Abstract
Background
The 2010 WHO classification recommends that pancreatic neuroendocrine tumors should be graded based on mitotic rate and Ki67 index, with grade 2 (G2) pancreatic neuroendocrine tumor (PanNET) defined as having a mitotic rate of 2–20 mitotic figures/10 high power fields (HPF) or a Ki67 index of 3–20%. Grade 3 (G3) pancreatic neuroendocrine carcinoma (NEC) is defined as having >20 mitotic figures/10 HPF or a Ki67 index of >20%. However, some PanNETs show discordance between the mitotic rate and Ki67 index, usually having a Ki67 index in the G3 range but a mitotic rate suggesting G2, prompting us to examine the clinical significance of the Ki67 index in a large series of clinically well characterized mitotic G2 PanNETs.
Design
Mitotic G2 well differentiated PanNETs, surgically resected at our institutions were reviewed. Of those, 19 cases had a Ki67 >20% and were selected as the study group of grade-discordant (mitotic count G2/Ki67 index G3) PanNETs. For comparison, 53 grade-concordant (both mitotic count and Ki67 index G2) PanNETs matched for presenting stage with the discordant group as well as 43 morphologically poorly differentiated (either small cell or large cell type) pancreatic NECs were also included. The percentage of Ki67 positive neoplastic cells was quantified by manual counting of at least 500 cells on printed photographic images of “hot spots”.
Results
The mean Ki67 index for grade-concordant and grade-discordant PanNETs and poorly differentiated NECs were 8.1% (range, 3–20), 40% (range, 24–80%) and 70% (range, 40–98), respectively. Overall, patients with grade-discordant PanNETs had significantly longer survival time compared to the patients with poorly differentiated NEC (median survival of 54.1 months vs 11 months and 5-year survival of 29.1% vs 16.1%; p=0.002). Also, the survival time of the patients with grade-discordant PanNETs was shorter than that of the patients with grade-concordant PanNETs (median survival of 67.8 months and 5-year survival of 62.4%); however, the difference was not statistically significant (p=0.2).
Conclusion
Our data support the notion that the mitotic rate and Ki67 index-based grades of PanNETs can be discordant, and when the Ki67 index indicates G3, the clinical outcome is slightly worse. More importantly, we demonstrate that well differentiated PanNETs that are G3 by Ki67 are significantly less aggressive than bona fide poorly differentiated NECs, suggesting that the current WHO G3 category is heterogeneous, contains two distinct neoplasms, and can be further separated into well differentiated PanNET with an elevated proliferation rate and poorly differentiated NEC.
Keywords: Pancreas, neuroendocrine tumor, neoplasm, carcinoma, grade, mitotic activity, Ki-67 antigen
BACKGROUND
A grading system for all gastroenteropancreatic neuroendocrine tumors (NETs) based on mitotic count and Ki67 labeling index was proposed in 2007 by the European Neuroendocrine Tumor Society (ENETS) 1. This system has since been modified and is now incorporated into the 2010 World Health Organization (WHO) classification of tumors of the digestive system, which divides neuroendocrine neoplasms of the tubular gastrointestinal tract, along with their pancreatic counterparts, into grade 1 (G1) and grade 2 (G2) NETs and grade 3 (G3) neuroendocrine carcinoma (NEC) 2. G1 NETs have <2 mitotic figures/10 high power fields (HPF) and a Ki67 labeling index of <3%, while G2 NETs have 2–20 mitotic figures/10 HPFs or a Ki67 labeling index of 3–20%. G3 NECs are defined by >20 mitotic figures/10 HPFs or a Ki67 labeling index >20% 2.
The mitotic rate and Ki67 index of a PanNET are not always concordant. The 2010 WHO classification recommends that when mitotic rate and Ki67 labeling index indicate different grades, then the higher of the two should be used to assign the grade. This recommendation is supported, at the lower end of the grading spectrum (G1/G2), by a recent study, which demonstrated that patients with grade-discordant (mitotic count G1/Ki67 index G2) PanNETs have larger tumors with more aggressive histologic features, more metastases, and shorter overall survival (12 years vs 16.7 years; p <0.01), compared with patients with grade-concordant (both mitotic count and Ki67 index G1) PanNETs 3. However, there is no study that has systematically assessed the clinical behavior of grade-discordant neuroendocrine tumors at the high-grade end of the spectrum (G2/G3).
In addition, tumor differentiation based on morphology is not explicitly considered in the 2010 WHO classification system. The terminology of “neuroendocrine carcinoma” for G3 neoplasms implies that they are histologically poorly differentiated, but some morphologically well differentiated NETs also have proliferative rates (usually the Ki67 index) that meet the threshold for G3 NECs. Also, a recent landmark study has shown that G3 NECs with a Ki67 index less than 55% do not respond to platinum-based chemotherapy, in contrast to G3 NECs with a Ki67 index greater than 55% 4, supporting the concept that the current WHO G3 category is heterogeneous, and the tumors at the lower end of the G3 range are in fact well differentiated NETs with an elevated proliferative rate (or “high-grade, well differentiated neuroendocrine tumors”).
The purpose of this study is to asses clinicopathologic features and survival outcomes of morphologically well differentiated PanNETs that are G2 by mitotic count but G3 by Ki67 index, and to compare these grade-discordant tumors to grade-concordant G2 PanNETs as well as to bona fide poorly differentiated (small cell or large cell type) pancreatic NECs.
MATERIALS AND METHODS
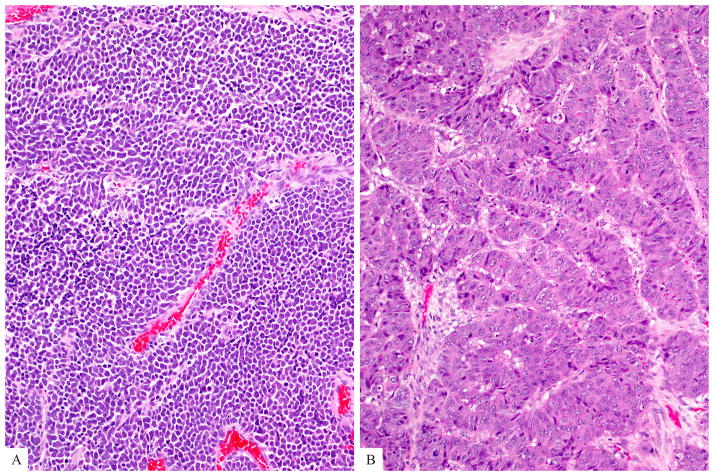
The surgical pathology databases of our institutions (including University of Pittsburgh Medical Center where cases were collected by A.M.K) were searched for cases with a diagnosis of well differentiated pancreatic neuroendocrine tumor/neoplasm/carcinoma from 1982 to 2013 and those with a mitotic rate ≥ 2/10 HPF, who had available clinical follow-up information, were identified. Within this group, 19 cases (all resections) had a Ki67 >20% and were selected as the study group of grade-discordant (mitotic count G2/Ki67 index G3) well differentiated PanNETs. To determine whether this discordance in mitotic grade and Ki67 index was significant, clinicopathologic features, including survival, of these grade-discordant PanNETs were contrasted with those of 53 grade-concordant (both mitotic count and Ki67 index G2) well differentiated PanNETs (all resections) matched for the presenting stage with the grade-discordant group as well as 43 morphologically poorly differentiated (either small cell or large cell type, Figures 2a and 2b) NECs (all resections). Detailed clinicopathologic features of the latter have been reported previously 5.
Figure 2.
Poorly differentiated neuroendocrine carcinoma (a) Small cell type is composed of relatively small tumor cells with a high nucleus-to-cytoplasm ratio, hyperchromatic nuclei, and nuclear molding. (b) Large cell type is characterized with cells that are often round to polygonal, and the nuclei have either vesicular chromatin or prominent nucleoli. Note multiple mitotic figures.
Available medical records including pathology reports were reviewed to obtain clinical data including age, gender, associated genetic syndromes, clinical functionality, and distant metastases (present at the time of resection or subsequently). All the H&E stained slides were then reassessed using the diagnostic and grading criteria put forth by the 2010 WHO classification of neuroendocrine neoplasms of the digestive system 2. The number of the tumor slides per case ranged between 5 and 18. The following histopathologic information was also re-evaluated: tumor size; lymphovascular and perineural invasion; extension into the peripancreatic soft tissue, duodenal wall, or other adjacent organs; margin status; and number of total and metastasis-containing lymph nodes.
The mitotic rate of each case was determined by counting mitotic figures in 50 high power fields (at 400× on an Olympus microscope), expressed as the rate per 10 high power fields.
A representative formalin-fixed paraffin-embedded tissue section of each tumor was immunolabeled using the standard avidin-biotin peroxidase method with an antibody against Ki67/MIB1 (DakoCytomation, Carpinteria, CA), using tonsillar tissue as the positive control. Each Ki67-labeled tumor slide was scanned at 10× to identify the most intensely labeling regions (“hot spots”). The hot spot was photographed at 20×, printed, and Ki67-positive and negative tumor cells were then visualized, manually counted and crossed off, using 2 different color markers as described previously 6. At least 500 tumor cells were counted (O.B.), non-neoplastic nuclei not included, and a labeling index was calculated: % Ki67 = positive nuclei / total nuclei.
All tumors were staged following the criteria defined by the 7th edition American Joint Cancer Committee (AJCC) cancer staging manual 7. Overall survival data were obtained from hospital records or the United States Social Security Death Index. Causes of death for determining disease-specific survival (DSS) were obtained from the National Death Index (National Center for Health Statistics, Hyattsville, MD), supplemented with medical chart review in cases for which a specific cause of death was not available in the National Death Index. Survival analyses were performed using the Kaplan-Meier method and log-rank (Mantel-Cox) comparison, with StatView software version 5.0 (SAS Institute, Cary, NC) and / or MedCalc version 13.1.2. (MedCalc Software bvba, Ostend, Belgium).
RESULTS
Clinical Features
The age and sex of the 21 patients with grade-discordant PanNETs (mean age = 54; male to female ratio = 1.7) were similar to those of the 53 patients with grade-concordant G2 PanNETs (mean age = 55; male to female ratio = 1.7) and those of the 43 patients with poorly differentiated NECs (mean age = 59; male to female ratio = 1.3); p=0.16 [one-way ANOVA for age] and p=0.88 [Chi-squared test for sex], respectively. In terms of the associated genetic syndromes; only four patients with PanNETs (one from grade-discordant group, three from grade-concordant group) had known MEN1 or von Hippel-Lindau syndromes (Table 1). None of the patients with poorly differentiated NEC had a known genetic syndrome except for one patient who carried a germline BRCA1 mutation.
Table 1.
Comparison of clinicopathologic characteristics
| Grade Concordant (Mitotic G2/Ki67 G2) PanNETs (n=53) | Grade Discordant (Mitotic G2/Ki67 G3) PanNETs (n=19) | Poorly Differentiated NECs (n=43) | P value | |
|---|---|---|---|---|
|
| ||||
| Mean Age (years) (range) | 55 (29–80) | 54 (25–84) | 59 (21–82) | 0.16 |
|
| ||||
| Sex (M:F) | 1.7 | 1.7 | 1.3 | 0.88 |
|
| ||||
| Syndromic | Multiple Endocrine Neoplasia 1 (n=2) Von Hippel-Lindau (n=1) |
Von Hippel-Lindau (n=1) | 0 | - |
|
| ||||
| Functional | Gastrinoma (n=1) Insulinoma (n=1) |
Ectopic ACTH (n=1) | 0 | - |
|
| ||||
| Location (head vs body or tail) | 25/28 | 6/13 | 26/14 | 0.04 |
|
| ||||
| Median tumor size (cm) (range) | 4.3 (1.2–17) | 4.5 (2–15) | 4.1 (2–18) | 0.20 |
|
| ||||
| Vascular invasion | 44/50 (88%) | 16/17 (94%) | 23/29 (79%) | 0.28 |
|
| ||||
| Perineural invasion | 29/44 (66%) | 8/16 (50%) | 20/28 (71%) | 0.35 |
|
| ||||
| pT | - | |||
| pT1 | 4 (8%) | 0 (0%) | 1 (2.5%) | |
| pT2 | 4 (8%) | 1 (6%) | 2 (5%) | |
| pT3 | 42 (84%) | 16 (94%) | 35 (92%) | |
|
| ||||
| LN metastasis | 27/52 (52%) | 8/17 (47%) | 34/40 (85%) | 0.79* 0.004** |
|
| ||||
| Distant metastasis | 25/36 (69%) | 10/15 (67%) | 11/25 (44%) | 1.0* 0.12** |
Grade-discordant PanNETs vs grade-concordant PanNETs
Grade-discordant PanNETs vs poorly differentiated NECs
Of those with available data, one grade-discordant (ectopic ACTH production) and two grade-concordant (1 gastrinoma, 1 insulinoma) PanNETs were functional. None of the poorly differentiated NECs were functional (Table 1).
Pathologic Features
The grade-discordant PanNETs, similar to the grade-concordant PanNETs, were located more common in the body or tail. In contrast, the majority of poorly differentiated NECs were located in the head the pancreas (p=0.04, Chi-squared test). The median tumor size of the grade-discordant PanNETs (4.5 cm) was similar to that of the grade-concordant PanNETs (4.3 cm) and of the poorly differentiated NECs (4.1 cm; p=0.78, one-way ANOVA).
Despite different growth patterns (diffuse, nested, trabecular, etc), overall morphology, cytologic features and distribution of mitotic figures were uniform throughout the tumors of grade-discordant group. Thus, no evidence of different grades within one tumor (i.e. tumor progression) was identified).
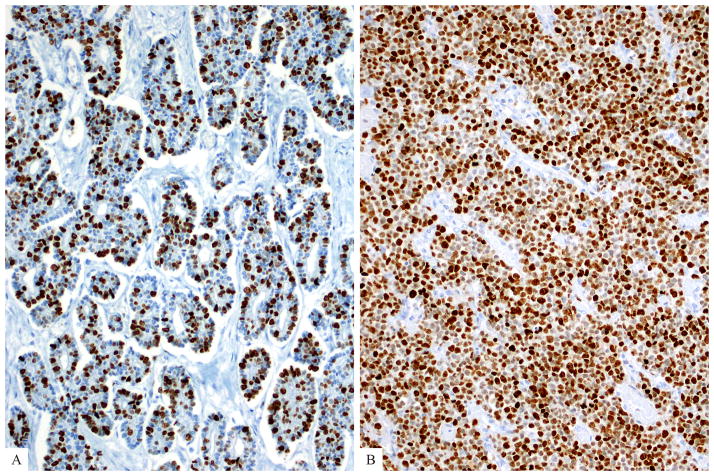
Average mitotic rates (per 10 HPF) as well as average Ki67 indices of grade-discordant PanNETs (Figure 3a) were higher than those of grade-concordant PanNETs (7.6/10 HPF vs 3.5/10 HPF; and 40% vs 8.1%), but only the latter reached statistical significance (p<0.05, ANOVA post-hoc Tukey-Kramer test). Of note, although the majority (71%) of grade-discordant PanNETs had ≥ 30% Ki67 index, only 2 had > 55% Ki67 index. However, the poorly differentiated NECs had much higher average mitotic rate and a higher average Ki67 index (Figure 3b) than the WHO-recommended minimum thresholds for the G3 category (51/10 HPF and 74% for small cell type and 37/10 HPF and 66% for large cell type) (Table 2).
Figure 3.
(a) Average Ki67 proliferation index of grade-discordant pancreatic neuroendocrine tumors was 40% (as opposed to 74% of small cell type and 66% of large cell type poorly differentiated neuroendocrine carcinomas). (b) A small cell carcinoma with Ki67 proliferation index of >95% is depicted here.
Table 2.
Comparison of mitotic rate and Ki67 proliferation index
| Grade Concordant (Mitotic G2/Ki67 G2) PanNETs (n=53) | Grade Discordant (Mitotic G2/Ki67 G3) PanNETs (n=19) | Poorly Differentiated NECs (n=43) | ||
|---|---|---|---|---|
| Small Cell Type (n=16) | Large Cell Type (n=27) | |||
| Average Mitotic Rate (10HPF) (range) | 3.5 (2–10) | 7.6 (2–20) | 51 (21–92) | 37 (21–83) |
| Average Ki67 index (%) (range) | 8.1 (3–20) | 40 (24–80) | 74 (50–98) | 66 (40–95) |
The majority of the neoplasms extended beyond the pancreas (pT3), usually into the peripancreatic soft tissue but also into the duodenum, portal vein, splenic vessels and spleen [94% of grade-discordant PanNETs, 84% of grade-concordant PanNETs, and 92% of poorly differentiated NECs (Table 1)].
There was no significant difference among the three groups in terms of prevalence of lymphovascular invasion and perineural invasion (Table 1, p=0.28 and p=0.35, respectively, Chi squared test). However, of cases with known lymph node status, 8 of 17 (47%) grade-discordant PanNETs had lymph node metastases, compared with 27 of 52 (52%) grade-concordant PanNETs (p=0.79, Fisher’s exact test), and 34 of 40 (85%) poorly differentiated NECs (p=0.004, Fisher’s exact test).
Distant metastasis rates were also similar among the three groups: Of cases in which information on distant metastases was available at the time of resection, 10 of 15 (67%) grade-discordant PanNETs had distant metastases, mostly to the liver, compared with 25 of 36 (69%) grade-concordant PanNETs (p=1.0, Fisher’s exact test), and 11 of 25 (44%) poorly differentiated NECs (p=0.12, Fisher’s exact test).
Clinical Course
Follow-up information was available for all patients. At the last follow-up, 10 patients (53%) with grade-discordant PanNET had died of disease. Five patients (26%) were alive with disease, and 4 (21%) were alive with no evidence of disease. Similarly, 26 patients (49%) with grade-concordant PanNET had died of disease, 3 (6%) died of unknown causes or other conditions. Thirteen patients (25%) with grade-concordant PanNET were alive with disease, and 11 (21%) were alive with no evidence of disease. The majority (n=33, 80%) of the patients with poorly differentiated NEC had died of disease. Of note, one patient died of surgical complications, and another patient with disease died of other conditions. Only eight (19%) were alive, and they all had disease.
Although the median survival of the patients with grade-discordant PanNETs was shorter than that of the patients with grade-concordant PanNETs (54.1 vs 67.8 months), the difference was not statistically significant (p=0.2; Table 3). However, the median survival of the patients with grade-discordant PanNETs was significantly longer than that of poorly differentiated NEC (54.1 vs 11 months, p=0.002). Similarly, 2-year and 5-year disease-specific survival rates of the patients with grade-discordant PanNETs were 74.9% and 29.1%, compared with 86.7% and 62.4% for grade-concordant PanNETs, and 22.5% and 16.1% of poorly differentiated NECs, respectively (Table 3 and Figure 4). Of note, when only the patients with distant metastases were analyzed, survival did not differ between groups but our ability to detect differences was limited by the small number of cases (p=0.10 for grade-discordant PanNETs vs grade-concordant PanNETs and p=0.085 for grade-discordant PanNETs vs poorly differentiated NEC; Figure 5).
Table 3.
Comparison of survival (all stages)
| Grade Concordant (Mitotic G2/Ki67 G2) PanNETs (n=53) | Grade Discordant (Mitotic G2/Ki67 G3) PanNETs (n=19) | Poorly Differentiated NECs (n=43) | |
|---|---|---|---|
| Median survival (months) (95% confidence interval) | 67.8 (51.8–93.8) | 54.1 (30.5–117.9) | 11 (6–18) |
| 2-yr survival (mean ± standard error) | 86.7 ± 5.1% | 74.9 ± 11% | 22.5 ± 6.9% |
| 5-yr survival (mean ± standard error) | 62.4 ± 8.3% | 29.1 ± 16% | 16.1 ± 6.3% |
| P value | 0.2 | 0.002 | |
Figure 4.
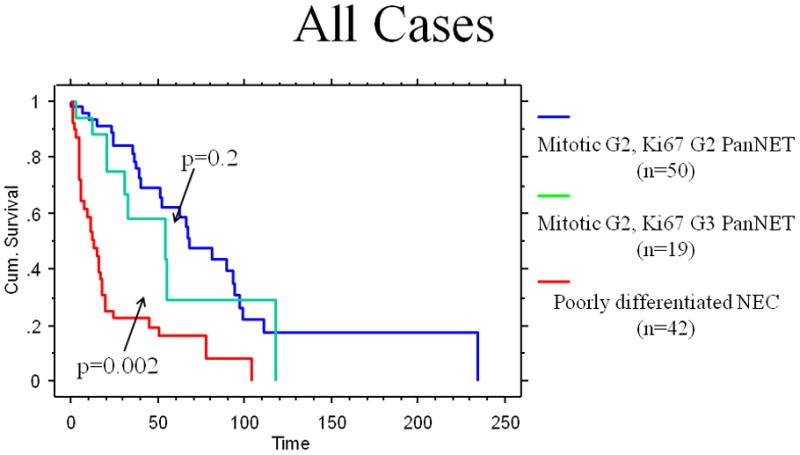
The Kaplan-Meier analysis comparing the overall disease-specific survivals of all grade-concordant pancreatic neuroendocrine tumors, grade-discordant pancreatic neuroendocrine tumors, and poorly differentiated neuroendocrine carcinomas (All cases)
Figure 5.
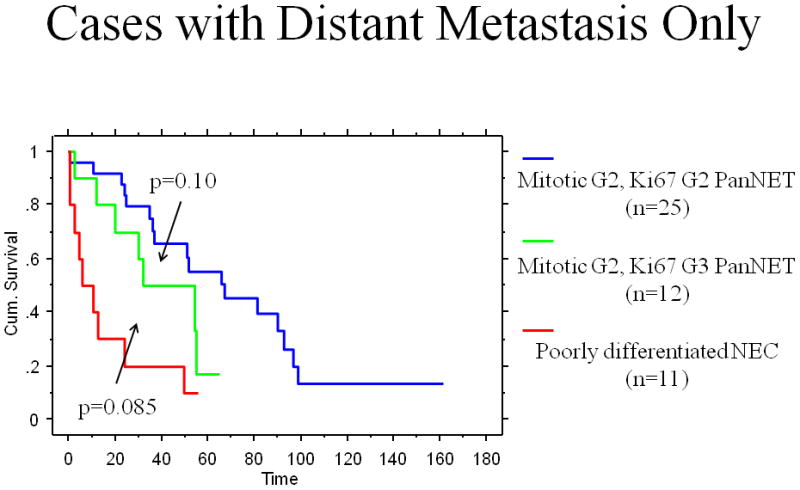
The Kaplan-Meier analysis comparing the overall disease-specific survivals of grade-concordant pancreatic neuroendocrine tumors, grade-discordant pancreatic neuroendocrine tumors, and poorly differentiated neuroendocrine carcinomas (Cases with distant metastasis only)
DISCUSSION
This study further supports the conclusion that the mitotic rate and Ki67 index-based grades of well differentiated pancreatic neuroendocrine tumors (PanNETs) can be discordant. In our series, nineteen cases that would have been G2 by mitotic rate alone were G3 by Ki67 index. It is noteworthy that none of the G2 by mitotic rate cases included in this study had a Ki67 index in the G1 range, further supporting the observation that when there is discordance in grade between the mitotic rate and the Ki67 index, it is the Ki67 index that typically points to the higher grade 3. We also demonstrate that morphologically well differentiated PanNETs that are classified as G3 based on the Ki67 labeling index alone are less aggressive than bona fide poorly differentiated (small cell or large cell type) pancreatic neuroendocrine carcinomas (NECs).
Although the current European Neuroendocrine Tumor Society (ENETS) / WHO classification suggests that G3 NECs are part of a continuum with G1 and G2 NETs, and that grade should be based entirely on proliferation rate, evolving evidence strongly suggests that morphologic differentiation also relevant, and that poorly differentiated NECs should be regarded as a completely separate entity from well differentiated PanNETs. Supporting this concept, it has been shown recently that pancreatic small cell carcinoma and large cell NEC are genetically related entities and that the genetic changes frequently seen in these poorly differentiated carcinomas, such as inactivation of the TP53 and the Rb/p16 pathways, are rarely observed in well differentiated PanNETs 8. Conversely, inactivating mutations in DAXX and ATRX and mutations in MEN1 are exclusively found in well differentiated PanNETs 8. Thus, the molecular genetic distinction of poorly differentiated NECs from well differentiated NETs along with our results confirms the impression that the G3 category actually includes 2 different groups: 1) well differentiated NET with an elevated proliferative rate, and 2) poorly differentiated NEC with small cell or large cell morphology. The former is characterized by a much lower average Ki67 index (40% versus 70%) and its outcome is not as poor as that of poorly differentiated NEC (2- and 5-year survivals of 74.9% and 29.1% vs 22.5% and 16.1%). Furthermore, the mitotic rate of the well differentiated G3 NETs appears to be mostly in the G2 range, although the current study design (in which only discordant cases were selected) does not allow firm conclusions whether some well differentiated PanNETs with a Ki67 index in the G3 range may also have a mitotic rate >20 per 10 HPF.
Supporting these conclusions, the “Nordic NEC Study” found that not all patients with 2010 WHO G3 NEC benefit from platinum-based chemotherapies typically employed for poorly differentiated NECs such as small cell carcinoma. G3 tumors with a Ki67 index less than 55% were less responsive than G3 NECs with a Ki67 index greater than or equal to 55%, although the latter group experienced early recurrence of shorter ultimate survival than the group with a Ki67 in the 20–55% range 4. However, the pathologic features of the tumors in the “Nordic NEC Study” were not evaluated in detail and it is unclear whether these two different groups strictly corresponded to well differentiated NETs and poorly differentiated NECs, respectively. Nevertheless, the current evidence suggests that if careful mitotic counting and immunolabeling for Ki67 are not performed, and if morphologic features (differentiation) are not considered, misclassification can occur, which may have important therapeutic consequences. It must also be acknowledged that neuroendocrine neoplasms exist for which the distinction of well differentiated versus poorly differentiated may be challenging, especially when the nuclear morphology is not that of small cell carcinoma. In some cases, the Ki67 index may fall in the 35–60% range and the morphologic features may also be intermediate. In such cases, evaluation of molecular characteristics may prove helpful to assign the most appropriate diagnosis, but definitive data linking the mutational profile to outcome and response to therapy have yet to be generated.
Other studies have attempted to address the optimal proliferation cut-points to stratify outcome at the lower end of the grading spectrum: one study of 24 patients with PanNETs found that a Ki67 labeling index over 10% predicted lymph node metastases and poorer overall survival, and suggests that a Ki67 index cut-point of 10% is better than 3% as in the 2010 WHO classification 9. Similarly, a larger study of 140 patients with PanNETs found that a Ki67 index of >9% predicted a higher likelihood of disease recurrence and poorer overall survival 10. Currently, however, there are limited options for therapeutic stratification within the well differentiated PanNET group, based on the G1 versus G2 distinction, and it is not clear that sufficient data exist to justify a change in the proliferation cut-points that separate G1 and G2 NETs.
Naturally, all of these findings call into question some aspects of the current grading system. Proposals for modifications to the system are currently being developed, but we believe that the current evidence suggests that mitotic G2, Ki67 G3 well differentiated PanNETs should be distinguished from true poorly differentiated NECs. One option would be to raise the Ki67 index threshold for the diagnosis of poorly differentiated NEC (e.g., from 20% to 50%). However, this change would place a very broad range of proliferative rates in the G2 category, so it is important to determine whether survival differences exist between the grade-concordant (both mitotic count and Ki67 index G2) NETs and the grade-discordant (mitotic count G2/Ki67 index G3) NETs. In our study, the patients with the grade-discordant PanNETs had worse clinical outcome than did patients with grade-concordant PanNETs (median survival of 54.1 months and 67.8 months, respectively) but the difference was not statistically significant. However, the Kaplan-Meier curves show separation between the groups, and the lack of statistical significance may be due to the small number of cases and the inability to accurately correct for variations in extent of disease, despite our attempt to stage-match the cases. Therefore, until additional data are accumulated, we advocate maintaining the separation of the pure G2 NETs from the well differentiated G3 NETs, which we now designate well differentiated NET with an elevated proliferative rate.
It is also noteworthy that the highly aggressive nature of the true poorly differentiated NECs appears to be independent of presenting stage. This group had a worse outcome than the grade-discordant well differentiated PanNETs, despite having a smaller proportion with metastases at presentation (44% vs 71%). However, when only the patients with distant metastasis were analyzed, survival could not be shown to differ statistically between the groups, again being limited by the small number of cases.
In summary, we report that patients with morphologically well differentiated PanNET that would be classified as grade 2 based on mitotic rate, and grade 3 based on Ki67 index have a significantly better clinical outcome than patients with morphologically poorly differentiated NEC. The outcome for patients with grade 2 by mitotic rate, and grade 3 by Ki67 index PanNET also appears to be slightly worse than that of patients with PanNET with concordant grade 2 mitotic rate, and Ki67 index. These findings, combined with the recent introduction of effective targeted therapies 4, suggest that it is not correct to consider all current WHO grade 3 neuroendocrine neoplasms as a single disease entity, and that a modified classification system distinguishing well differentiated neuroendocrine tumors with an elevated proliferative rate from poorly differentiated neuroendocrine carcinomas is needed for more accurate treatment decisions.
Figure 1.
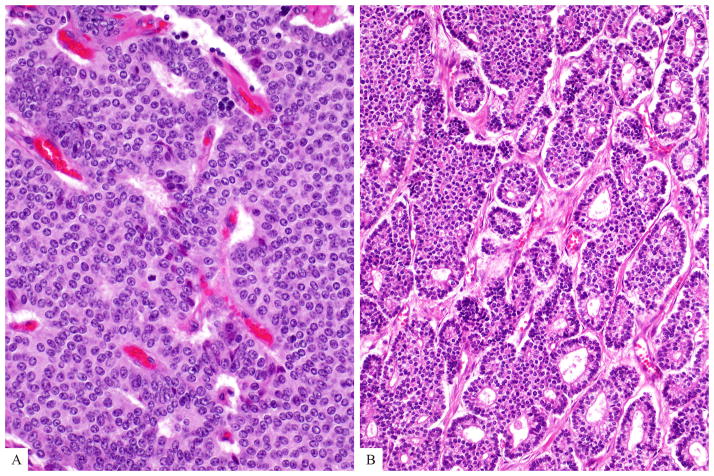
Well differentiated neuroendocrine tumors may reveal different growth patterns (a-diffuse and b-glandular growth patterns are depicted here). The cells vary in size but usually have moderate amount of eosinophilic cytoplasm and nuclei are uniform in size and shape. Mitotic figures are rare (by definition, between 2 and 20 per 10 HPF for Grade 2).
Acknowledgments
Zhaohai Yang has received honoraria from UpToDate, Inc. Ralph H. Hruban, N. Volkan Adsay and David S. Klimstra have a grant from National Institutes of Health (#5P50 CA62924). David S. Klimstra was on a speaker’s bureau for Novartis and has received honoraria from UpToDate. For the remaining authors none were declared.
Footnotes
This study was presented in part at the 102nd annual meeting of the United States and Canadian Academy of Pathology in Baltimore, MD, in March 2013, and supported in part by the National Cancer Institute Specialized Program in Research Excellence (SPORE) CA62924.
References
- 1.Rindi G, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosman FT, et al. WHO classification of tumors of Digestive System. Lyon: WHO Press; 2010. Neuroendocrine tumors of the GI tract. [Google Scholar]
- 3.McCall CM, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671–7. doi: 10.1097/PAS.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorbye H, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 5.Basturk O, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–47. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagci P, et al. Comparative Analysis of Different Counting Methodologies for Ki-67 in Pancreatic Neuroendocrine Tumors (Abstract) Modern Pathology. 2012;25:441A. [Google Scholar]
- 7.Edge S, et al. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 8.Yachida S, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–84. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe K, et al. Ki-67 labeling: a more sensitive indicator of malignant phenotype than mitotic count or tumor size? J Surg Oncol. 2012;106:724–7. doi: 10.1002/jso.23124. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton NA, et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152:107–13. doi: 10.1016/j.surg.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]