+ and LLP- individuals. A statistically significant (P<0.05) increase in proliferating (PCNA-positive) keratinocytes was detected in LLP+ individuals, but it remains unclear how meaningful this increase is considering that epidermal thickness did not change (Figure 2H). Normal skin turnover requires a degree of cycling keratinocytes to maintain homeostasis. The question is whether keratinocytes are compensating for increased melanin levels in the basal layer by exhibiting greater numbers of proliferating keratinocytes.
Data mining of the microarray dataset
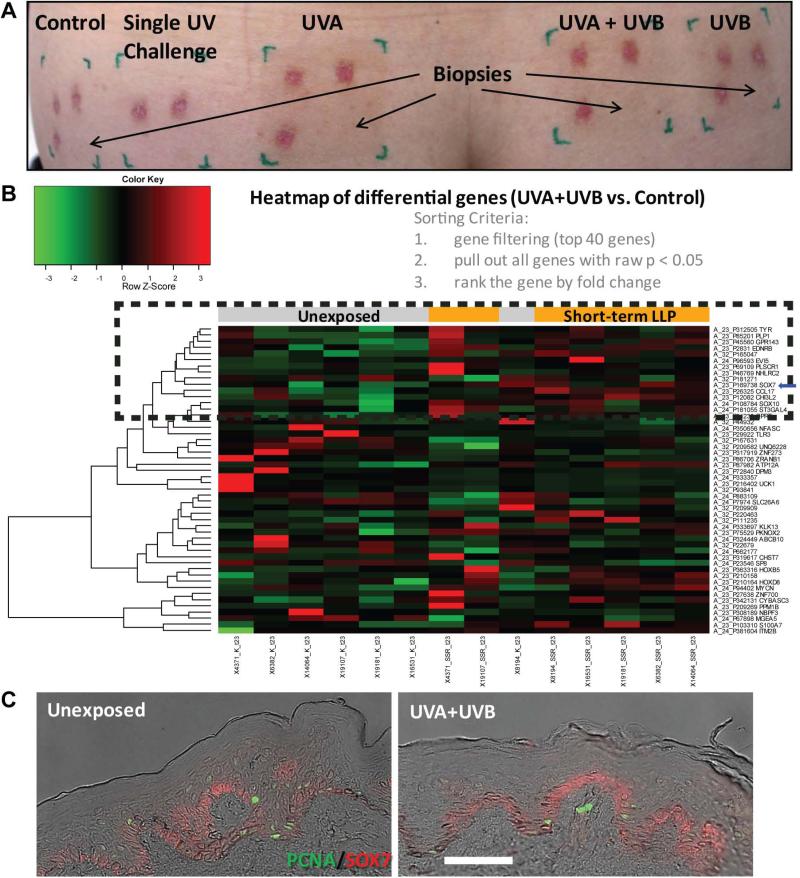
To further understand what might be the mechanism behind LLP, data mining of a microarray dataset from a different clinical study where individuals exhibited short-term LLP at 4 months post-UV exposure was examined (Table S1, Figure 3A). This clinical study was designed to evaluate UV photoprotection in fair skinned individuals by inducing pigmentation through 2 weeks of daily repetitive UV treatments (UVA, UVB and/or UVA+UVB). The microarray dataset analysis demonstrated that the top 50 differentially expressed genes (with a P<0.05) for the UV treatment compared to the control clustered two-dimensionally on a heatmap according to fold-change absolute values (Figure 3B). With the exception of one subject, the controls and UV-treated samples clustered together. Looking closely at the UVA+UVB versus control heatmap, known targets involved in melanogenesis can be found, such as the rate-limiting enzyme in melanogenesis (TYR), the endothelin B receptor EDNRB and SOX10. Considering that the type of UV used in the long-term LLP+ individuals was a combination of UVA and UVB, UVA+UVB versus the control was the focus of the evaluation, but this dataset also includes comparisons of UVA or UVB only (Figure S1). However, in the heatmaps for UVA versus the control (Figure S1A) or UVB versus the control (Figure S1B), the melanogenic targets were not differentially expressed. What became evident were the different expression patterns in genes among the different UV treatments.
Figure 3.
Short-term LLP hyperpigmentation and heatmap of UV-induced genes identify SOX7 expression changes. (A) Image of skin pigmentation resulting from different types of UV treatments at 4 months post-exposure on the lower back of one of the subjects. Red areas are a result of previously acquired biopsies at earlier time points. (B) Microarray analysis of short-term LLP at 4 months in UV-irradiated sites compared to unexposed control skin. Heatmap clustering was generated for UVA+UVB vs the unexposed control (red (max = +3) to green (min = -3) color gradient). The orange bars at the top of heatmap indicate UV-irradiated samples and gray bars indicate the control unexposed samples. The black-dashed box highlights a cluster of known pigment genes and the blue arrow highlights SOX7. (C) Expression and distribution of SOX7 (red) and proliferating/cycling keratinocytes (stained with PCNA in green) within the rete ridge and arch areas of the epidermis corroborating the UV-induced expression changes in the heatmap (subject #17972 shown; scale bar = 50 μm).
Due to the array of potential gene targets that could be evaluated, this analysis focused on gene expression changes that might modulate pigmentation and/or UV carcinogenesis. Therefore, to determine if any of the melanogenic or paracrine network pathways were over-represented in our dataset, this short-term LLP dataset was uploaded into Ingenuity Pathway Analysis (IPA, 2013 Ingenuity Systems, Redwood City, CA; www.ingenuity.com). The following cutoff filters were applied to the dataset: fold change = 1.2, Intensity = 3, P≤0.05 to offer a statistically significant target pool while at the same time allowing for the detection of subtle changes. Not surprisingly, the core analysis listed the top diseases and disorders with Dermatological Diseases and Conditions at the top of each respective repetitive UV regimen (Table S2 and Table S3). Of particular significance within the Dermatological Diseases and Conditions category, the UVA and UVB alone treatments identified molecules involved in skin cancer, skin carcinomas, but also senile lentigo (or age spots) (Table S3). Melanocyte Development and Pigmentation Signaling was one of the top canonical pathways for the suberythemal repetitive UVA+UVB exposure treatment regimen (Table S4). IPA visualization of this pathway indicated that the melanogenic network remained active even after 4 months in both the UVA+UVB and UVB alone datasets, while the UVA alone dataset showed a predicted inhibition state. These data corroborate previous findings that showed that suberythemal repetitive UVA irradiation did not increase de novo melanogenesis [11]. Essentially, the chemical analysis of the different components of melanin were reported for the different 2-week repetitive UV treatments (UVA, UVB and/or UVA+UVB) and the results showed that UVA had no effect on increasing melanin levels under the conditions tested [11]. In contrast, the UVA alone treatment highlighted the persistence of oxidative stress, specifically of two differentially expressed antioxidant genes involved in the Glutathione Redox Reactions I pathway (Table S4) [12,13]. UVA predominantly produces oxidative damage in the form of reactive oxygen species (ROS), i.e. hydrogen peroxide and superoxide, and to a lesser extent cyclobutane pyrimidine dimers compared to UVB [14,15]. Glutathione peroxidase-1 has been indicated to suppress the oxidative stress effects induced by UVA irradiation [16]. The significance of the antioxidant genes relates to UVA carcinogenesis because fair-skinned individuals with low amounts of melanin are more susceptible to ROS production [12]. With the exception of the endothelin B receptor (ENDRB), the top molecules that were up- or down-regulated with each respective UV treatment were not involved in pigmentation (Table S5). Therefore, further data mining was carried out to screen for the top upstream regulators predicted to be activated or inhibited in IPA, which provided upstream targets for several molecules found to be involved in skin cancer, skin carcinomas, and senile lentigo (Table S6). In the end this process identified SOX7 (discussed below) as a target of an activated upstream transcriptional regulator involved in cell-cell adhesion.
Upon close examination of the genes that clustered together with known melanogenic proteins (TYR, EDNRB and SOX10) after UVA+UVB treatment, another SRY-related high-mobility-group box (SOX) family member was identified (Figure 3B). Because other SOX family members such as SOX9 and SOX10 have been reported to play a role in pigmentation [17], our focus turned to SOX7. SOX7 is a member of the subgroup F of SOX proteins along with SOX17 and SOX18, all of which contain a noncanonical high-mobility-group (HMG) box domain and a transactivation domain [18]. The literature suggests that SOXF subgroup transcription factors are involved in hemidesmosome formation and that SOX18 mutant mice resemble the skin blistering disease epidermolysis bullosa [19]. In light of the literature and lack of studies involving SOX7, the potential role of SOX7 in destabilizing cell-to-cell junctions at the basement membrane required further inquiry.
The responses after UV treatment detected at the gene expression level in the microarray dataset were consistent with the SOX7 protein expression pattern in short-term LLP specimens (Figure 3C). SOX7 expression was strongest in the arch areas compared to the rete ridge areas (Figure 3C). Although, SOX proteins are generally nuclear, SOX9 cytoplasmic staining has been documented [20]. Furthermore, a recent review on the shuttling of SOX proteins (i.e. SOX9) highlighted a consensus nuclear export signal found in the HMG box that may suggest a mechanism of regulation [21]. This evidence suggested that SOX7 might be involved in regional differences between rete ridge and arch areas along the basal layer at the basement membrane that could potentially affect hemidesmosome formation.
Role of SOX7 at the epidermal-dermal junction along with hemidesmosomal components
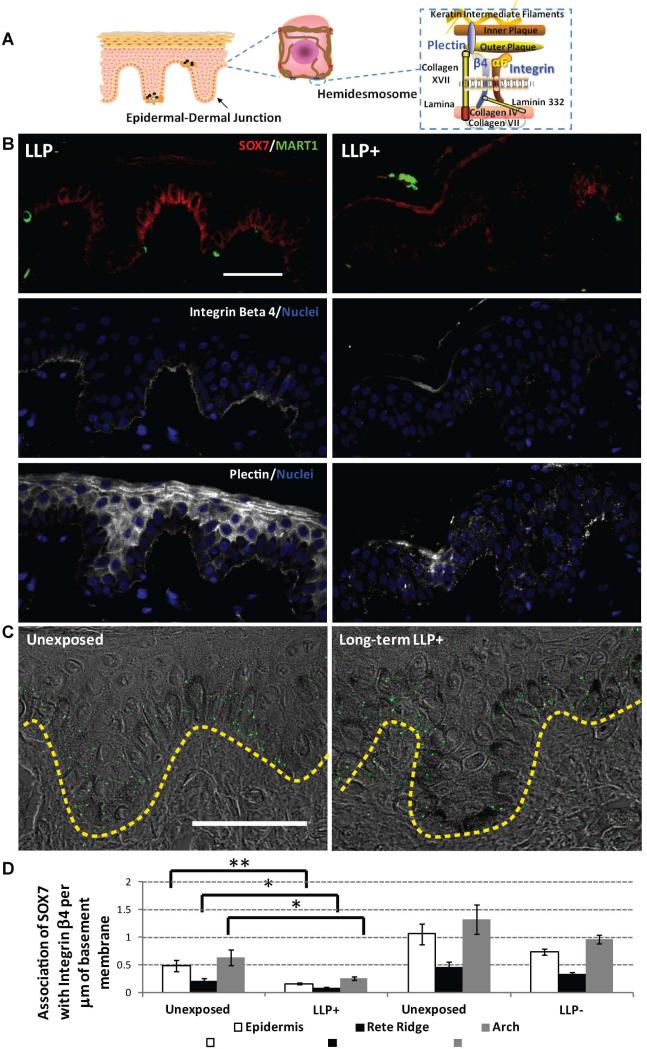
To further evaluate the role of SOX7 in LLP induction, long-term LLP+ and LLP- individuals were evaluated along with several components (integrin β4 and plectin) of the cytoplasmic plaque of the hemidesmosome (Figure 4A). The LLP- specimens showed strong staining for SOX7 along the basal layer that correlated with the strong basement membrane staining of integrin β4 (Figure 4B). The plectin protein is found both in desmosomes between keratinocytes and in hemidesmosomes at the basement membrane [22-25]. Staining for plectin along the basement membrane was consistent with the staining of integrin β4 for LLP- individuals (Figure 4B). However, for LLP+ individuals, the staining for SOX7, integrin β4 and plectin was markedly reduced. Specifically, the rete ridge areas seemed to lack any staining for SOX7 and the other hemidesmosomal components.
Figure 4.
Spatial disruption of hemidesmosomal components associated with SOX7. (A) Diagram of the epidermis of human skin with detailed components of hemidesmosomes found at the epidermal-dermal junction. (B) Decreases in the expression of epidermal-dermal junction components (integrin β4 and plectin) correlate with the decrease in SOX7 in long-term LLP samples >9 months after UV exposure. Representative micrographs for LLP- (#S70) and LLP+ (#S100) individuals show SOX7 (red) and the melanocyte marker MART1 (green) in the left column. Serial sections of the same subject were used to stain integrin β4 (white, middle column) and plectin (white, right column) along the basement membrane of epidermal skin sections with nuclei (blue) labeled using DAPI; scale bar = 50 μm. (C) Association between SOX7 and the hemidesmosomal component integrin β4 using proximity ligation assays in LLP+ individuals. The distribution of green puncta within the epidermis indicates an association of <40 nm between the two proteins along the epidermal-dermal junction (yellow dashed lines). scale bar = 50 μm. (D) Decreases in the total number of associations between SOX7 and integrin β4 were quantified along the basement membrane for both the rete ridge and the arch areas. At least 5 random micrographs across 2 sections were quantified for each individual. * = P<0.05, ** = P<0.005 compared to the unexposed control. No significant differences detected between LLP- areas versus unexposed control. Diagram in (A) manually created using available shapes in Microsoft PowerPoint, Redmond, WA and the add-in ScienceSlides, VisiScience, Inc., Chapel Hill, NC.
To quantify the presence of SOX7 associated with hemidesmosomes, proximity ligation assays were used to determine if SOX7 was associated with integrin β4 (<40 nm) within the basal layer of the long-term LLP individuals. This particular technique allows for the localization of SOX7- integrin β4 interactions at the single-molecule level in skin specimens. The proximity ligation assays showed that SOX7 associated quite well with integrin β4 along the basement membrane of the epidermis (shown as green puncta in Figure 4C). Generally, the entire epidermal area had statistically significant fewer associations of SOX7-integrin β4 per µm of basement membrane in the LLP+ compared to the unexposed control (P<0.005) (Figure 4D). When the rete ridge areas and arch areas were evaluated separately, both regions maintained a statistically significant reduction in SOX7-integrin β4 interactions compared to paired controls (P<0.05). There were no differences in SOX7-integrin β4 associations in LLP- samples compared to their respective controls. These results mirrored observations seen in Figure 4B, which seem to suggest a reduction of all targets of interest in the rete ridge and arch areas. Consequently, the proximity ligation assays also indicated that SOX7-integrin β4 associations were indeed denser in arch areas compared to rete ridge areas (Figure 4D). These data suggest that some leakiness might be occurring at the basement membrane and that fragility of hemidesmosomes in basal keratinocytes may be a contributing factor in the LLP phenomenon.
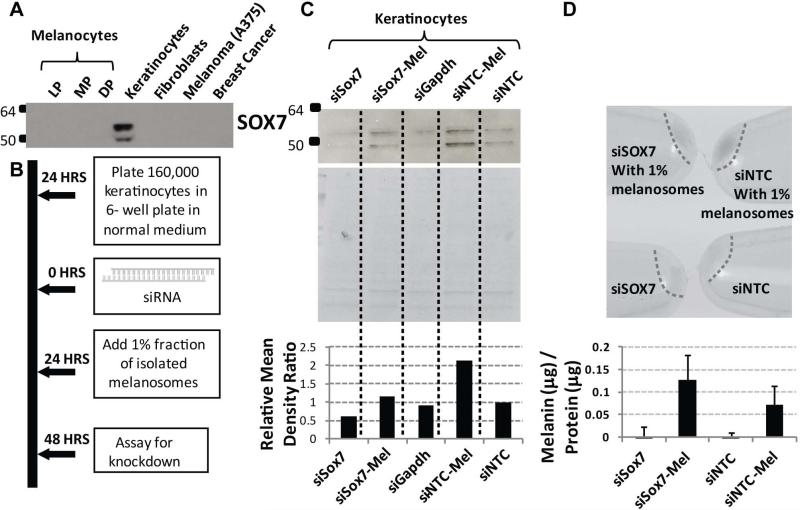
siRNA knockdown of SOX7 and effect on melanosome uptake
SOX7 was found to be specifically expressed by keratinocytes and is not expressed by human melanocytes (HEMn-LP, -MP, -DP), by human fibroblasts (Fb), by human melanoma cells (A375) or by breast cancer cells (T47D) (Figure 5A). To further evaluate if the attenuated SOX7 expression in LLP+ specimens had impaired melanosome uptake, a knockdown of SOX7 in primary human keratinocytes was evaluated. siRNA knockdown of SOX7 and NTC (non-targeting control) of keratinocytes was carried out and at 24 hours purified melanosomes were added to the culture (Figure 5B). SOX7 protein expression by keratinocytes was significantly reduced (Figure 5C). When normalized to the non-targeting control (siNTC), siSOX7 showed a ~40% reduction in SOX7, however this effect was reversed by the addition of melanosomes (Figure 5C). The cell pellets were darker for keratinocytes treated with melanosomes when measured spectrophotometrically against a known melanin standard, more specifically the siNTC pellet (Figure 5D). There are several limitations of evaluating keratinocytes in this 2D assay. Specifically, this in vitro tissue culture system (1) cannot fully model the basement membrane with rete ridge and arch features found in human skin and (2) does not represent keratinocytes with mature hemidesmosomes. Consequently, these results are only suggestive of a potential effect on melanosome uptake and would have to be further evaluated in a more robust model system.
Figure 5.
SOX7 knockdown indicates the potential to increases melanosome uptake by keratinocytes. (A) Western blot showing that SOX7 protein is expressed by human keratinocytes but not by human melanocytes (Lightly Pigmented: LP, Moderately Pigmented: MP, Darkly Pigmented: DP), fibroblasts, melanoma cells (A375) or breast cancer cells (T47D). (B) Workflow for SOX7 silencing and melanosome uptake assay by primary human keratinocytes. (C) Immunoblot, and corresponding Coomassie blue stained gel to show loading, indicate an effective knockdown of SOX7 as indicated by densitometry (NTC: Non-targeting control, Mel: with 1% melanosomes added). (D) Cell pellets after protein extraction show melanosome uptake following SOX7 knockdown. Tissue culture experiments were done on 3 separate occasions in duplicate, while the melanosome uptake assay was done on 2 separate occasions in duplicate. No significant differences detected.
Transmission electron microscopy of hemidesmosomes
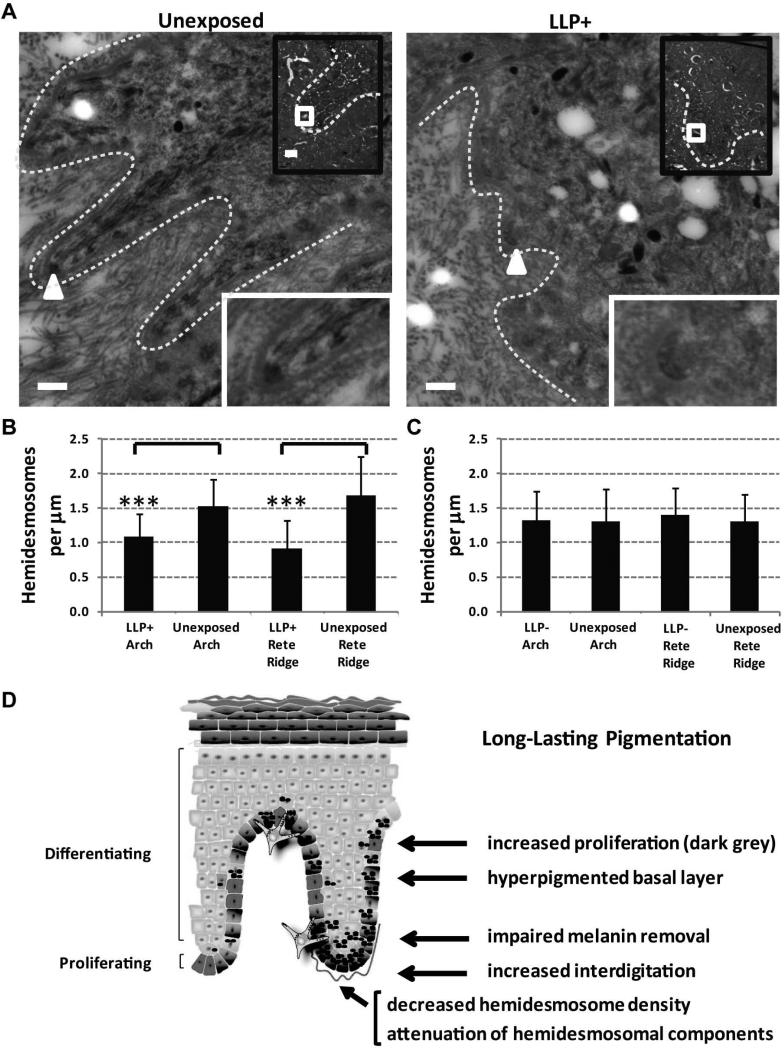
To further evaluate the integrity of the basement membrane in rete ridge areas versus arch areas, transmission electron microscopy was used to quantify the number of hemidesmosomes in long-term LLP specimens. The rete ridge areas quantified for the LLP+ specimen and its unexposed control are shown along the basement membrane area (Figure 6A). The number of hemidesmosomes on the basement membrane was significantly reduced (P<0.0005) in LLP+ rete ridge and arch areas versus the untreated corresponding areas (Figure 6B). The LLP- individuals showed no statistically significant differences in both rete ridge and arch areas of UV-treated and untreated areas (Figure 6C). Although significant, these results need to be considered with caution since only two LLP+ individuals versus two LLP- individuals could be analyzed by TEM given the sample limitations. Taken together, the SOX7 microarray results, the SOX7 long-term LLP expression patterns, the increased interdigitation index and the increased proliferation responses in long-term LLP+ specimens seem to suggest that hemidesmosome homeostasis may be affected in LLP+ individuals leading to long-term melanin retention in the basal layer of the epidermis.
Figure 6.
Electron microscopy highlights differences in hemidesmosome density in LLP+ individuals contributing to the UV-induced hyperpigmentation model for LLP. (A) Electron micrographs of long-term LLP+ specimens (#S100) depict rete ridge areas (white lines) within the epidermis as visualized in the black box insets. The white squares in the insets indicate regions of interest shown in the larger images. Hemidesmosomes are indicated by white arrowheads along the epidermal-dermal junction (white dashed line) and are further magnified 10X in the insets at the bottom right. Large image scale bars = 0.5 μm and small black box inset image scale bars = 5 μm. (B) Hemidesmosome densities in LLP+ individuals and (C) LLP- individuals were quantified for rete ridge and arch areas of UV-treated and adjacent unexposed controls. * = P<0.05, ** = P<0.005, *** = P<0.0005 compared to the control. (D) Scheme for UV-induced hyperpigmentation for LLP development highlighting spatial modifications within the skin. Scheme manually created using available shapes in Microsoft PowerPoint, Redmond, WA and the add-in ScienceSlides, VisiScience, Inc., Chapel Hill, NC.
Discussion
Long-term evaluation of single UV exposures of human skin has not been previously reported. Interestingly, there was no increase in melanocyte density or melanocyte proliferation with very little to no melanin in proliferating keratinocytes. Instead, the proposed UV-induced hyperpigmentation model for LLP (shown schematically in Figure 6D) suggests that this phenomenon may be caused by impaired melanin removal by keratinocytes leading to a hyperpigmented basal layer. There is a compensatory mechanism by other normally cycling keratinocytes in the basal layer to maintain normal skin homeostasis which may lead to a slight increase in interdigitation. In addition, the decreased hemidesmosome density due to the attenuation of hemidesmosomal components suggests a plasticity at the basement membrane potentially caused by the increases in ridge formation. Taken together, these long-term morphological changes after a single UV-induced sunburn seem to all play a role in the LLP phenomenon (Figure 6D). If indeed LLP development is a prelude to age spot formation, and since age spots are significantly associated with precancerous lesion formation [3], then the long-term LLP data of a single sunburn-inducing UV dose are compelling enough to warrant further exploration.
It is known that very subtle changes in melanin content can produce dramatic differences in visible skin color [26]. The increased visual pigmentation along with the histopathological features of a hyperpigmented basal layer and the increased interdigitation highlight the similarities of this long-term phenomenon with other forms of UV-induced hyperpigmentation, i.e. age spots or solar lentigines. Normal benign nevi also present with a hyperpigmented basal layer, but do not involve dermal solar elastosis or form the club-shaped rete ridges typically found in solar lentigines. This suggests that other etiological factors are involved in the hyperpigmented macule formation of nevi. In familial skin cancer, there are increased numbers of nevi present in family members who develop melanoma [27]. Generally, the total number of nevi increases an individual's risk for skin cancer [28,29], and the details of that relationship continue to be investigated [30,31].
The involvement of SOX7 at the epidermal-dermal junction may be linked to the impaired removal of melanin. The fact that the literature for SOX7 is suggestive of its role in destabilizing hemidesmosome formation [19] with a clinical manifestation of a skin blistering disease, epidermolysis bullosa simplex (EBS) [32], was particularly interesting. In some cases of EBS, hyperpigmented macules develop on the skin [33], which raises provocative questions about the basement membrane plasticity. The attenuation of SOX7 in long-term LLP+ specimens and of other hemidesmosomal components (integrin β4 and plectin) point to a slight fragility in the basement membrane that may provide the flexibility necessary to increase interdigitation to maintain the normal cycling of keratinocytes in LLP+ areas. The electron microscopic analysis was very provocative given the decreased hemidesmosome density displayed in rete ridge areas and arch areas. A reduction in hemidesmosome density along the basement membrane has also been documented in mice harboring a plectin mutation leading to a form of EBS [34]. The knockdown of SOX7 in primary human keratinocytes showed elevated melanosome uptake ability which correlates well with the attenuation of SOX7 in the hyperpigmented basal layer. In vitro data suggest that melanosomes inhibit keratinocyte proliferation and that melanosome uptake is increased in calcium treated keratinocytes [35]. This is particularly interesting since SOX7 is not expressed in the suprabasal keratinocyte layers in normal skin where calcium levels would be higher in terminally differentiating keratinocytes. This suggests that the SOX7 knockdown experiment showing increased melanosome uptake may be relevant to the physiological situation.
The involvement of the SOX family of proteins with melanocyte function was first discovered in mice with a truncation in SOX10 which led to a hypopigmentary phenotype and later became a model for Waardenburg-Shah syndrome type 4 [36]. It has been shown in human colon carcinoma cells that the C-terminal region with a 9 amino acid conserved motif allows SOX subgroup members, SOX7 and SOX17, to bind β-catenin [37,38]. If SOX subgroup F family members can attenuate proliferation spatially, as has been indicated in the repression of Drosophila Wg (Wnt) [39], a similar process of SOX-β-catenin/WNT signaling might be occurring regionally in the basal layer of the epidermis [38]. Interestingly, our extensive evaluation of top upstream regulators predicted β-catenin to be activated upstream of SOX7 after the repetitive UVB treatment (Table S6).
Taken together, the LLP phenomenon seems to provide early longitudinal information into other UV-induced hyperpigmentary lesions such as solar lentigines and merits further inquiry with respect to the role of SOX7 at the epidermal-dermal junction. This hyperpigmentation phenomenon may lead to increased interdigitation in order to maintain normal skin homeostasis in LLP+ individuals. These data provide a catalyzing framework upon which to better understand the formation of elongated rete ridges in solar lentigines, which are a known risk factor for precancerous lesions.
Supplementary Material
Acknowledgements
This research was supported in part by the Office of Science and the Center for Devices and Radiological Health, Food and Drug Administration, and in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. We would like to acknowledge Professors Ito and Wakamatsu for completing the melanosome melanin analysis.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Statement of author contributions
SGC conceived, performed and analyzed all immunohistochemistry, tissue culture and siRNA knockdown experiments; SGC analyzed all data and wrote the manuscript. SGC and JCV performed the siRNA knockdown experiments. SGC and LY analyzed the microarray dataset and performed statistical analyses. GZ carried out the EM processing and imaging. SGC and PML were involved in the design of the in vitro experiments. LK, VJH, SAM and JZB were involved in the clinical studies concept and design. SGC, SAM and JZB were involved in long-term clinical study coordination, irradiation and specimen acquisition for histology. CS and AM were involved in the short-term clinical study coordination and specimen acquisition for microarray and histology. All authors were involved in editing the paper and had final approval of the submitted and published versions.
References
- 1.Brooke MA, Nitoiu D, Kelsell DP. Cell-cell connectivity: desmosomes and disease. J Pathol. 2012;226:158–71. doi: 10.1002/path.3027. [DOI] [PubMed] [Google Scholar]
- 2.Sherratt MJ, Bayley CP, Reilly SM, et al. Low-dose ultraviolet radiation selectively degrades chromophore-rich extracellular matrix components. J Pathol. 2010;222:32–40. doi: 10.1002/path.2730. [DOI] [PubMed] [Google Scholar]
- 3.Bastiaens M, Hoefnagel J, Westendorp R, et al. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004;17:225–9. doi: 10.1111/j.1600-0749.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 4.Coelho SG, Zhou Y-C, Bushar HF, et al. Insights into UV-induced long-lasting pigmentation (LLP) in human skin. In: Jimbow K, editor. Proc. XXth Intl. Pigment Cell Conf. Medimond; Bologna, Italy: 2008. pp. 137–41. [Google Scholar]
- 5.Coelho SG, Zhou Y, Bushar HF, et al. Long-lasting pigmentation of human skin, a new look at an overlooked response to UV. Pigment Cell Melanoma Res. 2009;22:238–41. doi: 10.1111/j.1755-148X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadokoro T, Yamaguchi Y, Batzer J, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–32. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi W, Miyamura Y, Wolber R, et al. Regulation of human skin pigmentation in situ by repetitive UV exposure: molecular characterization of responses to UVA and/or UVB. J Invest Dermatol. 2010;130:1685–96. doi: 10.1038/jid.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res. 2011;24:136–47. doi: 10.1111/j.1755-148X.2010.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner M, Coelho SG, Beer JZ, et al. Long-lasting molecular changes in human skin after repetitive in situ UV irradiation. J Invest Dermatol. 2009;129:1002–11. doi: 10.1038/jid.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura R, Tsukamoto K, Harada K, et al. Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: role of SCF/KIT protein interactions and the downstream effector, MITF-M. J Pathol. 2004;202:463–75. doi: 10.1002/path.1538. [DOI] [PubMed] [Google Scholar]
- 11.Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21:487–91. doi: 10.1111/j.1755-148X.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haywood RM, Lee M, Andrady C. Comparable photoreactivity of hair melanosomes, eu- and pheomelanins at low concentrations: low melanin a risk factor for UVA damage and melanoma? Photochem Photobiol. 2008;84:572–81. doi: 10.1111/j.1751-1097.2008.00343.x. [DOI] [PubMed] [Google Scholar]
- 13.Phillipson RP, Tobi SE, Morris JA, et al. UV-A Induces Persistent Genomic Instability In Human Keratinocytes Through An Oxidative Stress Mechanism. Free Radical Biology and Medicine. 2002;32:474–80. doi: 10.1016/s0891-5849(01)00829-2. [DOI] [PubMed] [Google Scholar]
- 14.Mouret S, Baudouin C, Charveron M, et al. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA. 2006;103:13765–70. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol B. 2001;63:88–102. doi: 10.1016/s1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 16.Yu L, Venkataraman S, Coleman MC, et al. Glutathione peroxidase-1 inhibits UVA-induced AP-2alpha expression in human keratinocytes. Biochem Biophys Res Commun. 2006;351:1066–71. doi: 10.1016/j.bbrc.2006.10.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passeron T, Valencia JC, Bertolotto C, et al. SOX9 is a key player in ultraviolet B-induced melanocyte differentiation and pigmentation. Proc Natl Acad Sci U S A. 2007;104:13984–9. doi: 10.1073/pnas.0705117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de JJ, Stoop H, Gillis AJ, et al. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J Pathol. 2008;215:21–30. doi: 10.1002/path.2332. [DOI] [PubMed] [Google Scholar]
- 19.Oommen S, Francois M, Kawasaki M, et al. Cytoplasmic plaque formation in hemidesmosome development is dependent on SoxF transcription factor function. PLoS ONE. 2012;7:e43857. doi: 10.1371/journal.pone.0043857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morais da SS, Hacker A, Harley V, et al. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–8. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 21.Malki S, Boizet-Bonhoure B, Poulat F. Shuttling of SOX proteins. Int J Biochem Cell Biol. 2010;42:411–6. doi: 10.1016/j.biocel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Eger A, Stockinger A, Wiche G, et al. Polarisation-dependent association of plectin with desmoplakin and the lateral submembrane skeleton in MDCK cells. J Cell Sci. 1997;110(Pt 11):1307–16. doi: 10.1242/jcs.110.11.1307. [DOI] [PubMed] [Google Scholar]
- 23.Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208–16. doi: 10.1038/35043032. [DOI] [PubMed] [Google Scholar]
- 24.Castanon MJ, Walko G, Winter L, et al. Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem Cell Biol. 2013;140:33–53. doi: 10.1007/s00418-013-1102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buijsrogge JJ, de Jong MC, Kloosterhuis GJ, et al. Antiplectin autoantibodies in subepidermal blistering diseases. Br J Dermatol. 2009;161:762–71. doi: 10.1111/j.1365-2133.2009.09206.x. [DOI] [PubMed] [Google Scholar]
- 26.Beer JZ, Hearing VJ. Skin color, melanin, race/ethnicity and UV-induced DNA damage. In: Giacomoni PU, editor. Biophysical and Physiological Effects of Solar Radiation on Human Skin. Royal Society of Chemistry; Cambridge: 2007. pp. 99–126. [Google Scholar]
- 27.Clark WH, Jr., Reimer RR, Greene M, et al. Origin of familial malignant melanomas from heritable melanocytic lesions. ‘The B-K mole syndrome’. Arch Dermatol. 1978;114:732–8. [PubMed] [Google Scholar]
- 28.Chin L. The genetics of malignant melanoma: lessons from mouse and man. Nat Rev Cancer. 2003;3:559–70. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- 29.Richmond-Sinclair NM, van der Pols JC, Green AC. Melanocytic naevi and basal cell carcinoma: is there an association? J Eur Acad Dermatol Venereol. 2012;26:1092–6. doi: 10.1111/j.1468-3083.2011.04213.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhu G, Duffy DL, Eldridge A, et al. A major quantitative-trait locus for mole density is linked to the familial melanoma gene CDKN2A: a maximum-likelihood combined linkage and association analysis in twins and their sibs. Am J Hum Genet. 1999;65:483–92. doi: 10.1086/302494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu G, Montgomery GW, James MR, et al. A genome-wide scan for naevus count: linkage to CDKN2A and to other chromosome regions. Eur J Hum Genet. 2007;15:94–102. doi: 10.1038/sj.ejhg.5201729. [DOI] [PubMed] [Google Scholar]
- 32.Kopecki Z, Arkell RM, Strudwick XL, et al. Overexpression of the Flii gene increases dermal-epidermal blistering in an autoimmune ColVII mouse model of epidermolysis bullosa acquisita. J Pathol. 2011;225:401–13. doi: 10.1002/path.2973. [DOI] [PubMed] [Google Scholar]
- 33.Schumann H, Kiritsi D, Pigors M, et al. Phenotypic spectrum of epidermolysis bullosa associated with alpha6beta4 integrin mutations. Br J Dermatol. 2013;169:115–24. doi: 10.1111/bjd.12317. [DOI] [PubMed] [Google Scholar]
- 34.Walko G, Vukasinovic N, Gross K, et al. Targeted proteolysis of plectin isoform 1a accounts for hemidesmosome dysfunction in mice mimicking the dominant skin blistering disease EBS-Ogna. PLoS Genet. 2011;7:e1002396. doi: 10.1371/journal.pgen.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi HI, Sohn KC, Hong DK, et al. Melanosome uptake is associated with the proliferation and differentiation of keratinocytes. Arch Dermatol Res. 2014;306:59–66. doi: 10.1007/s00403-013-1422-x. [DOI] [PubMed] [Google Scholar]
- 36.Harris ML, Baxter LL, Loftus SK, et al. Sox proteins in melanocyte development and melanoma. Pigment Cell Melanoma Res. 2010;23:496–513. doi: 10.1111/j.1755-148X.2010.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinner D, Kordich JJ, Spence JR, et al. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27:7802–15. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dichtel-Danjoy ML, Caldeira J, Casares F. SoxF is part of a novel negative-feedback loop in the wingless pathway that controls proliferation in the Drosophila wing disc. Development. 2009;136:761–9. doi: 10.1242/dev.032854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.